Abstract
Compound ZJ-101, a structurally simplified analog of the marine natural product superstolide A, was previously developed in our laboratory. In the subsequent structure-activity relationship study, a new analog ZJ-109 was designed and synthesized to probe the importance of the lactone moiety of the molecule by replacing the lactone in ZJ-101 with a lactam. The biological evaluation showed that ZJ-109 is about 8–12 times less active against cancer cells in vitro than ZJ-101, suggesting that the lactone moiety of the molecule is important for its anticacner activity.
Keywords: Superstolide A analog, Anticancer agent, Structure-activity relationship, Drug design, Asymmetric synthesis
Graphical Abstract
Over 70% of the earth’s surface is covered by oceans, which contain numerous marine organisms with tremendous biological and chemical diversity. Over the last 30 years the marine environment has become the hot spot for the identification of biologically active natural products.1 With more drugs from marine natural products approved by regulatory agencies for clinical uses and more entering clinical trials,2 we are likely experiencing a 21st century renaissance of drug discovery based on marine natural products.3
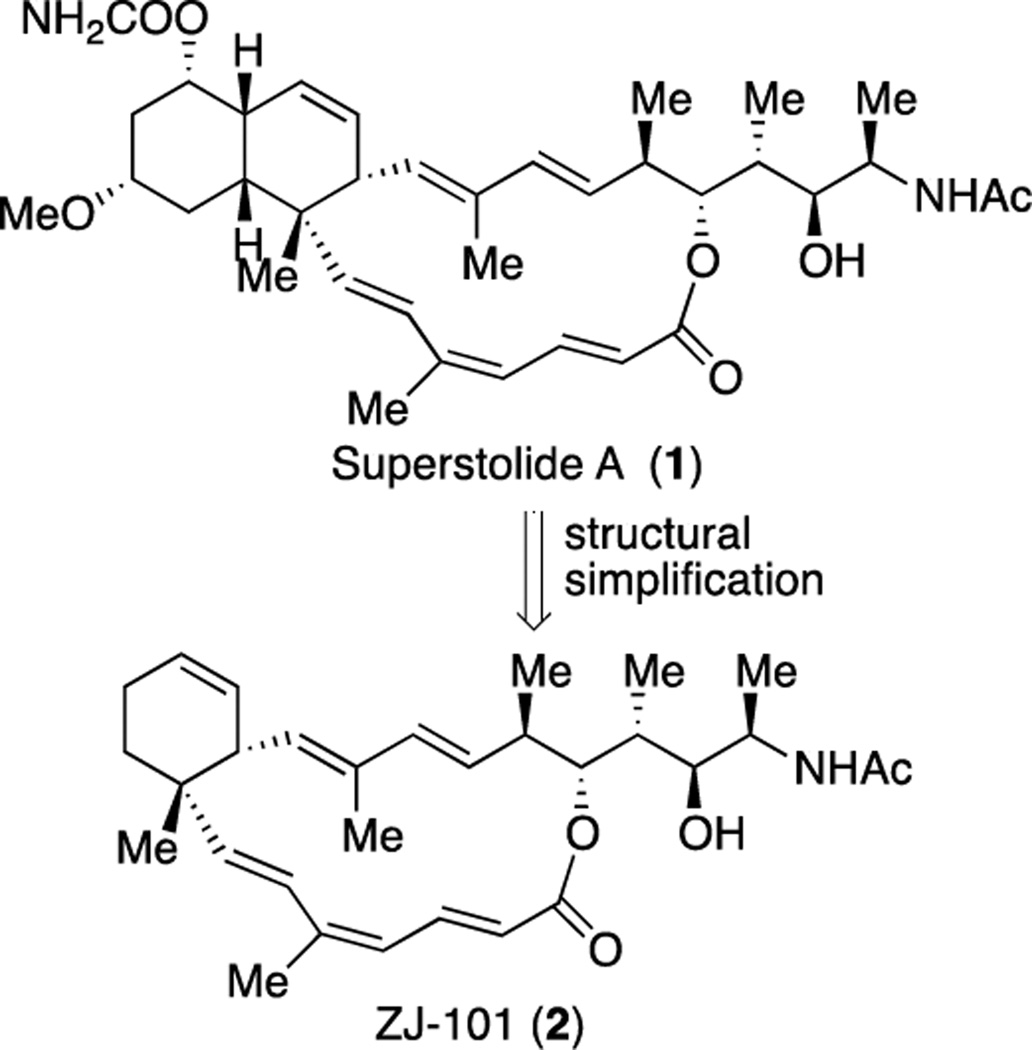
We recently designed and synthesized a truncated superstolide A (named as ZJ-101) that maintains the potent anticancer activity of the original natural product superstolide A4 that was isolated in minute amounts from the deep-water marine sponge Neosiphonia superstes (Figure 1).5 Because our synthetic approach is efficient and the synthesis can be readily scaled up, we have produced sufficient amounts of material for additional biological and mechanistic evaluation.
