Abstract
The Haller index is a ratio of thoracic width and height, measured from an axial CT image and used to describe the internal dimensions of the thoracic cage. Although the Haller index for a normal thorax has been established (Haller et al. 1987; Daunt et al. 2004), this is only at one undefined vertebral level in the thorax. What is not clear is how the Haller index describes the thorax at every vertebral level in the absence of sternal deformity, or how this is affected by age. This paper documents the shape of the thorax using the Haller index calculated from the thoracic width and height at all vertebral levels of the thorax between 8 and 18 years of age. The Haller Index changes with vertebral level, with the largest ratio seen in the most cranial levels of the thorax. Increasing age alters the shape of the thorax, with the most cranial vertebral levels having a greater Haller index over the mid thorax, which does not change. A slight increase is seen in the more caudal vertebral levels. These data highlight that a ‘one size fits all’ rule for chest width and depth ratio at all ages and all thoracic levels is not appropriate. The normal range for width to height ratio should be based on a patient's age and vertebral level.
Keywords: chest wall, CT, Haller index, paediatric, pectus excavatum, sternum, thoracic cage
Introduction
The dimensions of the thoracic cage alter as a child grows, changing in vertical height, transverse diameter and anterior posterior (AP) height.
It is not clear how this growth occurs. The relationship between the width and height of the thoracic cage between the most cranial and the most caudal thoracic levels is not clear. How this relationship alters with increasing age and the difference between males and females with the associated changes of the adolescent growth spurt are not defined.
A method for describing the relationship of thoracic height to width was described by Haller et al. (1987) as the Haller Index, and is a descriptive and decision making tool for the management of pectus excavatum. The index is determined from an axial CT scan slice at the level of maximum deformity in the thorax. It is calculated using the maximal internal thoracic width of the chest divided by the minimal AP height. The latter is measured from the posterior surface of the sternum to the anterior border of the vertebral body at the same level (Fig. 1. These two measurements are always perpendicular to each other even if the sternum is displaced laterally relative to the vertebral column and are measured from bone rather than any overlying soft tissue.
Figure 1.
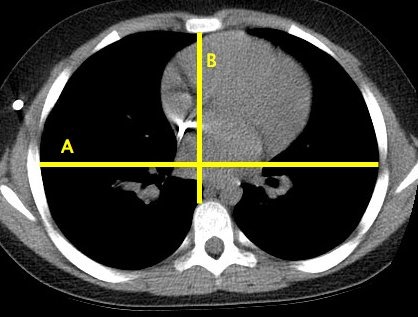
An example of an axial CT image with the measurements required for calculation of the Haller index, the ratio of A/B.
Haller et al. (1987) and subsequently Daunt et al. (2004) have used the index to describe thoracic dimensions in those without a pectus deformity, but this has only been at one vertebral level per subject, which is not always the same vertebral level.
The aim of this paper is to use the Haller methodology to describe the shape of the thorax across all vertebral levels in the non‐deformed thorax for a range of ages in both males and females.
Methods
The database of all CT scans previously performed as part of routine medical care in our institution was queried for scans labelled ‘chest, abdomen and pelvis’ or ‘chest’ for all ages and diagnoses between January 2010 and October 2015. In our institution these data are available from the age of 8 years of age through to adulthood. This group was then reduced to the age range of 8–18 years and these scans formed the study group.
The identified scans were then measured at every vertebral level from T3 to T12 using the Haller methodology. It was not possible to measure at the level of T1 and T2 as there was no identifiable anterior margin to measure from. This is because this was cranial to the jugular notch of the sternum (incisura jugularis) in the plane of the axial CT slice. When caudal to the sternum the AP measurement was defined as the minimum distance from the inside of the body cavity anteriorly (the linea alba between the rectus abdominus muscles of the anterior abdominal wall in lieu of a bony landmark) to the anterior border of the vertebral body.
All of the measurements were taken by the first author using digital measurements from the PACS system (GE systems, New York, NY, USA). The data were then analysed to describe the Haller index at every vertebral level between T3 and T12.
Ethical review was judged not necessary by the local ethics committee, as the study design is the analysis of previously collected anonymised data; however, institutional peer review was performed and approval granted.
Results
According to the database, 496 scans were available for analysis. Of these, 63 scans were excluded for a number of reasons: the scan was a duplicate, no images had been saved or there was evidence of either spinal or thoracic deformity, thoracic trauma or a chest wall resection. This left 433 scans for analysis, 230 male and 203 female. The mean age of the male group was 14.4 years (SD 2.65 years, range 8.2–18 years) and of the female group 13.7 years (SD 2.89 years, range 8.1–18 years).
