Abstract
In fish, the pectoral appendage is adjacent to the head, but during vertebrate evolution a long neck region emerged via caudal relocation of the pectoral appendage. The pectoral appendage is comprised of endochondral portions, such as the humerus and the scapula, and a dermal portion, such as the clavicle, that contributes to the shoulder girdle. In the search for clues to the mechanism of the caudal relocation of the pectoral appendage, the cell lineage of the rostral lateral plate mesoderm was analyzed in chickens. It was found that, despite the long neck region in chickens, the origin of the clavicle attached to the head mesoderm ranged between 1 and 14 somite levels. Because the pectoral limb bud and the endochondral pectoral appendage developed on 15–20 and 15–24 somite levels, respectively, the clavicle‐forming region corresponds to the embryonic neck, which suggests that the relocation would have been executed by the expansion of the source of the clavicle. The rostral portion of the clavicle‐forming region overlaps the source of the cucullaris muscle, embraces the pharyngeal arches caudally, and can be experimentally replaced with the head mesoderm to form the cucullaris muscle, which implies that the mesodermal portion could have been the head mesoderm and that the clavicle would have developed at the head/trunk boundary. The link between the head mesoderm and the presumptive clavicle appears to have been the developmental constraint needed to create the evolutionarily conserved musculoskeletal connectivities characterizing the gnathostome neck. In this sense, the dermal girdle of the ganathostomes would represent the wall of the branchial chamber into which the endochondral pectoral appendage appears to have attached since its appearance in evolution.
Keywords: clavicle, development, evolution, neck, paired appendage
Introduction
A vertebrate body is comprised of the head and trunk regions. Intervening between them, the neck region has not attracted as much attention in evolutionary developmental studies (reviewed by Ericsson et al. 2013).
The head and trunk regions developed under different developmental contexts, and the neck region appears to show a mixture of them (reviewed by Romer, 1972; Kuratani, 1997, 2008; Matsuoka et al. 2005; Sambasivan et al. 2011). Although the vertebrate body is characterized by segmental organization, the metamerism in the head originated from pharyngeal arches and rhombomeres, whereas that in the trunk derived from segmentally arranged paraxial mesoderm, somites. Segmental skeletons in the head, branchial skeletons, derived from cephalic neural crest cells; while those in the trunk, vertebrae and ribs, derived from somites (Burke & Nowicki, 2003; Nowicki et al. 2003; Mongera et al. 2013). Connective tissue in the head originated from the cephalic neural crest cells, and that in the trunk derived from the mesoderm (Noden, 1983; Köntges & Lumsden, 1996; Matsuoka et al. 2005; reviewed by Pu et al. 2015). The head muscles derived from the head mesoderm [unsegmented paraxial head mesoderm and head lateral plate mesoderm (LPM); also see below], while the trunk muscles derived from somites. Although myogenic determination and differentiation required myogenic regulatory factors (MRFs), such as Myf5 and MyoD, in the head and trunk muscles, the upstream transcription factors of MRFs differed between them (reviewed by Noden & Francis‐West, 2006; Buckingham & Vincent, 2009; Sambasivan et al. 2011; Tzahor & Evans, 2011; Ericsson et al. 2013; Tzahor, 2015). Head muscles associated with pharyngeal arches are innervated by branchial nerves sprouting from the dorsal roots, whereas trunk muscles are innervated by spinal somatic nerves from the ventral roots (reviewed by Goodrich, 1930). The motor nuclei of the branchial and spinal somatic nerves show the distinct expression patterns of marker genes during development (reviewed by Benninger & McNeil, 2010; Kobayashi et al. 2013; Tada & Kuratani, 2015).
With a typical trunk structure such as the vertebrae, the neck region is characterized by the presence of hypobranchial and cucullaris muscles (McGonnell, 2001; Kuratani, 2008; Ericsson et al. 2013). The hypobranchial muscle includes the tongue and infrahyoid muscles in humans, and is derived from somites (reviewed by Huang et al. 1999). These muscles are innervated by the hypoglossal nerve (cranial nerve XII) and the cervical nerves, which belong to the spinal somatic group (Kuratani et al. 1988). These features suggest that these muscles are of the trunk. However, its connective tissue (tendon and fascia) is derived from cephalic neural crest cells [i.e. circumpharyngeal (CP) crest cells; Noden, 1983; Matsuoka et al. 2005] like the head muscles.
The cucullaris muscle is a gnathostome‐specific muscle referred to as the trapezius and sternocleidomastoid muscles in humans. Although the somitic origin of the cucullaris muscle has been reported, (Noden, 1983; Couly et al. 1993; Huang et al. 1997, 2000; Noden et al. 1999; Piekarski & Olsson, 2007), its contribution is suggested to be minor (Theis et al. 2010). Instead, the muscle had a major contribution from the lateral plate mesoderm (LPM): mesodermal cells located lateral to the paraxial mesoderm (Theis et al. 2010). Moreover, the differentiation timing and gene expression patterns of the cucullaris muscle are not those of trunk muscles, but are those of head muscles (Theis et al. 2010; reviewed by Pu et al. 2015; for branchial nature of the cucullaris muscle, see the review by Diogo et al. 2015). The muscle is innervated by the spinal accessory nerve (cranial nerve XI) and the cervical spinal nerves. These sprouted from the dorsal roots like branchial nerves, and their motor nuclei expressed molecules of branchial motor neurons rather than those of somatic motor neurons (Kobayashi et al. 2013). Its connective tissue is also from the CP crest cells (Matsuoka et al. 2005; Theis et al. 2010). Thus, this muscle represents many features of the branchial muscles.
The hypobranchial and cucullaris muscles connect the skull to the shoulder girdle. The shoulder girdle is a component of the pectoral appendage, and is situated proximally to anchor distally localized fins proper, or free limbs, onto the axial skeleton. The skeleton of the free limbs, such as humerus and ulna, is made up of endochondral bones, while the girdle element includes both endochondral and dermal bones. The endochondral girdle consists of a dorsal scapula and a ventral coracoid, which are arranged rostral with a series of dermal bones, such as a cleithrum, a clavicle and a post‐temporal (Fig. 1A). In fishes, because the cleithrum and the clavicle are close to the branchial arches to construct the caudal wall of the branchial chamber, the neck region is very narrow (Fig. 1A,B; McGonnell, 2001; Clack, 2002; Kuratani, 2008; Ericsson et al. 2013). In tetrapods, however, because the pectoral appendage was relocated caudally during evolution, the long neck region emerged. For example, amphibians have one cervical vertebra, most mammals have seven, and chickens have 14 (Romer & Parsons, 1977; Feduccia & McCrady, 1991; McGonnell, 2001; Clack, 2002; Narita & Kuratani, 2005; Kardong, 2009). In chickens, the only dermal girdle remains as a chevron‐shaped bone referred to as the furcula, which has been homologized with the medially fused clavicle (Fig. 1C; Parker, 1868; Versluys, 1927; Nauck, 1938; Starck, 1979; Yates & Vasconcelos, 2005; Nesbitt et al. 2009; for another view, see Vickaryous & Hall, 2010; Tschopp & Mateus, 2013).
Figure 1.
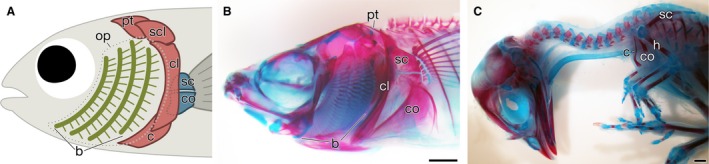
Neck and pectoral fin/limb. (A) A cartoon to show the pectoral girdle in fishes, modified from Feduccia & McCrady (1991). The dotted line shows the position of the removed operculum (op). (B,C) Skeletal preparation of adult Oryzias latipes (B) and Gallus gallus embryo (HH38) (C). In O. latipes, the operculum is removed to show the branchial arches (b). Note that the cleithrum (cl) forms the caudal wall of the branchial chamber in O. latipes. The supracleithrum (scl) and clavicle is lost in O. latipes, whereas the clavicle (c) is the only dermal girdle element in chicken. Abbreviations: co, coracoid; h, humerus; pt, post‐temporal; sc, scapula. Scale bars: 1 mm (B); 2 mm (C).
