Abstract
Objectives
To evaluate the safety and effectiveness of tocilizumab (TCZ) in patients with systemic juvenile idiopathic arthritis (sJIA) in real-world clinical settings in Japan.
Methods
Paediatric patients with sJIA initiating TCZ between April 2008 and February 2012 and those previously enrolled in clinical trials who initiated TCZ before April 2008 were enrolled in a Japanese registry surveillance programme. Safety and effectiveness parameters were collected for 52 weeks.
Results
Of 417 patients enrolled, mean age was 11.2 years and 48.0% were female. TCZ exposure was 407.0 patient-years (PYs). Baseline corticosteroid use was higher than in clinical trials. Rates of total adverse events (AEs) and serious AEs (SAEs) were 224.3/100 PYs and 54.5/100 PYs, respectively, with SAEs higher than previously reported. The most frequent AEs and SAEs were infections and infestations (69.8/100 PYs and 18.2/100 PYs, respectively). 74 serious infections occurred in 55 patients (18.2/100 PYs); higher than previously reported. 26 macrophage activation syndrome events were reported in 24 patients (6.4/100 PYs). Fever and rash symptoms improved from baseline to week 52 (54.6% to 5.6% and 43.0% to 5.6%, respectively). At 4 weeks, 8 weeks and 52 weeks, 90.5%, 96.2% and 99.0% of patients achieved normal C reactive protein levels (<0.3 mg/dL), respectively.
Conclusions
These first real-world data demonstrated that TCZ was well tolerated, with acceptable safety and effectiveness in patients with sJIA. Higher incidences of SAEs and serious infections may be due to differences, such as corticosteroid use and concomitant diseases, between patient populations enrolled in previously reported clinical trials and this study.
Keywords: Juvenile Idiopathic Arthritis, DMARDs (biologic), Treatment
Introduction
Systemic juvenile idiopathic arthritis (sJIA) is a severe category of JIA characterised by prominent systemic features, such as fever, rash and serositis, and an onset before age 16 years. Patients are initially treated with non-steroidal anti-inflammatory drugs. As symptoms persist, corticosteroids are indicated.1 2 However, an estimated half of all patients with JIA have been reported to have active disease after a 10-year period of observation, and long-term corticosteroid use has been associated with severe adverse effects, such as excessive weight gain, osteoporosis and growth suppression.3 4 Moreover, treatment with methotrexate has not been shown to be effective in improving systemic features in patients with JIA.5
Interleukin 6 (IL-6) is a proinflammatory cytokine that is elevated in peripheral and synovial fluid and signals through the inflammatory biomarker C reactive protein (CRP). In patients with sJIA, IL-6 expression has been correlated with the extent and severity of joint involvement, with fever, and with platelet counts.2 6 The humanised antihuman IL-6 receptor monoclonal antibody tocilizumab (TCZ) modulates IL-6 activity by blocking its binding to the soluble and membrane-bound IL-6 receptor and, consequently, lowers CRP levels. In clinical trials of patients with sJIA, including two phase II and two phase III trials, TCZ improved symptoms, such as fever and rash, and laboratory measurements, such as CRP, haemoglobin concentration and platelet counts, in patients with sJIA.7–12 Adverse events (AEs) reported with TCZ treatment in patients with sJIA included infections, neutropenia and abnormalities in liver function test results. Most AEs were mild or moderate in severity and typical of those noted with other biologic agents, such as abatacept and canakinumab.13 14 Notably, no or few cases, which resolved, of macrophage activation syndrome (MAS) were reported. On the basis of these results, TCZ was approved for the treatment of sJIA in Japan in 2008 and in the European Union and USA in 2011.
Clinical trials of TCZ in patients with sJIA had specific inclusion criteria and excluded patients with infections; concurrent medical or surgical conditions; leucopenia; thrombocytopenia; or concomitant diseases of the nervous, renal, endocrine or hepatic systems. Therefore, the data from these clinical trials may not fully represent the safety and effectiveness of TCZ for patients in real-world clinical settings. As a condition of approval of TCZ for the treatment of sJIA, the Japanese Health Authority required that an all-patient registry postmarketing surveillance (PMS) be conducted to investigate the safety and effectiveness of TCZ in real-world clinical settings in patients with sJIA. To our knowledge, this single-arm observational study is the first to evaluate the safety and effectiveness of TCZ in patients with sJIA in real-world clinical settings for as long as 52 weeks.
Methods
Patients
All paediatric patients with sJIA who initiated intravenous TCZ in real-world settings in Japan between April 2008, when TCZ was approved in this country for the treatment of sJIA, and February 2012, were enrolled and observed in this 52-week study. In addition, patients who were previously enrolled in a clinical trial received TCZ prior to its approval in April 2008. Safety parameters collected in these clinical trials were not assessed in this PMS study. Following the clinical trials and TCZ approval in 2008, these patients were enrolled in this study; safety parameters were collected for 52 weeks.