Figure 2 plots the mean Haller index per level for both males and females. This ratio is created from thoracic width and height as described earlier (Figs 3 and 4. A tabulated form of mean Haller index per level for age and sex can be found in Supporting Information Tables S1–S3.
Figure 2.
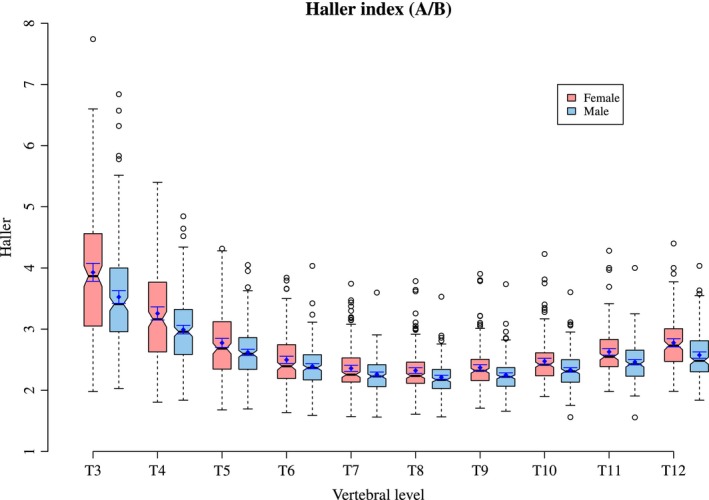
The Haller index per level in males and females. The median value is shown as a horizontal line at the level of the pinch in the box. The mean and 95% confidence interval of the mean shown as the solid dot and whiskers around that dot in each box.
Figure 3.
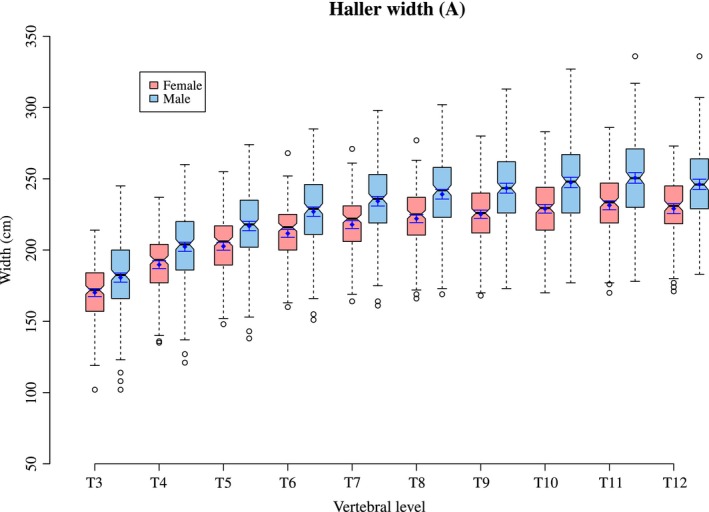
The thoracic width (A) per level in males and females.
Figure 4.
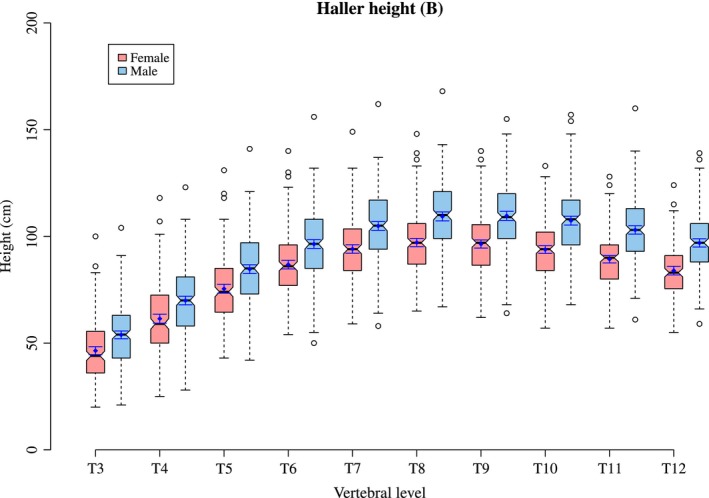
The thoracic height (B) per level in males and females.
When the Haller index is analysed for age at time of scan and then plotted longitudinally it can be seen that the Haller index increases with age in both the most cranial levels (T3–T5) and the most caudal levels (T11 and T12) of the thorax. In the middle levels of the thorax (T6–T10) there is relatively little change in the Haller Index. The increase with age is greater in the cranial levels than in the caudal levels of the thorax (Figs 5 and 6. This is due to an increase in width against little change in height, which is particularly noticeable at T3 and T4.