A major portion of the paired‐appendage skeletons derives from LPM (reviewed by Gumpel‐Pinot, 1984; also see Shearman et al. 2011). The mesoderm can be subdivided into the head and the trunk LPM, and further into the outer somatopleure mesoderm and the inner splanchnic mesoderm. The boundary between the head and the trunk LPM is assumed to be at the same rostrocaudal level as that between the paraxial head mesoderm and somites, but there is no obvious morphological border in the former, as there is in the latter (Fig. 2A; Sambasivan et al. 2011). In 1977, Chevalier found that, in chickens, the clavicle developed from LPM at the level of somite 10–15, and the endochondral pectoral appendage skeletons from LPM at the 15–24 somite levels (reviewed by Gumpel‐Pinot, 1984). Thus, the origin of the pectoral appendage is separated from the head mesoderm by nine somites, which would reflect the caudal relocation of the pectoral appendage and the establishment of the long neck region in the embryonic body level.
Figure 2.
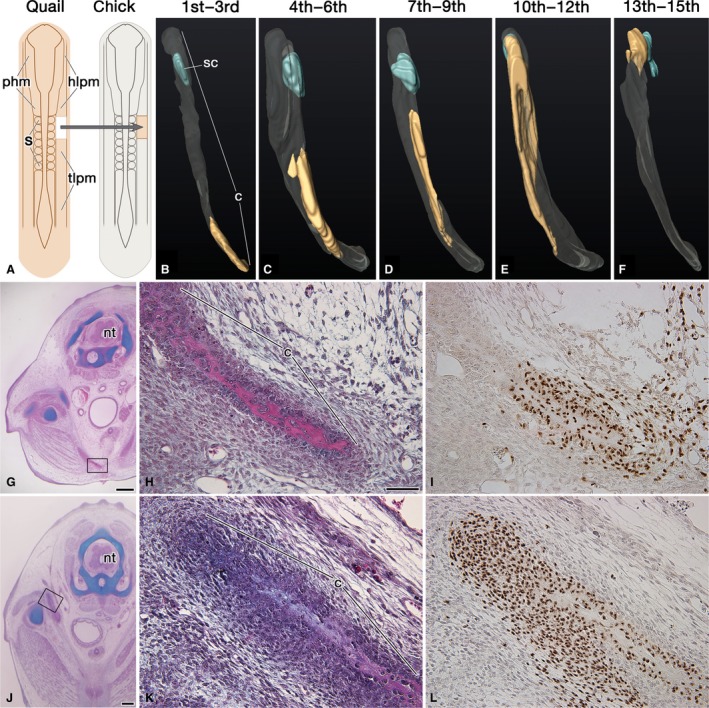
Developmental origin of the clavicle in chickens. (A) Diagram showing homotopic transplantation of the lateral plate mesoderm (LPM). (B–F) Three‐dimensional reconstruction of the clavicle in chimeras. Rostral view of the clavicle in a chimera, in which either LPM lateral to somites 1–3 (B), 4–6 (C), 7–9 (D), or the somatopleure mesoderm lateral to somites 10–12 (E), 13–15 (F) was homotopically transplanted. The clavicle is transparent, and the contribution of the graft is highlighted in yellow. (G–L) The transverse section of the chimera, in which either LPM lateral to somite 1 (G–I) or the somatopleure mesoderm lateral to somite 14 (J–L) was homotopically transplanted. (H and K) are higher magnifications of the boxes in (G and J), respectively. (I and L) are adjacent sections to (H and K), respectively, and show the distribution of QCPN‐positive quail cells. Note that quail cells contribute to the ventral (G–I) and dorsal (J–L) ends of the clavicle. Abbreviations: hlpm, head lateral plate mesoderm; nt, neural tube; phm, paraxial head mesoderm; s, somite; tlpm, trunk lateral plate mesoderm. Scale bars: 200 μm (G, J); 50 μm (H, I, K and L).
To obtain a clue for the mechanism of the caudal relocation of the pectoral appendage, cell lineage of the rostral LPM was analyzed. This analysis has shown that the clavicle originated from just caudal to the head mesoderm: 1–14 somite level LPM contributed to the clavicle. Because the pectoral limb bud and the endochondral pectoral appendage developed from LPM at the somite levels of 15–20 and 15–24, respectively, the origin of the clavicle corresponds to the neck in the embryonic level, which suggests that the relocation would have been achieved by the expansion of the clavicle‐forming domain. The rostral portion of the clavicle‐forming mesoderm was overlapped with a source of the cucullaris muscle (Theis et al. 2010), encircled the pharyngeal arch arteries caudally, and could be experimentally replaced with the head mesoderm to form the cucullaris muscle, implying that the mesodermal portion could be the head mesoderm. Thus, the clavicle would develop at the head/trunk boundary. Considering the long neck in a chicken, the link between the head mesoderm and the presumptive clavicle implies the presence of a developmental constraint, which would provide a basis for the evolutionarily conserved musculoskeletal connectivities characterizing the gnathostome neck. And, finally, the dermal girdle of amniotes also seems to represent the wall of the branchial chamber, which implies that the endochondral pectoral appendage would have evolved just caudal to the wall.
Materials and methods
Animals
Fertilized eggs of the chicken Gallus gallus and the Japanese quail Coturnix coturnix were obtained from local suppliers. The eggs were incubated at 38 °C, and the embryos were staged according to Hamburger and Hamilton (Hamburger & Hamilton, 1951; HH stages).
Japanese medaka Oryzias latipes were obtained from the National BioResource Project (NBRP) Medaka (www.shigen.nig.ac.jp/medaka/).
Scyliorhinus torazame embryos were obtained from the Niigata city aquarium Marinepia Nihonkai. Embryos were staged according to a procedure established by Ballard et al. (1993; B stages).
Grafting procedure
The grafting was performed on HH stage 9–11 chick and quail embryos. A window was created in a chick eggshell, and the embryo was visualized by injecting black watercolor diluted with 0.9% NaCl/distilled water into the subgerminal cavity. With a sharpened tungsten needle, an incision was made unilaterally at the lateral border of three newly developed somites (somite stages +I to +III; Roman numerals indicate the positions of somites counted rostrally from the most newly formed one that is called somite +I; Ordahl, 1993); thereby, the coelom was opened. One microliter of Dispase II (500 IU mL−1 in Tyrode's solution; Sanko Junyaku) was applied to the scar and, after a few minutes, the surface ectoderm over the LPM was peeled. The somatopleure mesoderm was then transversally cut 1.5 somites wide at the rostral level of the stage +III somite and caudal level of the stage +I somite. Finally, the lateral border of the somatopleure mesoderm was cut rostrocaudally, and the mesoderm was removed. A strip of somatopleure mesoderm of quail embryos was isolated at the same axial level in the same manner, and was placed into the scar of the chicken host along the same rostro‐caudal and medio‐lateral axes. For transplantation at the level of somites 1–3 and 4–6, seven somite embryos (HH stage 9) were used due to the high degree of lethality of the chimeras. Because the coelom did not develop in embryos younger than the 9‐somite stage, the splanchnic mesoderm was also transplanted together with the somatopleure mesoderm (Table 1). The chick hosts were reincubated for 3–7 days.
Table 1.
Numbers of homotopic transplantations
| Stage of surgery | Axial level | Type of transplantation | Number of chimeras | Contribution to clavicle |
|---|---|---|---|---|
| 7 somites | Head mesoderm | PHM, SM, SP | 5 | 1 |
| 7 somites | Somites 1 | SM, SP | 5 | 4 |
| 7 somites | Somites 1–3 | SM, SP | 2 | 2 |
| 7 somites | Somites 4–6 | SM, SP | 2 | 2 |
| 9 somites | Somites 7–9 | SM, SP | 2 | 2 |
| 12 somites | Somites 10–12 | SM | 2 | 2 |
| 15–16 somites | Somites 13–15 | SM | 2 | 2 |
| 14–15 somites | Somites 14 | SM | 4 | 3 |
| 15–16 somites | Somites 15 | SM | 4 | 1 |
| 18 somites | Somites 16–18 | SM | 1 | 0 |
PHM, paraxial head mesoderm; SM, somatopleure mesoderm; SP, splanchnic mesoderm.
