Abstract
Hyperostosis, excessive bone growth along bone that stems from bone, periosteum or articular or epiphyseal cartilage, occurs in at least 22 families of fishes most of which are tropical or subtropical marine species. While the presence of hyperostosis is well documented in fishes, the mechanism driving the development of the excessive bone growth is unclear. This study documented hyperostosis along the dorsal pterygiophores in both sexes of oarfish, Regalecus russellii; however, it was not present in all specimens examined. This is the second lampridiform fish with hyperostoses and the first case documented in a deeper‐water, epi‐mesopelagic fish. In oarfish, the majority of the dorsal pterygiophores tissues are poorly mineralized, anosteocytic bones with some fish displaying localized stiffened, hyperostotic growths near the distal edge. Oarfish lack a swim bladder so they must continuously beat their bi‐directional dorsal fin to maintain position within the water column while engaged in locomotory behavior. These fishes have areas of localized, hyperostotic skeletal elements along the dorsal pterygiophores that, presumably, function as a stiffened lever system to support fin undulation. It was noted that hyperossification was not present in all fish examined and was only documented in fish with total lengths greater than 3 m.
Keywords: bone growth, computed tomography, epipelagic fish, Tilly bones
Introduction
The five recent strandings of oarfish (Regalecus russellii; Cuvier & Valenciennes, 1817) along the California coast presented a rare opportunity to perform an anatomical assessment of a rarely seen Lampridiform (synonymous with Lampriform) fish. Preliminary static X‐rays indicated hyperostotic bone, along the dorsal axial skeleton. Hyperostotic bone (synonymous with ‘Tilly bones’, swollen bones, and sometimes referred to as exostosis although this term implies growth of a new bone or cartilage) is described as an excessive benign growth along bone that stems from bone, periosteum, or articular or epiphyseal cartilage (Schluter et al. 1992; Smith‐Vaniz et al. 1995). The hyperostotic bone appears externally swollen in appearance, though histological examination reveals that only the periosteum shows increased ossification, while the remaining cellular tissue undergoes resorption resulting in only trabecular bone remaining (Meunier & Desse, 1994; Jawad, 2013). In fishes, hyperostosis is a localized area of osteocytic bone, within an otherwise anosteocytic skeletal element (Smith‐Vaniz et al. 1995). The inside can be hollow, or filled with spongy bone (either avascular or vascular) or fat (Smith‐Vaniz et al. 1995; Meunier et al. 2010). The presence of hyperostotic bone is well characterized in mammalian systems, including: hyperostosis in the auditory canal (Glatt, 1933); in the mandible (Rouas & Midy, 1997; Restrepo et al. 2004); cranium (Lynch et al. 2002); or in various other bones including long bones (Duncan et al. 1996; Lynch et al. 2002). In fishes, the presence of hyperostotic bone has been described in at least 22 families (Schlumberger & Lucke, 1948; Konnerth, 1966; Olsen, 1969; Smith‐Vaniz et al. 1995), most of which are extant or fossilized marine species (Tiffany et al. 1980; Meunier et al. 2010). The location of hyperostotic bone along the skeleton varies, and is most commonly found in the dorsal pterygiophores, cleithrum and along the supraoccipital bone (Smith‐Vaniz et al. 1995; Meunier et al. 2010; Fig. 1).
Figure 1.
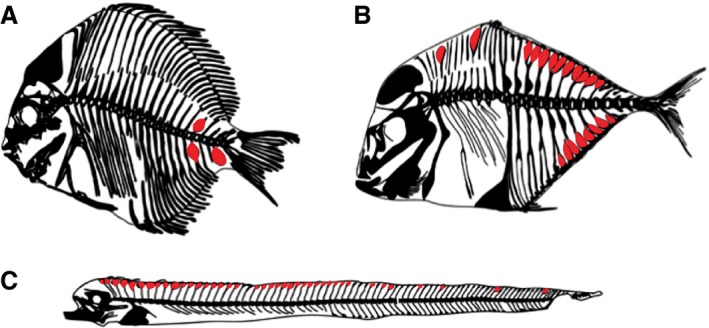
Representative examples of hyperostosis in fishes indicated in red. (A) Hyperostosis in Drepane longimana (modified from radiograph available on Australian Museum, http://australianmuseum.net.au/image/hyperostosis-in-a-banded-sicklefish; Jawad, 2013). (B) Alectis alexandrinus (modified from Smith‐Vaniz et al. 1995). (C) Lepidops caudatus (modified from Giarratana et al. 2012).
Hyperostotic growth in fishes was once presumed to be pathological, related to fungal growth (Grabda, 1982) or cancerous bone tumors (osteomas; Schlumberger & Lucke, 1948; William et al. 1980; Schluter et al. 2002; Capasso, 2005). While some cases of hyperostosis in fishes may be pathological deformities, a survey of silver scabbardfish (Lepidopus caudatus; Euphrasen, 1788) showed that nearly 40 of 50 specimens (80%) surveyed had some level of hyperostosis (Giarratana et al. 2012). The general consensus among ichthyologists studying this phenomena is that hyperostosis is a natural variant to the skeleton and, in some cases, appears to be an inevitable process (Olsen, 1971; Desse et al. 1981; Gauldie & Czochanska, 1990; Meunier et al. 2010). The presence of increased hyperostosis has been linked to genetic factors (Capasso, 2005) and/or ecological factors (Couch, 1979; Chang et al. 2008; Witten & Huysseune, 2009); however, based on previous data, this condition appeared to be a shallow‐water, mostly neoteleostean condition and has not been previously described in deeper‐water, mesopelagic fishes.
