Abstract
Objective
To determine safety and efficacy of cardiac rehabilitation (CR) initiated immediately following balloon pulmonary angioplasty (BPA) in patients with inoperable chronic thromboembolic pulmonary hypertension (CTEPH) who presented with continuing exercise intolerance and symptoms on effort even after a course of BPA; 2–8 sessions/patient.
Methods
Forty-one consecutive patients with inoperable CTEPH who underwent their final BPA with improved resting mean pulmonary arterial pressure of 24.7±5.5 mm Hg and who suffered remaining exercise intolerance were prospectively studied. Participants were divided into two groups just after the final BPA (6.8±2.3 days): patients with (CR group, n=17) or without (non-CR group, n=24) participation in a 12-week CR of 1-week inhospital training followed by an 11-week outpatient programme. Cardiopulmonary exercise testing, haemodynamics, and quality of life (QOL) were assessed before and after CR.
Results
No significant between-group differences were found for any baseline characteristics. At week 12, peak oxygen uptake (VO2), per cent predicted peak VO2 (70.7±9.4% to 78.2±12.8%, p<0.01), peak workload, and oxygen pulse significantly improved in the CR group compared with the non-CR group, with a tendency towards improvement in mental health-related QOL. Quadriceps strength and heart failure (HF) symptoms (WHO functional class, 2.2–1.8, p=0.01) significantly improved within the CR group. During the CR, no patient experienced adverse events or deterioration of right-sided HF or haemodynamics as confirmed via catheterisation.
Conclusions
The combination of BPA and subsequent CR is a new treatment strategy for inoperable CTEPH to improve exercise capacity to near-normal levels and HF symptoms, with a good safety profile.
Introduction
In patients with chronic thromboembolic pulmonary hypertension (CTEPH) who are not candidates for pulmonary endarterectomy (PEA), balloon pulmonary angioplasty (BPA) has become an alternative therapy to improve haemodynamics and functional capacity,1–5 including our own report demonstrating right ventricular (RV) reverse remodelling.6 Moreover, using cardiopulmonary exercise testing (CPX), we recently demonstrated that a course of BPA sessions consisting of two to seven sessions/patient (BPAs) alone ameliorates exercise intolerance, which is an important prognostic predictor in CTEPH,7 relative to haemodynamic improvements, early after the procedure (even within 1 week), with minimal postprocedural physical deconditioning.8
In spite of recent remarkable advances in outcome after PEA, pulmonary hypertension (PH)-specific therapies, and BPA, most patients with CTEPH remain symptomatic on effort, possibly causing long-term inactivity and physical deconditioning. Consequently, they often experience exercise intolerance despite an improved prognosis. Indeed, our recent study clearly highlights the fact that exercise capacity improvement immediately after the final BPA remains insufficient (per cent predicted peak oxygen uptake (VO2), 70.9±10.9%), despite remarkably improved resting haemodynamics (mean pulmonary arterial pressure (mPAP), 23.0±5.1 mm Hg).8 Thus, exercise intolerance and symptoms on effort that remain even after BPAs are ongoing and important issues for inoperable CTEPH. In contrast, exercise training significantly improves exercise capacity, quality of life (QOL), and heart failure (HF) symptoms as an add-on to PH-specific therapies in small cohorts of patients with CTEPH as well as pulmonary arterial hypertension (PAH).9–12 However, the effects of cardiac rehabilitation (CR) in patients treated with BPA, which may be a more powerful and curative treatment for inoperable CTEPH than medical therapy, remain unknown. Additionally, only one report has assessed the effects of CR on pulmonary haemodynamics in detail, using invasive right heart catheterisation (RHC).12
Therefore, the aim of this study was (1) to determine the safety and efficacy of CR initiated immediately following the final BPA in patients with inoperable CTEPH who continued to suffer exercise intolerance and symptoms on effort despite improved haemodynamics and (2) to directly measure changes in haemodynamics before and after CR via RHC.
Methods
Study population and design
This prospective, controlled study with control subjects included 43 consecutive patients with inoperable CTEPH without any exclusions, who underwent their final BPA between July 2013 and February 2015, with markedly improved haemodynamics to a level below the definition of PH (<25 mm Hg; mPAP, 39.0±11.3 mm Hg to 24.5±5.3 mm Hg) and improved exercise capacity (per cent predicted peak VO2, 61.3±11.9% to 70.2±11.0%). However, all patients had either remaining exercise intolerance defined as per cent predicted peak VO2 <80% and/or dyspnoea on effort (≥II, per WHO functional class (WHO-FC)). One of the 43 patients was excluded due to a musculoskeletal disorder before group assignment.
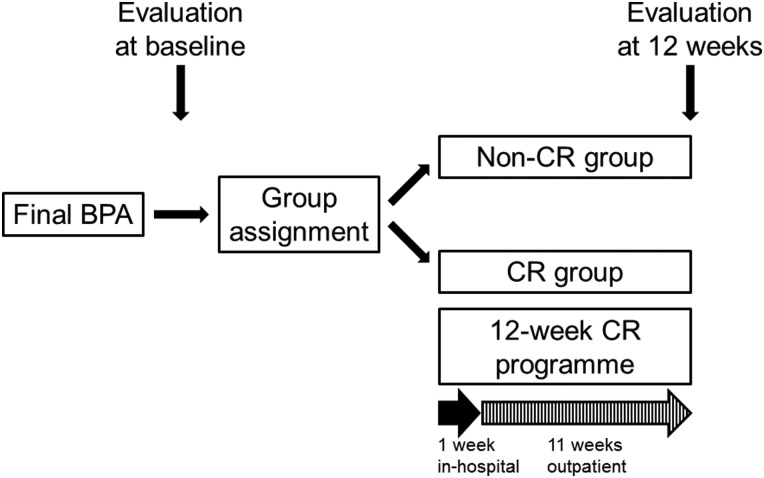
Details for the CTEPH diagnosis and BPA procedure are provided in the online supplementary material. The study protocol is summarised in figure 1.
Figure 1.
