Abstract
Objectives
In Italy, asbestos has been extensively used from 1945 to 1992. We evaluated the impact of exposure to asbestos on occurrence of malignant mesothelioma (MM) in the Lombardy Region, Northwest Italy, the most populated and industrialised Italian region.
Methods
From the Lombardy Mesothelioma Registry, we selected all incident cases of MM diagnosed between 2000 and 2012. We described sources of exposure to asbestos and examined time trends of MM rates. Using Poisson age-cohort models, we derived projections of burden of MM in the Lombardy population for the period 2013–2029.
Results
In 2000–2012, we recorded 4442 cases of MM (2850 men, 1592 women). Occupational exposure to asbestos was more frequent in men (73.6%) than in women (38.2%). Non-occupational exposure was found for 13.6% of women and 3.6% of men. The average number of cases of MM per year was still increasing (+3.6% in men, +3.3% in women). Incidence rates were still increasing in individuals aged 65+ years and declining in younger people. A maximum of 417 cases of MM (267 men, 150 women) are expected in 2019. We forecast there will be 6832 more cases (4397 in men, 2435 in women) in the period 2013–2029, for a total of 11 274 cases of MM (7247 in men, 4027 in women) in 30 years.
Conclusions
This study documented a high burden of MM in both genders in the Lombardy Region, reflecting extensive occupational (mainly in men) and non-occupational (mainly in women) exposure to asbestos in the past. Incidence rates are still increasing; a downturn in occurrence of MM is expected to occur after 2019.
Keywords: Mesothelioma incidence, Asbestos exposure, Mesothelioma projections, Age-cohort model, Cancer registry
What this paper adds.
Depending on the pattern of use of asbestos in the past, occurrence of mesothelioma is decreasing in some countries but is still increasing in others.
Italy has been using large quantities of asbestos till the 1992 ban, and is currently among those countries with the highest worldwide mortality of mesothelioma.
Using mesothelioma registry data, we evaluated the incidence of mesothelioma in the most populated (currently, 10 million people) and industrialised Italian region, and made future projections as of 2029.
In 2000–2012 we recorded more than 4400 cases, and have forecast that there will be a total of about 11 000 cases (7000 in men and 4000 in women) in a 30-year period (2000–2029).
Documenting occurrence of mesothelioma may help to increase awareness of dangers of exposure to asbestos in countries that still use it, but where its health effects are still overlooked.
Introduction
Asbestos is a known carcinogen to humans that causes malignant mesothelioma (MM), lung, laryngeal and ovarian cancer, while evidence regarding its association with other cancer sites (pharynx, stomach, colon and rectum) is considered limited.1 All forms of asbestos are recognised to possess carcinogenic properties, although with different potencies (with regard to MM, amphiboles are more potent than chrysotile). In addition, asbestos may cause non-neoplastic diseases, including asbestosis and pleural effusions and plaques. It has been estimated that, worldwide, 107 000 people die from MM, lung cancer, or asbestosis every year.2
Most MM are caused by asbestos (or other asbestiform fibres, like erionite and fluoro-edenite).3 Prognosis is very poor and has not improved in the last decades. Hence, MM, and/or incidence and mortality of pleural cancer have been largely used to monitor the health impact of exposure to asbestos worldwide. Currently, the largest burden of incidence and mortality in MM is in countries that began using asbestos a long time ago (in Western Europe, North America and Oceania). Most of them have banned the extraction, sale and use of asbestos. However, in most countries in Asia, Eastern Europe and South America, chrysotile is still in use and its health implications are overlooked due to the fact that MM is a rare cancer and its burden is currently apparently low for different reasons, including under-reporting and miscoding and, most importantly, the fact that in those countries the use of asbestos started later.2 4 Hence, given the long latency between exposure to asbestos and occurrence of MM, the burden of MM and other cancers in those countries is expected to increase in the coming years. For these reasons, many researchers and organisations have called for a global ban on asbestos.5
Several countries have established MM registries to monitor incidence of MM over time, identify sources of exposure to asbestos, provide medicolegal assistance to patients and their familiars, evaluate survival and forecast future trends of incidence of MM.6–9 Italy has been using large quantities of chrysotile and amphiboles from 1945 to the 1992 ban, and is currently among the countries with the highest frequency of MM worldwide.4 In Italy, a national MM registry (Registro Nazionale Mesoteliomi, ReNaM) has been implemented and organised as a network of regional registries.7
Our aim was to describe the results of the MM registry of the Lombardy Region (Registro Mesoteliomi Lombardia, RML), Northwest Italy, the most populated (currently 10 million inhabitants, one-sixth of the Italian population) and industrialised of the 20 Italian regions. For years 2000–2012, we assessed sources of exposure to asbestos, examined MM time patterns, and for 2013–2029 we derived projections of burden of MM on the Lombardy population using age-cohort models.
Methods
Ascertainment of mesothelioma cases
The RML is a population-based registry. Since 2000 it has been collecting all cases of MM reported among Lombardy residents at the time of diagnosis.10 The primary source is the reporting of MM (compulsory by law: 277/1991 and 81/2008) from every hospital. Completeness of ascertainment is periodically verified using several sources, notably pathology, hospital admission and mortality databases. Final diagnosis is established on a case-by-case basis considering all available clinical information, classifying cases as ‘certain MM’ (histological diagnosis of MM, possibly with immunohistochemical confirmation and imaging); ‘probable MM’ (usually, cytology suggesting MM plus imaging); ‘possible MM’ (positive imaging), or ‘non-MM’. Morphology is defined and coded according to WHO histological classification and the International Classification of Diseases for Oncology (ICD-O), Third Edition.