Figure 1.
Superstolide A and truncated superstolide A (ZJ-101)
ZJ-101 exhibited potent anticancer activity in the NCI-60 cell screen. The COMPARE pattern-recognition analysis of the NCI 60-cell mean graph screening profile of ZJ-101 does not show a strong correlation with any other known anticancer agents in the NCI’s database, suggesting it likely has a novel and, as yet, undefined mechanism of action.6
While the potent anticancer activity of ZJ-101 partially confirmed our original hypothesis that the 16-membered macrolactone is likely the pharmacophore responsible for interacting with its putative target,5 it is imperative to precisely characterize the role of several key functionalities in ZJ-101 on the anticancer activity. Our recent structure-activity relationship studies have shown that the cyclohexenyl group and the double bonds between C2–C3 and the C4–C5 of the trienyl conjugated lactone moiety are important for its anticancer activity.7
To investigate the role of the lactone moiety on the anticancer activity, we designed analog ZJ-109 (Figure 2) where the lactone is replaced with a lactam. This analog would not only enable us to gain insights on the structure-activity relationship of ZJ-101 but also reveal the significance of the lactone moiety on the anticancer activity. Moreover, ZJ-109 may have preferable DMPK properties over ZJ-101. Herein, we report our synthesis and biological evaluation of compound ZJ-109.
Figure 2.
Designed analog ZJ-109
The retrosynthetic analysis of ZJ-109 is analogous to that of ZJ-101 and is outlined in Scheme 1. This synthetic route is advantageous as it is flexible and convergent.
Scheme 1.
Retrosynthetic analysis of ZJ-109
The commercially available N-Boc-D-alanine methyl ester 7 was converted to compound 8 using reported procedures (Scheme 2).8 Compound 8 underwent Lemieux-Johnson oxidation9 to give aldehyde 9 in 89% yield. Crotylboration between compound 9 and (Z)-crotyldiisopinocampheylborane derived from (−)-B-methoxydiisopinocampheylborane ((−)-IpC2BOMe) afforded homoallylic alcohol 10 in 75% yield using Brown’s conditions10 and the workup-purification procedure developed in our laboratory.11 The stereochemistry of compound 10 was assigned on the basis of literature precedents.10 Compound 10 was converted to compound 11 in 92% yield under Mitsunobu conditions. It should be noted that hydrazoic acid is dangerously explosive and its solution should be handled with extra care. The reaction should be carried out behind a blast shield. Azide 11 was reduced by L-selectride and the resulting amine was protected by a trimethylsilylethoxy-carbonyl (Teoc) protecting group to provide compound 12 in 81% yield. Cross metathesis reaction between compound 12 and pinacol vinylboronate gave trans-vinyl boronate 5 in 49% yield.12
Scheme 2.
Synthesis of compound 12
Fragment 5 reacted with fragment 4 under Suzuki conditions to afford compound 13 in 43% yield with complete stereoselectivity (Scheme 3).5 Negishi coupling between vinyl bromide 13 and dimethylzinc provided the requisite trisubstituted olefin 14 in 82% yield with complete stereoselectivity.5 The triethyl silyl group in compound 14 was removed chemo- and regioselectively by 1 equivalent of TBAF to give compound 15, which underwent a regio- and stereoselective hydrostannylation to afford trans-vinyl stannane 16 in 70% yield. The trimethylsilylethoxycarbonyl (Teoc) protecting group was cleaved by TBAF and the resulting amine reacted with fragment 6 to furnish amide 17 in 93% yield. Compound 17 underwent an intramolecular Stille coupling to give the 16-membered lactam 18 in 64% yield.5 Removal of both Boc and oxazolidine protecting groups under acidic conditions followed by chemoselective acylation of the resulting amine provided ZJ-109 in 71% yield.
Scheme 3.
Synthesis of ZJ-109
The antiproliferative effects of ZJ-109 were determined in MDA-MB-231 (human breast cancer), SF-295 (human glioblastoma), MCF-7 (human breast cancer), HCT-116 (human colon cancer) and HeLa (human cervical cancer) cell lines using the Alamar Blue viability assay, along with ZJ-101 as the positive control.15 The IC50 values are shown in Table 1. The data showed that the antiproliferative activity of ZJ-109 is about 8–12 times less than ZJ-101 in these five cell lines, suggesting that the lactone moiety is important for its biological activity.