Protocol
Patients were registered before initiating TCZ treatment. Registration was centrally controlled, and data were collected and evaluated by Chugai Pharmaceutical in collaboration with the Chugai TCZ JIA Safety Evaluation Committee. Patients were prescribed to receive intravenous TCZ 8 mg/kg once every 2 weeks. Dosing schedules were adjusted, within prescription guidelines, to TCZ weekly depending on disease severity; this is limited to cases in which improvement of symptoms is insufficient. No restrictions on the use of concomitant non-biologic disease-modifying antirheumatic drugs or corticosteroids were imposed. Patients were observed for 52 weeks or until discontinuation of TCZ treatment. Patient characteristics, AEs and effectiveness parameters were collected using case report forms. MAS included events reported as MAS, suspected MAS and hemophagocytic syndrome including physician-reported virus -associated hemophagocytic syndrome. Infusion-related reactions (IRRs) were defined as events developing within 24 h of TCZ infusion and included potential allergic reactions. Anti- TCZ immunoglobulin E (IgE) antibody testing was required, if feasible, in all patients with events defined as IRRs. CRP was recorded every 4 weeks. The systemic feature score was based on the number of systemic manifestations present from eight parameters of sJIA: rash; fever; cervical, axial or inguinal lymphadenopathy; hepatomegaly; splenomegaly; and serositis.5
Statistical analysis
AEs were coded and classified using system organ classes and preferred terms according to the Medical Dictionary for Regulatory Activities, V.16.1. Data from patients previously enrolled in a clinical trial who initiated TCZ prior to April 2008 were included in the safety analyses and excluded from the effectiveness analyses. Data were analysed to report the proportion (95% CI) of patients experiencing an event, and incidence rates were presented as the number of events per 100 patient-years (PYs). Each parameter of the systemic feature score was given a value of 1 (present) or 0 (absent), and a total score indicating the number of parameters present was calculated as previously described (range, 0 to 8).5 Paired t test was used to assess changes in systemic feature score from baseline to week 52.
Results
Patient disposition and baseline characteristics
A total of 417 patients, 52.0% (217/417) male and 48.0% (200/417) female, were enrolled. One hundred and twenty-seven patients (30.5%) who were previously enrolled in a clinical trial who received TCZ were included. The mean (median) age was 11.2 (10.0) years and the mean (median) age at disease onset was 5.4 (4.0) years. Patient demographics and baseline characteristics are summarised in table 1. The most characteristic systemic features of sJIA, fever and rash, were reported at baseline in approximately one-third of patients. No systemic manifestations were observed at baseline in 61.2% (255/417) of the patients. The proportion of patients who continued to receive TCZ until the end of the observation period was 86.6% (361/417), and the cumulative exposure to TCZ was 407.0 PYs. A total 9.6% of patients (40/417) discontinued TCZ treatment, including discontinuation because the treatment objective was met (2.6% (11/417)). The most common reasons for discontinuation for the remaining patients were AEs (4.1% (17/417)) and insufficient response (1.4% (6/417)); 3.8% (16/417) were lost to follow-up.
Table 1.
Patient demographics and baseline characteristics
| Characteristics | Patients (N=417) |
|---|---|
| Sex, n (%) | |
| Male | 217 (52.0) |
| Female | 200 (48.0) |
| Age, mean (±SD)/median (min, max), years | 11.2 (7.2)/10.0 (0, 53) |
| Age at disease onset,* mean (±SD)/median (min, max), years | 5.4 (3.9)/4.0 (0, 17) |
| Disease duration,* mean (±SD)/median (min, max), years | 5.8 (5.9)/4.1 (0, 35) |
| Patients presenting with disease symptoms (baseline), n (%) | |
| Fever | 149 (35.7) |
| Rash | 119 (28.5) |
| Cervical lymphadenopathy | 55 (13.2) |
| Axillary lymphadenopathy | 16 (3.8) |
| Inguinal lymphadenopathy† | 13 (3.1) |
| Hepatomegaly† | 33 (7.9) |
| Splenomegaly† | 27 (6.5) |
| Serositis† | 28 (6.7) |
| Systemic feature score,† n (%) | |
| 0 | 255 (61.2) |
| 1 | 35 (8.4) |
| 2 | 53 (12.7) |
| ≥3 | 73 (17.5) |
| Concomitant conditions, n (%) | |
| Respiratory disease‡ | 33 (7.9) |
| Cardiac functional disorders | 4 (1.0) |
| Liver disorder | 44 (10.6) |
| Renal dysfunction | 13 (3.1) |
| Gastrointestinal tract disturbance | 42 (10.1) |
| Diabetes mellitus | 10 (2.4) |
| Prior biologic use, n (%) | 13 (3.1) |
| Prior DMARD use, n (%) | 181 (43.4) |
| Methotrexate | 108 (25.9) |
| Prior corticosteroids use, n (%) | 367 (88.0) |
| Corticosteroid dosage, mean (±SD), mg/kg/day | 0.8 (2.7) |
*N=398.
†N=416.