Summary of the present study protocol. Consecutive patients with inoperable chronic thromboembolic pulmonary hypertension who underwent a course of balloon pulmonary angioplasty (BPA) sessions were divided into the two groups just after the final BPA: those who did (cardiac rehabilitation (CR) group) or did not (non-CR group) participate in a 12-week CR programme consisting of 1-week inhospital training and a subsequent 11-week outpatient programme. Clinical assessment to evaluate efficacy and safety of CR was performed both at the beginning and end of CR.
heartjnl-2015-309230supp.pdf (124.2KB, pdf)
Immediately following the final BPA, patients were divided into two groups: patients who did (CR group, n=17) or did not (non-CR group, n=25) participate in a 12-week CR programme. Although we uniformly invited all patients to participate in CR, those who were unwilling to participate or lived too far to regularly attend the outpatient programme were assigned to the non-CR group. PH-specific therapies remained unchanged during the study period.
Outcome measures
Patients underwent clinical assessments to evaluate the efficacy and safety of the 12-week CR, both immediately following the final BPA (at the beginning of CR) and 3 months after the final BPA (at the end of CR), including CPX, measurements of muscle strength, RHC, QOL questionnaires, functional status (WHO-FC), brain natriuretic peptide (BNP) level, 6-min walk distance (6MWD), and respiratory function including arterial blood gas analysis at room air and spirometry, as previously described.6 8 13 14 Details for the outcome measures are provided in the online supplementary material.
Exercise training programme
The CR programme started just after the final BPA with daily inhospital training for 1 week, followed by an 11-week outpatient programme that combined home exercise and once or twice weekly outpatient sessions, which were closely monitored and supervised by physical therapists, cardiologists and PH experts with expertise in exercise training (figure 1).8 13–15 Inhospital training consisted of walking, bicycle ergometer and low-intensity resistance training of the lower limbs, but without specific training of respiratory muscles or upper limb muscles. Details for the exercise training programme are provided in the online supplementary material.
Statistical analysis
Quantitative results are expressed as mean±SD or medians (25% and 75%) when appropriate. Comparison of baseline characteristics between groups was performed using t tests or the Mann–Whitney U test for continuous variables as appropriate and χ2 tests for categorical variables. Between-group differences from baseline to week 12 were examined using an analysis of covariance (ANCOVA) adjusted for baseline measures, or the Mann–Whitney U test comparing the changes from baseline, as appropriate, except WHO-FC, which was assessed using ordinal logistic regression analysis. Within-group differences, including haemodynamics, CPX, and other clinical parameters before and after CR, were examined using paired t tests or Wilcoxon signed-rank tests, as appropriate. Spearman's correlation coefficients were used to evaluate the relationships between variables. Two-sided p values <0.05 were considered statistically significant. Instances of missing data, including cases where either the preinterventional or postinterventional data were missing, were excluded from the corresponding analysis. All statistical analyses were performed using JMP V.12.0 (SAS Institute, Cary, North Carolina, USA).
Results
Study population
The non-CR group included one patient who discontinued the CR early owing to familial problems. All of the remaining 17 patients with CR tolerated and completed the CR. One non-CR patient was excluded due to modification of the PH-specific therapy during the study period. Thus, the final study population was 41 patients, consisting of the 24 patients in the non-CR group and the 17 patients in the CR group. Two non-CR patients were unable to undergo either the preinterventional or postinterventional CPX owing to social problems.
Baseline patient characteristics and follow-up
No significant differences were found between the groups at baseline (just after the final BPA) (table 1). At baseline, 30/40 patients for whom preinterventional CPX recordings were available had exercise intolerance (per cent predicted peak VO2 <80%); the remaining 10 patients without initial exercise intolerance and 1 patient without a preinterventional CPX remained symptomatic on effort at WHO-FC II–III.
Table 1.
Baseline characteristics of patients with inoperable chronic thromboembolic pulmonary hypertension, based on participation in cardiac rehabilitation
| Non-CR group | CR group | p Value | |
|---|---|---|---|
| Patients (n) | 24 | 17 | |
| Age (years, range) | 66±10 (49–85) | 70±7 (53–82) | 0.16 |
| Sex (female/male) | 20/4 | 10/7 | 0.08# |
| Body mass index (kg/m2) | 22.4±3.8 | 22.2±3.3 | 0.85 |
| WHO-FC (I/II/III/IV) | 1/21/2/0 | 0/14/3/0 | 0.26## |
| Patients previously treated with PEA (n, %) | 3 (13) | 1 (6) | 0.48# |
| Disease duration before the first BPA (months) | 66 (29–127) | 53 (20–86) | 0.23## |
| Pulmonary hypertension-specific therapy (n, %) | 15 (63) | 10 (59) | 0.81# |
| 6-min walk distance (m) | 468±102 | 498±96 | 0.34 |
| Brain natriuretic peptide level (pg/mL) | 28 (16–39) | 22 (15–41) | 0.56## |
| Haemodynamic data | |||
| Mean PAP (mm Hg) | 24.9±4.9 | 24.5±6.4 | 0.80 |
| Mean RAP (mm Hg) | 1.5 (0.0–3.0) | 1.0 (0.5–2.0) | 0.17## |
| Cardiac index (L/min/m2) | 2.54±0.53 | 2.62±0.50 | 0.63 |
| TPR (dyne.s/cm5) | 544±189 | 507±178 | 0.53 |
| CPX parameters | n=22 | n=17 | |
| Peak work load (W) | 81±19 | 85±23 | 0.53 |
| Peak VO2 (mL/min/kg) | 17.8±2.6 | 17.4±2.6 | 0.61 |
| Peak VO2% predicted (%) | 69.7±12.3 | 70.7±9.4 | 0.78 |
| VE-VCO2 slope | 37.6±6.8 | 42.1±10.2 | 0.11 |
Results are expressed as mean±SD or median (25% and 75% centile) unless otherwise indicated.
p Values from #χ2 tests, ##the Mann–Whitney U test, or otherwise t tests.