The oarfish is a deep epipelagic to shallow mesopelagic fish usually found between 30 and 200 m, although they can be found as deep as 1000 m (Eschmeyer et al. 1983; Heemstra, 1986). It is considered the longest known bony fish, reportedly reaching sizes of up to 8 m TL (Total Length), and has inspired folklore around the world including the existence of sea serpents (Roberts, 2012). One might predict from the depth profile of oarfish that finding repeating, hyperossified elements is unlikely compared with other fishes that display prominent hyperostoses. Their systematic classification as a pre‐percomorph fish suggests that the majority of bones in the oarfish are anosteocytic (Parenti, 1986) – a skeletal feature that unifies all fish with documented hyperostosis. The axial body is flaccid in nature due, in large part, to the poorly mineralized skeleton that is associated with adaptation to a deeper epipelagic to mesopelagic lifestyle, similar to other mesopelagic fishes like lanternfish, hatchetfishes and stomiids (Randall & Farrell, 1997). Thus, the oarfish lacks the stiffened skeleton generally associated with the shallow‐water, perciform fishes (Parenti, 1986; Kranenbarg et al. 2005a; Cohen et al. 2012) that usually display hyperossified bone, particularly the carangids (Smith‐Vaniz et al. 1995).
The goal of this study is to document hyperossification in R. russellii and to discuss the functional implications of regional hyperossification on an otherwise poorly mineralized skeleton.
Materials and methods
Oarfish specimens were collected following natural stranding events (five separate stranding events occurred between 2013 and 2015) and also from examination of museum specimens ranging from juveniles to adult size (verified by the gonadal tissue examined for a separate study). The approximate age of the fish was not determined as otoliths are difficult to obtain and are not a reliable method for aging as they are not well mineralized (Midway & Wagner, 2015). The first oarfish was found stranded in Oceanside, CA, USA on 18 October 2013. The fish was transferred to the Southwest Fisheries Science Center (La Jolla, CA, USA) for a preliminary necropsy, and removal of the stomach, gonads, heart and left portion of the head. The remaining tissues were cut into a total of nine sections, weighed and transferred to California State University Fullerton. The entire fish was frozen in a −80 °C freezer for 4 weeks prior to computed tomography (CT) scanning. CT imaging is now widely available, which enabled visualization of the internal anatomy of this rare species prior to performing large‐scale gross necropsies. This is an unusual specimen of fish that is rarely available for dissection, therefore it was an ideal opportunity to analyze the anatomy of the intact skeleton prior to skeletal disarticulation.
Following the initial freezing of 2013 oarfish, CT scans were performed at the University of California, Los Angeles Translation Research Imaging Center using a 20‐slice, multi‐detector row CT scanner (Definition AS, Siemens, Healthcare, Forchheim, Germany) with 0.6‐mm slice thickness. Each fish (n = 4; two oarfish) was scanned twice, once at 120 kV and once at 80 kV, to ensure good contrast due to poor bone mineralization. Two comparison Lampridiform specimens [ribbonfish (Trachipterus altivelis), provided by NOAA Southwest Fisheries; and opah (Lampris guttatus), available in the authors’ freezer], were CT scanned as well. CT scans were then reconstructed from 8‐bit TIFF stacks as three‐dimensional images using ITK‐Snap software (version 3.x free open source software). The June 2015 oarfish was scanned as a fresh sample instead of as a frozen specimen also at UCLA.
The additional specimens (n = 4) of oarfish were stranded in: June (Catalina, #2); August (Catalina, #3); September (La Jolla, #4); and November (Palos Verdes Peninsula, #5) 2015. In addition to salvaged fishes, several preserved specimens from the LA Natural History Museum (n = 3) and from Scripps Institute of Oceanography (n = 3) collections were examined; methods of sampling are listed in Table 1. Radiographs of juvenile oarfish were performed to illustrate the anatomy of the skeleton prior to the development of hyperossification while also preserving the relatively rare specimens (Fig. 2). Hyperostotic dorsal pterygiophores, the bones along the body with which the median, elongated dorsal fin rays connect, were photographed after dissection from the body in stranded specimens and also examined in previously excised, preserved specimens (Fig. 3A). The same dorsal pterygiophores were photographed again following clearing and staining to visualize the skeletal elements (Song & Parenti, 1995) using Alizarin red for staining bone and Alcian blue for cartilage (Fig. 3B). Additionally, a single hyperossified pterygiophore from oarfish #1 was visualized via high‐resolution micro‐CT imaging (Bruker SKYSCAN 1272) scanned at 60 kV, 38.9 μm resolution at the University of Washington Friday Harbor Laboratories by Adam Summers (Fig. 4A–C). This allowed to: (i) get a better sense of the relationship between the ascending pterygiophore and the hyperostosis; and (ii) visualize the entire internal and external structure of the hyperostosis.