Heterotopic transplantations were conducted by replacing chick splanchnic and somatopleure mesoderm lateral to somites 1–3 of the seven somite stage embryos with the head mesoderm of seven somite stage quail embryos (Table 2). The head mesoderm was three somites in length and 1.5 somites wide. To avoid contamination of the somites and the LPM at the somite 1–3 levels, a caudal border of the graft was one somite rostral to the first somite. Thus, the rostral border was lateral to the caudal mesencephalon (Fig. 3D). With a sharpened tungsten needle, an incision was made unilaterally between the neural tube and the head mesoderm. After the application of Dispase II, the surface ectoderm was peeled. Then the head mesoderm was transversally cut 1.5 somites wide. The mesoderm was separated from the endoderm, and the lateral border of the mesoderm was cut rostrocaudally. Because there is no boundary between the paraxial and LPM (Noden & Francis‐West, 2006), the graft contained both parts. The chicken hosts were reincubated for 7 days.
Table 2.
Numbers of heterotopic transplantations
| Stage of surgery | Donor level | Host level | Number of chimeras | Labels in clavicle | Labels in cucullaris |
|---|---|---|---|---|---|
| 6–7 somites | Head mesoderm | Somites 1–3 | 5 | 4 | 5 |
Figure 3.
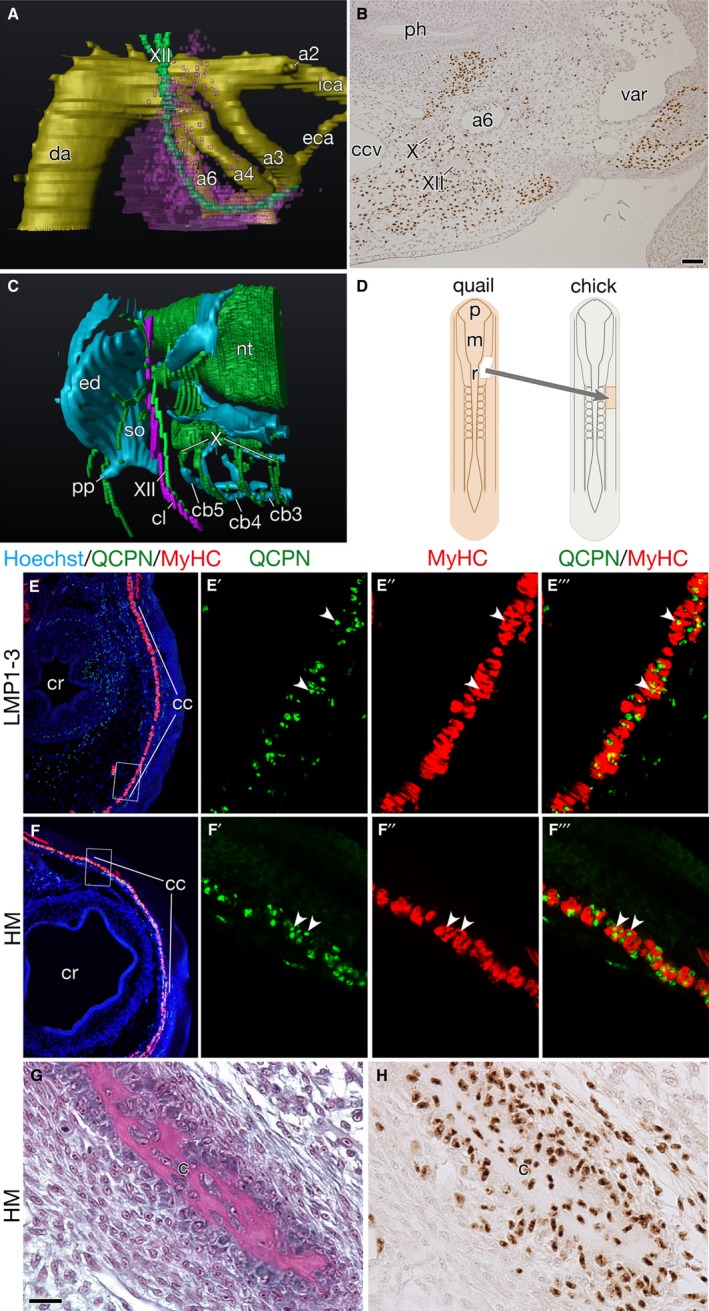
Developmental characteristics of the clavicle‐forming mesoderm. (A,B) Distribution of cells derived from LPM adjacent to somites 1–3 at HH stage 24. (A) Three‐dimensional reconstruction of the chick–quail chimera showing the distribution of quail cells (pink) around the pharyngeal arch arteries (a2–a6). (B) Horizontal section of the chimera immunostained with QCPN antibody. Note that quail cells are found along the hypoglossal nerve (XII) and close to the sixth pharyngeal arch artery (a6). (C) Three‐dimensional reconstruction of the head–trunk interface in an Oryzias latipes hatchling. Note that the cleithrum develops attached to the hypoglossal nerve. (D) Schematic drawing shows the heterotopic transplantation. The quail head mesoderm was transplanted into the place of LPM adjacent to somites 1–3 in the chicken host. (E–E''') Transverse section of chick–quail chimera at HH stage 34+, in which LPM beside somites 1–3 were homotopically replaced with that of the donor quail, showing the distribution of the cucullaris muscle (cc). (E'–E''') are higher magnifications of the box in (E). Note that QCPN‐positive quail nuclei (arrow heads) are found in the myosin heavy chain (MyHC) positive cucullaris myofibers (E'–E'''). (F–F''') Transverse section of chick–quail chimera at HH stage 34+, in which the quail head mesoderm was heterotopically transplanted, as shown in (D). (F'–F''') are higher magnifications of the box in (F). Note that the head mesoderm formed the cucullaris muscle with normal morphology (F), and that QCPN‐positive quail nuclei (arrowheads) are found in the MyHC‐positive cucullaris myofibers (F'–F'''). (G,H) Transverse section of the clavicle in the heterotopic head mesoderm chimera. (H) is an adjacent section to (G). Note that QCPN‐positive quail cells contribute to the clavicle. Abbreviations: cb3–5, ceratobranchials 3–5; ccv, common cardinal vein; cr, crop; da, dorsal aorta; eca, external carotid artery; ed, endoskeletal disc; ica, internal carotid artery; m, mesencephalon; p, prosencephalon; ph, pharynx; pp, postcoracoid process; r, rhombomere; so, scapulocoracoid; var, ventral aortic root; X, vagus nerve. Scale bars: 50 μm (B); 20 μm (G and H).
Histology, immunohistochemistry and 3D reconstruction
Most of the chick–quail chimeras were fixed with Serra's fixative (Serra, 1946). Hematoxylin, eosin and 0.1% alcian blue were used to stain the 6‐μm‐thick paraffin sections. Immunohistochemistry was performed using quail‐specific antibody QCPN (Developmental Studies Hybridoma Bank, DSHB). A Vectastain ABC Elite kit (Vector Laboratories) was used to visualize the immunoreaction. Images were recorded with a DP70 digital camera (Olympus) attached to a light microscope. Histological sections were reconstructed with AVIZO® (Visualization Sciences Group).
Some of the chick–quail chimeras were fixed with 4% paraformaldehyde/phosphate‐buffered saline and processed for cryosectioning (8 μm). The sections were subjected to immunohistochemistry using QCPN and anti‐myosin heavy chain antibodies (MF20, DSHB) followed by goat‐anti‐mouse IgG1 488 and goat anti‐mouse IgG2b 546 antibodies (Life Technologies). Images were taken with a confocal microscope (LSM710NLO, Carl Zeiss) after staining the nuclei with Hoechst 33342 (Life Technologies).
