Abstract
Objective
To analyse the role of multibiomarker disease activity (MBDA) score in predicting disease relapses in patients with rheumatoid arthritis (RA) in sustained remission who tapered disease modifying antirheumatic drug (DMARD) therapy in RETRO, a prospective randomised controlled trial.
Methods
MBDA scores (scale 1–100) were determined based on 12 inflammation markers in baseline serum samples from 94 patients of the RETRO study. MBDA scores were compared between patients relapsing or remaining in remission when tapering DMARDs. Demographic and disease-specific parameters were included in multivariate logistic regression analysis for defining predictors of relapse.
Results
Moderate-to-high MBDA scores were found in 33% of patients with RA overall. Twice as many patients who relapsed (58%) had moderate/high MBDA compared with patients who remained in remission (21%). Baseline MBDA scores were significantly higher in patients with RA who were relapsing than those remaining in stable remission (N=94; p=0.0001) and those tapering/stopping (N=59; p=0.0001). Multivariate regression analysis identified MBDA scores as independent predictor for relapses in addition to anticitrullinated protein antibody (ACPA) status. Relapse rates were low (13%) in patients who were MBDA−/ACPA−, moderate in patients who were MBDA+/ACPA− (33.3%) and MBDA−ACPA+ (31.8%) and high in patients who were MBDA+/ACPA+ (76.4%).
Conclusions
MBDA improved the prediction of relapses in patients with RA in stable remission undergoing DMARD tapering. If combined with ACPA testing, MBDA allowed prediction of relapse in more than 80% of the patients.
Trial registration number
EudraCT 2009-015740-42.
Introduction
Reaching a state of disease remission in rheumatoid arthritis (RA) is considered as the primary treatment goal in RA.1 2 Due to earlier diagnosis, more efficient use of disease modifying antirheumatic drugs (DMARDs) and a broader drug armamentarium, an increasing number of patients with RA experience disease remission. For instance, data from the Norvegian-DMARD study show doubling of remission rates in the last decade.3 Moreover, many patients maintain their remission state over two subsequent visits.4 Hence, strategies to manage patients with RA in remission, including the possibility to taper or even stop treatment, gain growing importance.5–8 Especially, predictive markers are needed for identifying patients, in which treatment can be successfully tapered without facing a high risk for relapse of RA.
We have recently conducted a randomised prospective study in patients with RA in remission.10 In this RETRO study, we aimed to define the likelihood for disease recurrence in patients with RA when tapering and/or stopping DMARD treatment. While recurrence of disease was higher in patients tapering or stopping DMARDs than in those remaining on treatment, it was stunning that more than half of the patients still kept their remission state despite tapering the DMARDs. These data suggested that tapering or stopping antirheumatic drugs may be a feasible option in a subset of patients with RA, raising the question on the respective patient profile, which is able to taper or stop DMARD treatment.
Presence of anticitrullinated protein antibodies (ACPA) has shown to increase the likelihood for relapse of disease when tapering DMARDs.10 Still, prediction models for disease relapse based on ACPA need further improvement. A more comprehensive assessment of inflammation, which (a) allows detection of residual subclinical inflammation and (b) extends beyond mere detection of acute phase responses, may help to improve prediction of disease relapse. Notably, absence of clinical joint swelling does not necessarily mean true absence of inflammation. In accordance, imaging of joints of patients with RA in remission revealed a rather high prevalence of inflammatory lesions despite absence of respective clinical changes.11–13 While the meaning of subclinical inflammation is still under debate, these findings indicate that local inflammatory changes are still present in patients with RA in remission and may precipitate disease relapses.
To address this concept, we performed a comprehensive assessment of inflammation by a multibiomarker disease activity (MBDA) test in the serum of patients with RA who were in sustained remission and were included into the prospective RETRO study undergoing DMARD tapering. We hypothesised that patients with higher levels of inflammation markers may face an increased risk for relapses in comparison with those showing overall low markers of inflammation.
Methods
Patients and inclusion criteria
RETRO is phase-3, multicentre, randomised, open, prospective, controlled, parallel-group study (EudraCT number 2009-015740-42). Details of the study are described elsewhere.10 The primary objective of the study is to evaluate the possibility of tapering or stopping DMARDs in patients fulfilling the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2010 classification criteria for RA.14 To be enrolled, patients had to have RA for at least 12 months and sustained clinical remission with a disease activity score (DAS)-28 based on erythrocyte sedimentation rate (ESR) of less than 2.6 for at least 6 months.15 16 In addition, patients had to receive stable treatment with conventional and/or biological DMARDs without alteration in dose for at least 6 months. The study was approved by the ethic committee of the Friedrich-Alexander-University of Erlangen-Nuremberg, all local ethical committees of the external centres as well as the Paul Ehrlich Institute (PEI) and was conducted according to the ethical principles of the Declaration of Helsinki.