BPA, balloon pulmonary angioplasty; CPX, cardiopulmonary exercise testing; CR, cardiac rehabilitation; PAP, pulmonary arterial pressure; PEA, pulmonary endarterectomy; RAP, right atrial pressure; TPR, total pulmonary resistance; VCO2, carbon dioxide production; VO2, oxygen uptake; VE, minute ventilation; WHO-FC, WHO functional class.
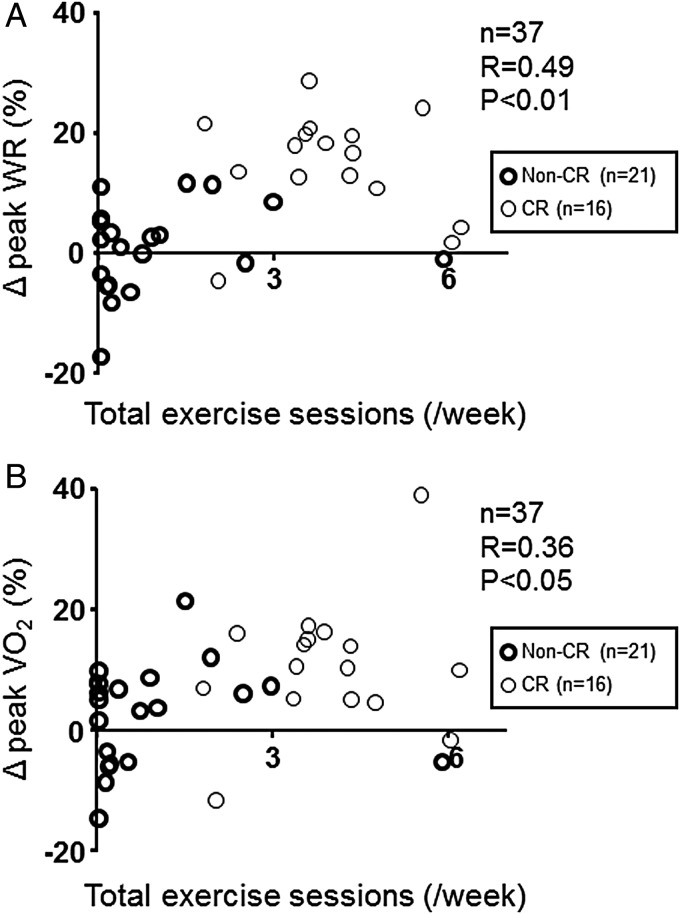
As a whole, patients had low per cent predicted peak VO2 (70.2±11.0%) at baseline (table 1). Patients with CR participated in CR 6.8±2.3 (range, 3–11) days after the final BPA. During the mean follow-up of 12.8±6.1 (range, 3–22) months from the final BPA, any cardiac events such as deaths or hospitalisation due to exacerbation of HF did not occur. Among 37 patients (90%) who recorded the time and frequency of home exercise throughout the study period, the CR group more frequently implemented both inhospital and home exercise training (median, 3.7 (3.3–4.6); range, 1.8–6.2 days/week) compared with the non-CR group (median, 0.2 (0.0–1.2); range, 0–5.9 days/week; p<0.01), when only training lasting ≥30 min/day was included.
Exercise capacity, muscle strength and heart failure symptoms
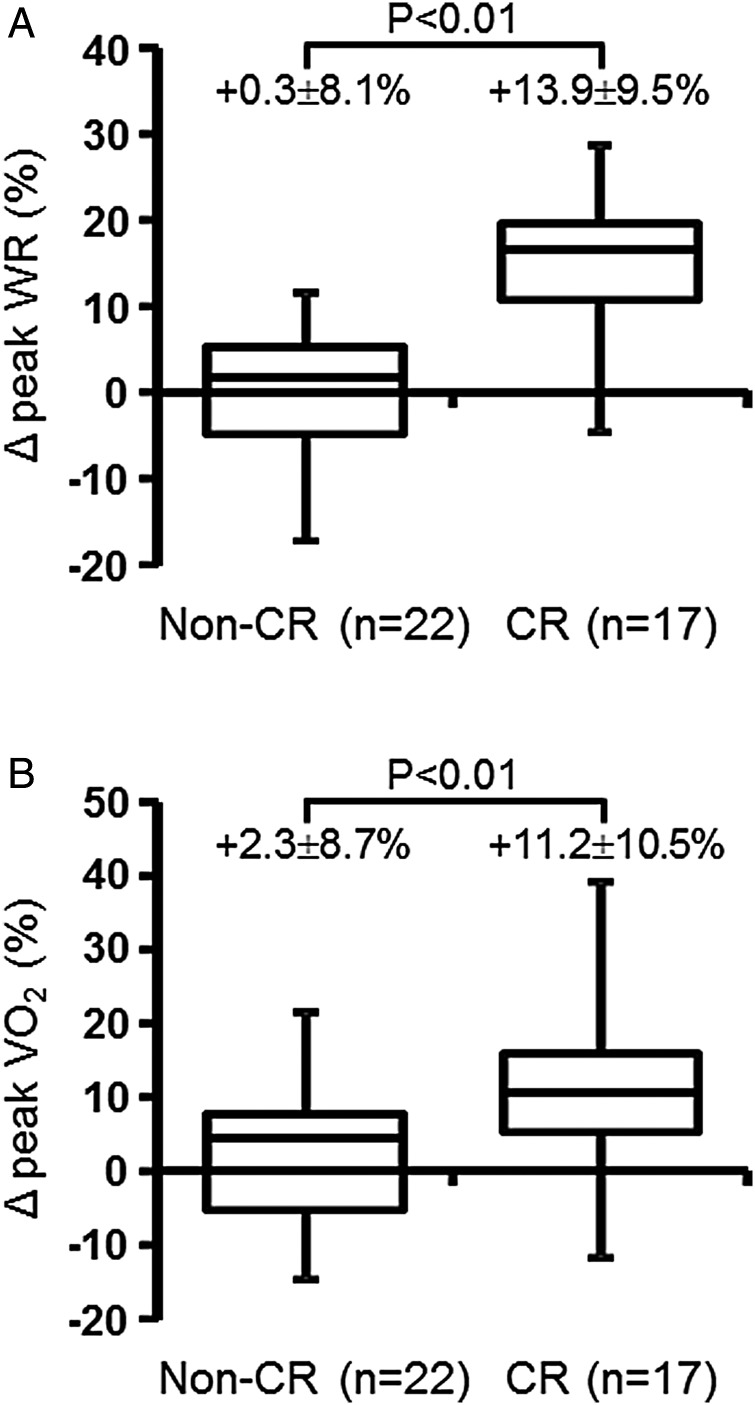
At 12 weeks, the per cent increase in peak workload (%Δ peak WR) and %Δ peak VO2 from baseline were significantly greater in the CR group than in the non-CR group (figure 2A, B). Moreover, the improvement in most exercise capacity indices, such as peak VO2, per cent predicted peak VO2, peak WR and oxygen pulse, was significantly greater in the CR group than in the non-CR group, except VO2 at the anaerobic threshold (AT) (table 2). Particularly, per cent predicted peak VO2 in the CR group increased to near-normal levels (78.2±12.8%). Both %Δ peak WR and %Δ peak VO2 were significantly correlated with the number of total exercise sessions/week during the study period (figure 3A, B). Quadriceps isometric strength, but not maximal handgrip strength, significantly increased in the CR group, both of which were unavailable in the non-CR group (table 2). The average WHO-FC, already at 2.2 via BPA, further improved to 1.8 after CR. The increase in 6MWD, another index of exercise capacity, improved non-significantly in the CR group.