Table 1.
List of oarfish specimens. Stranded specimens are listed by the date of stranding. Other examined specimens are collected from Scripps Institute of Oceanography (SIO) or LA County Natural History Museum (LACNHM)
| Specimen | Method of examination | Hyperostosis | Age range |
|---|---|---|---|
| Oct 2013 | CT, dissection | Yes | Adult |
| June 2015 | CT, dissection | Yes | Adult |
| Aug 2015 | Sub sample dissection | Yes | Adult |
| Sept 2015 | Sub sample dissection | Yes | Adult |
| Nov 2015 | Sub sample dissection | Yes | Adult |
| SIO 77‐3 | X‐ray | N | Juv |
| SIO 11‐380 | X‐ray | N | Juv |
| SIO W58‐267 | X‐ray | N | Juv |
| LACNHM 37292‐1 | X‐ray | N | Juv |
| LACNHM 58180‐1 | X‐ray | N | Juv |
| LACNHM 57292‐1 | X‐ray | N | Juv |
Figure 2.
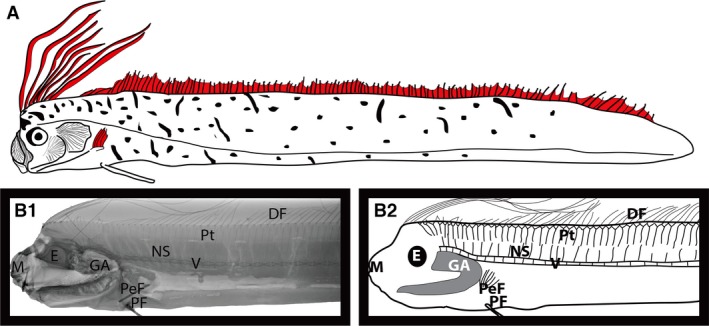
Radiograph image showing the anatomy of a non‐hyperossified oarfish (juvenile). DF, dorsal fin; E, eye; GA, gill arch; M, mouth; NS, neural spine; PeF, pectoral fin; PF, pelvic fin; Pt, pterygiophore; V, vertebrae.
Figure 3.
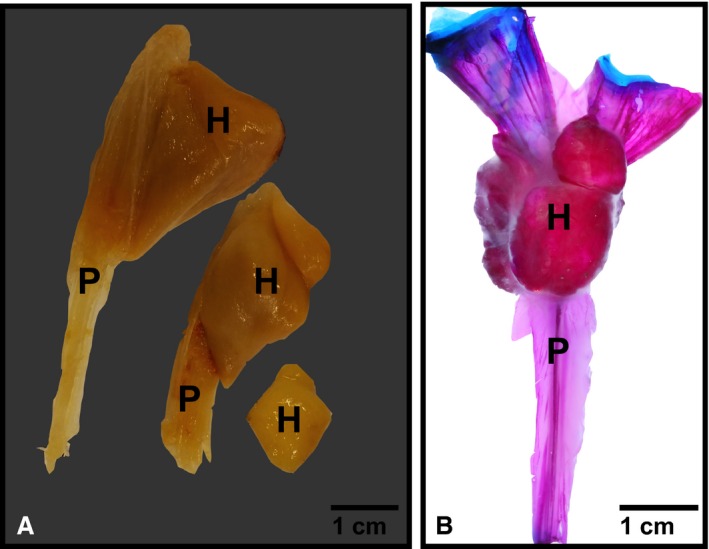
Hyperostosis in oarfish. (A) Hyperostosis along individual dorsal pterygiophores in a museum specimen (compliments of Scripps Institution of Oceanography Marine Vertebrate Collection). Specimens were already excised from the oarfish. Anterior view. (B) Cleared and stained dorsal pterygiophore from oarfish 1 (Oct 2013 specimen), lateral view. Red is mineralized tissue stained with alizarin red, blue is cartilage stained with alcian blue. Note the ascending pterygiophore (P) is poorly mineralized acellular bone compared with the cellular hyperostotic portion (H) at the distal tip. Blue stain is articular cartilage.
Figure 4.
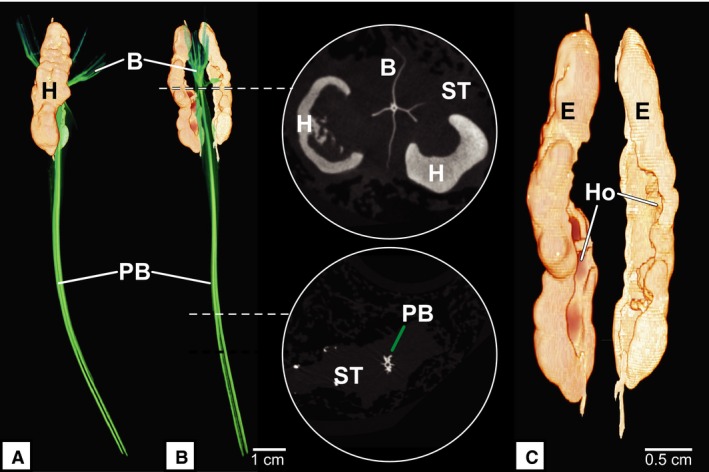
Micro‐CT of ascending pterygiophore and hyperostosis. (A) Lateral view of pterygiophore. (B) Anterior view of pterygiophore with corresponding slice scans. (C) Anterior view of hyperostosis. (B) Cross‐section of micro‐CT slice data showing the branching pterygiophore and relationship to hyperostosis. (C) The inside of the hyperostosis appears hollow. Pterygiophore shown in green (A and B), hyperostosis shown in tan. B, branched elements; E, external hyperostosis; H, hyperostosis; Ho, internal hollow center; PB, pterygiophore body; ST, soft tissue. Images by Adam Summers.