Treatment and follow-up
Patients were randomised into three different trial arms: Arm 1 (continuation) kept existing conventional and/or biological DMARD regimen at full dose for 12 months. Arm 2 (tapering) reduced the dose of all conventional and/or biological DMARDs by 50%. Arm 3 (stop) reduced the dose of all conventional and/or biological DMARDs by 50% for the first 6 months before entirely stopping all DMARDs. Details on the mode of tapering DMARDs are outlined elsewhere.10 Primary efficacy parameter was disease activity, which was assessed at baseline and after months 3, 6, 9 and 12 using the DAS28-ESR. Relapse of disease was defined as leaving DAS28 remission corresponding to a DAS28-ESR score of >2.6.
Assessment of demographic and disease-specific parameters
Age, sex and body mass indices were recorded in all patients. With respect to disease-specific parameters, disease duration, remission duration, tender joint count 68, swollen joint count 66, visual analogue scales (VAS) for pain and patient global were assessed. In addition, C reactive protein (CRP) level, ESR and the presence of rheumatoid factor (RF) as well as ACPA were measured. Composite scores including DAS28-ESR, DAS28-CRP, ACR/EULAR Boolean remission16 and Health Assessment Questionnaire (HAQ-DI) were calculated at baseline and each follow-up visit, Antirheumatic therapy and any other concomitant treatment were recorded at baseline and at follow-up visits.
Serum analyses
Baseline serum was available from 94 of the first 101 patients of the RETRO study. Twelve inflammation markers were measured in the baseline serum samples of all these 94 patients using the MBDA blood test, which is commercially available in the USA (Vectra DA, Crescendo Bioscience, Myriad Genetics, Salt Lake City, Utah, USA). In developing the MBDA test, these markers were selected from 130 candidates in prior studies by a combination of objective regression modelling and assay performance criteria.17 18 Serum was stored at –80°C before the analysis. All analyses were performed in blinded fashion by the central laboratory of Crescendo Bioscience (South San Francisco, California, USA). The 12 inflammation markers included epidermal growth factor (EGF), vascular endothelial growth factor A (VEGF-A), interleukin 6 (IL-6), serum amyloid A (SAA), CRP, vascular cell adhesion molecule 1 (VCAM-1), matrix metalloproteinase 1 (MMP-1), matrix metalloproteinase 3 (MMP-3), tumour necrosis factor receptor 1 (TNF-RI), human cartilage glycoprotein 39 (YKL-40), leptin and resistin. Measurement of serum concentrations was done by immunoassays using three custom multiplex panels on the Meso Scale Discovery Sector Imager 6000: panels A (for EGF, IL-6, leptin, and VEGF-A), B (for CRP, SSA, and VCAM-1) and C (for MMP-1, MMP-3, resistin, TNF-RI, and YKL-40). Concentrations were calculated using standard curves using four-parameter logistic regression curve fits.18
Calculation of MBDA scores
The algorithm for calculating MBDA scores based on the serum levels of the aforementioned 12 serum proteins has been validated and described previously.16 17 The algorithm was the same as those used in the previously developed and validated MBDA test, Vectra DA (Crescendo Bioscience). Briefly, this algorithm used serum biomarker concentrations to separately estimate TJC28, SJC28 and VAS-GH. The estimates for TJC28, SJC28 and VAS-GH were combined with a CRP test result to calculate an overall MBDA score: a whole number on a scale of 1–100, using a validated formula analogous to that of the DAS28-CRP.16 The algorithm was previously developed and trained using independent serum samples from different studies.18–20 The categories for MBDA scores are less than 30 units for low disease activity, 30–44 units for moderate and more than 44 units for high disease activity.17
Statistical analysis
In descriptive analyses treatment, arms were analysed for demographical and disease-related parameters. Descriptive results are stated in medians and interquartile ranges due to deviation from normal distribution. Corresponding inferential comparisons of subgroups were calculated using Kruskal–Wallis or Mann–Whitney U-tests for numerical variables and exact χ2-tests for nominal characteristics. A multivariate logistic regression model using ‘enter’ method was used to predict the occurrence of disease relapses from the following set of baseline characteristics including an intercept term: age, sex, duration of disease, duration of remission, RF status, ACPA status, ACR/EULAR remission status, randomisation arm (treatment continuation was designated the reference category), biological DMARDs use and MBDA score. Kaplan–Meier plots were used to illustrate relapses over the 12 months of the study with respect to (a) low versus moderate-to-high MBDA scores and (b) ACPA positivity. SPSS software V.21 was used for calculations. p Values ≤0.05 were considered statistically significant.