Figure 5.
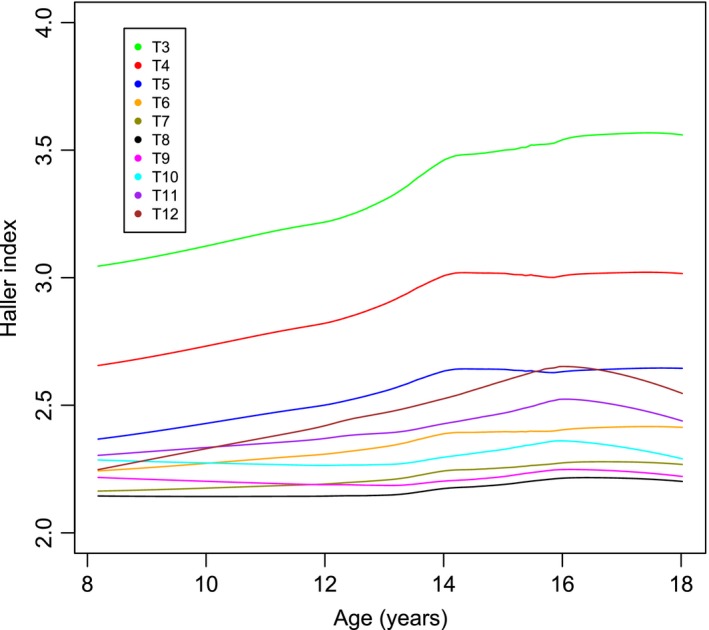
The Haller index against age per level in males.
Figure 6.
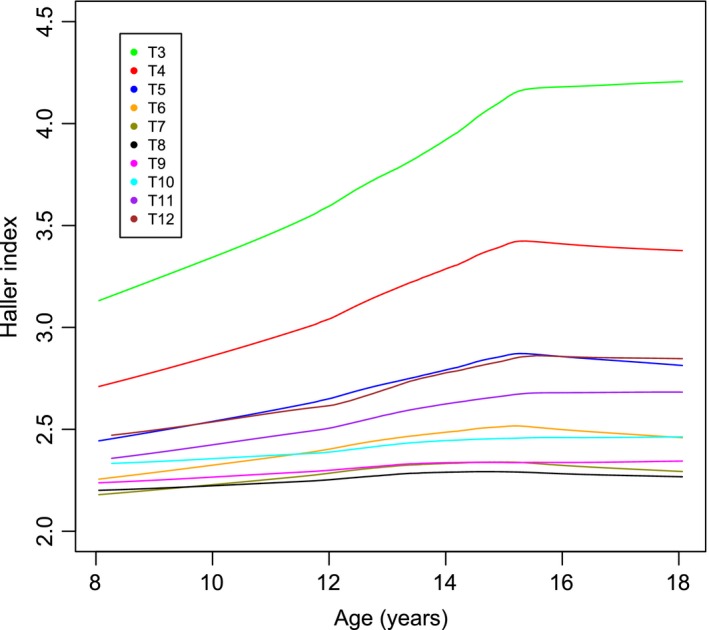
The Haller index against age per level in females.
Discussion
The thoracic cage is a complex three‐dimensional structure that reflects the intrathoracic contents and the function of the thorax. The most cranial ribs form the rim of the thoracic inlet which, given the downward slope of the ribs, may be thought of as not being part of the true thoracic cage. In the cranial thorax, the anterior border is formed by the sternum. Caudal to the sternum, the anterior border of the thorax is formed by the abdominal wall musculature. A view of the thoracic cage from the lateral aspect reveals that the posterior border is longer than the anterior if viewed as between the 1st and 12th thoracic vertebrae posteriorly and between the superior and inferior ends of the sternum anteriorly.
When the thoracic cage is viewed with CT in the axial plane, the thoracic cage commences at the level of T3. Cranial to this is the thoracic inlet. No anterior border can be reliably identified. Caudal to the end of the sternum there is a clear demarcation of the anterior border formed as the rectus abdominus muscles come together in the midline.
The Haller index is described as the ratio of the thoracic width to the thoracic height within the thoracic cage used in the assessment of pectus excavatum (Haller et al. 1987). This ratio is calculated from measurements from a single axial CT image per patient that is not referenced to a vertebral level. In the small sample from the original paper by Haller et al. (1987), the mean Haller index of those without deformity was 2.56.
A large review of CT scans in children obtained for reasons not related to thoracic shape, between the ages of 0 and 18 years of age, was performed by Daunt et al. (2004). The level in the chest used for the measurement was that which gave the largest value to the Haller index for that particular thorax and thus was not always at the same vertebral level. The paper demonstrated that the normal value was less than that from the Haller paper and that it depended on the age of the subject, as the Haller index changes with age as the thorax develops.