The relative amount of hyperossified bone for each pterygiophore in specimen #1 was estimated by uploading the sectioned CT images into Image J software and calculating surface area by measuring the external perimeter of the hyperostosis slice by slice. This is similar to the measurements taken on fossil hyperossified pterygiophores reported in Meunier et al. (2010). Three parameters were calculated: pterygiophore total surface area (PTSA; cm2), which included the entire length of the pterygiophore including the hyperossified tissue; the hyperossified surface area (HSA), which only included areas of hyperossification; and the pterygiophore surface area (PSA), which did not include any hyperossified tissue. Of the 126 available pterygiophores in this specimen, it was possible to determine the percent surface area covered by hyperostosis from 100 individual pterygiophores. For pterygiophores with no data, either there was no evidence of hyperostosis present or the bone mineralization was too poor to isolate the pterygiophore in the CT scan with confidence (Table 1). The anatomy is reported throughout using anatomy described by Roberts (2012), where the distal pterygiophore articulates with the dorsal fin ray and the proximal pterygiophore is closer to the neural spine.
Results
All of the stranded oarfish examined showed the presence of repeating, hyperossified structures along the distal portion of the dorsal pterygiophores (Fig. 5). Three fish had regular repeating hyperostoses running the entire length of the body (> 80% of the pterygiophores were hyperossified, specimens #1, #3, #5) that were well developed in size (up to 15 mm in diameter). The most pronounced hyperostoses were present within the dorsal ends of the anterior‐most pterygiophores, just rostral to the cristophore (the first six pterygiophores fuse to form the cristophore, which supports the paired dorsal fin crests; Fig. 6).
Figure 5.
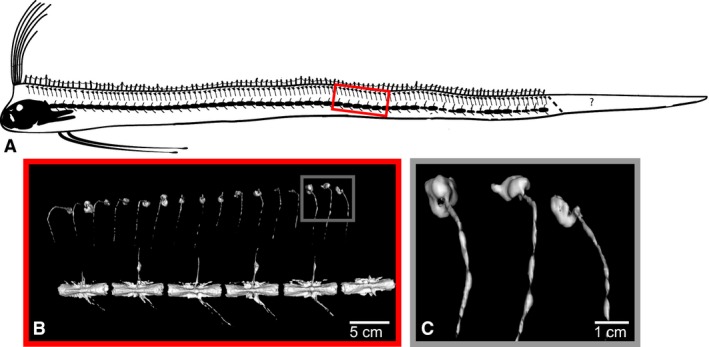
Repeating, hyperossified, distal pterygiophores in adult oarfish. (A) Schematic of the scan of oarfish 1. The ‘?’ indicates the area of the tail distal to the point of autotomy. All adult scavenged fish examined were missing part of their tail. Fish 1 (this scan) shows evidence of healing on the tail suggesting the loss of the tail was not related to the stranding. (B) CT rendering of the pterygiophore hyperostoses. Some pterygiophores are bent as an artifact of freezing. (C) CT rendering of pterygiophore hyperostoses near the anal vent. The degree of hyperostosis along the pterygiophores is similar to the anterior (rostral) pterygiophores.
Figure 6.
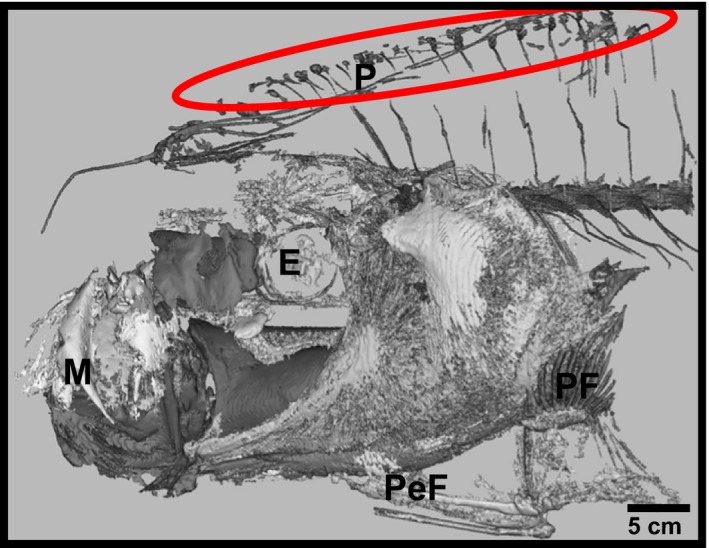
Evidence of dorsal pterygiophores in the anterior‐most portion of the axial skeleton. CT scan of the head and anterior vertebrae in oarfish 2. The skeleton is poorly mineralized and hence difficult to separate out specific cranial bones. However, it was noted that the hyperostosis extends to the anterior‐most pteygiophores. E, eye; M, mouth (protruded); P, pterygiophores; PeF, pelvic fin; PF, pectoral fin. Sectioned by Callie Crawford.