Results
Baseline characteristics of the patients
Baseline characteristics of the 94 RETRO patients analysed are described in table 1. Briefly, 35 of the 94 patients had been randomised into arm 1 continuing full-dose DMARD treatment over 1 year. The other 59 patients had been randomised into the two tapering arms, with arm 2 reducing all DMARDs by 50% for 1 year (N=32) and arm 3 stopping all DMARDs after a 6 months tapering interval (N=27). Patients had established RA and were in sustained remission with a median duration of remission of 12 months. More than two-thirds of the patients were also fulfilling the ACR/EULAR remission criteria. Levels for CRP (median: 0.28 mg/L) and ESR (median: 13 mm) were normal and like MBDA scores evenly distributed among the three strategy arms. HAQ-DI was generally low and not significantly different among the different treatment arms. The majority of patients were receiving methotrexate (84%), while biological DMARDs were used by 37% of the patients with TNF inhibitors the most frequently used entity. After 1 year, 63 of the 94 patients remained in remission, whereas 31 patients relapsed.
Table 1.
Baseline characteristics of the patients
| Characteristics | Total population (n=94) | Continuation | Tapering | Stopping | p Values |
|---|---|---|---|---|---|
| Arm 1 (n=35) | Arm 2 (n=32) | Arm 3 (n=27) | |||
| Age, years | 55.0 (19) | 55.0 (19.5) | 54.0 (18) | 54.5 (19) | 0.90 |
| Females, % (N) | 59.6 (56) | 57.1 (20) | 56.2 (18) | 66.7 (18) | 0.67 |
| Disease duration, years | 5.0 (7) | 5.0 (7) | 6.0 (6) | 3.0 (5) | 0.13 |
| Remission duration, months | 12.0 (12.0) | 12.0 (27) | 9.0 (12) | 12.0 (10) | 0.13 |
| DAS-28, units | 1.9 (0.8) | 1.9 (1.1) | 1.9 (0.7) | 2.0 (0.9) | 0.56 |
| ACR/EULAR remission, % (N) | 77.6 (73) | 77.1 (27) | 81.2 (26) | 74.0 (20) | 0.80 |
| ESR, mm | 13.0 (15) | 11.0 (17) | 13.0 (13) | 14.5 (8.5) | 0.79 |
| CRP, mg/dL | 0.28 (0.23) | 0.27 (0.24) | 0.28 (0.33) | 0.26 (0.21) | 0.57 |
| MBDA score | 21.5 (22.0) | 25.0 (13.0) | 19.0 (22.0) | 18.0 (23.0) | 0.69 |
| HAQ, units | 0.0 (0.13) | 0.0 (0.13) | 0.0 (0.25) | 0.0 (0.16) | 0.77 |
| Positive RF, % (N) | 60.6 (57) | 51.4 (18) | 71.8 (23) | 59.2 (16) | 0.23 |
| Positive ACPA, % (N) | 56.3 (53) | 57.1 (20) | 59.3 (19) | 51.8 (14) | 0.84 |
| Methotrexate use, % (N) | 84.0 (79) | 85.7 (30) | 71.8 (23) | 96.3 (26) | 0.003 |
| Other DMARD* use, % (N) | 9.5 (9) | 8.5 (3) | 12.5 (4) | 7.4 (2) | 0.78 |
| Biological DMARD† Use, % (N) | 37.2 (35) | 42.8 (15) | 50.0 (16) | 14.8 (4) | 0.014 |
*Leflunomide, sulfasalazine, hydroxychloroquine.
†Tumour necrosis factor inhibitors and tocilizumab; descriptive results in median (IQR) due to deviation from normal distribution, if not stated otherwise.
ACPA, anticitrullinated protein antibody; ACR, American College of Rheumatology; CRP, C reactive protein; DAS-28, disease activity score-28 (based on ESR); DMARDs, disease modifying antirheumatic drugs; ESR, erythrocyte sedimentation rate; EULAR, European League Against Rheumatism; HAQ, health assessment questionnaire; MBDA, multibiomarker disease activity; RF, rheumatoid factor; VAS, visual analogue scale.
Distribution of MBDA scores
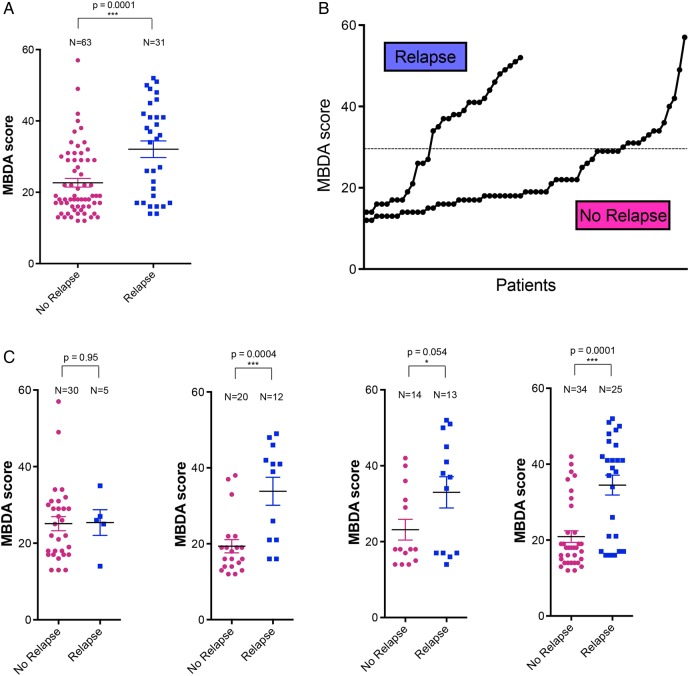
MBDA scores were calculated from the analysis of baseline serum samples of 94 RETRO patients. Distribution of MBDA scores showed that 63 patients (67%) had low MDBA scores (<30 units), while 31 patients (33%) had moderate to high scores (≥30 units) according to the previously established cut-offs (figure 1A). In patients who maintained their remission status over 1 year, MDBA scores were shifted to low scores (79%), while moderate-to-high score were less frequent (21%) (figure 1B). In contrast, the frequency of moderate-to-high MBDA scores was more than twice as high (58%) in patients subsequently experiencing a relapse (figure 1C).
