Abstract
Background
Family carers play a significant role in managing pain and associated medicines for people with advanced cancer. Research indicates that carers often feel inadequately prepared for the tasks involved, which may impact on carers’ and patients’ emotional state as well as the achievement of optimal pain control. However, little is known about effective methods of supporting family carers with cancer pain medicines.
Aims
To systematically identify and review studies of interventions to help carers manage medicines for pain in advanced cancer. To identify implications for practice and research.
Method
A systematic literature search of databases (MEDLINE, CINAHL, PsycINFO and AMED) was carried out to identify studies of pain medication management interventions that involved family carers of patients with advanced cancer, and reported specific outcomes for family carers. Patient pain outcomes were also sought. Studies were quality appraised; key aspects of study design, interventions and outcomes were compared and a narrative synthesis of findings developed.
Results
8 studies were included; all had significant methodological limitations. The majority reported improvements in family carer knowledge and/or self-efficacy for managing pain medicines; no effect on patient pain outcomes; and no adverse effects. It was not possible to discern any association between particular intervention characteristics and family carer outcomes.
Conclusions
Current evidence is limited, but overall suggests face-to-face educational interventions supported by written and/or other resources have potential to improve carers’ knowledge and self-efficacy for pain management. Further research is needed to identify how best to help family carers manage pain medicines for patients with advanced cancer.
Keywords: Cancer, Carers, Pain, Medicines, Education
Introduction
Family carers provide much of the essential care that enables many people with advanced cancer to live at home as their illness progresses.1 2 Fear of pain is one of the most significant concerns for those approaching the end of life, and 71% of patients with advanced cancer experience pain, often requiring treatment with multiple analgesics, including opioids.3 4 There is increasing recognition of family carers’ role in managing patients’ symptoms and medications, a crucial but often challenging aspect of providing palliative care at home.5 Medication management requires knowledge and practical skills and involves carers in selecting, administering and evaluating the effectiveness of medicines,6 7 tasks that become more exacting when complex drug regimens are prescribed to control cancer pain.4 Internationally, research has repeatedly described that family carers experience problems with medication management as a consequence of their beliefs about pain and worries about analgesics, particularly opioids;8 9 knowledge deficits;10 and insufficient information and support from health professionals.4 11 Recent UK studies found that family carers report receiving little information or education about end-of-life medications, and perceived managing medicines as a demanding and burdensome responsibility that can provoke anxiety.5 12 Identifying family carers’ difficulties helps explain why analgesic use may be inadequate and cancer pain control less than optimal.4 13 Deficiencies in pain and medications management have also been linked with unnecessary hospital admission.14
Qualitative research has articulated cancer carers’ concerns and needs in relation to pain management,5 11 15 and there is consensus that health professionals should provide family carers with more information, training and continuing support for this aspect of their role.5 11 12 16 However, little is known about the best methods of supporting carers to manage pain medicines. Most cancer pain interventions have focused on the patient, and systematic reviews have confirmed their benefits on pain outcomes.17–19 Few studies have involved family carers or reported discrete outcomes for carers.20 Systematic reviews have considered interventions that address a variety of cancer and palliative carers’ needs,21–25 but none has specifically or comprehensively appraised cancer pain medicines management interventions. We therefore undertook this systematic review of published studies evaluating interventions for family carers managing pain medication for patients with advanced cancer. The specific questions addressed were (1) What are the pain medication management interventions for family carers of patients with advanced cancer that have been evaluated? (2) What were their effects, positive or otherwise, on family carers and on patients’ pain? (3) Were any particular intervention characteristics or components (eg, intensity, tailoring, timing, underpinning theoretical framework) associated with improved outcomes?