Of the 126 pterygiophores sampled from specimen #1, 22 did not have any discernable hyperostoses. There were no clear patterns as to whether certain pterygiophores lacked hyperossified tissue as it occurred along the entire length of the axial skeleton (e.g. anterior, medial and posterior pterygiophores). The location of the hyperostosis was always in the distal 1/3 portion of the pterygiophores. The average PTSA, HSA and PSA were 0.89, 0.50 and 0.49 cm2, respectively. The average percent surface area covered by hyperostosis was 54.22%, and ranged from 97% of the total pterygiophore covered by hyperostotic tissue to just 17% coverage.
The other two stranded specimens (specimens #2, #4) displayed variable levels of hyperostotic bone (54–78% of the pterygiophores had hyperostosis). Instead the hyperostoses were relatively small, often not much bigger than 5 mm in diameter. Hyperostosis was present along the entire length of these two fish; however, it was more evident in the mid‐posterior portion of the fish. Additional museum specimens confirmed that the current specimens were not unique in the presence of hyperossified bones within the pterygiophores (Fig. 2). Hyperostoses were not evident in CT scans from the two other Lampridiform fishes, opah and ribbonfish (Fig. 7). It was qualitatively noted that both opah and ribbonfish had more bone mineralization in the axial skeleton compared with oarfish.
Figure 7.
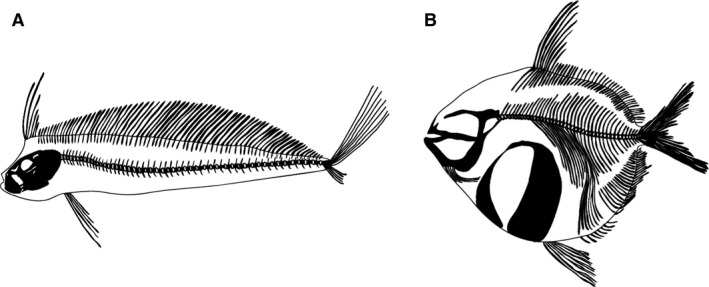
Comparison of Lampridiformes: (A) king of the salmon; and (B) opah. No evidence of hyperostosis. Redrawn from CT data.
Clearing and staining of the pterygiophore complex showed that the body of the pterygiophore was composed of poorly mineralized bone. The distal tip of the pterygiophore was composed of articular cartilage shown in blue (Fig. 3B). The hyperostotic area contained far more mineralization than the ascending process, as was evident by the deep red color at the bulbous bone (Fig. 3B). The distal‐most edge of the pterygiophore was composed of highly branched bony elements encased within a soft, unmineralized tissue element (Fig. 4A and B), while the internal structure of the hyperostosis appeared to be hollow (Fig. 4C). It was noted that the ‘soft’ tissue surrounding the bone appears to be cartilaginous; however, this has not yet been verified with histology.
Static radiographs of small oarfish showed no evidence of hyperostoses in the pterygiophores, even in the anterior‐most region near the cristophore.
Discussion
To the authors’ knowledge, this is the first example of a deep‐water, epi‐mesopelagic, temperate marine fish displaying prominent, hyperossified pterygiophores. It is especially rare to find these present along the entire length of the axial skeleton. To date, only one other lampridiform fish, family Veliferidae, is documented exhibiting hyperostosis. The veliferid fishes, or moonfishes, are morphologically and ecologically different from the oarfishes as they are generally deep and small bodied fishes (< 30 cm), and live in shallow, coastal water habitats. As such, their morphology and ecology are more similar to other fishes with known hyperostosis (e.g. Carangids, Sparids and Sciaenids; Desse et al. 1981; Meunier & Desse, 1986).
When examining the list of fishes possessing known hyperostotic regions of their skeleton, it was noted that the majority of fishes with prevalent hyperostoses live in tropical, shallow‐water conditions (Smith‐Vaniz et al. 1995), and tend to be very active (i.e. highly locomotory fishes). However, it is important to emphasize that the list of species having some level of hyperostosis is likely far from comprehensive, and may be a reflection of relative knowledge of nearshore fishes vs. those from habitats that are harder to access. The presence of hyperostosis in fishes appears to be species‐specific within any particular clade (Smith‐Vaniz et al. 1995), though not every individual will display hyperossified bone, nor will the degree of hyperostoses between individuals or even within one specimen be consistent (Giarratana et al. 2012). However, Smith‐Vaniz et al. (1995) noted that fish hyperostoses appear predictably in larger individuals. It is perhaps not surprising that hyperostoses were not found in opah or ribbonfish (both adult individuals), or in the smaller oarfish specimens. It is important to consider that hyperostosis may occur in opah and ribbonfishes, but simply were not present in the two specimens available to this study.