‡Respiratory disease included asthma, atelectasis, chronic bronchitis, bronchopulmonary dysplasia, interstitial lung disease, pleurisy, pulmonary hypertension, allergic rhinitis and sleep apnoea syndrome.
DMARD, disease-modifying antirheumatic drug; max, maximum; min, minimum.
Safety
The overall incidence rate per 100 PYs for all AEs was 224.3 (table 2). After excluding patients preliminarily treated with TCZ in a clinical trial, the incidence rate per 100 PYs for all AEs was 233.8. AEs that led to TCZ discontinuation occurred in 4.1% of patients (17/417). The most common AEs were infections and infestations, with a rate of 69.8/100 PYs. The second most common AEs were respiratory, thoracic and mediastinal disorders, with a rate of 34.9/100 PYs. Other frequently reported (>10%) AEs were investigations, musculoskeletal and connective tissue disorders, blood and lymphatic system disorders, and gastrointestinal disorders, with incidence rates of 19.7, 17.0, 14.0 and 13.8/100 PYs, respectively. Frequently reported AEs classified as investigations were decreased platelet count, with an incident rate of 2.9/100 PYs, and decreased white cell count, with an incident rate of 4.2/100 PYs.
Table 2.
Overview of adverse events and serious adverse events (SOC and preferred term >5%)
| Tocilizumab exposure (N=417; 407.0 PYs) |
||||||
|---|---|---|---|---|---|---|
| Adverse events* | Serious adverse events* | |||||
| Patients, n (%) |
Events, n |
Rate/100 PYs | Patients, n (%) |
Events, n |
Rate/100 PYs | |
| Total events | 289 (69.3) | 913 | 224.3 | 117 (28.1) | 222 | 54.5 |
| Infections and infestations | 171 (41.0) | 284 | 69.8 | 55 (13.2) | 74 | 18.2 |
| Gastroenteritis | 30 (7.2) | 32 | 7.9 | 8 (1.9) | 9 | 2.2 |
| Influenza | 30 (7.2) | 33 | 8.1 | 3 (0.7) | 3 | 0.7 |
| Bronchitis | 24 (5.8) | 34 | 8.4 | 4 (1.0) | 6 | 1.5 |
| Pharyngitis | 22 (5.3) | 37 | 9.1 | 1 (0.2) | 1 | 0.2 |
| Nasopharyngitis | 22 (5.3) | 29 | 7.1 | 0 (0.0) | 0 | 0.0 |
| Respiratory, thoracic and mediastinal disorders | 82 (19.7) | 142 | 34.9 | 8 (1.9) | 9 | 2.2 |
| Upper respiratory tract inflammation | 71 (17.0) | 118 | 29.0 | 1 (0.2) | 1 | 0.2 |
| Investigations | 44 (10.6) | 80 | 19.7 | 13 (3.1) | 18 | 4.4 |
| Musculoskeletal and connective tissue disorders | 59 (14.1) | 69 | 17.0 | 15 (3.6) | 17 | 4.2 |
| Juvenile idiopathic arthritis | 36 (8.6) | 40 | 9.8 | 14 (3.4) | 16 | 3.9 |
| Blood and lymphatic system disorders | 46 (11.0) | 57 | 14.0 | 34 (8.2) | 40 | 9.8 |
| Haematophagic histiocytosis | 24 (5.8) | 26 | 6.4 | 24 (5.8) | 26 | 6.4 |
| Gastrointestinal disorders | 42 (10.1) | 56 | 13.8 | 10 (2.4) | 15 | 3.7 |
| Skin and subcutaneous tissue disorders | 38 (9.1) | 43 | 10.6 | 3 (0.7) | 3 | 0.7 |
| Hepatobiliary disorders | 35 (8.4) | 41 | 10.1 | 9 (2.2) | 9 | 2.2 |
| Hepatic function abnormal | 29 (7.0) | 33 | 8.1 | 6 (1.4) | 6 | 1.5 |
| General disorders and administration site conditions | 24 (5.8) | 32 | 7.9 | 7 (1.7) | 9 | 2.2 |
| Injury, poisoning and procedural complications | 27 (6.5) | 32 | 7.9 | 9 (2.2) | 11 | 2.7 |
| Eye disorders | 12 (2.9) | 18 | 4.4 | 0 (0.0) | 0 | 0.0 |
| Vascular disorders | 14 (3.4) | 17 | 4.2 | 3 (0.7) | 4 | 1.0 |
| Metabolism and nutrition disorders | 12 (2.9) | 14 | 3.4 | 0 (0.0) | 0 | 0.0 |
| Nervous system disorders | 12 (2.9) | 12 | 2.9 | 7 (1.7) | 7 | 1.7 |
| Renal and urinary disorders | 7 (1.7) | 7 | 1.7 | 4 (1.0) | 4 | 1.0 |
| Immune system disorders | 5 (1.2) | 5 | 1.2 | 1 (0.2) | 1 | 0.2 |
| Cardiac disorders | 3 (0.7) | 3 | 0.7 | 1 (0.2) | 1 | 0.2 |
| Surgical and medical procedures | 1 (0.2) | 1 | 0.2 | 0 (0.0) | 0 | 0.0 |
*Adverse events and serious adverse events were classified using the Medical Dictionary for Regulatory Activities (MedDRA, V.16.1).