Figure 1.
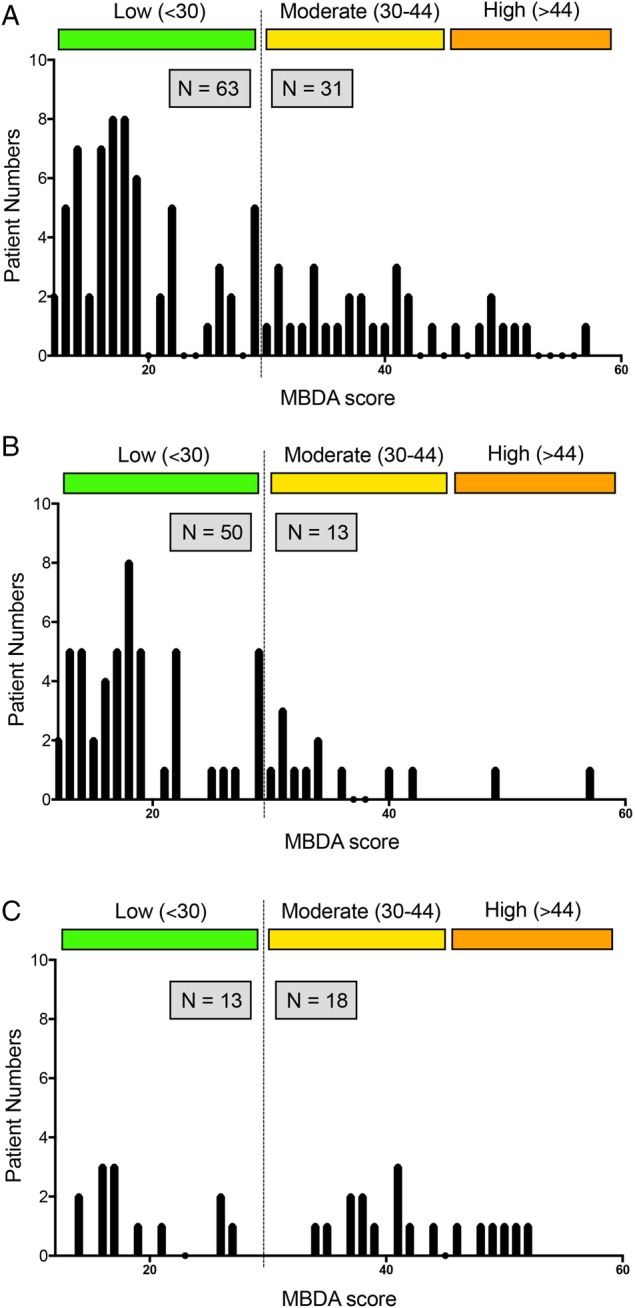
Distribution of multibiomarker disease activity (MBDA) scores. (A) Distribution of baseline MBDA scores in patients with rheumatoid arthritis in remission. Colours indicate low (green), moderate (yellow) and high levels (orange). Dashed line indicates the cut-off between low MBDA scores and moderate-to-high MBDA score. (A) entire population; (B) patients remaining in remission and (C) patients experiencing relapse of disease.
MBDA scores in patients with RA relapsing and remaining in sustained remission
We found that levels of baseline MBDA scores were significantly (p=0.0001) higher in patients experiencing a relapse (mean±SEM: 32.0±2.3 units) than those remaining in sustained remission (22.6±1.2) (figure 2A). MBDA values indicating moderate (30–44 units) and high (over 44 units) inflammatory activity were clearly increased in patients experiencing a subsequent disease relapse (figure 2B). Subgroup analysis showed that MBDA scores did not differ (p=0.95) between relapsing and sustained remission patients when full-dose DMARD treatment was continued (arm 1) (figure 1C). However, in the subgroups of patients tapering (arm 2; p=0.0004) and stopping (arm 3; p=0.05) DMARD treatment MBDA scores were significantly higher in patients experiencing disease relapse. Significant (p=0.0001) differences in baseline MBDA scores between relapsing and sustained remission patients were also found, when groups 2 and 3 resembling all patients tapering their DMARD, were analysed (figure 2C). Analysis of individual components of MBDA showed trends to higher levels of parameters linked to acute phase response (SAA, CRP, IL-6), tissue inflammation (EGF, VEGF, MMP-1 and -3) and energy metabolism (leptin) in patients with relapse (see online supplementary figure S1). Hence, the aforementioned significant differences in MBDA scores between relapsing and non-relapsing patients are not built on one single but several different serum parameters, underscoring the strength of a composite score like MBDA.