The mere presence of hyperossified bone is inherently interesting in oarfish as previous descriptions of R. russellii have described the dorsal pterygiophores as cartilaginous rather than as bony structures (Nishimura, 1960), and hyperostosis has not been documented before on their pterygiophores. The cleared and stained samples of pterygiophores in this study show that the structures are mostly comprised of bone (or possibly mineralized cartilage), except at the distal articular cartilage that connects the pterygiophore to the dorsal fin ray. The oarfish has a poorly mineralized and bendable skeleton in general, except in the localized areas of hyperossified bones; thus, the functional implications of hyperostoses may be very different in oarfish compared with hyperostosis in shallow‐water, well‐mineralized fishes (Tiffany et al. 1980; Smith‐Vaniz et al. 1995).
The main function of vertebrate skeletons is to form a load‐bearing structure that works in conjunction with skeletal muscle contractions to perform as a lever system (Dean & Shahar, 2012). Indeed this holds true for fishes that experience loading on their skeletons from two sources: (i) through muscle attachments; and (ii) through the resistive force of living in a water environment (Summers & Long, 2006). However, poorly mineralized or unmineralized bone is mechanically compliant compared with highly mineralized cartilage or bone, making it less effective in this load‐bearing capacity. While the skeletons of many mid‐ and deep‐water fishes are less stiff, and therefore presumably less able to resist load‐bearing forces, a reduction in ossification does potentially offer certain advantages. The lighter‐weight skeleton likely plays an important functional role in maintaining buoyancy – especially in fishes that have reduced or absent swim bladders, like oarfish. Hyperostotic bone has been suggested to assist with buoyancy/equilibrium (Meunier & Desse, 1986); however, there are clearly a large number of congeneric, non‐hyperostotic species living in the same habitat as species with documented hyperostoses (Smith‐Vaniz et al. 1995). This would seem to suggest that buoyancy cannot be the primary role of hyperostoses. It certainly cannot be the case that hyperostoses are required for buoyancy in oarfishes given the complete lack of this character in previously examined specimens, the smaller individuals examined for this study, and the reduced size in three of the stranded specimens. Further, their relative density compared with the remainder of the oarfish skeleton is counter‐intuitive to the notion that they could provide additional lift. This supports Breder's (1952) argument that the specific gravity of the fat‐filled hyperostoses makes them an unlikely candidate for assisting with either balance or buoyancy. Thus, it was suggested that the poorly mineralized skeleton is likely the most important contributor to maintaining position in the water column, along with the oscillations of the elongate dorsal fin.
Given that oarfish have a skeleton that is less stiff, functionally it would make sense that localized hyperossified structures along a dorsal pterygiophore would help to provide some element of support to the pterygiophore during dorsal fin undulations. The need for a stiffened skeletal lever makes sense, as there are at least two muscular attachments at the distal edge of the pterygiophore: the dorsal fin levator and the dorsal fin undulator (Roberts, 2012). It was noted that the fish moves via undulation of the dorsal fin and, therefore, it was speculated that this bone ‘thickening’ is a direct result of locomotion of this fin to move an increasingly large body. Indeed, histological examination of the structure of hyperostoses in other documented species indicates that the spongiosa‐like structure of the tissue likely provides additional mechanical support with a minimal investment of bone mass (Parfitt, 1988; Witten & Hall, 2002).
However, if this is the case, why then is the occurrence of hyperossified bone in oarfish so far and few between? It was noted that of the five stranded specimens of oarfish examined, only three specimens displayed any degree of regular, large hyperossified structures (in fish larger than 4 m; females), though small, irregular hyperostoses were present in smaller stranded fish (between 3 and 4 m TL; two males). Interestingly, the individuals that displayed regular hyperostosis were visually the most robust specimens, though they were all very nearly the same total length. Unfortunately, their condition when salvaged after stranding events makes it difficult to obtain accurate measures of mass or similar metrics. The sheer mass of the individuals could be contributing to the need for reinforcement along the base of the dorsal fin. Indeed, Konnerth (1966) postulated that the presence of hyperostoses could be linked with age, and Smith‐Vaniz et al. (1995) similarly found that, when there was variation within a species, hyperostoses were only present in relatively large and mature individuals – which is supported by the current findings. While no study yet has been able to provide conclusive evidence that hyperostoses are linked with age, these findings taken together are strongly compelling. It may be that as oarfish reach mature sizes, they develop localized areas of hyperossification to provide a stiffened region for stress concentration along their axial skeleton that is capable of remodeling.
Histology of hyperostotic bone has shown that there is strong evidence for localized remodeling, including the presence of osteoblasts, osteoclasts and even occasionally osteocytes that are not present within the skeleton of an otherwise anosteocytic fish (Smith‐Vaniz et al. 1995; Meunier et al. 2010). The localized areas of remodeling would combat microfractures that would certainly occur from repeated loading events (e.g. dorsal fin undulations; Currey, 1999). Oarfish have been repeatedly documented to orient themselves vertically within the water column, maintaining position (station holding) through bi‐directional undulations of their elongated dorsal fin (Clarke & Haedrich, 1968; Roberts, 2012), presumably for extended periods of time. These localized areas of support could serve as an inexpensive solution to providing much needed support for locomotion and/or station holding while maintaining the otherwise poorly mineralized (e.g. reduced density and/or increased water content in the bone) skeletal plan deployed by the vast majority of deep‐water fishes (Randall & Farrell, 1997).