PYs, patient-years, SOC, system organ class.
The overall incidence rate per 100 PYs for serious AEs (SAEs) was 54.5 (tables 2 and 3). Excluding patients preliminarily treated with TCZ in a clinical trial, the incidence rate per 100 PYs of all SAEs was 62.3. SAEs that led to TCZ discontinuation occurred in 3.4% of patients (14/417). The most common SAEs were infections and infestations, with an incidence rate of 18.2/100 PYs. The second most common SAEs were blood and lymphatic system disorders, with a rate of 9.8/100 PYs. Other frequent SAEs were investigations, musculoskeletal and connective tissue disorders, and gastrointestinal disorders, with incidence rates of 4.4, 4.2 and 3.7/100 PYs, respectively (table 2). A total of 55 patients reported serious infections (18.2/100 PYs), with the most common being bacterial pneumonia (10 patients (2.9/100 PYs); table 3). Eight patients developed gastroenteritis (2.2/100 PYs). There were no reports of tuberculosis. Two deaths (one due to vasculitis and cardiac failure and one due to Pseudomonas infection, interstitial lung disease and sepsis) were reported during the 52-week observation period.
Table 3.
Overview of commonly reported serious adverse events
| Serious adverse events | Tocilizumab exposure (N=417; 407.0 PYs) | ||
|---|---|---|---|
| Patients, n (%) | Events, n | Rate/100 PYs | |
| Serious infections | 55 (13.2) | 74 | 18.2 |
| Bacterial pneumonia | 10 (2.4) | 12 | 2.9 |
| Gastroenteritis | 8 (1.9) | 9 | 2.2 |
| Bronchitis | 4 (1.0) | 6 | 1.5 |
| Cellulitis | 3 (0.7) | 3 | 0.7 |
| Sepsis | 3 (0.7) | 3 | 0.7 |
| Herpes zoster | 3 (0.7) | 3 | 0.7 |
| Influenza | 3 (0.7) | 3 | 0.7 |
| Pneumocystis jirovecii pneumonia | 2 (0.5) | 2 | 0.5 |
| Varicella | 2 (0.5) | 2 | 0.5 |
| Macrophage activation syndrome | 24 (5.8) | 26 | 6.4 |
| Serious infusion-related reactions | 8 (1.9) | 14 | 3.4 |
PYs, patient-years.
There were 26 MAS events reported in 24 patients (6.4/100 PYs), 3 of whom had a history of MAS (table 3). Of these 26 MAS events, 2 were classified as definite MAS, 15 were probable MAS, 3 events were virus -associated hemophagocytic syndrome and 6 events were possible or non-MAS. Based on physician reports, other than treatment with TCZ, sJIA itself was considered a potential contributing factor in 21 of the MAS events. Infections were suspected to contribute to seven MAS events, including three virus-associated hemophagocytic syndrome, and treatment with a reduced corticosteroid dose contributed to two MAS events. One MAS event did not require treatment and 25 required treatment, including intravenous corticosteroids and ciclosporine. Of the 26 MAS events, 22 (84.6%) resolved, 2 did not resolve, 1 improved and 1 had an unknown outcome.
A total of 7.2% of patients (30/417) reported IRRs at a rate of 11.3/100 PYs. Symptoms associated with IRRs included decreased or increased blood pressure, vomiting, flushing, fever, urticaria, headache, rash, tachycardia, facial puffiness and chills. Eight patients experienced 14 serious IRRs at a rate of 3.4/100 PYs. All events occurred between the second and fourth TCZ infusion. All patients received additional treatment, including antihistamines and corticosteroids; three patients received epinephrine. All patients recovered or improved, one patient discontinued and seven (87.5%) continued TCZ treatment. However, three of the seven patients experienced a subsequent IRR reported as urticaria, decreased blood pressure, facial puffiness and fever, which led to discontinuation in two of the three patients. Of the eight patients who experienced serious IRRs, six were tested for anti-TCZ antibodies (IgE) and five (83.3%) were positive. Of the three patients who discontinued due to serious IRRs, two developed anti-TCZ antibodies.