Skeletal staining
Embryos were fixed with 20% formalin for several days, and dehydrated with a series of methanol. The pigment was removed using Dent's fixative (hydrogen peroxide : methanol : dimethyl sulfoxide = 5 mL : 36 mL : 9 mL). Samples were stained overnight with Alizarin red (0.005% in 70% ethanol). After dehydration with an ethanol series, the cartilage was stained with Alcian blue (95% ethanol : acetic acid : alcian blue 8GX = 800 mL : 200 mL : 20 mg) for several days. The samples were digested with tripsin (1 g dissolved in 30 mL saturated sodium tetraborate and 70 mL water), cleared using a glycerol series and stored in 80% glycerol.
Results
Clavicle progenitor is adjacent to the head mesoderm
To trace the cell lineage of the LPM, a chick–quail homotopic transplantation was conducted (Fig. 2A; Table 1). The LPM at somite levels 1–3 formed the ventromedial extremity of the clavicle (Fig. 2B). For a LPM caudal to the somite‐3 level, there was a tendency for a more rostral LPM to contribute to the more ventromedial portion of the clavicle (Fig. 2C–E). The dorsolateral edge of the clavicle was derived from the somatopleure mesoderm adjacent to somites 13–15 (Fig. 2F). While the LPM at the somite‐1 level formed the ventral edge of the clavicle (Fig. 2G–I), the 1‐somite length of the head mesoderm (paraxial mesoderm and LPM) just rostral to somite 1 did not contribute to the formation of the bone in four of five chimeras (data not shown). While the somatopleure mesoderm at the somite‐14 level developed into the dorsolateral extremity of the clavicle (Fig. 2J–L), that at the somite‐15 level did not form the bone in three of four chimeras (data not shown). The quail cell contribution found in the head mesoderm and the somite‐15 level chimeras (Table 1) appeared to be due to contamination of the cells in the neighboring clavicle‐forming region, because their cell numbers were very few compared with that in the other chimeras. Also, the absence of quail cells in somite levels 1 and 14 chimeras (Table 1) was due to a miscounting of the somite number, as the first somite is sometimes hard to discern. The somatopleure mesoderm lateral to somites 16–18 did not take part in the clavicle formation (data not shown). These observations explain how the clavicle is developmentally originated from the LPM at somite levels 1–14.
Developmental characteristics of the clavicle‐forming mesoderm
The LPM at somite 1–3 levels is a known source of cucullaris muscle that represents gene expression patterns characteristic to head muscles during its development (Theis et al. 2010). In order to investigate the developmental relationship that this particular mesoderm has with the head, the distribution of its cells was observed at HH stage 24 (Hamburger & Hamilton, 1951), the point at which the caudal pharyngeal arch arteries fully develop (Hiruma & Hirakow, 1995). A 3D reconstruction of the chimera showed that the mesodermal cells were distributed along the hypoglossal nerve (cranial nerve XII) to reach the pharyngeal floor and encircled the segmentally arranged pharyngeal arch arteries caudally (Fig. 3A; Video S1). In particular, the quail cells were found close to the sixth pharyngeal arch artery (Fig. 3B). The distribution pattern of the cells is reminiscent of the developing dermal girdle, the cleithrum, in the teleost fish medaka O. latipes, in which the bone develops along the XII nerve caudal to the branchial skeleton (Fig. 3C; Video S2).
For the development of cucullaris muscle, the importance of both embryonic environment and developmental competence is suggested, as the LPM beside somites 1–3 could not form the muscle at the somite 21–23 levels, and because the LPM beside somites 10–12 could not form the muscle at the somite 1–3 levels (Theis et al. 2010). Then, to confirm whether the head mesoderm could respond to the embryonic signals, it was heterotopically transplanted at the somite 1–3 levels (Fig. 3D; Table 2). The caudal limit of the graft was one somite length rostral to the first somite to prevent contamination of mesodermal cells at the somite 1–3 levels. After 7 days of operation (HH stage 34+), quail cells derived from the graft formed cucullaris muscle comparable to that in the control chimera, in which the LPM at the somite 1–3 levels was replaced homotopically with that of a quail donor (Fig. 3E–F'''). In the heterotopic chimera, the cells that originated from the head mesoderm also contributed to the ventromedial tip of the clavicle (Fig. 3G,H).
Discussion
Head/trunk interface as a source of clavicle and cucullaris muscle
The contribution of somite 1–9 levels of LPM to the appendage skeleton has not been investigated (Chevallier, 1977). The current results show that LPM at the 1–14 somite levels contributed to the clavicle (Fig. 4A,B). The mesodermal portion at the somite 1–3 levels overlapped the major source of the cucullaris muscle (Theis et al. 2010), which shows many traits of the head muscle. These cells were distributed along the arc made by the XII nerve, and caudally circumscribed the pharyngeal arch arteries (Fig. 3A,B; Video S1). The horseshoe‐shaped distribution corresponds to a CP ridge (Kuratani & Kirby, 1991, 1992; reviewed by Kuratani, 1997). The ridge represents the caudal portion of cephalic neural crest cells that are associated with pharyngeal arches. Thus, although the caudal limits of the 6th pharyngeal arch are not obvious morphologically, at least a portion of these mesodermal cells would comprise the head mesoderm (also see Ericsson et al. 2013). Supporting this assumption, although the cucullaris muscle development required both environmental signals and an inherent competence that was associated with its axial level (Theis et al. 2010), both the LPM at the somite 1–3 levels and the head mesoderm had the potential to respond to the environmental signals and differentiate into the cucullaris muscle (Fig. 3E–F'''). Thus, the chicken dermal girdle appears to include the head/trunk interface.
Figure 4.