Assessment of exposure to asbestos
Patients of MM (or their next-of-kin) are interviewed (mostly face-to-face) by trained personnel using a standardised questionnaire to collect information on lifetime occupational history (industry, occupation, work environment characteristics). In addition, the patient is asked to provide information on each cohabitant, including longest occupation, years lived with them, and brushing or washing of dirty work clothes at home. Home-related activities involving potential exposure to asbestos are also investigated, including ironing on asbestos-coated ironing-boards, small repair works, thermal insulation, use of asbestos gloves, and use of any asbestos-containing objects. Finally, the questionnaire also contains a section on lifetime residential history, including questions on house type, presence of asbestos-cement tiles or water tanks, and presence of industries in the vicinity (eg, asbestos-cement, petrochemical, railroad, or shipbuilding industries). On the basis of this information, the lifetime asbestos exposure is classified as ‘occupational’ (certain, probable, or possible), ‘para-occupational’ (exposure through the cohabitants), ‘home-related’, or ‘environmental’.
Individuals may have been exposed to asbestos from more than one source. Patients ever exposed to asbestos at the workplace (where levels of airborne asbestos were usually of orders of magnitudes higher than in other settings), are classified as occupationally exposed irrespective of other sources of exposure (para-occupational, home-related, or environmental). For patients never occupationally exposed to asbestos, we usually follow this hierarchy of exposure: para-occupational>home-related>environmental. However, the final decision is taken on a case-by-case basis, considering also information on time since first exposure and length of exposure to each source.
Statistical analysis
We selected from the RML database all cases of MM diagnosed between 1 January 2000 and 31 December 2012, the period in which activities of case ascertainment, evaluation and interview have been completed. For each year from 2000 to 2012, we calculated crude and standardised rates for men and women using the Italian 2001, the European and the World (Segi's) standard populations. We fitted crude and age-adjusted Poisson regression models to calculate MM case and rate change (%) per year. We plotted incidence rates by age at diagnosis, period of diagnosis and birth cohort, for men and women separately, after combining the extreme classes of age (ages 20–34 years) and birth cohorts (cohorts 1900–1909 and 1970–1989) because of small numbers. We evaluated possible differences in time patterns between men and women by including gender-age and/or gender-cohort interaction terms in the Poisson models. Model comparisons were performed using likelihood ratio (LR) tests between nested models. In addition, we fitted separate Poisson models using cases classified as occupationally and non-occupationally exposed to asbestos, respectively (individuals without information were excluded). Figures calculated in this way are not interpretable in absolute terms (rates) because population data (denominators) are not stratified by exposure to asbestos, but they are, nonetheless, useful for relative (rate ratios) time pattern comparisons.
To make projections, we fitted categorical Poisson age-cohort models using 5-year categories for age at diagnosis (reference: 70–74 years) and birth cohort (reference: cohort 1920–1924).11 The gender-specific age and cohort regression coefficients were then applied to population data to calculate projections of the numbers of cases of MM and their 90% CIs in the years 2013–2029. Actual (2000–2015) and estimated (2016–2029, intermediate scenario) population data, by year, gender and age were downloaded from the National Institute of Statistics website (http://demo.istat.it/index_e.html). We also made projections stratified by exposure to asbestos. Data management and statistical analyses were performed with Stata V.13.
Results
Characteristics of mesothelioma patients and exposure to asbestos
In 2000–2012, we identified 4442 persons with MM, 2850 men (64.2%) and 1592 women (35.8%), with a male/female ratio of 1.79 (table 1). Median age at diagnosis was 70.4 years in men and 73.8 years in women. Pleura was the site of origin of MM in more than 90% of cases. The number of pleural MM was much higher in men than in women, while the number of peritoneal MM was similar. Diagnosis was evaluated as certain in more than three-quarters of cases. Morphology was obtained in more than 80% of patients. The most represented morphology was the epithelioid. Pleural plaques were detected in 13.8% of men and 8.0% of women. Interviews were obtained for more than 90% of affected persons, either from the patients themselves or from one of their relatives.
Table 1.
Characteristics of persons with malignant mesothelioma by gender, Lombardy Region Mesothelioma Registry, 2000–2012
| Men | Women | |||
|---|---|---|---|---|
| N | Per cent | N | Per cent | |
| Total | 2850 | 100 | 1592 | 100 |
| Age at diagnosis (years) | ||||
| 20–49 | 113 | 4.0 | 49 | 3.1 |
| 50–54 | 118 | 4.1 | 45 | 2.8 |
| 55–59 | 212 | 7.4 | 91 | 5.7 |
| 60–64 | 396 | 13.9 | 150 | 9.4 |
| 65–69 | 523 | 18.4 | 238 | 15.0 |
| 70–74 | 608 | 21.3 | 295 | 18.5 |
| 75–79 | 461 | 16.2 | 311 | 19.5 |
| 80–84 | 257 | 9.0 | 247 | 15.5 |
| 85+ | 162 | 5.7 | 166 | 10.4 |
| Site | ||||
| Pleura | 2693 | 94.5 | 1462 | 91.8 |
| Peritoneum | 134 | 4.7 | 125 | 7.9 |
| Pericardium | 6 | 0.2 | 5 | 0.3 |
| Tunica vaginalis testis | 17 | 0.6 | – | – |
| Diagnosis | ||||
| Certain | 2340 | 82.1 | 1203 | 75.6 |
| Probable | 245 | 8.6 | 157 | 9.9 |
| Possible | 265 | 9.3 | 232 | 14.6 |
| Morphology (ICD-O code) | ||||
| MM not otherwise specified (90503) | 169 | 5.9 | 107 | 6.7 |
| Fibrous/sarcomatoid/desmoplastic MM (90513) | 241 | 8.5 | 76 | 4.8 |
| Epithelioid MM (90523) | 1707 | 59.9 | 967 | 60.7 |
| Biphasic MM (90533) | 391 | 13.7 | 152 | 9.6 |
| Unknown | 342 | 12.0 | 290 | 18.2 |
| Presence of pleural plaques | 394 | 13.8 | 127 | 8.0 |
| Interview | ||||
| Patient | 1658 | 58.2 | 706 | 44.4 |
| Relative | 1061 | 37.2 | 771 | 48.4 |
| Not performed | 131 | 4.6 | 115 | 7.2 |
| Asbestos exposure source | ||||
| Occupational | 2099 | 73.6 | 608 | 38.2 |
| Para-occupational | 16 | 0.6 | 63 | 4.0 |
| Home-related | 24 | 0.8 | 75 | 4.7 |
| Environmental | 62 | 2.2 | 78 | 4.9 |
| Unknown* | 493 | 17.3 | 624 | 39.2 |
| No information/not classified† | 156 | 5.5 | 144 | 9.0 |
| Occupational asbestos exposure: industry‡ | 2099 | 100 | 608 | 100 |
| Metalmechanic and metallurgy | 731 | 34.8 | 51 | 8.4 |
| Building construction | 705 | 33.6 | 0 | 0.0 |
| Textile and clothing production | 188 | 9.0 | 398 | 65.5 |
| Chemical | 122 | 5.8 | 18 | 3.0 |
| Motor vehicle production | 136 | 6.5 | 7 | 1.1 |
| Food and beverage | 98 | 4.7 | 21 | 3.5 |
| Rubber | 71 | 3.4 | 23 | 3.8 |
| Military | 87 | 4.1 | 0 | 0.0 |
| Asbestos-cement | 65 | 3.1 | 12 | 2.0 |
| Health and social services | 39 | 1.9 | 38 | 6.3 |
| Transport | 76 | 3.6 | 0 | 0.0 |
| Railroad production and maintenance | 51 | 2.4 | 3 | 0.5 |
| Energy production | 51 | 2.4 | 0 | 0.0 |
*Individuals without any identified asbestos exposure.