Effectiveness
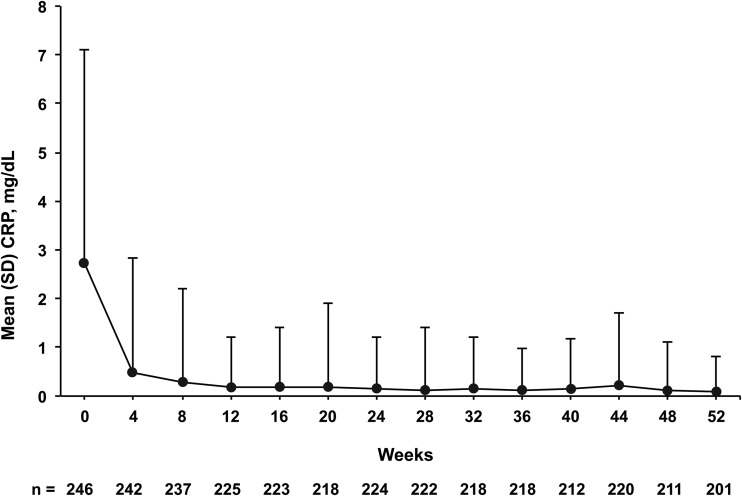
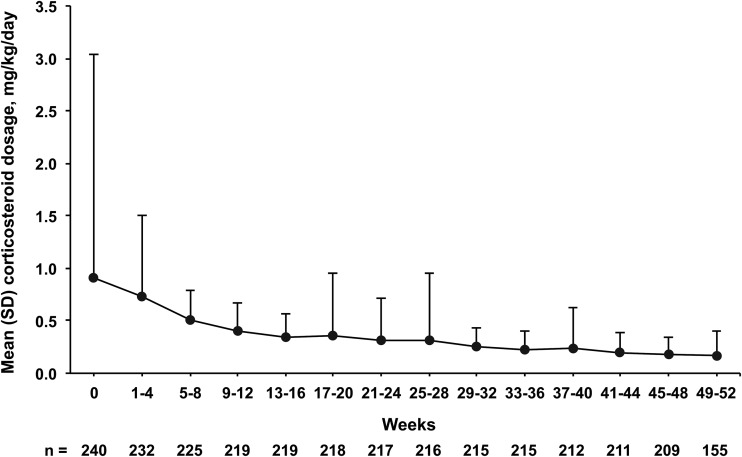
Mean CRP levels decreased from a baseline level of 2.7 mg/dL to 0.5 mg/dL 4 weeks after TCZ initiation. Levels remained within normal range below 0.3 mg/dL from week 8 to week 52 (figure 1). At 4 weeks, 8 weeks and 52 weeks, 90.5%, 96.2% and 99.0% of patients achieved normal CRP levels (<0.3 mg/dL), respectively. The mean daily corticosteroid dosage decreased from 0.9 mg/kg/day at baseline to 0.7 mg/kg/day after 4 weeks of TCZ treatment and to 0.5 mg/kg/day after 8 weeks. The mean daily dosage continued to decrease gradually thereafter, to 0.2 mg/kg/day at week 52 (figure 2). Of 155 patients who were receiving corticosteroids at baseline and received TCZ for 48 weeks, 19 (12.3%) discontinued corticosteroids.
Figure 1.
Mean CRP levels in patients with sJIA treated with TCZ from baseline to week 52. Bars represent SD. CRP, C reactive protein; sJIA, systemic juvenile idiopathic arthritis; TCZ, tocilizumab.
Figure 2.
Mean daily corticosteroid use from baseline to week 52. Bars represent SD.
Overall, 251 patients reported systemic features at baseline and week 52 (table 4). The proportion of patients who reported fever, the most common systemic feature associated with sJIA, decreased from 54.6% (137/251) at baseline to 5.6% (14/251) at week 52. In addition, 125 of these patients had fever resolve by week 52. The proportion of patients who reported rash, the second most common sJIA symptom, decreased from 43.0% (108/251) at baseline to 5.6% (14/251) at week 52. The mean (±SD) systemic feature score significantly decreased from 1.6 (1.7) at baseline to 0.2 (0.6) at week 52 (p<0.0001).
Table 4.
Overview of systemic feature symptoms associated with systemic juvenile idiopathic arthritis before and after tocilizumab treatment
| Symptom | Patients reporting symptoms (N=251) | |
|---|---|---|
| Baseline, n (%) | Week 52, n (%) | |
| Fever | 137 (54.6) | 14 (5.6) |
| Rash | 108 (43.0) | 14 (5.6) |
| Cervical lymphadenopathy | 47 (18.7) | 2 (0.8) |
| Hepatomegaly | 27 (10.8) | 10 (4.0) |
| Serositis | 23 (9.2) | 0 (0.0) |
| Splenomegaly | 21 (8.4) | 8 (3.2) |
| Axillary lymphadenopathy | 14 (5.6) | 1 (0.4) |
| Inguinal lymphadenopathy | 11 (4.4) | 0 (0.0) |
Discussion
This observational PMS study demonstrated for the first time that intravenous TCZ was effective, with a tolerable safety profile, in patients with sJIA treated in a real-world clinical setting. The findings in this PMS were consistent with observations from a Japanese PMS study of patients with rheumatoid arthritis treated with TCZ, with the most common AEs and SAEs being infections and infestations.15 16 In addition, the most frequently reported AEs were similar to those reported in clinical trials of TCZ in patients with sJIA in Japan.10 11 17 The incidence rates of SAEs (54.5/100 PYs) and serious infections (18.2/100 PYs), however, were higher than those reported in clinical trials of TCZ for sJIA by De Benedetti et al (SAEs: 25/100 PYs; serious infections: 11/100 PYs) and Yokota et al (SAEs: 34.7/100 PYs; serious infections: 13.2/100 PYs).12 17
Differences in patient populations between the current study and clinical trials may account for the higher observed incidence of SAEs and serious infections. High corticosteroid dosages can contribute to increased risks of SAEs, especially infections, and the mean corticosteroid dosages used at baseline were higher in PMS patients than those in patients in clinical trials (0.8 mg/kg/day in PMS vs 0.3 mg/kg/day and 0.5 mg/kg/day in clinical trials).12 17 In addition, although the reported incidence rates in the clinical trials were likely more accurate because AEs were more closely monitored and therefore more rigorously recorded, patients with previous treatment history and a number of conditions were excluded. In contrast, the PMS enrolled all patients with sJIA, regardless of severity in disease activity or concomitant diseases. This may explain the findings from a subanalysis that excluded the 127 patients preliminarily treated with TCZ in a clinical trial and indicated that the incidence of SAEs and serious infections was higher in patients who initiated TCZ (62.3/100 PYs and 20.9/100 PYs, respectively) than in patients who continued TCZ after participation in the clinical trial (38.8/100 PYs and 12.7/100 PYs, respectively).