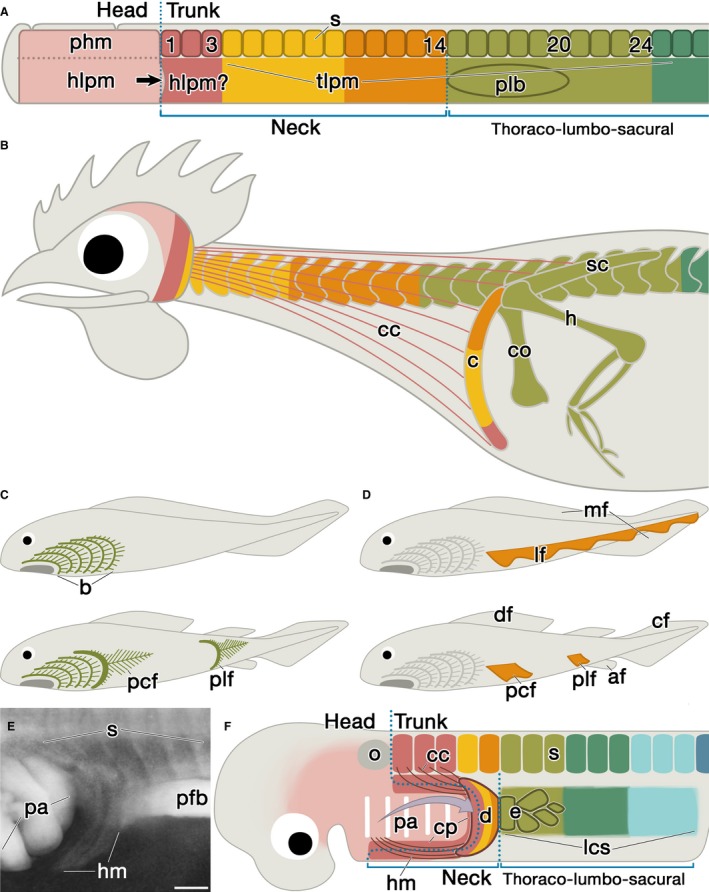
Development and evolution of the neck and the pectoral appendage. (A,B) Cartoons show chickens in early embryonic (A) and adult (B) stages. Colors indicate the axial position in the early stage. Whereas paraxial head mesoderm and somites form the axial skeletons (Noden, 1984; Huang et al. 2000), LPM develops into the appendicular skeletons. The embryonic body can be divided into head and trunk regions, and the latter further into the limb‐incompetent neck region and the limb‐competent thoraco‐lumbo‐sacral region. The pectoral limb bud (plb) develops at the rostral margin of the thoraco‐lumbo‐sacral region. Note that the embryonic neck corresponds to the presumptive clavicle region in this early stage (A). The rostral part of the neck LPM could be the head mesoderm. (C,D) Classic theories on the evolution of paired appendages. The gill‐arch theory (C; Gegenbaur, 1876, 1878) posits that the appendage is made up of the transformed posterior branchial arches. The pelvic fins (plf) are assumed to have migrated caudally. The lateral fin‐fold theory (D; Thacher, 1877; Mivart, 1879; Balfour, 1881) posits that the ancestral animal possessed a paired lateral fin‐fold (lf) along the length of the trunk, and the structure divided rostrocaudally to form both pectoral (pcf) and pelvic appendages. (E) The circumpharyngeal ridge in the Scyliorhinus torazame embryo (B 27). Note that the hypobranchial muscle anlagen (hm) grow ventrorostrally between the pharyngeal arches (pa) and the pectoral fin bud (pfb). Rostral is to the left. (F) Schematic drawing showing the embryonic architecture of the neck region in late embryonic stage. The head LPM expands caudally to form the pharyngeal arches (arrow in A; Kuratani, 1997). As a result, the neck region contains the presumptive clavicle domain, the caudal pharyngeal arches, and the horseshoe‐shaped circumpharyngeal ridge (cp; reviewed by Kuratani, 1997) as their border. In the CP ridge, the caudal margin of the cephalic neural crest cells and LPM adjacent to the most rostral somites overlap. From the pharyngeal arches, the parathyroid glands develop in tetrapods and the internal gill buds in fishes (Okabe & Graham, 2004). In the CP ridge, the cucullaris and hypobranchial muscles develop (viz. circumpharyngeal muscles; Kusakabe et al. 2011). Note that the dermal shoulder girdle (d) includes the CP ridge, and forms the caudal wall of the branchial chamber. The endochondral pectoral appendage (e) develops only in the rostral margin of the lateral competence stripe (lcs; Yonei‐Tamura et al. 2008), which adjoins the presumptive dermal girdle. The arrow indicates growth of the second pharyngeal arch, which develops into the operculum in fishes and the platysma muscle in amniotes (reviewed by Graham & Richardson, 2012). Abbreviations: af, anal fin; cf, caudal fin; df, dorsal fin; mf, medial fin‐fold; o, otic vesicle. Scale bar: 200 μm (E).
Dermal girdle as the caudal margin of the branchial chamber
During evolution, the long neck region was formed by the caudal relocation of the pectoral appendage (Feduccia & McCrady, 1991; McGonnell, 2001; reviewed by Clack, 2002; Kuratani, 2008; Kardong, 2009). Thus, it would be natural to assume that it was achieved either by caudal transposition of the presumptive whole pectoral appendage or by intercalation of the neck domain between the head mesoderm and the presumptive pectoral appendage. However, this is actually not the case. In chickens, the pectoral limb bud and the endochondral pectoral appendage developed from the LPM at the somite levels of 15–20 and 15–24, respectively (Chevallier, 1977; Tickle, 2015). Because the clavicle‐forming LPM (1–14 somite levels) intervenes between the head mesoderm and these LPM regions (Fig. 4A,B), the neck can be defined as the source of the clavicle in the embryonic level. These observations suggest that the caudal relocation would have been attained by the expansion of the clavicle‐forming domain. Because the connection between the head mesoderm and the presumptive clavicle is not lost even in this long‐neck animal, there would be some cell‐to‐cell interactions between them, which would be a prerequisite in some developmental contexts known as a developmental constraint (Wagner, 1994; Nagashima et al. 2013).
One of the causes for the constraint could be the CP ridge (Fig. 3A; also see above). The ridge harbors the progenitor cells of the cucullaris muscle (Fig. 3A; Froriep, 1885; Theis et al. 2010), the hypobranchial muscle (i.e. hypoglossal cord; Froriep, 1885; Hazelton, 1970; O'Rahilly & Müller, 1984; Huang et al. 1999; Lours‐Calet et al. 2014), the connective tissue (i.e. CP crest cells; Kuratani, 1997; McGonnell et al. 2001; Matsuoka et al. 2005; Theis et al. 2010) and the clavicle (this study). Moreover, the area is adjacent to the origin of the occipital bone of the skull (1–5 somites; Huang et al. 2000), which provides an attachment for the cucullaris muscle. Hence, all the materials of the structures characterizing the neck were already set in the CP ridge in early development. And the latest finding showed that the progenitor cells of the cucullaris muscle are shared by the cardiac muscle in mice (Lescroart et al. 2015). Because the development of these many structures would require complicated inductive interactions between the CP crest cells, the head mesoderm, the clavicle‐forming LPM, somites and presumably pharyngeal endoderm under appropriate spatiotemporal conditions, it would be almost impossible to change the developmental program established in the common ancestor from which all the vertebrates with paired appendages should have originated. As a result, the program seems to form the constraint, which would help to explain the evolutionarily conserved connectivities between the skeletons (skull and shoulder girdle) and the muscles (hypobranchial and cucullaris muscles) among gnathostomes (Fig. 4F; McGonnell, 2001; Kuratani, 2008; Ericsson et al. 2013).
Against the evolutionary process, it would be possible to deduce that if the cervical domain were shortened by shrinking the clavicle‐forming region to less than three somites long, the whole clavicle would develop from the cells along the nerve XII as shown in Fig. 3A, and the pectoral appendage skeletons would be situated close to the pharyngeal arches, which is similar to the morphological relationships between the nerve XII, the pectoral appendage and the branchial arches in the teleost fish (Figs 1B and 3C). Hence, the dermal girdle of chickens also seems to represent a part of the caudal wall of the branchial chamber. This assumption agrees with the fact that in both teleost fishes and amniotes the dermal girdle is attached rostrally by the second pharyngeal arch derivative (the operculum and the platysma muscle, respectively), which is formed by the growth of the second pharyngeal arch over the subsequent pharyngeal arches (Fig. 4F; reviewed by Graham & Richardson, 2012; Richardson et al. 2012).
In this respect, it is important to note that the paired appendage equipped with girdle elements originated exclusively from around the branchial chamber in stem gnathostomes (Janvier, 1996; Min & Schultze, 2001; Coates, 2003; Wilson et al. 2007). For example, the earliest pectoral appendage is found in osteostracans, in which the endochondral fin proper was articulated to the dermal plates circumscribing the branchial arches via an endochondral girdle (Janvier, 1978, 1996; Coates, 2003; Janvier et al. 2004). Although the homology between each dermal plate and each dermal girdle is yet to be unveiled (McGonnell, 2001), if the dermal girdle in amniotes represents the caudal wall of the branchial chamber, the endochondral pectoral appendage would have always been associated with the branchial wall since its appearance in evolution.
Implications for the evolutionary origin of the paired appendage
Since the 1870s, the evolutionary origin of paired appendages in vertebrates has remained an open question (reviewed by Goodrich, 1906, 1930; Jarvik, 1965, 1980; Bemis & Grande, 1999; Coates, 2003; Gillis et al. 2009; Gillis & Shubin, 2009; Pieretti et al. 2015). There were two classic theories on this enigma. The gill‐arch hypothesis (Fig. 4C; Gegenbaur, 1876, 1878) proposes that the two caudal gill skeletons would have transformed into the pectoral and pelvic appendages. In this hypothesis, the appendage skeleton was regarded as the head structure. This hypothesis, however, has become somewhat of a ‘historical curiosity’ (Coates, 2003) due to some problems. For example, Goodrich (1906, 1930) argued that the gill skeletons developed inwardly in the wall of the alimentary canal, while the appendage skeletons were formed outside in the body wall.