†Not interviewed or with insufficient information at interview.
‡Only industries with at least 50 cases are listed; a person may have been exposed to asbestos in more than one industrial sector in his/her occupational history.
ICD-O, International Classification of Diseases for Oncology, Third Edition; MM, malignant mesothelioma.
Occupational exposure was documented in 73.6% of men and 38.2% in women. Para-occupational, home-related and environmental asbestos exposures were more frequent in women (13.6% in total) than in men (3.6%). For 493 men (17.3%) and 624 women (39.2%) no evidence of asbestos exposure was found at interview. For 156 men (5.5%) and 144 women (9.0%) information on exposure to asbestos was lacking (no interview) or insufficient (non-informative interview). Median length of occupational exposure was 28.0 years in men and 13.0 years in women, while median time since first occupational exposure was 49.5 years (men) and 56.2 years (women). In men, about one-third of cases had been exposed to asbestos in metalmechanic and metallurgy industries, one-third in building construction, and the remaining in various sectors. The majority of women (65.5%) had been exposed to asbestos in the non-asbestos textile (cotton, wool and silk manufacture) and clothing production sectors. We compared gender-specific case distributions across the same industries reported in table 1 between those classified as occupationally exposed to asbestos (2099 men and 608 women), and for whom we were not able to identify any (occupational, para-occupational, home-related, or environmental) exposure to asbestos (493 men and 624 women) (see online supplementary table S1, upper half). Except for building construction (men), textile (women) and asbestos-cement and railroad production/maintenance industries, case distributions were similar between asbestos-exposed and non-exposed persons. However, there remains a substantial proportion of individuals who have been ever employed in other industrial sectors (eg, trade, agriculture and livestock, education, bank, insurance and mail) for which we did not find any evidence of exposure to asbestos at interview (see online supplementary table S1, lower half).
oemed-2016-103652supp.pdf (935.9KB, pdf)
Exposure to asbestos (any) was similar across the MM site. In those interviewed, 2083 (81.0%) men with pleural MM, 102 (81.0%) with peritoneal MM, 4 (80.0%) with pericardial MM, and 12 (75.0%) with MM of tunica vaginalis testis had ever been exposed to asbestos (p value=0.99). In women, exposure to asbestos was documented for 767 (56.4%) with pleural MM, 55 (49.1%) with peritoneal MM, and 2 (40.0%) with pericardial MM (p value=0.40). When restricting analyses to certain MM diagnoses and individuals directly interviewed (patients), the numbers (proportions) of men with past exposure to asbestos were 1235 (84.5%) for pleura, 50 (82.0%) for peritoneum, 1 (50%) for pericardium and 8 (100%) for tunica vaginalis testis (p value=0.31). The corresponding figures in women were 348 (58.1%) for pleura and 24 (58.5%) for peritoneum (p value=0.90).
Time patterns, 2000–2012, and future projections, 2013–2029
The number of cases of MM increased from 277 in 2000 to 403 in 2012 (table 2). On average, over the 13-year period, there was a 3.6% per year increase of cases of MM in men and 3.3% in women. Crude rates (per 100 000 person-years) went from 4.0 to 5.8 in men (average crude and age-adjusted increase/year 2.6% and 1.3%), and from 2.2 to 2.6 in women (average crude and age-adjusted increase/year 2.5% and 1.6%). Pleural MM increased from 262 cases in 2000 to 389 cases in 2012. Peritoneal MM went from 14 cases in 2000 to 29 in 2011, with a drop in 2012 (13 cases). Trends in pericardial and cases of tunica vaginalis testis are difficult to recognise, because the number of cases was very small (from 1 to 4 cases per year).
Table 2.