MAS is a severe complication of sJIA with high incidence and poor outcomes, and is difficult to diagnose in the context of sJIA because systemic features such as fever, hepatomegaly and splenomegaly are common to both conditions.18 In published studies, the incidence of MAS reported in patients with sJIA who did not receive biologic treatment varied from 6.8% to 13%.19–21 The rate in our study was lower, although the result of this study included all the MAS events which were reported by physicians including suspected MAS. MAS has a poor prognosis, with mortality rates of 8% to 22%.19 22 No patients died due to MAS in the present study, indicating that early detection and biologic treatment may have prevented fatalities. Notably, most patients were asymptomatic and were diagnosed with MAS based on test results. TCZ inhibition of IL-6 activity may suppress clinical symptoms of MAS; therefore, patients should be carefully monitored for clinical symptoms and for changes in laboratory parameters, such as reduced platelet counts.23 Moreover, patients should be monitored for infections, especially viral infections, which are known risk factors for MAS24 and were suspected in seven of the MAS events reported. Detailed information regarding MAS in patients with sJIA treated with TCZ was previously reported.25
A total of eight patients experienced serious IRRs. All events occurred between the second and the fourth TCZ infusion, and three patients experienced a subsequent IRR during continuous TCZ treatment. Of the eight patients with serious IRRs, six were tested and five were positive for anti-TCZ antibodies. In this study, patients without IRRs were not tested for anti-TCZ antibodies. In the two phase III clinical trials performed by De Benedetti et al and Yokota et al, patients were tested for anti-TCZ antibodies independent of IRRs, and only 1.8% of patients (2/112) and 7.5% (5/67), respectively, developed anti-TCZ antibodies.12 17 These results suggest a higher positive rate for anti-TCZ antibodies in patients with IRRs. Healthcare providers should monitor patients with serious IRRs and vigilantly evaluate patients for IRRs that can occur with continuous treatment and subsequent to the first TCZ infusion.
TCZ effectiveness was assessed by CRP, daily corticosteroid doses and systemic feature score. CRP levels decreased significantly 4 weeks after initiation of TCZ treatment and remained within normal limits throughout the observation period. TCZ treatment was also conducive to corticosteroid tapering. The mean daily corticosteroid dose decreased over 52 weeks, similar to that observed in clinical trials.12 The systemic feature score improved significantly from baseline to week 52. The proportion of patients reporting symptoms associated with sJIA, such as fever; rash; cervical, axillary and inguinal lymphadenopathy; hepatomegaly; splenomegaly; and serositis decreased from baseline to week 52 of TCZ treatment. To our knowledge, this is the first study to demonstrate the effectiveness of TCZ regarding the improvement of clinical symptoms associated with sJIA that was maintained throughout the 52-week observation period.
Clinical trials are generally restricted to evaluating specific interventions with a focus on assessing the efficacy and safety of therapies. They are designed to assess treatment effects in a highly selected and homogeneous population.26 Healthcare providers often underuse treatments due to the lack of data in patients who do not meet the parameters of the clinical trial inclusion criteria.27 While collecting data in real-world settings can be challenging, understanding the effectiveness and safety of therapies in sicker and more complicated patients can provide reassurance for clinicians and offer valuable insights in regards to treatment choices. Although clinical trials have demonstrated high efficacy and a favourable safety profile for TCZ in patients with sJIA, until the present study, data regarding its effectiveness in real-world settings have been lacking. This PMS study had an observation period of 52 weeks, which allowed for long-term real-world data in patients who may have not been included in a clinical trial. This study also included 417 patients with sJIA, which allowed for a high degree of heterogeneity in the patient population.