Acknowledgements
The authors are greatly indebted to Michael McNitt‐Gray, Anthony Smithson, D. Enzmann and the excellent staff at UCLA Translational Research Imaging Center for CT scanning. The authors thank Suzanne Kohin, Antonella Preti, Nick Wegner and Russ Vetter (NOAA South West Fisheries Science Center), and Catalina Conservancy for the oarfish and ribbonfish specimens as well as static X‐rays. The authors thank HJ Walker (SIO Marine Vertebrate Collection at Scripps Institute of Oceanography) and Rick Feeny (LA County Natural History Museum) for their insight into hyperossified fish skeletons and for providing museum specimens. Special thanks to Adam Summers and the Karel F. Liem Biovisualization Center for the micro‐CT data, and to Callie Crawford for sectioning the oarfish head CT scan. The authors thank Mason Dean who provided feedback on the figures, and the two anonymous reviewers who greatly improved this manuscript.
References
- Breder CM Jr (1952) The problem of directives to cellular proliferation as illustrated by ontogenetic processes in certain fishes. Growth 16, 189–198. [PubMed] [Google Scholar]
- Capasso LL (2005) Antiquity of cancer. Int J Cancer 113, 2–13. [DOI] [PubMed] [Google Scholar]
- Chang M, Wang X, Liu H, et al. (2008) Extraordinarily thick‐boned fish linked to the aridification of the Qaidam Basin (northern Tibetan Plateau). Proc Natl Acad Sci USA 105, 13 246–13 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke WD, Haedrich RL (1968) Dive 218 In: Gulfview Diving Log. (eds Gaul RD, Clarke WD.), pp. 14–15. College Station, TX: Gulf Universities Research Corporation. [Google Scholar]
- Cohen L, Dean MN, Shipov A, et al. (2012) Comparison of structural, architectural and mechanical aspects of cellular and acellular bone in two teleost fish. J Exp Biol 215, 1983–1993. [DOI] [PubMed] [Google Scholar]
- Couch JA (1979) Vertebral dysplasia in young fish exposed to the herbicide trifluralin. J Fish Dis 2, 35–42. [Google Scholar]
- Currey JD (1999) The design of mineralized hard tissues for their mechanical functions. J Exp Biol 202, 3285–3294. [DOI] [PubMed] [Google Scholar]
- Cuvier G, Valenciennes A (1817) Le Regne Animal Distribute D'apres son Organization: Por Sevir de Base Al'histoire Naturelle des Animax et D'introduction a L'anatomie Compare: Avec Figures, Dessinees D'apres Nature, Vol. 2, xviii + 532 pp., pls 9–10 in Vol. 4. Paris: Deterville. [Google Scholar]
- Dean MN, Shahar R (2012) The structure‐mechanics relationship and the response to load of the acellular bone of neoteleost fish: a review. J Appl Ichthyol 28, 320–329. [Google Scholar]
- Desse G, Meunier FJ, Peron M, et al. (1981) Hyperostose vertébrale chez l'animal. Rhumatologie 33, 105–119. [Google Scholar]
- Duncan M, Crawshaw GJ, Mehren KG, et al. (1996) Multicentric hyperostosis consistent with fluorosis in captive fruit bats (Pteropus giganteus, P. poliocephalus, and Rousettus aegyptiacus). J Zoo Wildl Med 27, 325–338. [Google Scholar]
- Eschmeyer WN, Herald ES, Hammann H (1983) A Field Guide to Pacific Coast Fishes of North America, 336 pp.Boston, MA, USA: Houghton Mifflin Company. [Google Scholar]
- Gauldie RW, Czochanska Z (1990) Hyperostotic bones from the New Zealand snapper Chrysophys auratus (Sparidae). Fish Bull 88, 201–206. [Google Scholar]
- Giarratana F, Ruolo A, Muscolino D, et al. (2012) Occurance of hyperostotic pterygiophores in the silver scabbardfish, Lepidopus caudatus (Actinopterygii: Perciformes: Trachiuridae). Acta Ichthyologica et Piscatoria 42, 233–237. [Google Scholar]
- Glatt MA (1933) Hyperostosis‐exostosis of the external auditory canal. Laryngoscope 43, 558–562. [Google Scholar]
- Grabda E (1982) Fungi‐related outgrowths on pterygophores of single fins of Lepidopus caudatus (Euphrasen, 1788) (Pisces: Trichiuridae). Acta Ichthyologica et Piscatoria 12, 87–103. [Google Scholar]
- Heemstra PC (1986) Regalecidae In: Smiths’ Sea Fishes. (eds Smith MM, Heemstra PC.), p. 403 Berlin: Springer. [Google Scholar]
- Jawad LA (2013) Hyperostosis in three fish species collected from the Sea of Oman. Anat Rec 296, 1145–1147. [DOI] [PubMed] [Google Scholar]
- Konnerth A (1966) Tilly bones. Oceanus 12, 6–9. [Google Scholar]
- Kranenbarg S, van Cleynenbreugel T, Schipper H, et al. (2005a) Adaptive one formation in acellular vertebrae of sea bass (Dicentrarchus labrax L.). J Exp Biol 208, 3493–3502. [DOI] [PubMed] [Google Scholar]