Number of cases, person-years, and crude and age-standardised rates (per 100 000 person-years, age 0–99 years) of malignant mesothelioma (MM) by gender and year of diagnosis, Lombardy Region Mesothelioma Registry, 2000–2012
| Year | Men | Women | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Person-years | Rate | Cases | Person-years | Rate | |||||||
| Crude | Italy* | Europe† | World‡ | Crude | Italy* | Europe‡ | World‡ | |||||
| 2000–2012 | 2850 | 59 685 638 | 4.8 | 5.3 | 3.5 | 2.4 | 1592 | 62 922 196 | 2.5 | 2.2 | 1.4 | 0.9 |
| 2000 | 174 | 4 340 561 | 4.0 | 4.9 | 3.3 | 2.2 | 103 | 4 630 593 | 2.2 | 2.0 | 1.5 | 1.0 |
| 2001 | 182 | 4 358 338 | 4.2 | 4.8 | 3.4 | 2.3 | 99 | 4 645 746 | 2.1 | 2.0 | 1.4 | 1.0 |
| 2002 | 193 | 4 374 405 | 4.4 | 5.1 | 3.4 | 2.3 | 116 | 4 659 197 | 2.5 | 2.2 | 1.5 | 1.0 |
| 2003 | 209 | 4 417 259 | 4.7 | 5.5 | 3.7 | 2.5 | 104 | 4 691 386 | 2.2 | 2.0 | 1.3 | 0.9 |
| 2004 | 193 | 4 497 954 | 4.3 | 4.9 | 3.3 | 2.2 | 103 | 4 748 842 | 2.2 | 1.8 | 1.2 | 0.8 |
| 2005 | 219 | 4 579 992 | 4.8 | 5.3 | 3.7 | 2.5 | 120 | 4 813 100 | 2.5 | 2.1 | 1.4 | 0.9 |
| 2006 | 196 | 4 624 741 | 4.2 | 4.8 | 3.2 | 2.2 | 127 | 4 850 461 | 2.6 | 2.2 | 1.5 | 1.0 |
| 2007 | 227 | 4 660 352 | 4.9 | 5.4 | 3.6 | 2.4 | 130 | 4 885 089 | 2.7 | 2.3 | 1.5 | 1.0 |
| 2008 | 221 | 4 711 487 | 4.7 | 5.1 | 3.3 | 2.2 | 134 | 4 930 919 | 2.7 | 2.3 | 1.5 | 0.9 |
| 2009 | 252 | 4 762 370 | 5.3 | 5.6 | 3.8 | 2.5 | 107 | 4 980 306 | 2.1 | 1.8 | 1.1 | 0.7 |
| 2010 | 254 | 4 802 363 | 5.3 | 5.7 | 3.7 | 2.4 | 155 | 5 023 778 | 3.1 | 2.6 | 1.7 | 1.2 |
| 2011 | 255 | 4 844 524 | 5.3 | 5.6 | 3.5 | 2.3 | 166 | 5 073 190 | 3.3 | 2.6 | 1.6 | 1.0 |
| 2012 | 275 | 4 711 292 | 5.8 | 6.1 | 3.8 | 2.5 | 128 | 4 989 589 | 2.6 | 1.9 | 1.2 | 0.8 |
| Change/year | +3.6 | +2.6 | +1.3§ | +3.3 | +2.5 | +1.6§ | ||||||
*Rates standardised on the Italian population, 2001.
†Rates standardised on the European population.
‡Rates standardised on the world (Segi's) population.
§From age-adjusted Poisson regression models.
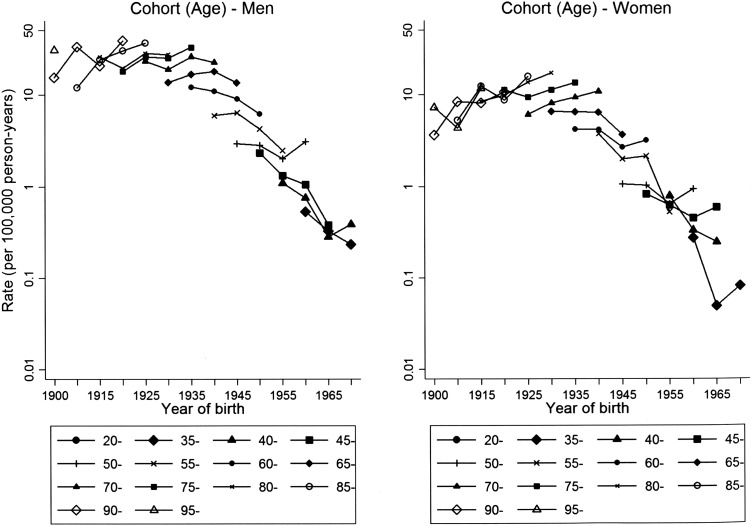
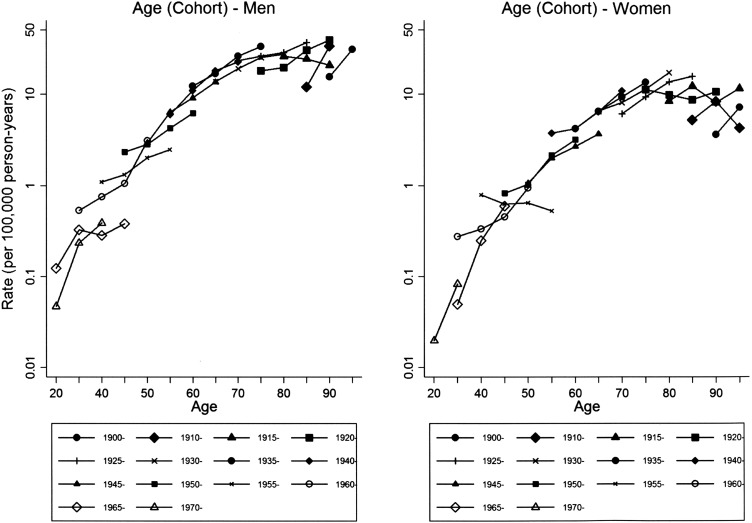
Age-specific MM rates were higher and increased over the study period in men over 65 years in men and women, while a decrease was observed for younger persons (see online supplementary table S2). In particular, from age-adjusted Poisson regression models, the annual rate changes in individuals aged 65+ years were +3.4% in men and +3.5% in women, while for those aged <65 years they were −3.0% in men and −4.3% in women. Incidence rates ranged from 22.7 to 28.1 per 100 000 person-years between 70 years and 94 years of age at diagnosis in men (see online supplementary table S3, rightmost column). In women, rates between 10.3 and 12.2 per 100 000 person-years were recorded for women aged 75–89 years (see online supplementary table S4, rightmost column). In men, the highest burden of MM, 2278 cases (79.9%), was among those born between 1925 and 1949 (men) (see online supplementary table S3, penultimate row). The highest rates were found for men born between 1905 and 1949 (figure 1 and online supplementary table S3, last row). In women, 1080 cases of MM (67.8%) occurred in those born between 1925 and 1944 (see online supplementary table S4, penultimate row). The highest rates were found for women born between 1900 and 1944 (figure 1 and see online supplementary table S4, last row). Incidence rates by birth cohort show similar patterns of increase by age class, except for some recent cohorts with few cases (1965–1969 in men and 1955–1959 in women) (figure 2 and see online supplementary tables S3 and S4). Accordingly, we did not find statistical evidence of different patterns between men and women (LR tests for interaction terms yielded the following results: gender-age plus gender-cohort interactions: p value=0.47; gender-age interaction: p value=0.31, gender-cohort interaction: p value=0.40). When we stratified rates by occupational exposure to asbestos (see online supplementary figures S1–S4), relative patterns were roughly similar across gender, with p values for interactions ranging from 0.21 to 0.89.