Figure 2.
Comparison of changes in peak work load (WR) and peak oxygen uptake (VO2) from baseline. At 12 weeks, the percentages of change from baseline in peak WR (Δ peak WR, %) (A) and peak VO2 (Δ peak VO2, %) (B) were significantly greater in the cardiac rehabilitation (CR) group (n=17) than in the non-CR group (n=22). p Values are given for the differences in Δ peak WR and Δ peak VO2 from baseline between the non-CR and CR groups using ANCOVA. Results are expressed as mean±SD.
Table 2.
Cardiopulmonary exercise testing (CPX) parameters, muscle strength and heart failure symptoms at baseline and 12 weeks
| Non-CR group (n=22) | CR group (n=17) | Between-group p value |
|||||
|---|---|---|---|---|---|---|---|
| Baseline | 12 weeks | Within-group p value |
Baseline | 12 weeks | Within-group p value |
||
| Peak respiratory exchange ratio | 1.25±0.10 | 1.23±0.10 | 0.18 | 1.32±0.11 | 1.31±0.10 | 0.57 | 0.10 |
| Peak WR (W) | 81±19 | 82±23 | 0.49 | 85±23 | 97±27 | <0.01 | <0.01 |
| Peak VO2 (mL/min/kg) | 17.8±2.6 | 18.1±3.4 | 0.45 | 17.4±2.6 | 19.1±3.5 | <0.01 | <0.01 |
| Peak VO2% predicted (%) | 69.7±12.3 | 70.9±13.5 | 0.41 | 70.7±9.4 | 78.2±12.8 | <0.01 | <0.01 |
| VO2 at AT (mL/min/kg)* | 10.8±1.6 | 11.4±2.7 | 0.12 | 10.2±1.6 | 10.8±2.2 | 0.25 | 0.98 |
| VO2 at AT % predicted (%)* | 61.6±10.4 | 65.6±15.3 | 0.09 | 61.7±10.6 | 65.6±11.9 | 0.24 | 0.99 |
| Resting heart rate (beats/min) | 80±14 | 80±16 | 0.79 | 75±12 | 75±11 | 0.71 | 0.45 |
| Peak heart rate (beats/min) | 141±25 | 141±26 | 0.66 | 145±15 | 147±15 | 0.31 | 0.73 |
| Δ VO2/Δ WR (mL/min/W) | 8.2±1.3 | 8.0±1.5 | 0.47 | 7.6±1.4 | 7.6±1.9 | 0.88 | 0.75 |
| Oxygen pulse (mL/min/beat) | 6.9±1.7 | 7.0±1.7 | 0.39 | 6.6±1.5 | 7.3±1.7 | <0.01 | 0.01 |
| VE-VCO2 slope | 37.6±6.8 | 36.6±6.5 | 0.33 | 42.1±10.2 | 39.7±9.8 | 0.17 | 0.94 |
| Lowest SpO2 (%) | 90.2±4.7 | 91.3±3.9 | 0.09 | 89.0±5.1 | 89.2±4.8 | 0.80 | 0.21 |
| Muscle strength | |||||||
| Forearm (kg) | NA | NA | NA | 28 (23–37) | 27 (25–37) | 0.74# | NA |
| Quadriceps (kg) | NA | NA | NA | 26.4±8.1 | 29.1±8.1 | <0.01 | NA |
| Other clinical parameters | n=24 | n=24 | n=17 | n=17 | |||
| WHO-FC (I/II/III/IV, mean) | 1/21/2/0, 2.0 | 4/19/1/0, 1.9 | 0.05# | 0/14/3/0, 2.2 | 4/12/1/0, 1.8 | 0.01# | 0.28## |
| 6-min walk distance (m) | 468±102 | 476±97 | 0.43 | 498±96 | 510±98 | 0.10 | 0.53 |
Results are expressed as mean±SD or median (25% and 75% centile) unless otherwise indicated. Within-group p values from #Wilcoxon signed-rank tests or otherwise paired t tests. Between-group p values from ##ordinal logistic regression analysis or otherwise ANCOVA.
*21 patients in the non-CR group and 12 patients in the CR group for this data point, because we could not accurately determine the AT level in preinterventional and/or postinterventional CPX in 6 patients due to ventilatory oscillation-like changes or increased ventilatory drives even at rest.
AT, anaerobic threshold; CR, cardiac rehabilitation; NA, not available; SpO2, oxygen saturation; VCO2, carbon dioxide production; VE, minute ventilation; VO2, oxygen uptake; WHO-FC, WHO functional class; WR, work load.
Figure 3.