This study was limited by the absence of a control group; therefore, further analyses will be necessary to confirm the benefit-risk of TCZ treatment in patients with sJIA. Second, the present study was not a clinical trial with a specific protocol for dosage of TCZ and comedications, but rather an observational study to investigate the safety and effectiveness of TCZ in real-world clinical settings in patients with sJIA. These limitations in the PMS observational study affect the possibility of generalising the results.
In conclusion, the results of this study demonstrated that TCZ was well tolerated and its safety profile was within an acceptable range for patients with sJIA in real-world clinical settings. Future studies, with a follow-up period beyond 52 weeks, are needed in order to further establish the long-term use of TCZ in patients with sJIA.
Acknowledgments
The authors thank all investigators and patients who participated in the study and all members of the study team. Support for third-party writing assistance for this manuscript, furnished by Denise Kenski, PhD, of Health Interactions, was provided by F Hoffmann-La Roche.
Footnotes
Contributors: All authors participated in the design of the study, analysis and interpretation of the data. SY, YI, TM, HO, NS and SM are members of the Chugai Tocilizumab Juvenile Idiopathic Arthritis Safety Evaluation Committee. The Committee was created in response to a request for assistance from the Ministry of Health, Labor and Welfare (MHLW) of Japan. The role of the committee is to provide independent advice to Chugai Pharmaceutical on conducting the PMS programme mandated by the MHLW and on the results thereof.
Funding: This study was funded by Chugai Pharmaceutical. Chugai sponsored the study and participated in the design of the study, as well as in the collection, analysis and interpretation of the data. This manuscript was reviewed by Chugai, but the decision to submit and publish this manuscript was contingent only upon the approval of the lead author and coauthors, including those employed by Chugai.
Competing interests: SY has received honoraria from AbbVie, Bristol-Myers Squibb, Taisho Toyama Pharmaceutical and Chugai Pharmaceutical; has patents, royalties or other intellectual property with Chugai Pharmaceutical; and been a consultant for Asahi Kasei Pharma, Japan Blood Products Organization, Novartis, Santen Pharmaceutical and Chugai Pharmaceutical. YI has received honoraria from Astellas Pharma, Mitsubishi Tanabe Pharma Corporation, Eisai, Asahi Kasei Pharma Corporation and Santen Pharmaceutical; received research funding from Morinaga Milk Industry, Mitsubishi Tanabe Pharma Corporation, Eisai, Asahi Kasei Pharma Corporation, Santen Pharmaceutical and Japan Blood Products Organization; and been a consultant for Chugai Pharmaceutical. TM has received honoraria from AbbVie, Astelas, CSL Behring, Daiichi Sankyo Pharmaceutical, Dainippon Sumitomo Pharma, Japan Blood Products Organization, Janssen Pharma, Kaketsuken, Meiji Pharma, Pfizer and Teijin Pharmaceutical; received research funding from CSL Behring, Japan Blood Products Organization, Pfizer and Teijin Pharmaceutical; and been a consultant for Chugai Pharmaceutical. HO has been a consultant for Chugai Pharmaceutical., Astellas Pharma and UCB Japan. NS has received honoraria from Daiichi Sankyou, Ezai Pharmaceutical and MSD; received research funding from Ezai Pharmaceuticals; and been a consultant for Chugai Pharmaceutical, Ono pharmaceuticals and AbbVie. MT is an employee of Chugai Pharmaceutical. KT is an employee of Chugai Pharmaceutical. SM has received honoraria from Eisai, Takeda Pharmaceutical, Asahi Kasei Pharma, Santen Pharmaceutical, Mitsubishi Tanabe Pharma, Pfizer, Astellas Pharma, Daiichi Sankyo, Janssen Pharma, AbbVie, Actelion, Bristol-Myers Squibb, Eli Lilly, Ono Pharmaceutical, GlaxoSmithKline and has patents, royalties or other intellectual property with Chugai Pharmaceutical.
Patient consent: Obtained.