Figure 1.
Incidence rates (per 100 000 person-years) of malignant mesothelioma (20–99 years of age) (no cases were recorded in persons <20 years of age. Owing to small number of cases, ages 20–24, 25–29, and 30–34 and birth cohorts 1900–1904, 1905–1909, 1970–1974, 1975–1979, 1980–1984, and 1985–1989 were combined) by birth cohort for different categories of age at diagnosis, Lombardy Region Mesothelioma Registry, 2000–2012.
Figure 2.
Incidence rates (per 100 000 person-years) of malignant mesothelioma by age at diagnosis (20–99 years) (no cases were recorded in persons <20 years of age. Owing to small number of cases, ages 20–24, 25–29, and 30–34 and birth cohorts 1900–1904, 1905–1909, 1970–1974, 1975–1979, 1980–1984, and 1985–1989 were combined) for different birth cohorts, Lombardy Region Mesothelioma Registry, 2000–2012.
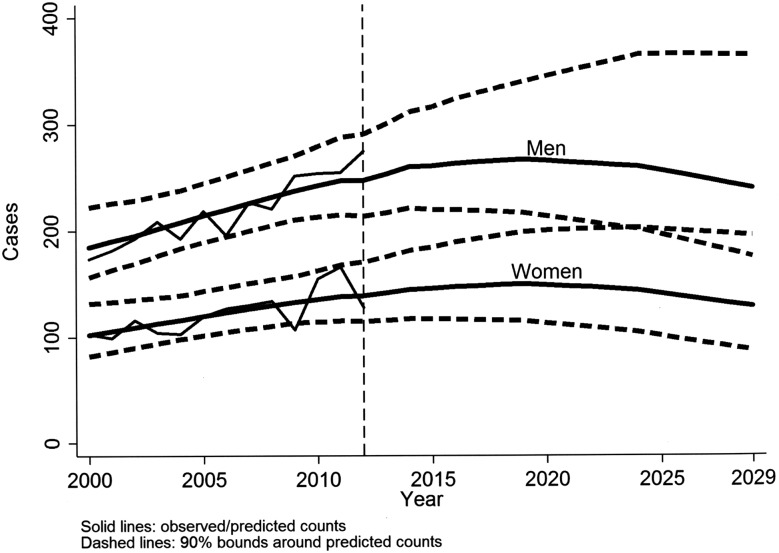
Poisson age-cohort models showed a good fit (p values=0.39/0.63 for men/women). Fitted number of cases of MM were quite close to the observed (results not shown). These models confirmed the higher rates for those born 1925–1949 (men) and 1925–1944 (women). Application of gender-specific age and birth cohort regression coefficients to population data yielded the projection curves for the annual number of cases of MM shown in figure 3. The peak in cases of MM is expected to occur in 2019 for both genders (267 in men and 150 in women). The projected number of cases in 2012–2030 is 6832 (90% CI 5333 to 9184), 4397 (90% CI 3505 to 5860) in men and 2435 (90% CI 1828 to 3324) in women, for a total over the period 2000–2029 of 11 274 (90% CI 9157 to 14 411) MM cases, 7247 (90% CI 5998 to 9156) in men, 4027 (90% CI 3159 to 5255) in women. Observed and predicted numbers of occupational and non-occupational cases by gender follow a time pattern similar to total cases of MM (see online supplementary figure S5).
Figure 3.
Observed (2000–2012), model predicted and projected (2013–2029, based on Poisson age-cohort models) numbers of malignant mesothelioma cases per year, by gender, Lombardy Region Mesothelioma Registry.
Discussion
In this study, we have documented a large impact of past use of asbestos in the Lombardy Region, with over 4400 cases of MM in both genders in a 13-year period (2000–2012), representing about one-fourth of all cases of MM occurring in Italy. Occupational exposure was more frequent in men (about 75%), than in women (<40%). Incidence of MM is still increasing in Lombardy, with the peak expected around 2019. We forecast there will be almost 7000 cases of MM in the period 2013–2029, for a total of more than 11 000 cases of MM in 2000–2029.
The study was made possible by the high-quality population registry of MM patients (RML).10 The performance of the RML was recently evaluated via comparison of its data with that of cancer registries covering four Lombardy provinces (Brescia, Mantova, Milan, Sondrio) in 2000–2004: no case of MM was missed by the RML. Identification of sources of exposure to asbestos was made possible in a large percentage of cases, thanks to high rates of interview.