- Lynch M, McCracken H, Slocombe R (2002) Hyperostotic Bone Disease in Red Pandas (Ailurus fulgens). J Zoo Wildl Med 33, 263–271. [DOI] [PubMed] [Google Scholar]
- Meunier FJ, Desse D (1986) Les hyperostoses chez les Teleosteons: description, histologie et problemes etiologiques. Ichthyophysiol Acta 10, 130–141. [Google Scholar]
- Meunier FJ, Desse J (1994) Histological structure of hyperostotic cranial remains of Pomadasys hasta (Osteichthyes, Perciformes, Haemulidae) from archeological sites of the Arabian Golf and the Indian Ocean. In: Fish Exploitation in the Past, Proceedings of the 7th Meeting ICAZ Fish Remains Working Group. (ed. Van Neer W.). Ann Mus R Afr Cent Sci Zool 274, 47–53. [Google Scholar]
- Meunier FJ, Gaudant J, Bonelli E (2010) Morphological and histological study of the hyperostoses of Lepidopus albyi (Sauvage, 1870), a fossil Trichiuridae from the Tortonian (Upper Miocene) of Piedmont (Italy). Cybium 34, 293–301. [Google Scholar]
- Midway SR, Wagner T (2015) The first description of oarfish (Regalecus russellii Cuvier 1816) (Regalecidae) aging structures. Appl Ichthyol 32, 113–116. [Google Scholar]
- Nishimura S (1960) A record of Regalecus russellii from the Sato Straits in Japan Sea. Ann Rep Reg Japan Sea Fish Res Lab 6, 58–68. [Google Scholar]
- Olsen SJ (1969) Hyperostosic fish bones from archaeological sites. Archaeol Soc New Jersey Bull 24, 17–20. [Google Scholar]
- Olsen SJ (1971) Swollen bones in the Atlantic cutlass fish Trichiurus lepturus Linnaeus. Copeia 1, 174–175. [Google Scholar]
- Parenti LR (1986) The phylogenetic significance of bone types in euteleost fishes. Zool J Linn Soc 87, 37–51. [Google Scholar]
- Parfitt AM (1988) Bone remodeling: relationship to amount and structure of bone, and the pathogenesis and prevention of fractures In: Osteoporosis, Etiology, Diagnosis, and Management. (eds Riggs BL, Melton LJ.), pp. 45–93. New York, NY: Raven Press. [Google Scholar]
- Randall DJ, Farrell AP (1997) Deep Sea Fishes. San Diego, CA: Academic Press. [Google Scholar]
- Restrepo S, Sanchez AM, Pelacios E (2004) Infantile cortical hyperostosis of the mandible. Ear Nose Throat J 83, 454–455. [PubMed] [Google Scholar]
- Roberts TR (2012) Systematics, Biology, and Distribution of the Species of the Oceanic Oarfish Genus Regalecus (Teleostei, Lampridiformes, Regalecidae), p. 268 Paris: Mémoires du Muséum national d'histoire naturelle Tome 202. [Google Scholar]
- Rouas A, Midy D (1997) About a mandibular hyperostosis: the torus mandibularis. Surg Radiol Anat 19, 41–43. [DOI] [PubMed] [Google Scholar]
- Schlumberger HG, Lucke B (1948) Tumors of fishes, amphibians, and reptiles. Cancer Res 8, 657–753. [PubMed] [Google Scholar]
- Schluter T, Kohring R, Mehl J (1992) Hyperostotic fish bones (“Tilly bones”) from presumably Pliocene phosphorites of the Lake Manyara area, northern Tanzania. Paläontologische Zeitschrift 66, 129–136. [Google Scholar]
- Schluter T, Kohring R, Palaeo Schluter T, et al. (2002) Fish bones from phosphorites of the Lake Manyara area, Northern Tanzania – fossil evidence of a physiological response to survival in an extreme biocenosis. Environ Geochem Health 24, 131–140. [Google Scholar]
- Smith‐Vaniz WF, Kaufman LS, Glovacki J (1995) Species‐specific patterns of hyperostosis in marine teleost fishes. Mar Biol 121, 573–580. [Google Scholar]
- Song J, Parenti LR (1995) Clearing and staining whole fish specimens for simultaneous demonstration of bone, cartilage, and nerves. Copeia 1, 114–118. [Google Scholar]
- Summers AP, Long JHJr (2006) Skin and bones, sinew and gristle: the mechanical behavior of fish skeletal tissues In: Fish Biomechanics, Vol. 23. (eds Shadwick RE, Lauder GV.), pp. 141–178. New York, NY: Elsevier; (Fish Physiology Series). [Google Scholar]
- Tiffany WJ, Pelham RE, Howell FW (1980) Hyperostosis in Florida fossil fishes. Fla Sci 43, 44–49. [Google Scholar]
- Witten PE, Hall BK (2002) Differentiation and growth of kype skeletal tissues in anadromous male Atlantic salmon (Salmo salar). Int J Biol 46, 719–730. [PubMed] [Google Scholar]
- Witten PE, Huysseune A (2009) A comparative view on mechanisms and functions of skeletal remodeling in teleost fish, with special emphasis on osteoclasts and their function. Biol Rev 84, 315–346. [DOI] [PubMed] [Google Scholar]