This study reveals that Haller index is largest at the first measureable level of T3 in both males and females, falling to the lowest value at T8 and T9 before increasing again slightly in the more caudal vertebral levels (Fig. 2). As both sexes increase in age there are corresponding increases in the Haller index, particularly in the more cranial thorax and, to a lesser degree, the more caudal thorax. This is because of a greater increase in thoracic width relative to thoracic height. The mid thorax demonstrates little change with increasing age. There is some difference between the sexes, with the increase in the Haller index greatest in females. In both sexes there is a slowing in the increase of the index, seen at age 14 in males and 15 in females. It is reasonable to suspect that this tailing off may be related to the adolescent growth spurt.
The results are of relevance because this is the first description of the growth and development of the thoracic cage of this type providing the normative data for any future investigations. The clinical relevance is related to the management of pectus excavatum and to the management of scoliosis. Pectus excavatum is now invariably treated using a minimally invasive technique with a Nuss bar (Nuss et al. 1998) in the teenage years (Pilegaard, 2015; Zhang et al. 2015). Scoliosis presents with a rotation of the thoracic cage and the development of a rib hump (Hong et al. 2011) and current operative techniques aim to reduce this. It is imperative that the surgeon knows what is ‘normal’ in any surgical technique where the aim is to return the patient to ‘normal’.
The weakness of these data for the caudal levels of the thorax is that the numbers used to analyse each group are smaller than the ‘N’ stated (with N reflecting the number of scans measured across all levels of the thorax), as some of the CT scans were not caudal enough to include the last vertebral levels of the thoracic spine. This is the case in both the male and female group.
The Haller index, as a measurement, is not without problems, as there is evidence that differences exist within the Haller index dependent on whether CT images are captured during inspiration or expiration (Birkemeier et al. 2011; Albertal et al. 2013). Although a retrospective study, it is routine practice to obtain CT scans of the thorax with a ‘breath hold in maximal inspiration’. There is no reason to suspect this is not the case for the scans used in this series.
This study documents the mean Haller index per vertebral level in both males and females between the ages of 8 and 18 years in the non‐deformed thorax and will provide normative data of thoracic dimensions for the future.
Conflict of interest
None declared.
Funding
None declared.
Supporting information
Table S1. The mean and standard deviation for the Haller index per level.
Table S2. The mean and standard deviation for the Haller index per level by age in years in males.
Table S3. The mean and standard deviation for the Haller index per level by age in years in females.
References
- Albertal M, Vallejos J, Bellia G, et al. (2013) Changes in chest compression indexes with breathing underestimate surgical candidacy in patients with pectus excavatum: a computed tomography pilot study. J Pediatr Surg 48, 2011–2016. [DOI] [PubMed] [Google Scholar]
- Birkemeier K, Podberesky D, Salisbury S, et al. (2011) Breathe in… breathe out… stop breathing: does phase of respiration affect the Haller index in patients with pectus excavatum? AJR Am J Roentgenol 197, W934–W939. [DOI] [PubMed] [Google Scholar]
- Daunt S, Cohen J, Miller S (2004) Age‐related normal ranges for the Haller index in children. Pediatr Radiol 34, 326–330. [DOI] [PubMed] [Google Scholar]
- Haller J, Kramer S, Leitman S (1987) Use of CT scans in selection of patients for pectus excavatum surgery: a preliminary report. J Pediatr Surg 22, 904–906. [DOI] [PubMed] [Google Scholar]
- Hong J‐Y, Suh S‐W, Easwar T, et al. (2011) Evaluation of the three‐dimensional deformities in scoliosis surgery with computed tomography: efficacy and relationship with clinical outcomes. Spine 36, E1259–E1265. [DOI] [PubMed] [Google Scholar]
- Nuss D, Kelly R Jr, Croitoru D (1998) A 10‐year review of a minimally invasive technique for the correction of pectus excavatum. J Paediatr Surg 33, 545–552. [DOI] [PubMed] [Google Scholar]
- Pilegaard H (2015) Nuss technique in pectus excavatum: a mono‐institutional experience. J Thorac Dis 7(Suppl 2), S172–S176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Tang J, Ben X, et al. (2015) Surgical correction of 639 pectus excavatum cases via the Nuss procedure. J Thorac Dis 7, 1595–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The mean and standard deviation for the Haller index per level.
Table S2. The mean and standard deviation for the Haller index per level by age in years in males.
Table S3. The mean and standard deviation for the Haller index per level by age in years in females.