Table 1.
Antiproliferative effect of ZJ-105 and ZJ-106 on various malignant tumor cells (Alamar Blue assay)
| Entry | Cell Line | ZJ-109 IC50 (nM) |
ZJ-101 IC50 (nM) |
|---|---|---|---|
| 1 | MDA-MB-231 | 584.2 | 63.04 |
| 2 | SF295 | 716.3 | 63.48 |
| 3 | MCF-7 | 447.7 | 36.54 |
| 4 | HCT-116 | 746.5 | 94.02 |
| 5 | HeLa | 596.7 | 75.44 |
In conclusion, we have designed and synthesized compound ZJ-109 by replacing the lactone moiety in ZJ-101 with a lactam. The biological testing has confirmed our original hypothesis that the trienyl conjugated lactone moiety of the molecule is indeed the pharmacophore and future structural optimization should keep the chemical structure of this region intact. Research in this direction is currently underway and will be reported in due course.
Acknowledgments
This work was made possible by the generous support of the GAP funding from the Office of the Vice President for Research and Economic Development (OVPRED) at the University of Iowa, funding from InnoBioPharma, LLC (D2015070002) and a grant (1R21CA204836-01) from the National Institutes of Health.
Conflict of interest statement
Dr. Zhendong Jin is the founder and a shareholder of InnoBioPharma, LLC that sponsored the project, “Development of novel anticancer agents based on natural products” at the University of Iowa.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Newman DJ, Cragg GM. J. Nat. Prod. 2012;75:311. doi: 10.1021/np200906s. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J-W, Wu Q-H, Rowley DC, Al-Kareef AMQ, Wang H. J. Asian Nat Prod Res. 2015;17:199. doi: 10.1080/10286020.2014.996140. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 3.Montaser R, Luesch H. Future Med. Chem. 2011;3:1475. doi: 10.4155/fmc.11.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) D’Auria MV, Debitus C, Paloma LG, Minale L, Zampella A. J. Am. Chem. Soc. 1994;116:6658. [Google Scholar]; (b) D’Auria MV, Debitus C, Paloma LG, Minale L, Zampella A. J. Nat. Prod. 1994;57:1595. doi: 10.1021/np50113a024. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Riaz Ahmed KB, Huang P, Jin Z. Angewandte Chemie Int. Ed. 2013;52:3446. doi: 10.1002/anie.201209300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin Z. Unpublished results. [Google Scholar]
- 7.(a) Shah AK, Qian S, Head SA, Liu JO, Jin Z. Bioorg. Med. Chem. Lett. 2016;26:2890. doi: 10.1016/j.bmcl.2016.04.044. [DOI] [PubMed] [Google Scholar]; (b) Qian S, Shah AK, Head SA, Liu JO, Jin Z. Bioorg. Med. Chem. Lett. 2016;26:3411. doi: 10.1016/j.bmcl.2016.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zampella A, D’Auria MV. Tetrahedron: Asymmetry. 2001;12:1543. [Google Scholar]
- 9.Yu W, Mei Y, Kang Y, Hua Z, Jin Z. Org. Lett. 2004;6:3217. doi: 10.1021/ol0400342. [DOI] [PubMed] [Google Scholar]
- 10.(a) Brown HC, Ramachandran PV. AdV. Asym. Synth. 1995;1:147. and references therein. [Google Scholar]; (b) Brown HC, Singaram B. Acc. Chem. Res. 1988;21:287. and references therein. [Google Scholar]; (c) Brown HC, Bhat KS, Randad RS. J. Org. Chem. 1989;54:1570. and references therein. [Google Scholar]
- 11.Hua Z, Jin Z. Tetrahedron Lett. 2007;48:7695. doi: 10.1016/j.tetlet.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Morrill C, Grubbs RH. J. Org. Chem. 2003;68:6031. doi: 10.1021/jo0345345. [DOI] [PubMed] [Google Scholar]; (b) Morrill C, Funk TW, Grubbs RH. Tetrahedron Lett. 2004;45:7733. [Google Scholar]
- 13.Zampella A, D’Auria MV. Tetrahedron: Asymmetry. 2001;12:1543. [Google Scholar]
- 14.Lipshutz BH, Clososki GC, Chrisman W, Chung DW, Ball DB, Howell J. Org. Lett. 2005;7:4561. doi: 10.1021/ol051406p. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed SA, Gogal RM, Walsh JE. J. Immunol. Methods. 1994;170(2):211. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]