The Lombardy Region is the most populated (currently 10 million people), and has been the region with the largest number of men employed in many industrial sectors in which asbestos has been widely used, including mechanic (motor vehicle construction and repair), metallurgy (iron and steel foundries), chemical and rubber. In Lombardy, there were also asbestos-cement factories. The second largest Italian asbestos-cement factory was located in Broni, Pavia Province, Southeast of Lombardy.12 For these reasons, a high burden of cases of MM in men was largely expected. The high number of cases of MM among women is partly explained by the presence of asbestos in the textile and clothing production industries. Investigations in this industrial sector had been prompted when the registry started noting a very large number of cases of MM among women. Industrial hygiene surveys showed that large amounts of asbestos had been regularly used on the ceilings and also on the walls of factories, in order to avoid condensation of steam and reflection of noise. In addition, asbestos had also been widely used to insulate water and steam pipes. The braking systems of most machines also had asbestos gaskets, and on several looms some brakes operated continuously.13 Asbestos fibres have been found in necropsy lung samples of textile workers with MM.14
However, for a large proportion (39.2%) of women, we were not able to identify any source of exposure to asbestos at interview. Failure to identify occupational exposure to asbestos does not necessarily imply that occupation did not play a role. Rather, it may indicate that interview is an incomplete tool to uncover exposure to asbestos (eg, many persons might not be aware of the presence of asbestos at their workplace). Another possible explanation for the high number of cases in women is the widespread low-level environmental exposure to asbestos from industries using asbestos, and from asbestos-containing materials, most importantly asbestos-cement roofs in buildings. A recent study found detectable levels of asbestos fibres (mainly amphiboles) in necroscopic lung samples taken from the general population of Milan.15 However, effects of environmental exposure to asbestos are even more difficult to study16 and can be usually evaluated only in areas with heavy environmental contamination.17 For example, using RML data, we were able to document a high impact of exposure to environmental asbestos on occurrence of MM in women living near the asbestos-cement factory in Broni.12
In Italy, until recently, only individuals with MM with recognised occupational exposure to asbestos were entitled to file claims for compensation. However, it has been estimated that only about 50% of them were compensated in 1994–2006, although an increasing trend in the more recent years has been noted. The risk of not seeking and obtaining compensation is higher for women, the elderly and in Central and South Italy.18 Cases of MM non-occupationally exposed, were not compensated until 2014, when a law was passed to compensate affected persons with para-occupational or environmental exposure and first diagnosis between 2015 and 2017.17
Our findings in women (high incidence of MM and low proportion of occupationally exposed) are in line with results regarding Italy as a whole: in the period 1993–2012, among 21 463 cases of MM, ReNaM recorded 38.4% women (male/female ratio 2.5).7 17 To investigate more in depth the role of occupation in women, a case–control study is in progress in five Italian regions, including Lombardy. Also, other countries (eg, Denmark, France and Spain) recorded a relatively high number of affected women,19–21 while still others (eg, UK and USA) showed a higher male/female ratio,22 23 reflecting different male–female patterns of past exposure to asbestos.
Projections of occurrence of MM (incidence or mortality) have been made in many countries in Europe (Britain, France, Germany, Italy, the Netherlands and Switzerland),3 Australia,24 25 New South Wales (Australia),26 Brazil,27 Canada and Quebec,28 Denmark,19 France,20 29 30 Germany,31 Italy,32 Veneto (Italy),33 Japan,34 35 the Netherlands,36 Spain,21 37 UK,22 38 39 South East England (UK),40 and USA.23 41–43 In several of those studies, projections regarded only men. Definitions of mesothelioma differed between studies: some considered pleural cancer, some mesotheliomas of pleura plus other sites, relying on different versions of the International Classification of Disease (usually the Ninth and Tenth Revisions). The statistical model most often used to make projections was the Poisson age-cohort, either classical3 19 23 28 29 31 32 34 36 38 40–42 or Bayesian.33 37 Others used log-linear or generalised additive models relating incidence of MM or mortality to past consumption of asbestos.20 21 22 24 26 27 32 35 39 44 Some authors found quite different predictions between the two approaches (eg, in France and UK), showing that the Poisson age-cohort model may overestimate future burden of MM compared with that obtained taking into account past use of asbestos.20 22 Other authors, using more recent data than those previously used to make projections in six European countries,3 also pointed out the overestimation of Poisson age-cohort models.45 However, still others (eg, in New South Wales (Australia) and Italy) found a substantial agreement between the two approaches.26 32
In this study, we used Poisson age-cohort models for two main reasons; its relative simplicity and, most importantly, the lack of information on past use of asbestos in the Lombardy Region. In Italy, predictions of male pleural cancer mortality were similar using different age-cohort, age-period-cohort and models based on national asbestos production, import and export patterns.32 All approaches indicated a peak of MM cases between 2012 and 2024. Our results, which indicates a maximum of MM occurrence in 2019 (417 cases) for men (267 cases) and women (150 cases), are consistent with those predictions. Conversely, in other Italian regions, the peak may have already occurred.33
Conclusions
MM registries are important for documentation of the impact of use of asbestos on incidence of MM. Thanks to the existence of the Lombardy Mesothelioma Registry, in this study, we showed the continuing increase of MM in the region, reflecting extensive occupational (mainly in men) and non-occupational (mainly in women) exposure to asbestos in the past. More than 4000 cases were recorded in the first 13 years of activity of the registry (2000–2012), and almost 7000 are expected to occur in the following years (2013–2029), for a total of more than 11 000 cases of MM in 30 years. Contrarily to other countries in which the number of men diagnosed with MM was largely predominant, in Lombardy (as in the rest of Italy), the number of affected women is quite large. We hope these results help to increase awareness of dangers of asbestos exposure in countries that still use it but where its health effects are still overlooked.
Acknowledgments
The authors wish to thank the personnel of the regional hospital Occupational Health Departments (UOOML) and of the Occupational Prevention and Safety Departments of the Local Health Units (SPSAL) for their collaboration in notifying and interviewing individuals affected by mesothelioma; the personnel of the regional hospital Medical, Surgical and Pathology Departments for their collaboration in providing clinical documentation; Luana Garlati, Lombardy Mesothelioma Registry, for her valuable secretarial assistance; and the individuals affected by mesothelioma and their family members for granting interviews.