Ethics approval: Registration was centrally controlled and data were collected and evaluated by Chugai Pharmaceutical in collaboration with the Chugai Tocilizumab JIA Safety Evaluation Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet 2011;377:2138–49. 10.1016/S0140-6736(11)60244-4 [DOI] [PubMed] [Google Scholar]
- 2.Martini A. Systemic juvenile idiopathic arthritis. Autoimmun Rev 2012;12:56–9. 10.1016/j.autrev.2012.07.022 [DOI] [PubMed] [Google Scholar]
- 3.Wallace CA, Levinson JE. Juvenile rheumatoid arthritis: outcome and treatment for the 1990s. Rheum Dis Clin North Am 1991;17:891–905. [PubMed] [Google Scholar]
- 4.Beukelman T. Treatment advances in systemic juvenile idiopathic arthritis. F1000Prime Rep 2014;6:21,21 eCollection 2014 10.12703/P6-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo P, Southwood TR, Prieur AM, et al. Randomized, placebo-controlled, crossover trial of low-dose oral methotrexate in children with extended oligoarticular or systemic arthritis. Arthritis Rheum 2000;43:1849–57. [DOI] [PubMed] [Google Scholar]
- 6.Gurion R, Lehman TJ, Moorthy LN. Systemic arthritis in children: a review of clinical presentation and treatment. Int J Inflam 2012;2012:271569 10.1155/2012/271569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokota S, Miyamae T, Imagawa T, et al. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 2005;52:818–25. 10.1002/art.20944 [DOI] [PubMed] [Google Scholar]
- 8.Yokota S, Miyamae T, Imagawa T, et al. Clinical study of tocilizumab in children with systemic-onset juvenile idiopathic arthritis. Clin Rev Allergy Immunol 2005;28:231–8. 10.1385/CRIAI:28:3:231 [DOI] [PubMed] [Google Scholar]
- 9.Woo P, Wilkinson N, Prieur AM, et al. Open label phase II trial of single, ascending doses of MRA in Caucasian children with severe systemic juvenile idiopathic arthritis: proof of principle of the efficacy of IL-6 receptor blockade in this type of arthritis and demonstration of prolonged clinical improvement. Arthritis Res Ther 2005;7:R1281–8. 10.1186/ar1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokota S, Imagawa T, Mori M, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet 2008;371:998–1006. 10.1016/S0140-6736(08)60454-7 [DOI] [PubMed] [Google Scholar]
- 11.Yokota S, Kishimoto T. Tocilizumab: molecular intervention therapy in children with systemic juvenile idiopathic arthritis. Expert Rev Clin Immunol 2010;6:735–43. 10.1586/eci.10.41 [DOI] [PubMed] [Google Scholar]
- 12.De Benedetti F, Brunner HI, Ruperto N, et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med 2012;367:2385–95. 10.1056/NEJMoa1112802 [DOI] [PubMed] [Google Scholar]
- 13.Ruperto N, Lovell DJ, Quartier P, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet 2008;372:383–91. 10.1016/S0140-6736(08)60998-8 [DOI] [PubMed] [Google Scholar]
- 14.Ruperto N, Brunner HI, Quartier P, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med 2012;367:2396–406. 10.1056/NEJMoa1205099 [DOI] [PubMed] [Google Scholar]
- 15.Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis 2011;70:2148–51. 10.1136/ard.2011.151092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koike T, Harigai M, Inokuma S, et al. Effectiveness and safety of tocilizumab: postmarketing surveillance of 7901 patients with rheumatoid arthritis in Japan. J Rheumatol 2014;41:15–23. 10.3899/jrheum.130466 [DOI] [PubMed] [Google Scholar]
- 17.Yokota S, Imagawa T, Mori M, et al. Longterm safety and effectiveness of the anti-interleukin 6 receptor monoclonal antibody tocilizumab in patients with systemic juvenile idiopathic arthritis in Japan. J Rheumatol 2014;41:759–67. 10.3899/jrheum.130690 [DOI] [PubMed] [Google Scholar]
- 18.Minoia F, Davi S, Horne A, et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, multicenter study of 362 patients. Arthritis Rheumatol 2014;66:3160–9. 10.1002/art.38802 [DOI] [PubMed] [Google Scholar]
- 19.Sawhney S, Woo P, Murray KJ. Macrophage activation syndrome: a potentially fatal complication of rheumatic disorders. Arch Dis Child 2001;85:421–6. 10.1136/adc.85.5.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behrens EM, Beukelman T, Paessler M, et al. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol 2007;34:1133–8. [PubMed] [Google Scholar]
- 21.Moradinejad MH, Ziaee V. The incidence of macrophage activation syndrome in children with rheumatic disorders. Minerva Pediatr 2011;63:459–66. [PubMed] [Google Scholar]
- 22.Stephan JL, Kone-Paut I, Galambrun C, et al. Reactive haemophagocytic syndrome in children with inflammatory disorders. A retrospective study of 24 patients. Rheumatology (Oxford) 2001;40:1285–92. 10.1093/rheumatology/40.11.1285 [DOI] [PubMed] [Google Scholar]
- 23.Kostik MM, Dubko MF, Masalova VV, et al. Identification of the best cutoff points and clinical signs specific for early recognition of macrophage activation syndrome in active systemic juvenile idiopathic arthritis. Semin Arthritis Rheum 2015;44:417–22. 10.1016/j.semarthrit.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 24.Kelly A, Ramanan AV. Recognition and management of macrophage activation syndrome in juvenile arthritis. Curr Opin Rheumatol 2007;19:477–81. 10.1097/BOR.0b013e32825a6a79 [DOI] [PubMed] [Google Scholar]
- 25.Yokota S, Itoh Y, Morio T, et al. Macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis under treatment with tocilizumab. J Rheumatol 2015;42:712–22. 10.3899/jrheum.140288 [DOI] [PubMed] [Google Scholar]
- 26.Nallamothu BK, Hayward RA, Bates ER. Beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation 2008;118:1294–303. 10.1161/CIRCULATIONAHA.107.703579 [DOI] [PubMed] [Google Scholar]
- 27.Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?” Lancet 2005;365:82–93. 10.1016/S0140-6736(04)17670-8 [DOI] [PubMed] [Google Scholar]