Figure 2.
Baseline multibiomarker disease activity (MBDA) scores in patients with relapsing and non-relapsing rheumatoid arthritis (RA). (A) Baseline MBDA scores in patients with RA experiencing a relapse or remaining in remission (no relapse). (B) Probability plot of MBDA scores in patients with RA experiencing a relapse or remaining in remission (no relapse). (C) MBDA scores in patients with relapsing and non-relapsing RA, who continued treatment (study arm 1), tapered treatment (study arm 2) or stopped treatment (study arm 3). Combined results for tapering and stopping treatment (arms 2 and 3) are shown in the last graph (from left to right). *** indicate a p value of less than 0.05 (unpaired Student's t test).
Relation between MBDA scores and ACPA
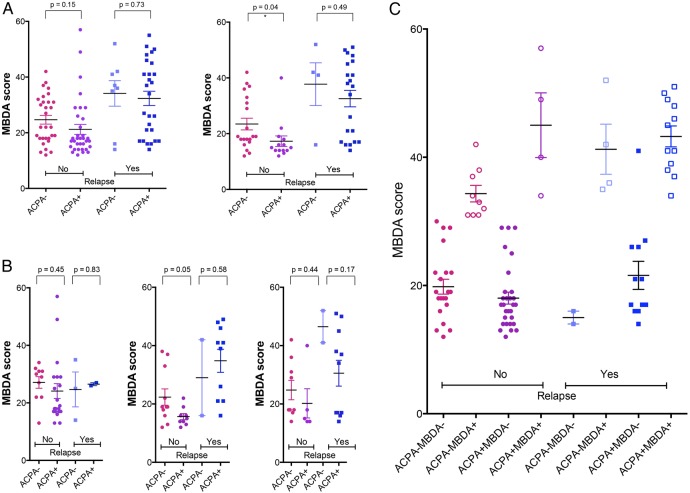
In our previous analyses, ACPA emerged as the only predictive factor for disease relapse in patients with RA tapering or stopping their DMARD treatment.9 We therefore addressed the relation between MBDA and ACPA to test whether there is a potential to refine the prediction of disease relapse by combining MBDA and ACPA measurements. Separate analysis of MBDA scores in patients who were ACPA-negative (ACPA−) and ACPA-positive (ACPA+) revealed virtually identical differences between relapsing and non-relapsing patients (figure 3A). As previously mentioned, differences in MBDA scores between patients relapsing and those remaining in remission were confined to patients tapering and stopping their DMARD treatment and this observation was true for both patients who were ACPA− and ACPA+ (figure 3B). Importantly, the majority of patients who were ACPA+MBDA+ were in the relapse group, while they were extremely rare (N=3) in the sustained remission group. Conversely, patients who were ACPA−MBDA− were enriched in the sustained remission group, but very rarely found in the relapse group (N=4) (figure 3C).
Figure 3.
Influence of anticitrullinated protein antibody (ACPA) status on multibiomarker disease activity (MBDA) scores in relapsing and non-relapsing patients. (A) Baseline MBDA scores in patients with rheumatoid arthritis experiencing a relapse or remaining in remission (no relapse) and relation to ACPA status. (A) entire population, study arms 1–3 (left); patients tapering or stopping treatment, study arms 2 and 3 (right); (B) patients continuing treatment, study arm 1 (left); patients tapering treatment, study arm 2 (middle) and patients stopping treatment, study arm 3 (right); (C) Distribution of MBDA scores based on relapse status, the presence of ACPA, and category of MBDA score (low(−) or moderate-to-high (+)) p values were calculated by unpaired Student's t test.