Footnotes
Contributors: CM and DC conceived the study. CM, BD and LR collected and evaluated clinical and interview data. DC performed statistical analyses and drafted the paper. CM, SDM, BD, LR and PAB contributed to interpretation of findings and discussion. All authors revised and approved the manuscript for intellectual content.
Funding: This study was partially funded by: the Lombardy Region ‘Attività Epidemiologiche per lo Studio dei Rischi e Programmazione di Servizi per la Salute della Popolazione Lombarda’ programme (14013-1/5/2010, 8956-7/6/2006); the Ministry of Health and the Istituto Nazionale per l'Assicurazione contro gli Infortuni (INAIL) (PMS/42/06).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.IARC. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 100C: arsenic, metals, fibres, and dusts. Lyon, France: International Agency for Research on Cancer, 2012. [PMC free article] [PubMed] [Google Scholar]
- 2.Stayner L, Welch LS, Lemen R. The worldwide pandemic of asbestos-related diseases. Annu Rev Public Health 2013;34:205–16. 10.1146/annurev-publhealth-031811-124704 [DOI] [PubMed] [Google Scholar]
- 3.Peto J, Decarli A, La Vecchia C, et al. . The European mesothelioma epidemic. Br J Cancer 1999;79:666–72. 10.1038/sj.bjc.6690105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park EK, Takahashi K, Hoshuyama T, et al. . Global magnitude of reported and unreported mesothelioma. Environ Health Perspect 2011;119:514–18. 10.1289/ehp.1002845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sim MR. A worldwide ban on asbestos production and use: some recent progress, but more still to be done. Occup Environ Med 2013;70:1–2. 10.1136/oemed-2012-101290 [DOI] [PubMed] [Google Scholar]
- 6.Goldberg M, Imbernon E, Rolland P, et al. . The French National Mesothelioma Surveillance Program. Occup Environ Med 2006;63:390–5. 10.1136/oem.2005.023200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marinaccio A, Binazzi A, Marzio DD, et al. . Pleural malignant mesothelioma epidemic: incidence, modalities of asbestos exposure and occupations involved from the Italian National Register. Int J Cancer 2012;130:2146–54. 10.1002/ijc.26229 [DOI] [PubMed] [Google Scholar]
- 8.Neumann V, Gunthe S, Mulle KM, et al. . Malignant mesothelioma—German mesothelioma register 1987–1999. Int Arch Occup Environ Health 2001;74:383–95. 10.1007/s004200100240 [DOI] [PubMed] [Google Scholar]
- 9.Yeung P, Rogers A, Johnson A. Distribution of mesothelioma cases in different occupational groups and industries in Australia, 1979–1995. Appl Occup Environ Hyg 1999;14:759–67. 10.1080/104732299302189 [DOI] [PubMed] [Google Scholar]
- 10.Mensi C, Termine L, Canti Z, et al. . The Lombardy Mesothelioma Register, Regional Operating Centre (ROC) of National Mesothelioma Register: organizational aspects (Italian). Epidemiol Prev 2007;31:283–9. [PubMed] [Google Scholar]
- 11.Clayton D, Schifflers E. Models for temporal variation in cancer rates. I: Age-period and age-cohort models. Stat Med 1987;6:449–67. [DOI] [PubMed] [Google Scholar]
- 12.Mensi C, Riboldi L, De Matteis S, et al. . Impact of an asbestos cement factory on mesothelioma incidence: global assessment of effects of occupational, familial, and environmental exposure. Environ Int 2015;74:191–9. 10.1016/j.envint.2014.10.016 [DOI] [PubMed] [Google Scholar]
- 13.Mensi C, Macchione M, Termine L, et al. . Asbestos exposure in the non-asbestos textile industry: the experience of the Lombardy Mesothelioma Registry. Epidemiol Prev 2007;31(Suppl 1):27–30. [PubMed] [Google Scholar]
- 14.Barbieri PG, Somigliana A, Tironi A. [Lung asbestos fibre burden in textile workers with malignant mesothelioma]. Med Lav 2010;101:199–206. [PubMed] [Google Scholar]
- 15.Casali M, Carugno M, Cattaneo A, et al. . Asbestos lung burden in necroscopic samples from the general population of Milan, Italy. Ann Occup Hyg 2015;59:909–21. 10.1093/annhyg/mev028 [DOI] [PubMed] [Google Scholar]
- 16.Goldberg S, Rey G, Luce D, et al. . Possible effect of environmental exposure to asbestos on geographical variation in mesothelioma rates. Occup Environ Med 2010;67:417–21. 10.1136/oem.2009.050336 [DOI] [PubMed] [Google Scholar]
- 17.Marinaccio A, Binazzi A, Bonafede M, et al. . Malignant mesothelioma due to non-occupational asbestos exposure from the Italian national surveillance system (ReNaM): epidemiology and public health issues. Occup Environ Med 2015;72:648–55. 10.1136/oemed-2014-102297 [DOI] [PubMed] [Google Scholar]
- 18.Marinaccio A, Scarselli A, Merler E, et al. . Mesothelioma incidence surveillance systems and claims for workers’ compensation. Epidemiological evidence and prospects for an integrated framework. BMC Public Health 2012;12:314 10.1186/1471-2458-12-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjaergaard J, Andersson M. Incidence rates of malignant mesothelioma in Denmark and predicted future number of cases among men. Scand J Work Environ Health 2000;26:112–17. 10.5271/sjweh.520 [DOI] [PubMed] [Google Scholar]
- 20.Goldberg S, Rey G. Modélisation de l’évolution de la mortalité par mésothéliome de la plèvre en France. Projections à l'horizon 2050. Saint-Maurice: Institut de Veille Sanitaire, 2012. [Google Scholar]