Methods
Information sources and search strategy
Searches of MEDLINE, CINAHL, PsycINFO and AMED were initially carried out in 2013 and updated on 16 September 2014, with no date restrictions. The MEDLINE search strategy (see online supplementary appendix 1.1) combined relevant keywords, including intervention, pain management, pain medicine, carer, family carer, caregiver, cancer. The MEDLINE search strategy was adapted for other databases. The electronic searches were supplemented by scanning citations in related systematic reviews, and in the articles selected for full text review. Research team members identified one potentially eligible article that was not retrieved electronically by networking with colleagues working in the field of supportive care. There is no registered protocol for this review, which was undertaken to inform the development of a cancer pain medicines management intervention for family carers as part of a phase I–II feasibility study.26
Eligibility criteria and study selection
Two investigators (EL and JAH) independently read the titles and abstracts of all records retrieved and assessed them against the criteria agreed previously by the research team, shown in table 1. We were interested in articles reporting evaluation (quantitative or qualitative) of a pain medication management intervention that included unpaid family carers of adult patients with advanced cancer, in which data on carer outcomes had been gathered from the carers themselves. Where there was uncertainty about eligibility, typically because abstracts provided limited information about composition of the patient group or carer outcomes, the article was included at this stage of the search. The few disagreements about selection were resolved by a third investigator (SL). The full text of potentially eligible publications (n=19) was independently reviewed by at least two authors (SL, JBH and JAH). At this stage, close attention was paid to the patient population to verify inclusion of patients with advanced cancer, for example, from information provided about diagnosis or stage (metastases) or treatment (palliative or hospice care), and to results reporting (to confirm family carer outcomes were included). Final decisions about inclusion of articles were made by consensus.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Data extraction and analysis
To enable comparison, standardised formats were developed for extracting information from the selected studies. Details of the studies charted were: author, year of publication, country, study population (patients and family carers), sample size, attrition rate, study design, intervention characteristics, fidelity assessment, control group, outcomes specified with associated measurement instruments, and results reported for carers and patients. Where articles did not report patient outcomes we accessed referenced articles to obtain patient data from the study. Interventions were categorised according to format (mode of delivery and follow-up, eg, face-to-face, video, and resources provided to carers); duration and intensity (number and length of sessions, total duration and time period); delivery (provider, recipient and location); detail of intervention components and content; and the theoretical basis of the intervention. Information was extracted by one author (JAH) and reviewed by two authors (SL and JBH). Discussion of the comparative charts (SL, AR, JBH and JAH) identified key points to include in descriptive overviews of the studies and interventions. We also interrogated the charts for any patterns that might help explain the results obtained by the studies. The narrative synthesis of our findings presented in this paper was developed through an iterative process of scrutinising the data, articulating and testing interpretations, drawing on relevant literature and revising written drafts.
All the included studies were quantitative evaluations, and none had any substantial qualitative components. Not all studies were randomised, therefore we used the quality assessment checklist developed by Downs and Black,27 which is applicable to randomised and non-randomised studies. It has five subscales for quality of reporting, and factors that could systematically bias results, and provides an overall quality score. Following other reviews, the question concerning statistical power calculation was modified to a yes/no answer.28 The checklist has 27 items, with a maximum score of 28. We used Samoocha et al's28 method of grouping scores into four quality levels: excellent (26–28), good (20–25), fair (15–19) and poor (≤14). One author (DE) assessed and scored all the full text articles, and a second author (JAH) independently assessed 50% of them, without blinding to authorship or journal. Differences in scores (a total of 3 items in 2 studies) were resolved by a third author (SL).