As the more accepted model, the lateral fin‐fold theory (Thacher, 1877; Mivart, 1879; Balfour, 1881) claims that, as medial fin‐folds divided into unpaired medial fins during development, a pair of continuous longitudinal fin‐folds on the lateral flank of the ancestral animal would have divided into rostral and caudal versions to form the pectoral and pelvic appendages, respectively (Fig. 4D). As opposed to the gill‐arch theory, this hypothesis regarded the appendage skeleton as the trunk structure. According to this hypothesis, the paired appendages could emerge everywhere on the lateral trunk. Actually, in early‐stage chicken embryos, the neck and interlimb levels of the LPM with its associated ectoderm, endoderm, somites and intermediate mesoderm were reported to be able to differentiate into limbs when they were implanted into the body cavity (Stephens et al. 1989). Even in late developmental stages, the application of certain tissues or substances can induce supernumerary limb initiation in the interlimb region in various osteichthyan species (Balinsky, 1974; Hornbruch & Wolpert, 1991; Cohn et al. 1995; Ohuchi et al. 1995; Abud et al. 1996; Tanaka et al. 2000; Yonei‐Tamura et al. 2008). Because gene expressions in chondrichthyes imply the presence of a limb‐forming field in the lateral flank as well, the appendage‐forming competence is assumed to have allowed various rostrocaudal positions of paired appendages in early gnathostomes (Yonei‐Tamura et al. 2008).
The current study, however, found that the relative position of the endochondral pectoral appendage has been fixed at the rear of the branchial chamber since its emergence. This perspective is consistent with the observation that the pectoral appendage is only at the cervical/thoracic transition as characterized by the particular Hox expressions in various gnathostome species (Burke et al. 1995; Cohn et al. 1997; reviewed by Burke, 2000; Duboc & Logan, 2011; Tanaka, 2013; Tickle, 2015). One point that makes it difficult to understand the position of the pectoral appendage is the presence of the neck. Unlike the classic study (Stephens et al. 1989), Lours & Dietrich (2005) showed that the neck region (1–14 somite levels) and the interlimb region (21–24 somite levels) were not equivalent for the developmental competency of the limb bud, and the former was ‘limb‐incompetent’. Because the pectoral and pelvic limb buds appear at the level of somites 15–20 and 25–30, respectively, in chickens, the LPM at somite levels 15–30 appears to possess limb‐competence (i.e. lateral competence stripe; Yonei‐Tamura et al. 2008), and the pectoral limb bud is induced at its rostral margin.
In adult teleost fish, the neck region is difficult to find. However, as the hypobranchial and cucullaris muscles are found in various gnathostome species (McGonnell, 2001; Ericsson et al. 2013), the limb‐incompetent neck LPM and adjacent limb‐competent thoraco‐lumbo‐sacral LPM are expected to be found in the teleost fish embryos, although the former would be very narrow. As an outgroup species, although chondrichthyans secondarily lost the dermal girdle (Romer & Parsons, 1977), the CP ridge is found as the route of the ventrorostrally growing hypobranchial muscle anlage, to which the pectoral fin bud is adjoined (Fig. 4E; also see Kuratani, 1997). Thus, the pectoral fin/limb bud and the endochondral pectoral appendage would be structures presumably induced only at the rostral margin of the lateral competence stripe adjacent to the presumptive branchial wall (Fig. 4F).
Seemingly, the current proposal might be reminiscent of the gill‐arch hypothesis. However, the two differ in that the gill‐arch hypothesis supposed that the appendage skeleton was made up entirely of the head structure, while here it is presumed that the endochondral portion is the structure of the trunk. However, such dichotomy would not be applicable for the dermal girdle. As mentioned above, the hypobranchial and cucullaris muscles more or less possess features of both the head and the trunk. And, the dermal girdle would also fall into such transitional structures, which is supported by the contribution of the CP crest cells to the clavicle (McGonnell et al. 2001; Matsuoka et al. 2005). The transitional features appear to originate from development associated with the CP ridge (Fig. 4F). The developmental origin of the chicken clavicle would help to fill the morphological gap between the fishes and the tetrapods, and could serve to explain the unique features of the neck and the evolutionary origin of the pectoral appendage.
Author contributions
H.N. and F.S. designed the research. H.N., F.S., K.W., M.S. and A.C. performed the experiments. H.N., F.S. and N.S. wrote the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Supporting information
Video S1. Three‐dimensional reconstruction of the stage‐24 chick–quail chimera embryo shown in Fig. 3A. Rostral is to the right.
Video S2. Three‐dimensional reconstruction of the hatchling of O. latipes shown in Fig. 3C. Rostral is to the right.
Acknowledgements
The authors deeply thank Tomoaki Suzuki and Kozue Shibuya at the Niigata city aquarium Marinepia Nihonkai for collecting and providing the shark embryos. The authors also acknowledge Kiyoshi Naruse of the National Institute for Basic Biology for providing us with medaka fish through the National BioResource Project (NBRP) Medaka of MEXT, Japan. The monoclonal antibodies (MF20 developed by Donald A. Fischman; QCPN by Bruce M. Carlson and Jean A. Carlson) were obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the National Institute of Child Health and Human Development, and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, USA. This work was supported by Takeda Science Foundation, and JSPS KAKENHI Grant Numbers 22790196, 24590233 and 15K08130 to H.N.
References
- Abud HE, Skinner JA, McDonald FJ, et al. (1996) Ectopic expression of Fgf‐4 in chimeric mouse embryos induces the expression of early markers of limb development in the lateral ridge. Dev Genet 19, 51–65. [DOI] [PubMed] [Google Scholar]
- Balfour FM (1881) On the development of the skeleton of the paired fins of Elasmobranchii, considered in relation to its bearings on the nature of the limbs of the Vertebrata. Proc Zool Soc London 1881, 656–671. [Google Scholar]
- Balinsky BI (1974) Supernumerary limb induction in the Anura. J Exp Zool 188, 195–201. [DOI] [PubMed] [Google Scholar]
- Ballard WW, Mellinger J, Lechenault HA (1993) A series of normal stages for development of Scyliorhinus canicula, the lesser spotted dogfish (Chondrichthyes: Scyliorhinidae). J Exp Zool 267, 318–336. [Google Scholar]
- Bemis WE, Grande L (1999) Development of the median fins of the North American Paddlefish (Polyodon spatula), and a reevaluation of the lateral fin‐fold hypothesis In: Mesozoic Fishes 2. (eds Arratia G, Schultze HP.), pp. 41–68. München: Verlag. [Google Scholar]
- Benninger B, McNeil J (2010) Transitional nerve: a new and original classification of a peripheral nerve supported by the nature of the accessory nerve (CN XI). Neurol Res Int 2010, 476 018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M, Vincent SD (2009) Distinct and dynamic myogenic populations in the vertebrate embryo. Curr Opin Genet Dev 19, 444–453. [DOI] [PubMed] [Google Scholar]
- Burke AC (2000) Hox genes and the global patterning of the somitic mesoderm. Curr Top Dev Biol 47, 155–181. [PubMed] [Google Scholar]
- Burke AC, Nowicki JL (2003) A new view of patterning domains in the vertebrate mesoderm. Dev Cell 4, 159–165. [DOI] [PubMed] [Google Scholar]
- Burke AC, Nelson CE, Morgan BA, et al. (1995) Hox genes and the evolution of vertebrate axial morphology. Development 121, 333–346. [DOI] [PubMed] [Google Scholar]
- Chevallier PA (1977) Origine des ceintures scapulaires et pelviennes chez l'embryon d'oiseau. J Embryol Exp Morphol 42, 275–292. [Google Scholar]