The correlation of the number of total exercise sessions with the percentages of change in peak work load (WR) and peak oxygen uptake (VO2). As a whole including both the non-cardiac rehabilitation (CR) and CR groups (n=21 and 16, respectively), the number of total exercise sessions/week performed during the study period, including both inhospital and home exercise, was significantly correlated with the percentages of change from baseline in both peak WR (Δ peak WR, %) (A) and peak VO2 (Δ peak VO2, %) (B). p Values are derived from Spearman's correlation coefficients test.
QOL scores and respiratory function
The mental health-related QOL scores on the Short Form Health Survey at 12 weeks tended to improve in the CR group compared with the non-CR group (table 3). Depressive symptoms assessed with the Patient Health Questionnaire-9 remained unchanged in both groups at 12 weeks. The diffusing capacity of the lung for carbon monoxide improved in the CR group but not in the non-CR group, whereas other respiratory functional parameters including vital capacity, forced expiratory volume in 1 s and the three measured arterial blood gas parameters were unchanged in either group.
Table 3.
Quality of life (QOL) scores and respiratory functional parameters at baseline and 12 weeks
| Non-CR group (n=24) | CR group (n=17) | Between-group p value |
|||||
|---|---|---|---|---|---|---|---|
| Baseline | 12 weeks | Within-group p value |
Baseline | 12 weeks | Within-group p value |
||
| QOL questionnaire SF-36* | n=24 | n=24 | n=15 | n=15 | |||
| Physical functioning | 75 (54–90) | 80 (61–94) | 0.11# | 75 (50–85) | 85 (65–94) | 0.17# | 0.68## |
| Role-physical | 78 (53–94) | 81 (50–100) | 0.96# | 69 (44–94) | 69 (38–100) | 1.00# | 0.94## |
| Bodily pain | 100 (62–100) | 79 (65–100) | 0.21# | 100 (80–100) | 100 (62–100) | 0.55# | 0.91## |
| General health perception | 62 (37–67) | 56 (38–72) | 0.48# | 52 (35–77) | 62 (52–72) | 0.37# | 0.37## |
| Vitality | 75 (56–92) | 63 (50–81) | 0.11# | 75 (50–81) | 75 (50–88) | 0.87# | 0.21## |
| Social functioning | 88 (66–97) | 88 (75–100) | 0.17# | 63 (50–100) | 63 (50–100) | 0.46# | 0.52## |
| Role-emotional | 83 (50–100) | 88 (75–100) | 0.13# | 100 (67–100) | 100 (75–100) | 0.66# | 0.99## |
| Mental health | 80 (58–85) | 75 (60–85) | 0.57# | 80 (55–95) | 90 (60–100) | 0.10# | 0.09## |
| Depression questionnaire† | n=22 | n=22 | n=15 | n=15 | |||
| PHQ-9 | 3.5 (1.0–7.5) | 5.0 (1.8–8.3) | 0.20# | 1.0 (0.0–4.0) | 2.0 (0.0–5.0) | 0.93# | 0.20## |
| Respiratory function‡ | n=24 | n=24 | n=14 | n=14 | |||
| VC % predicted (%) | 103±11 | 103±12 | 0.96 | 117±13 | 117±14 | 1.00 | 0.46 |
| FEV1.0% predicted (%) | 97±15 | 93±20 | 0.09 | 112±16 | 112±17 | 0.85 | 0.41 |
| DLCO % predicted (%) | 72±13 | 75±11 | 0.09 | 71±12 | 75±16 | 0.04 | 0.69 |
| Arterial blood gas analysis§ | n=22 | n=22 | n=17 | n=17 | |||
| PaO2 (mm Hg) | 65.6±9.8 | 65.0±10.2 | 0.85 | 67.3±8.8 | 65.9±9.7 | 0.58 | 0.90 |
| PaCO2 (mm Hg) | 39.5±4.4 | 39.3±3.4 | 0.79 | 38.0±3.8 | 39.8±3.3 | 0.07 | 0.27 |
| AaDO2 (mm Hg) | 34.8±9.1 | 35.6±8.0 | 0.75 | 34.9±9.9 | 34.0±11.3 | 0.68 | 0.58 |
Results are expressed as mean±SD or median (25% and 75% centile). Within-group p values from #Wilcoxon signed-rank tests or otherwise paired t tests. Between-group p values from ##the Mann–Whitney U test comparing the changes from baseline or otherwise ANCOVA.
*15 patients in the CR group for this data point, because we could not obtain data for 2 patients.
†22 patients in the non-CR group and 15 patients in the CR group for this data point, because we could not obtain data for 4 patients.
‡14 patients in the CR group for this data point, because we could not obtain data on preinterventional respiratory function for 3 patients.
§22 patients in the non-CR group for this data point, because we could not obtain completely reliable data for 2 patients.
AaDO2, alveolar-arterial PO2 difference; CR, cardiac rehabilitation; DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 s; PaCO2, arterial carbon dioxide tension; PaO2, arterial oxygen tension; PHQ-9, Patient Health Questionnaire-9; SF-36, Short Form Health Survey; VC, vital capacity.
Safety profile and non-responders
During the study period, no patients with CR experienced adverse events, such as syncope, respiratory infection, arrhythmia, severe hypotension or haemoptysis requiring any treatment; moreover, there was no deterioration in haemodynamics or right-sided HF (table 4). Two (12%) of 17 patients in the CR group exhibited a decrease in peak VO2 from baseline and were considered to be non-responders to the present CR treatment. Except for one borderline responder (+4.7% in peak VO2), the remaining 14 patients exhibited significant improvements in peak VO2 (≥5%). Details for the non-responders are provided in the online supplementary material.
Table 4.