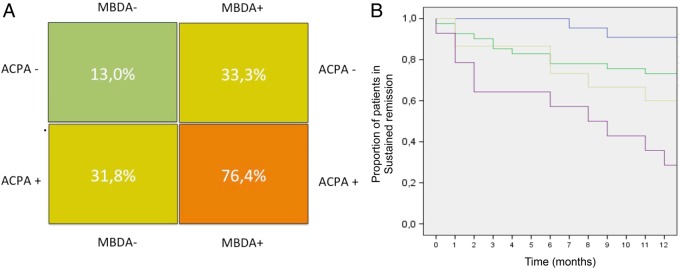
Based on these findings, which suggested that MBDA scores and ACPA status independently predict the risk for relapse, we separately calculated the relapse rates in double-negative (ACPA−MBDA−), double-positive (ACPA+MBDA+) and single-positive (ACPA+MBDA−:, ACPA−MBDA+) patients. While the risk for relapse in patients with double-negative RA was very low (13.0%), the large majority (76.4%) of double-positive patients relapsed (χ2-test; p=0.0001) (figure 4A). Relapse risks of single-positive patients (ACPA+MBDA−: 31.8%, ACPA−MBDA+: 33.3%) were in between these two extremes, indicating that MBDA scores and ACPA status independently contribute to relapse risk in patients with RA tapering DMARD treatment. Further evidence is provided by Kaplan–Meier curves showing a rather rapid loss of remission status in double-positive patients, while double-negative patients mostly remained in remission (figure 4B). No significant differences were observed when replacing MBDA by either CRP (p=0.15), ESR (p=0.09), DAS28 (p=0.11), simplified disease activity index (SDAI) (p=0.27) or clinical disease activity index (CDAI) scores (p=0.38) (see online supplementary figure S2A–E). Furthermore, we tested whether replacing ACPA by RF in an MBDA based prediction model was significant. While overall the groups showed significant differences in relapse rates (χ2-test; p=0.001), no separation between double-negative and single-positive groups was observed (see online supplementary figure S2F).
Figure 4.
Relapse rates of rheumatoid arthritis (RA) dependent on anticitrullinated protein antibody (ACPA) and multibiomarker disease activity (MBDA) score. (A) Risk chart for cumulative disease relapse over 1 year dependent on ACPA status and MBDA score. (−) indicates no ACPA or a low MBDA score (<30). (+) indicates presence of ACPA or moderate-to-high MBDA score (≥30). Values indicate the incidence of relapse over 1 year for that patient group. (B) Kaplan–Meier curves indicate loss of remission over 12 months in patients with RA in relation to ACPA status and MBDA score: (blue) ACPA/MBDA double-negative, (green) ACPA+/MBDA−, (yellow) ACPA−/MBDA+ and (purple) ACPA/MBDA double positive. Y-axis indicates the percentage of patients with RA in sustained remission (100% at baseline). X-axis indicates time.
Predictors for disease relapse
To further test whether MBDA scores are independent predictors for relapses, we set up a multivariate logistic regression model. In a model containing demographic variables (age, sex), autoantibody status (ACPA, RF), randomisation arm (treatment continuation was designated the reference category), remission status (ACR/EULAR Boolean remission) and other disease-specific variables (disease duration, remission duration, biological DMARD exposure), only ACPA positivity (Wald χ2=9.1, p=0.002, OR=24.4) and MBDA score (Wald χ2=8.3, p=0.004, OR=8.5) were identified as predictors beside the underlying randomisation arm (Wald χ²Arm2=5.4, p=0.02, OR=5.94; Wald χ²Arm3=4.3, p=0.04, OR=5.41) for subsequent relapse of disease (table 2). Overall, the model allowed correct prediction in 81.8% of the patients.
Table 2.
Results of multivariate logistic regression for prediction of relapse
| B | Wald | p Value | OR | OR (95% CI) | |
|---|---|---|---|---|---|
| Age | 0.70 | 3.75 | 0.05 | 1.05 | 0.99 to 1.10 |
| Sex | 0.95 | 1.28 | 0.26 | 2.01 | 0.60 to 6.76 |
| ACR/EULAR remission | 0.30 | 0.17 | 0.17 | 1.35 | 0.32 to 5.58 |
| Disease duration | −0.001 | 0.001 | 0.90 | 0.99 | −0.91 to 1.10 |
| Remission duration | 0.011 | 0.27 | 0.60 | 1.01 | 0.97 to 1.06 |
| Positive RF | −1.50 | 0.88 | 0.09 | 0.22 | 0.04 to 1.26 |
| Positive ACPA | 3.20 | 1.06 | 0.002 | 24.47 | 3.09 to 194.04 |
| Biological DMARD | 0.22 | 0.10 | 0.75 | 1.24 | 0.33 to 4.65 |
| MBDA score | 2.14 | 8.34 | 0.004 | 8.54 | 2.00 to 36.43 |
| Continuation vs tapering | 1.78 | 5.40 | 0.025 | 5.94 | 1.32 to 26.72 |
| Continuation vs stopping | 1.69 | 4.27 | 0.043 | 5.41 | 1.09 to 26.87 |
| Intercept | −8.18 | 12.33 | 0.000 | 0.000 | – |
Bold typeface indicates significant predictors.
ACPA, Anticitrullinated protein antibodies; ACR, American College of Rheumatology; DMARD, disease modifying antirheumatic drugs; EULAR, European League Against Rheumatism; MBDA, multibiomarker disease activity; RF, rheumatoid factor.