- Clack JA (2002) Gaining Ground. The Origin and Evolution of Tetrapods. Bloomington: Indiana University Press. [Google Scholar]
- Coates M (2003) The evolution of paired fins. Theory Biosci 122, 266–287. [Google Scholar]
- Cohn MJ, Izpisua‐Belmonte JC, Abud H, et al. (1995) Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell 80, 739–746. [DOI] [PubMed] [Google Scholar]
- Cohn MJ, Patel K, Krumlauf R, et al. (1997) Hox9 genes and vertebrate limb specification. Nature 387, 97–101. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM (1993) The triple origin of skull in higher vertebrates: a study in quail‐chick chimeras. Development 117, 409–429. [DOI] [PubMed] [Google Scholar]
- Diogo R, Kelly RG, Christiaen L, et al. (2015) A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature 520, 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboc V, Logan MP (2011) Regulation of limb bud initiation and limb‐type morphology. Dev Dyn 240, 1017–1027. [DOI] [PubMed] [Google Scholar]
- Ericsson R, Knight R, Johanson Z (2013) Evolution and development of the vertebrate neck. J Anat 222, 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feduccia A, McCrady E (1991) Torrey's Morphogenesis of the Vertebrates, 5th edn New York: John Wiley. [Google Scholar]
- Froriep A (1885) Über anlagen von sinnesorganen am facialis, glossopharyngeus und vagus, über die genetische stellung des vagus zum hypoglossus, und über die herkunft der zungenmuskulatur. Arch Anat Physiol 1885, 1–55. [Google Scholar]
- Gegenbaur C (1876) Zur morphologie der gliedmassen der wirbelthiere. Morph Jahrb 2, 396–420. [Google Scholar]
- Gegenbaur C (1878) Elements of Comparative Anatomy. London: MacMillan. [Google Scholar]
- Gillis JA, Shubin N (2009) The evolution of gnathostome development: insight from chondrichthyan embryology. Genesis 47, 825–841. [DOI] [PubMed] [Google Scholar]
- Gillis JA, Dahn RD, Shubin NH (2009) Shared developmental mechanisms pattern the vertebrate gill arch and paired fin skeletons. Proc Natl Acad Sci USA 106, 5720–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich ES (1906) Notes on the development, structure and origin of median and paired fins of fish. Q J Microsc Sci 50, 24–376. [Google Scholar]
- Goodrich ES (1930) Studies on the Structure and Development of Vertebrates. London: MacMillan. [Google Scholar]
- Graham A, Richardson J (2012) Developmental and evolutionary origins of the pharyngeal apparatus. Evodevo 3, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumpel‐Pinot M (1984) Muscle and skeleton of limbs and body wall In: Chimaeras in Developmental Biology. (eds Le Douarin N, McLaren A.), pp. 281–310. London: Academic Press. [Google Scholar]
- Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88, 49–91. [PubMed] [Google Scholar]
- Hazelton RD (1970) A radioautographic analysis of the migration and fate of cells derived from the occipital somites in the chick embryo with specific reference to the development of the hypoglossal musculature. J Embryol Exp Morphol 24, 455–466. [PubMed] [Google Scholar]
- Hiruma T, Hirakow R (1995) Formation of the pharyngeal arch arteries in the chick embryo. Observations of corrosion casts by scanning electron microscopy. Anat Embryol 191, 415–423. [DOI] [PubMed] [Google Scholar]
- Hornbruch A, Wolpert L (1991) The spatial and temporal distribution of polarizing activity in the flank of the pre‐limb‐bud stages in the chick embryo. Development 111, 725–731. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Ordahl CP, et al. (1997) The fate of the first avian somite. Anat Embryol 195, 435–449. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Izpisua‐Belmonte JC, et al. (1999) Origin and development of the avian tongue muscles. Anat Embryol 200, 137–152. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Patel K, et al. (2000) Contribution of single somites to the skeleton and muscles of the occipital and cervical regions in avian embryos. Anat Embryol 202, 375–383. [DOI] [PubMed] [Google Scholar]
- Janvier P (1978) Les nageoires paires des ostéostracés et la position systématique des céphalaspidomorphes. Ann Paleontol 64, 113–142. [Google Scholar]
- Janvier P (1996) Early Vertebrates. Oxford: Clarendon Press. [Google Scholar]
- Janvier P, Arsenault M, Desbiens S (2004) Calcified cartilage in the paired fins of the osteostracan Escuminaspis laticeps (Traquair 1880), from the Late Devonian of Miguasha (Québec, Canada), with a consideration of the early evolution of the pectoral fin endoskeleton in vertebrates. J Vertebr Paleontol 24, 773–779. [Google Scholar]
- Jarvik E (1965) On the origin of girdles and paired fins. Isr J Zool 14, 141–172. [Google Scholar]
- Jarvik E (1980) Basic Structure and Evolution of Vertebrates. London: Academic Press. [Google Scholar]
- Kardong KV (2009) Vertebrates: Comparative Anatomy, Function, Evolution, 5th edn New York: McGraw‐Hill. [Google Scholar]
- Kobayashi N, Homma S, Okada T, et al. (2013) Elucidation of target muscle and detailed development of dorsal motor neurons in chick embryo spinal cord. J Comp Neurol 521, 2987–3002. [DOI] [PubMed] [Google Scholar]
- Köntges G, Lumsden A (1996) Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development 122, 3229–3242. [DOI] [PubMed] [Google Scholar]
- Kuratani S (1997) Spatial distribution of postotic crest cells defines the head/trunk interface of the vertebrate body: embryological interpretation of peripheral nerve morphology and evolution of the vertebrate head. Anat Embryol 195, 1–13. [DOI] [PubMed] [Google Scholar]
- Kuratani S (2008) Evolutionary developmental studies of cyclostomes and the origin of the vertebrate neck. Dev Growth Differ 50, S189–S194. [DOI] [PubMed] [Google Scholar]
- Kuratani SC, Kirby ML (1991) Initial migration and distribution of the cardiac neural crest in the avian embryo: an introduction to the concept of the circumpharyngeal crest. Am J Anat 191, 215–227. [DOI] [PubMed] [Google Scholar]
- Kuratani SC, Kirby ML (1992) Migration and distribution of the circumpharyngeal crest cells in the avian embryo. Anat Rec 234, 263–280. [DOI] [PubMed] [Google Scholar]
- Kuratani S, Tanaka S, Ishikawa Y, et al. (1988) Early development of the hypoglossal nerve in the chick embryo as observed by the whole‐mount nerve staining method. Am J Anat 182, 155–168. [DOI] [PubMed] [Google Scholar]
- Kusakabe R, Kuraku S, Kuratani S (2011) Expression and interaction of muscle‐related genes in the lamprey imply the evolutionary scenario for vertebrate skeletal muscle, in association with the acquisition of the neck and fins. Dev Biol 350, 217–227. [DOI] [PubMed] [Google Scholar]
- Lescroart F, Hamou W, Francou A, et al. (2015) Clonal analysis reveals a common origin between nonsomite‐derived neck muscles and heart myocardium. Proc Natl Acad Sci USA 112, 1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lours C, Dietrich S (2005) The dissociation of the Fgf‐feedback loop controls the limbless state of the neck. Development 132, 5553–5564. [DOI] [PubMed] [Google Scholar]
- Lours‐Calet C, Alvares LE, El‐Hanfy AS, et al. (2014) Evolutionarily conserved morphogenetic movements at the vertebrate head–trunk interface coordinate the transport and assembly of hypopharyngeal structures. Dev Biol 390, 231–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, et al. (2005) Neural crest origins of the neck and shoulder. Nature 436, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonnell IM (2001) The evolution of the pectoral girdle. J Anat 199, 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonnell IM, McKay IJ, Graham A (2001) A population of caudally migrating cranial neural crest cells: functional and evolutionary implications. Dev Biol 236, 354–363. [DOI] [PubMed] [Google Scholar]
- Min Z, Schultze HP (2001) Interrelationships of basal osteichthyans In: Major Events in Early Vertebrate Evolution. (ed. Ahlberg PE.), pp. 289–314. London: Taylor & Francis. [Google Scholar]