Safety evaluation including brain natriuretic peptide (BNP) and haemodynamic data at baseline and 12 weeks
| Non-CR group (n=24) | CR group (n=17) | Between-group p value |
|||||
|---|---|---|---|---|---|---|---|
| Baseline | 12 weeks | Within-group p value |
Baseline | 12 weeks | Within-group p value |
||
| BNP level (pg/mL) | 28 (16–39) | 22 (16–48) | 0.96# | 22 (15–41) | 35 (20–45) | 0.52# | 0.76## |
| Haemodynamic data | |||||||
| Mean PAP (mm Hg) | 24.9±4.9 | 25.2±5.3 | 0.73 | 24.5±6.4 | 24.8±4.1 | 0.77 | 0.91 |
| Mean RAP (mm Hg) | 1.5 (0.0–3.0) | 3.0 (2.0–3.0) | 0.33# | 1.0 (0.5–2.0) | 2.0 (1.0–3.5) | 0.01# | 0.23## |
| Cardiac index (L/min/m2) | 2.54±0.53 | 2.52±0.54 | 0.86 | 2.62±0.50 | 2.48±0.55 | 0.19 | 0.52 |
| TPR (dyne.s/cm5) | 544±189 | 561±194 | 0.38 | 507±178 | 545±154 | 0.08 | 0.61 |
Results are expressed as mean±SD or median (25% and 75% centile). Within-group p values from #Wilcoxon signed-rank tests or otherwise paired t tests. Between-group p values from ##the Mann–Whitney U test comparing the changes from baseline or otherwise ANCOVA.
CR, cardiac rehabilitation; PAP, pulmonary arterial pressure; RAP, right atrial pressure; TPR, total pulmonary resistance.
Discussion
To the best of our knowledge, this is the first study to prospectively investigate the impact of CR on exercise intolerance and symptoms on effort that remain even after BPAs in patients with inoperable CTEPH.
An ongoing major problem exists after balloon pulmonary angioplasty
BPA has become an alternative therapy for inoperable CTEPH to improve haemodynamics, functional capacity and RV function,1–3 5 6 likely leading to an improved prognosis because these are all important prognostic predictors in CTEPH.7 16 Indeed, in the present study, any deaths or hospitalisation due to exacerbation of HF did not occur for 19.5±6.8 months from the first BPA, indicating 100% event-free survival. However, the patients still suffered exercise intolerance and dyspnoea on effort even after BPAs, despite remarkably restored resting haemodynamics. Therefore, we consider this as an ongoing, major problem that requires resolution.
Exercise intolerance and combined therapies for inoperable CTEPH
Patients with inoperable CTEPH usually have a long disease duration under deteriorated haemodynamics, resulting in long-term inactivity and deconditioning. Exercise intolerance is a key feature of PH/PAH and is more highly correlated with outcomes of patients with PH/PAH than haemodynamic variables.17–19 Patients with PH reportedly have both respiratory and peripheral skeletal muscle abnormalities, termed PH/PAH-related myopathy, with subsequent muscle atrophy and reduced contractility.19 In general, full recovery from deconditioning requires haemodynamic improvements and peripheral adaptations such as enhanced quadriceps muscle capillarisation and oxidative enzyme activity,20 that are impossible to restore with BPA alone. Indeed, we have previously reported the importance of peripheral adaptations after PEA.14
Therefore, we tested the combination of BPAs and subsequent CR, the latter of which targets the exercise intolerance and dyspnoea on effort that remain after BPA. We also expected a combination of BPA-induced haemodynamic improvements followed by CR-induced peripheral adaptations. Surprisingly, first, BPA alone significantly ameliorated exercise intolerance and haemodynamics to the level below the definition of PH. Second, the 12-week CR improved residual exercise intolerance to near-normal levels and further improved HF symptoms, with maintenance of improved haemodynamics. Moreover, the present patients had longer average disease duration (74 months) than in our previous reports, indicating serious long-term deconditioning before BPA. Collectively, these indicate that the combination of BPAs and subsequent CR additively ameliorated exercise intolerance to near-normal levels even in patients with long-term deconditioning, suggesting the promising effects of this new treatment strategy for inoperable CTEPH.
Differences from previous reports
In some previous reports, exercise training has been reported to significantly improve exercise capacity and QOL as an add-on to PH-specific therapies in patients with CTEPH.9–12 21–23 Patients with CTEPH were typically included as a small sample of mixed cohorts, with various forms of PH.9 10 12 21–23 Only one report described the efficacy of exercise training for cohorts purely consisting of CTEPH, but without control subjects.11 Thus, there has been no report describing the effects of CR in patients treated with BPA, which may be more powerful than medical therapy.
Compared with these previous studies, this study includes several important differences. First, the present patients had a remarkably lower mPAP (24.7±5.5 mm Hg) at the beginning of CR than in previous studies (41–57 mm Hg), suggesting a better safety profile for exercise.9–12 21–23 Second, the initial inhospital part of the training in this study was 1 week, shorter than that in most previous studies (3 weeks).9–12 22 23 Importantly, this outpatient-based programme did not result in low compliance, as evidenced by only one dropout and the number of total exercise sessions/week performed in the CR group (>three sessions/week).9 Third, compared with most previous studies, except one recent report,12 this study more accurately evaluated haemodynamics with invasive RHC both before and after CR. The present results indicate that CR did not further improve nor did it worsen the improved haemodynamics following BPA, inconsistent with the findings of Ehlken et al,12 who demonstrated that exercise training improved peak VO2 and haemodynamics and RV function. This discrepancy may be largely attributed to the differences in the severity of baseline haemodynamics between the two study populations (mPAP, 24.7±5.5 mm Hg vs 41 mm Hg).12 Finally, the improvement in per cent predicted peak VO2 after CR was higher (78.2±12.8%) in this study than in two previous studies (59–60.3%) in which the corresponding data were available,9 23 although direct comparisons are difficult due to methodological differences such as body position during CPX. Collectively, these differences suggest that CR as an add-on therapy to BPA may be a safer and more effective method to ameliorate exercise capacity than PH-specific therapies alone as an add-on therapy.