Discussion
We show that the presence of residual inflammation assessed by a standardised panel of biomarkers is associated with a higher relapse rate of RA when DMARDs are tapered or stopped. Our data suggest that (a) markers of inflammation are elevated in a subset of patients with RA in clinical remission and (b) that these patients are at higher risk for relapse if their anti-inflammatory treatment is reduced. Furthermore, assessment of residual inflammatory activity allows refinement of prediction models for relapses during DMARD tapering, if combined with the assessment of ACPA status. Hence, more than 80% of the relapses could be predicted when using MBDA scores in conjunction with ACPA testing.
MBDA scores are based on the serum levels of 12 different proteins, which provide a more comprehensive picture of inflammation than mere assessment of CRP levels. Notably, inflammation does not only reflect acute phase responses, but also comprises other aspects such as local tissue inflammation, tissue remodelling and the interphase between inflammation and metabolism. While the MBDA score includes elements of the acute phase response, such as CRP, IL-6 and SAA, it also contains markers resembling local tissue inflammation (TNFRI, EGF, VEGF-A and VCAM-1) expressed by activated synovial fibroblasts. Furthermore, MBDA score includes MMP-1, MMP-3 and YKL-40 indicating local tissue remodelling of the synovium and the cartilage, whereas resistin and leptin are adipokines, controlling the interaction between fat metabolism and inflammation. Increased prevalence of disease relapses in patients with elevated MBDA scores may reflect subclinical inflammation at the target tissue level such as residual synovitis or osteitis. It is conceivable that inflammation in these patients is effectively suppressed by DMARDs at the clinical level but has not completely resolved. Hence, tapering of DMARDs may permit recurrence of inflammatory disease activity associated with clinical relapse. In contrast, patients having low MBDA scores may indeed experience true resolution of inflammation, which lowers the risk for disease relapse if DMARDs are tapered. Our data suggest that MBDA scores are more effective than clinical scores in prediction of disease relapses. As reported previously, baseline DAS28 scores were not predictive for relapses in the RETRO study.10 This situation may be based on the overall very low mean DAS28 scores in our cohort, which may hamper discrimination between patients with and without residual inflammation by clinical instruments. It will therefore be important to see the performance of MBDA scores in predicting relapses in other cohorts, where DMARDs were tapered or stopped. For instance, Tanaka and colleagues have shown, that in the specific case of TNF inhibitor withdrawal patients with a very low DAS28 score (≤1.98 units) were more likely to stay in low disease activity than patients with a higher baseline DAS28 (1.98–2.6 units).21
In our previous analysis, ACPA was the only independent predictor for relapse, supporting the role of autoimmunity in the disease course of RA. Similar findings were also reported from other cohorts tapering or stopping DMARD treatment.22–24 In the combined analysis of ACPA status and MDBA score, prediction of relapses fundamentally improved. Hence, double-negative patients had a very low chance for relapse (13%), which was similar to patients remaining on full-dose DMARD treatment. Relapse rates increased to more than 30% in patients with either positive ACPA or elevated MBDA scores and to more than 75% with concomitant presence of ACPA and elevated MBDA scores. ACPA and MBDA appear to act independently from each other in precipitating relapses, which was supported by multiple logistic regression models. Hence, both autoimmunity and residual inflammation appear to influence relapse risk of RA.
In summary, we show that elevated MBDA scores independently predict relapse of RA in the process of tapering and stopping DMARDs. Together with the assessment of ACPA status, MBDA score improves the prediction of relapse risk in patients with RA, allowing defining patient patterns with low and high relapse risks during DMARDs tapering.
Supplementary Material
Acknowledgments
We thank Rebecca Bolce, Eric Sasso and Oscar Seguardo (all Crescendo Biosciences) for complementary measurement and calculation of MBDA scores.
Footnotes
Contributors: AJH, JHa, BM, AK, MRe, JFC, CF, H-PT, SK, JW, FS, MRo, MFe, MFl, KM, WO, MS-H, H-ML, HN, RA, JHe and KK collected and analysed the data. SF, AJH, JR and GS designed the study. ME performed statistical analyses. JR and GS wrote the manuscript.