- Mivart SG (1879) Notes on the fins of elasmobranchs, with considerations on the nature and homologues of vertebrate limbs. Trans Zool Soc Lond 10, 439–484. [Google Scholar]
- Mongera A, Singh AP, Levesque MP, et al. (2013) Genetic lineage labeling in zebrafish uncovers novel neural crest contributions to the head, including gill pillar cells. Development 140, 916–925. [DOI] [PubMed] [Google Scholar]
- Nagashima H, Hirasawa T, Sugahara F, et al. (2013) Origin of the unique morphology of the shoulder girdle in turtles. J Anat 223, 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita Y, Kuratani S (2005) Evolution of the vertebral formulae in mammals: a perspective on developmental constraints. J Exp Zool B304, 91–106. [DOI] [PubMed] [Google Scholar]
- Nauck ET (1938) Extremitätenskelett der Tetrapoden In: Handbuch der Vergleichenden Anatomie der Wirbeltiere. (eds Bolk L, Göppert E, Kallius E, Lubosch W.), Bd 5, pp. 71–248. Berlin: Urban & Schwarzenberg. [Google Scholar]
- Nesbitt SJ, Turner AH, Spaulding M, et al. (2009) The theropod furcula. J Morphol 270, 856–879. [DOI] [PubMed] [Google Scholar]
- Noden DM (1983) The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am J Anat 168, 257–276. [DOI] [PubMed] [Google Scholar]
- Noden DM (1984) The use of chimeras in analyses of craniofacial development In: Chimaeras in Developmental Biology. (eds Le Douarin N, McLaren A.), pp. 241–280. London: Academic Press. [Google Scholar]
- Noden DM, Francis‐West P (2006) The differentiation and morphogenesis of craniofacial muscles. Dev Dyn 235, 1194–1218. [DOI] [PubMed] [Google Scholar]
- Noden DM, Marcucio R, Borycki AG, et al. (1999) Differentiation of avian craniofacial muscles: I. Patterns of early regulatory gene expression and myosin heavy chain synthesis. Dev Dyn 216, 96–112. [DOI] [PubMed] [Google Scholar]
- Nowicki JL, Takimoto R, Burke AC (2003) The lateral somitic frontier: dorso–ventral aspects of anterior–posterior regionalization in avian embryos. Mech Dev 120, 227–240. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Nakagawa T, Yamauchi M, et al. (1995) An additional limb can be induced from the flank of the chick embryo by FGF4. Biochem Biophys Res Commun 209, 809–816. [DOI] [PubMed] [Google Scholar]
- Okabe M, Graham A (2004) The origin of the parathyroid gland. Proc Natl Acad Sci USA 101, 17 716–17 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rahilly R, Müller F (1984) The early development of the hypoglossal nerve and occipital somites in staged human embryos. Am J Anat 169, 237–257. [DOI] [PubMed] [Google Scholar]
- Ordahl CP (1993) Myogenic lineages within the developing somite In: Molecular Basis of Morphogenesis. (ed. Bernfield M.), pp. 165–176. New York: John Wiley. [Google Scholar]
- Parker WK (1868) A Monograph on the Structure and Development of the Shoulder‐Girdle and Sternum in the Vertebrates. London: Ray Society. [Google Scholar]
- Piekarski N, Olsson L (2007) Muscular derivatives of the cranialmost somites revealed by long‐term fate mapping in the Mexican axolotl (Ambystoma mexicanum). Evol Dev 9, 566–578. [DOI] [PubMed] [Google Scholar]
- Pieretti J, Gehrke AR, Schneider I, et al. (2015) Organogenesis in deep time: a problem in genomics, development, and paleontology. Proc Natl Acad Sci USA 112, 4871–4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Q, Patel K, Huang R (2015) The lateral plate mesoderm: a novel source of skeletal muscle In: Veretebrate Myogenesis. Stem Cells and Precursors. (ed. Brand‐Saberi B.), pp. 143–163. Berlin: Springer. [DOI] [PubMed] [Google Scholar]
- Richardson J, Shono T, Okabe M, et al. (2012) The presence of an embryonic opercular flap in amniotes. Proc Biol Sci 279, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer AS (1972) The vertebrate as a dual animal – somatic and visceral. Evol Biol 6, 121–156. [Google Scholar]
- Romer AS, Parsons TS (1977) The Vertebrate Body. Philadelphia: Saunders. [Google Scholar]
- Sambasivan R, Kuratani S, Tajbakhsh S (2011) An eye on the head: the development and evolution of craniofacial muscles. Development 138, 2401–2415. [DOI] [PubMed] [Google Scholar]
- Serra JA (1946) Histochemical tests for protein and amino acids: the characterization of basic proteins. Stain Technol 21, 5–18. [DOI] [PubMed] [Google Scholar]
- Shearman RM, Tulenko FJ, Burke AC (2011) 3D reconstructions of quail‐chick chimeras provide a new fate map of the avian scapula. Dev Biol 355, 1–11. [DOI] [PubMed] [Google Scholar]
- Starck D (1979) Vergleichende Anatomie der Wirbeltiere. Heidelberg: Springer. [Google Scholar]
- Stephens TD, Beier RL, Bringhurst DC, et al. (1989) Limbness in the early chick embryo lateral plate. Dev Biol 133, 1–7. [DOI] [PubMed] [Google Scholar]
- Tada MN, Kuratani S (2015) Evolutionary and developmental understanding of the spinal accessory nerve. Zoological Lett 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M (2013) Molecular and evolutionary basis of limb field specification and limb initiation. Dev Growth Differ 55, 149–163. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Cohn MJ, Ashby P, et al. (2000) Distribution of polarizing activity and potential for limb formation in mouse and chick embryos and possible relationships to polydactyly. Development 127, 4011–4021. [DOI] [PubMed] [Google Scholar]
- Thacher JK (1877) Median and paired fins, a contribution to the history of the vertebrate limbs. Trans Connect Acad Arts Sci 3, 281–310. [Google Scholar]
- Theis S, Patel K, Valasek P, et al. (2010) The occipital lateral plate mesoderm is a novel source for vertebrate neck musculature. Development 137, 2961–2971. [DOI] [PubMed] [Google Scholar]
- Tickle C (2015) How the embryo makes a limb: determination, polarity and identity. J Anat 227, 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp E, Mateus O (2013) Clavicles, interclavicles, gastralia, and sternal ribs in sauropod dinosaurs: new reports from diplodocidae and their morphological, functional and evolutionary implications. J Anat 222, 321–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahor E (2015) Head muscle development. Results Probl Cell Differ 56, 123–142. [DOI] [PubMed] [Google Scholar]
- Tzahor E, Evans S (2011) Pharyngeal mesoderm development during embryogenesis: implications for both heart and head myogenesis. Cardiovasc Res 91, 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versluys J (1927) Das skelet In: Vergleichende Anatomie der Wirbeltiere. (eds Ihre JEW, Kampen PN, Nierstrasz HF, Versluys J.), pp. 58–328. Berlin: Verlag von Julius Springer. [Google Scholar]
- Vickaryous MK, Hall BK (2010) Comparative development of the crocodylian interclavicle and avian furcula, with comments on the homology of dermal elements in the pectoral apparatus. J Exp Zool B314, 196–207. [DOI] [PubMed] [Google Scholar]
- Wagner GP (1994) Homology and the mechanisms of development In: Homology: the Hierarchial Basis of Comparative Biology. (ed. Hall BK.), pp. 273–299. San Diego: Academic Press. [Google Scholar]
- Wilson MVH, Hanke GF, Marss T (2007) Paired fins of jawless vertebrates and their homologies across the “agnathan”‐gnathostome transition In: Major Transition in Vertebrate Evolution. (eds Anderson JS, Sues HD.), pp. 122–149. Bloomington: Indiana University Press. [Google Scholar]
- Yates AM, Vasconcelos CC (2005) Furcula‐like clavicles in the prosauropod Massospondylus . J Vertebr Paleontol 25, 466–468. [Google Scholar]
- Yonei‐Tamura S, Abe G, Tanaka Y, et al. (2008) Competent stripes for diverse positions of limbs/fins in gnathostome embryos. Evol Dev 10, 737–745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Three‐dimensional reconstruction of the stage‐24 chick–quail chimera embryo shown in Fig. 3A. Rostral is to the right.
Video S2. Three‐dimensional reconstruction of the hatchling of O. latipes shown in Fig. 3C. Rostral is to the right.