Safety of the current CR protocol for inoperable CTEPH
There had been serious concerns about the risk of exercise in patients with PH.24 However, exercise training in patients with PH has an acceptable safety profile in recent studies, where exercise training was performed in a highly supervised setting only for patients who were stable on optimised PH-specific therapies.9–12 21–23 In this study, patients with CR, all of whom were closely supervised by PH experts and cardiologists with expertise in exercise training,8 13 14 did not experience adverse events and had no deterioration in haemodynamics or right-sided HF compared with the non-CR group, as evidenced by unchanged mPAP, cardiac index, total pulmonary resistance, and BNP level before and after CR. Importantly, the safety profile of CR for haemodynamics was confirmed directly with RHC. Thus, these results indicate that the current protocol of CR initiated immediately following the final BPA did not increase the risk, compared with previously reported protocols.9–12 21–23
Exercise training protocols in the present study
In this study, exercise training protocols did not include upper limb muscle training or respiratory training, to focus on the effects of CR on a single training component (lower limbs), rather than a mixture of different training components. Consequently, quadriceps isometric strength, but not maximal handgrip force, significantly increased in the CR group; this is likely associated with unchanged respiratory function and blood gas analysis. This is because forearm muscle strength is reported to directly correlate with respiratory muscle weakness.25 In most previous reports with patients with PH, the training programme included both low-dose endurance exercise and respiratory training.9–12 22 23 Mereles et al9 demonstrated that the combination of endurance exercise and respiratory training showed more improvements in 6MWD than endurance exercise alone. In addition, respiratory muscles are predominantly impaired, rather than general skeletal muscle, in patients with PH.26 In future studies, we need to clarify whether combined endurance exercise with upper and lower limb muscle training and respiratory training is more beneficial, especially on respiratory function and arterial blood oxygenation, for patients with CTEPH treated with BPA.
Limitations of the study
There are several limitations of this study. First, this study lacked randomisation during group assignment, although it was prospectively designed with a control group. Therefore, we cannot exclude the possibility that selection bias affected the present results. However, no significant between-group differences were found in any baseline characteristics, which might strengthen the value of the present results. Second, this study was implemented in a single centre, although our centre is one of the largest PH centres in Japan with experienced rehabilitation centres.8 13–15 These results should be confirmed in a large, randomised, multicentre study. Third, the increase in 6MWD, a convenient index of exercise capacity, in the CR group did not reach statistical significance—this is inconsistent with previous studies with patients with CTEPH.9–12 21–23 It is possible that our patients with CR walked much better in the baseline 6MWD examination (498±96 m) than the patients with CTEPH in previous studies (353–453 m),9–12 21–23 because our patients had already undergone BPAs before group assignment, in addition to PH-specific therapies. This is also supported by the findings that exercise training might be more effective in patients with a lower 6MWD, rather than those who have a near-normal 6MWD (>550 m)10 and that 6MWD is less sensitive to increases in peak VO2 at distances >500 m.27 Fourth, VO2 at AT did not significantly improve after CR, consistent with the findings of Yuan et al,28 who conducted a systematic review and meta-analysis on exercise training for PH. In addition, we could not accurately determine the AT level in the preinterventional and/or postinterventional CPX in 5 of 17 patients in the CR group due to ventilatory oscillation-like changes or increased ventilatory drives even at rest, implying that the AT level is unreliable in this population. Finally, physical-related QOL scores were unchanged after CR, which is inconsistent with previous studies.9–11 23 We could explain this discrepancy by our preliminary data that physical-related QOL scores in our patients had already improved to a certain degree before CR via BPA alone (data not shown), as well as haemodynamics and functional capacity.
Conclusions
Exercise intolerance, a key feature of CTEPH, remains even after BPAs, which continues to be an important issue that should be addressed. The combination of BPAs and subsequent CR for inoperable CTEPH additively ameliorates exercise intolerance to near-normal levels and improves HF symptoms, with a tendency towards improvement in mental health-related QOL. There were no adverse events or deterioration in haemodynamics or right-sided HF. The good safety profile of CR regarding haemodynamics was confirmed directly with RHC. This promising new treatment strategy does not require a prolonged hospital stay for initial inhospital training and did not lower patient compliance. Further large, randomised, multicentre studies are needed to confirm the present results.
Key messages.
What is already known on this subject?
A course of balloon pulmonary angioplasty sessions alone ameliorates exercise intolerance early after the last session (even within 1 week).
Exercise training significantly improves exercise capacity, quality of life and heart failure (HF) symptoms as an add-on to pulmonary hypertension-specific therapies in patients with inoperable chronic thromboembolic pulmonary hypertension.
What might this study add?
Exercise intolerance and symptoms on effort persist even after a course of balloon pulmonary angioplasty sessions, despite remarkably restored resting haemodynamics.
A 12-week cardiac rehabilitation programme initiated immediately following balloon pulmonary angioplasty improved residual exercise intolerance to near-normal levels and further improved HF symptoms, with maintenance of improved haemodynamics.
How might this impact on clinical practice?
Cardiac rehabilitation should be considered to further improve exercise intolerance and symptoms on effort that remain after balloon pulmonary angioplasty.
Acknowledgments
The authors thank Seiko Horiike, Harumi Konishi and Kazuya Yamamoto for their technical assistance.
Footnotes
Contributors: Acquisition of data/analysis and interpretation: SF, TO, JU, AT, YM, RK, TA, MN, TF and YG. Conception, hypothesis delineation and design of study: SF, TO, HT, SY, HO, NN and YG. Writing and revising the manuscript: SF, TO, SY and YG.
Competing interests: None declared.