Funding: This study was supported by the Deutsche Forschungsgemeinschaft (SPP1468-IMMUNOBONE), the Bundesministerium für Bildung und Forschung (BMBF; project METARTHROS), the Marie Curie project OSTEOIMMUNE, the TEAM and MASTERSWITCH projects of the European Union and the IMI funded project BTCure.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: University Clinic Erlangen.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–19. 10.7748/phc2011.11.21.9.29.c8797 [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010;69: 631–7. 10.1136/ard.2009.123919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aga AB, Lie E, Uhlig T, et al. Time trends in disease activity, response and remission rates in rheumatoid arthritis during the past decade: results from the NOR-DMARD study 2000–2010. Ann Rheum Dis 2015;74:381–8. 10.1136/annrheumdis-2013-204020 [DOI] [PubMed] [Google Scholar]
- 4.Prince FH, Bykerk VP, Shadick NA, et al. Sustained rheumatoid arthritis remission is uncommon in clinical practice. Arthritis Res Ther 2012;14:R68 10.1186/ar3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatzidionysiou K, van Vollenhoven RF. When to initiate and discontinue biologic treatments for rheumatoid arthritis? J Intern Med 2011;269:614–25. 10.1111/j.1365-2796.2011.02355.x [DOI] [PubMed] [Google Scholar]
- 6.O'Mahony R, Richards A, Deighton C, et al. Withdrawal of disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2010;69:1823–6. 10.1136/ard.2008.105577 [DOI] [PubMed] [Google Scholar]
- 7.van den Broek M, Huizinga TW, Dijkmans BA, et al. Drug-free remission: is it already possible? Curr Opin Rheumatol 2011;23:266–72. 10.1097/BOR.0b013e32834563e3 [DOI] [PubMed] [Google Scholar]
- 8.Allaart CF, Lems WF, Huizinga TW. The BeSt way of withdrawing biologic agents. Clin Exp Rheumatol 2013;31:S14–18. [PubMed] [Google Scholar]
- 9.Emery P, Hammoudeh M, FitzGerald O, et al. Sustained remission with etanercept tapering in early rheumatoid arthritis. N Engl J Med 2014;37:1781–92. 10.1056/NEJMoa1316133 [DOI] [PubMed] [Google Scholar]
- 10.Haschka J, Englbrecht M, Hueber AJ, et al. Relapse rates in patients with rheumatoid arthritis in stable remission tapering or stopping anti-rheumatic therapy—interim results from the prospective randomized controlled RETRO study. Ann Rheum Dis 2016;75:45–51.. 10.1136/annrheumdis-2014-2064 [DOI] [PubMed] [Google Scholar]
- 11.Brown AK, Conaghan PG, Karim Z, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum 2008;58:2958–67. 10.1002/art.23945 [DOI] [PubMed] [Google Scholar]
- 12.Gandjbakhch F, Conaghan PG, Ejbjerg B, et al. Synovitis and osteitis are very frequent in rheumatoid arthritis clinical remission: results from an MRI study of 294 patients in clinical remission or low disease activity state. J Rheumatol 2011;38:2039–44. 10.3899/jrheum.110421 [DOI] [PubMed] [Google Scholar]
- 13.Brown AK, Quinn MA, Karim Z, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum 2006;54:3761–73. 10.1002/art.22190 [DOI] [PubMed] [Google Scholar]
- 14.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. 10.1136/ard.2010.138461 [DOI] [PubMed] [Google Scholar]
- 15.Prevoo ML, van ‘t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. 10.1002/art.1780380107 [DOI] [PubMed] [Google Scholar]
- 16.Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis 2011;70:404–13. 10.1136/ard.2011.149765 [DOI] [PubMed] [Google Scholar]
- 17.Curtis JR, van der Helm-van Mil AH, Knevel R, et al. Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res 2012;64:1794–803. 10.1002/acr.21767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centola M, Cavet G, Shen Y, et al. Development of a multi-biomarker disease activity test for rheumatoid arthritis. PLoS ONE 2013;8:e60635 10.1371/journal.pone.0060635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleischmann RM, Curtis JR, Hamburger MH, et al. RA population characteristics in InFoRM, a longitudinal observational study. Ann Rheum Dis 2010;69:657. [Google Scholar]
- 20.Bakker MF, Cavet G, Jacobs JW, et al. Performance of a multi-biomarker score measuring rheumatoid arthritis disease activity in the CAMERA tight control study. Ann Rheum Dis 2012;71:1692–7. 10.1136/annrheumdis-2011-200963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka Y, Hirata S, Kubo S, et al. Discontinuation of adalimumab after achieving remission in patients with established rheumatoid arthritis: 1-year outcome of the HONOR study. Ann Rheum Dis 2015;74:389–95. 10.1136/annrheumdis-2013-204016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Woude D, Young A, Jayakumar K, et al. Prevalence of and predictive factors for sustained disease-modifying antirheumatic drug-free remission in rheumatoid arthritis: results from two large early arthritis cohorts. Arthritis Rheum 2009;60:2262–71. 10.1002/art.24661 [DOI] [PubMed] [Google Scholar]
- 23.Klarenbeek NB, van der Kooij SM, Güler-Yuksel M, et al. Discontinuing treatment in patients with rheumatoid arthritis in sustained clinical remission: exploratory analyses from the BeSt study. Ann Rheum Dis 2011;70:315–9. 10.1136/ard.2010.136556 [DOI] [PubMed] [Google Scholar]
- 24.van der Kooij SM, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. Drug-free remission, functioning and radiographic damage after 4 years of response-driven treatment in patients with recent-onset rheumatoid arthritis. Ann Rheum Dis 2009;68:914–21. 10.1136/ard.2008.092254 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.