- 21.Lopez-Abente G, Garcia-Gomez M, Menendez-Navarro A, et al. . Pleural cancer mortality in Spain: time-trends and updating of predictions up to 2020. BMC Cancer 2013;13:528 10.1186/1471-2407-13-528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodgson JT, McElvenny DM, Darnton AJ, et al. . The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br J Cancer 2005;92:587–93. 10.1038/sj.bjc.6602307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol 2009;39:576–88. 10.1080/10408440903044928 [DOI] [PubMed] [Google Scholar]
- 24.Leigh J, Driscoll T. Malignant mesothelioma in Australia, 1945–2002. Int J Occup Environ Health 2003;9:206–17. 10.1179/oeh.2003.9.3.206 [DOI] [PubMed] [Google Scholar]
- 25.Soeberg MJ, Leigh J, Driscoll T, et al. . Incidence and survival trends for malignant pleural and peritoneal mesothelioma, Australia, 1982–2009. Occup Environ Med 2016;73:187–94. 10.1136/oemed-2015-103309 [DOI] [PubMed] [Google Scholar]
- 26.Clements M, Berry G, Shi J, et al. . Projected mesothelioma incidence in men in New South Wales. Occup Environ Med 2007;64:747–52. 10.1136/oem.2006.031823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Algranti E, Saito CA, Carneiro AP, et al. . The next mesothelioma wave: mortality trends and forecast to 2030 in Brazil. Cancer Epidemiol 2015;39:687–92. 10.1016/j.canep.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 28.Krupoves A, Camus M, De Guire L. Incidence of malignant mesothelioma of the pleura in Quebec and Canada from 1984 to 2007, and projections from 2008 to 2032. Am J Ind Med 2015;58:473–82. 10.1002/ajim.22442 [DOI] [PubMed] [Google Scholar]
- 29.Ilg AG, Bignon J, Valleron AJ. Estimation of the past and future burden of mortality from mesothelioma in France. Occup Environ Med 1998;55:760–5. 10.1136/oem.55.11.760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banaei A, Auvert B, Goldberg M, et al. . Future trends in mortality of French men from mesothelioma. Occup Environ Med 2000;57:488–94. 10.1136/oem.57.7.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schonfeld SJ, McCormack V, Rutherford MJ, et al. . Regional variations in German mesothelioma mortality rates: 2000–2010. Cancer Causes Control 2014;25:615–24. 10.1007/s10552-014-0368-4 [DOI] [PubMed] [Google Scholar]
- 32.Marinaccio A, Montanaro F, Mastrantonio M, et al. . Predictions of mortality from pleural mesothelioma in Italy: a model based on asbestos consumption figures supports results from age-period-cohort models. Int J Cancer 2005;115:142–7. 10.1002/ijc.20820 [DOI] [PubMed] [Google Scholar]
- 33.Girardi P, Bressan V, Merler E. Past trends and future prediction of mesothelioma incidence in an industrialized area of Italy, the Veneto Region. Cancer Epidemiol 2014;38:496–503. 10.1016/j.canep.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 34.Murayama T, Takahashi K, Natori Y, et al. . Estimation of future mortality from pleural malignant mesothelioma in Japan based on an age-cohort model. Am J Ind Med 2006;49:1–7. 10.1002/ajim.20246 [DOI] [PubMed] [Google Scholar]
- 35.Myojin T, Azuma K, Okumura J, et al. . Future trends of mesothelioma mortality in Japan based on a risk function. Ind Health 2012;50:197–204. 10.2486/indhealth.MS1184 [DOI] [PubMed] [Google Scholar]
- 36.Segura O, Burdorf A, Looman C. Update of predictions of mortality from pleural mesothelioma in the Netherlands. Occup Environ Med 2003;60:50–5. 10.1136/oem.60.1.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitarque S, Cleries R, Martinez JM, et al. . Mesothelioma mortality in men: trends during 1977–2001 and projections for 2002–2016 in Spain. Occup Environ Med 2008;65:279–82. 10.1136/oem.2007.034769 [DOI] [PubMed] [Google Scholar]
- 38.Peto J, Hodgson JT, Matthews FE, et al. . Continuing increase in mesothelioma mortality in Britain. Lancet 1995;345:535–9. 10.1016/S0140-6736(95)90462-X [DOI] [PubMed] [Google Scholar]
- 39.Tan E, Warren N, Darnton AJ, et al. . Projection of mesothelioma mortality in Britain using Bayesian methods. Br J Cancer 2010;103:430–6. 10.1038/sj.bjc.6605781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riaz SP, Coupland VH, Luchtenborg M, et al. . Mesothelioma incidence projections in South East England. Eur Respir J 2012;40:965–8. 10.1183/09031936.00168111 [DOI] [PubMed] [Google Scholar]
- 41.Price B. Analysis of current trends in United States mesothelioma incidence. Am J Epidemiol 1997;145:211–18. 10.1093/oxfordjournals.aje.a009093 [DOI] [PubMed] [Google Scholar]
- 42.Price B, Ware A. Mesothelioma trends in the United States: an update based on Surveillance, Epidemiology, and End Results Program data for 1973 through 2003. Am J Epidemiol 2004;159:107–12. 10.1093/aje/kwh025 [DOI] [PubMed] [Google Scholar]
- 43.Moolgavkar SH, Meza R, Turim J. Pleural and peritoneal mesotheliomas in SEER: age effects and temporal trends, 1973–2005. Cancer Causes Control 2009;20:935–44. 10.1007/s10552-009-9328-9 [DOI] [PubMed] [Google Scholar]
- 44.Banaei A, Auvert B, Goldberg M. Computer modeling of population exposure to a carcinogen: the example of asbestos and mesothelioma mortality in France. Comput Biomed Res 2000;33:97–109. 10.1006/cbmr.1999.1536 [DOI] [PubMed] [Google Scholar]
- 45.Pelucchi C, Malvezzi M, La Vecchia C, et al. . The Mesothelioma epidemic in Western Europe: an update. Br J Cancer 2004;90:1022–4. 10.1038/sj.bjc.6601638 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
oemed-2016-103652supp.pdf (935.9KB, pdf)