Ethics approval: Our institution’s ethics committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Feinstein JA, Goldhaber SZ, Lock JE, et al. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001;103:10–13. 10.1161/01.CIR.103.1.10 [DOI] [PubMed] [Google Scholar]
- 2.Mizoguchi H, Ogawa A, Munemasa M, et al. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012;5:748–55. 10.1161/CIRCINTERVENTIONS.112.971077 [DOI] [PubMed] [Google Scholar]
- 3.Sugimura K, Fukumoto Y, Satoh K, et al. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J 2012;76:485–8. 10.1253/circj.CJ-11-1217 [DOI] [PubMed] [Google Scholar]
- 4.Kataoka M, Inami T, Hayashida K, et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012;5:756–62. 10.1161/CIRCINTERVENTIONS.112.971390 [DOI] [PubMed] [Google Scholar]
- 5.Andreassen AK, Ragnarsson A, Gude E, et al. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart 2013;99:1415–20. 10.1136/heartjnl-2012-303549 [DOI] [PubMed] [Google Scholar]
- 6.Fukui S, Ogo T, Morita Y, et al. Right ventricular reverse remodelling after balloon pulmonary angioplasty. Eur Respir J 2014;43:1394–402. 10.1183/09031936.00012914 [DOI] [PubMed] [Google Scholar]
- 7.Condliffe R, Kiely DG, Gibbs JS, et al. Prognostic and aetiological factors in chronic thromboembolic pulmonary hypertension. Eur Respir J 2009;33:332–8. 10.1183/09031936.00092008 [DOI] [PubMed] [Google Scholar]
- 8.Fukui S, Ogo T, Goto Y, et al. Exercise intolerance and ventilatory inefficiency improve early after balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Int J Cardiol 2015;180:66–8. 10.1016/j.ijcard.2014.11.187 [DOI] [PubMed] [Google Scholar]
- 9.Mereles D, Ehlken N, Kreuscher S, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation 2006;114:1482–9. 10.1161/CIRCULATIONAHA.106.618397 [DOI] [PubMed] [Google Scholar]
- 10.Grünig E, Lichtblau M, Ehlken N, et al. Safety and efficacy of exercise training in various forms of pulmonary hypertension. Eur Respir J 2012;40:84–92. 10.1183/09031936.00123711 [DOI] [PubMed] [Google Scholar]
- 11.Nagel C, Prange F, Guth S, et al. Exercise training improves exercise capacity and quality of life in patients with inoperable or residual chronic thromboembolic pulmonary hypertension. PLoS ONE 2012;7:e41603 10.1371/journal.pone.0041603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehlken N, Lichtblau M, Klose H, et al. Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo-embolic pulmonary hypertension: a prospective, randomized, controlled trial. Eur Heart J 2016;37:35–44. 10.1093/eurheartj/ehv337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuda H, Ogino H, Minatoya K, et al. Long-term recovery of exercise ability after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Ann Thorac Surg 2006;82:1338–43. 10.1016/j.athoracsur.2006.03.105 [DOI] [PubMed] [Google Scholar]
- 14.Iwase T, Nagaya N, Ando M, et al. Acute and chronic effects of surgical thromboendarterectomy on exercise capacity and ventilatory efficiency in patients with chronic thromboembolic pulmonary hypertension. Heart 2001;86:188–92. 10.1136/heart.86.2.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki S, Takaki H, Yasumura Y, et al. Assessment of quality of life with 5 different scales in patients participating in comprehensive cardiac rehabilitation after acute myocardial infarction. Circ J 2005;69:1527–34. 10.1253/circj.69.1527 [DOI] [PubMed] [Google Scholar]
- 16.Chin KM, Kim NH, Rubin LJ. The right ventricle in pulmonary hypertension. Coron Artery Dis 2005;16:13–18. 10.1097/00019501-200502000-00003 [DOI] [PubMed] [Google Scholar]
- 17.Wensel R, Opitz CF, Anker SD, et al. Assessment of survival in patients with primary pulmonary hypertension: importance of cardiopulmonary exercise testing. Circulation 2002;106:319–24. 10.1161/01.CIR.0000022687.18568.2A [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med 2000;161:487–92. 10.1164/ajrccm.161.2.9906015 [DOI] [PubMed] [Google Scholar]
- 19.Marra AM, Arcopinto M, Bossone E, et al. Pulmonary arterial hypertension-related myopathy: an overview of current data and future perspectives. Nutr Metab Cardiovasc Dis 2015;25:131–9. 10.1016/j.numecd.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 20.de Man FS, Handoko ML, Groepenhoff H, et al. Effects of exercise training in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2009;34:669–75. 10.1183/09031936.00027909 [DOI] [PubMed] [Google Scholar]
- 21.Fox BD, Kassirer M, Weiss I, et al. Ambulatory rehabilitation improves exercise capacity in patients with pulmonary hypertension. J Card Fail 2011;17:196–200. 10.1016/j.cardfail.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 22.Ehlken N, Verduyn C, Tiede H, et al. Economic evaluation of exercise training in patients with pulmonary hypertension. Lung 2014;192:359–66. 10.1007/s00408-014-9558-9 [DOI] [PubMed] [Google Scholar]
- 23.Grünig E, Ehlken N, Ghofrani A, et al. Effect of exercise and respiratory training on clinical progression and survival in patients with severe chronic pulmonary hypertension. Respiration 2011;81:394–401. 10.1159/000322475 [DOI] [PubMed] [Google Scholar]
- 24.Rubin LJ. Exercise training for pulmonary hypertension: another prescription to write? Eur Respir J 2012;40:7–8. 10.1183/09031936.00070312 [DOI] [PubMed] [Google Scholar]
- 25.Bauer R, Dehnert C, Schoene P, et al. Skeletal muscle dysfunction in patients with idiopathic pulmonary arterial hypertension. Respir Med 2007;101:2366–9. 10.1016/j.rmed.2007.06.014 [DOI] [PubMed] [Google Scholar]
- 26.de Man FS, van Hees HW, Handoko ML, et al. Diaphragm muscle fiber weakness in pulmonary hypertension. Am J Respir Crit Care Med 2011;183:1411–18. 10.1164/rccm.201003-0354OC [DOI] [PubMed] [Google Scholar]
- 27.Deboeck G, Van Muylem A, Vachiéry JL, et al. Physiological response to the 6-minute walk test in chronic heart failure patients versus healthy control subjects. Eur J Prev Cardiol 2014;21:997–1003. 10.1177/2047487313482283 [DOI] [PubMed] [Google Scholar]
- 28.Yuan P, Yuan XT, Sun XY, et al. Exercise training for pulmonary hypertension: a systematic review and meta-analysis. Int J Cardiol 2015;178:142–6. 10.1016/j.ijcard.2014.10.161 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2015-309230supp.pdf (124.2KB, pdf)