Results
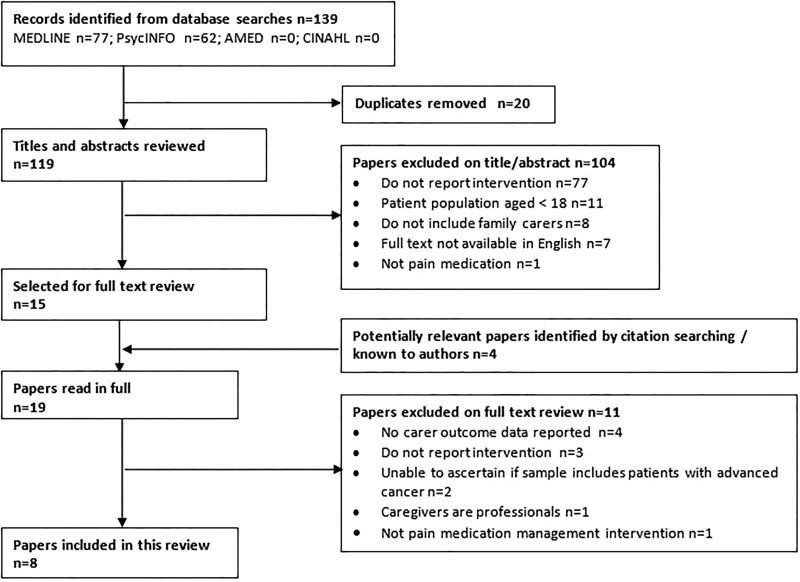
The database search found 139 citations, published 1985 to August 2014, from which 20 duplicate records were discarded. A further 104 records were excluded following assessment of abstracts, most because they were descriptive and did not report a specific intervention (n=77). The remaining 15 articles from the database search, along with 4 articles known to the research team and/or identified by citation searching, comprised the 19 papers read in full. Eight papers, each reporting a different study, met the criteria for inclusion in the review. A flowchart summarising the selection process is shown in figure 1.
Figure 1.
Systematic selection process.
Study characteristics
The characteristics of the eight studies are shown in table 2. The studies were published from 1995 to 2013, five were conducted in the USA, and one each in Taiwan, UK and Norway. Patients were recruited from hospital outpatient clinics, hospice care and home nursing services. All the studies included patients who reported current cancer-related pain; patient populations were heterogeneous in terms of cancer diagnoses. Three studies selected only patients with advanced cancer, and five also included patients at various stages of illness. Family carers were nominated by patients as the person most involved in their care. Carer demographics and information about the nature of patient-carer dyads were not consistently provided. All the studies delivered educational interventions to patients and family carers together, and included evaluation of the effect on carers: five in randomised control trials (RCT) and three in single-group prestudies and poststudies, one of which subsequently randomised participants to three different types of follow-up. Most RCTs compared the intervention to usual care; one used attention placebo control and written information.
Table 2.
Characteristics of studies included in the review
| Lead author | Year | Country | Description of sample | Study design | Number of family carers at baseline in | |
|---|---|---|---|---|---|---|
| Intervention | Control (UC or APC) | |||||
| Ferrell | 1995 | USA | Family carers of hospital outpatients aged 60+ years with cancer-related pain duration ≥3 months; prescribed opioid analgesia | SGPP | 50 | – |
| Wells | 2003 | USA | Family carers of hospital outpatients with cancer pain of onset or escalation in last 3 months; managed by analgesia; life expectancy >6 months | SGPP then 3-arm RCT of follow-up a. Patient-initiated b. Nurse-initiated |
64 a. 21 b. 19 |
−24 (UC) |
| Keefe | 2005 | USA | Family carers of hospice and hospital outpatients with advanced cancer diagnosis; disease-related pain (worst >3 BPI); life expectancy <6 months | 2-arm RCT | 41 | 37 (UC) |
| Lin | 2006 | Taiwan | Family carers of hospital outpatients with cancer pain taking oral analgesics | 2-arm RCT | 31 | 30 (UC) |
| Ward | 2009 | USA | Family carers of hospital outpatients with cancer diagnosis, reporting moderate to severe pain in past 2 weeks; performance status score indicating out of bed >50% of waking hours | 3-arm RCT a. Dyad b. Patient only |
a. 51 b. 53 |
57 (UC) |
| Capewell | 2010 | UK | Family carers of patients receiving palliative care from hospital outpatient clinics or community teams; living at home; pain from active cancer rated ≥3 on 0–10 scale | SGPP | 10 | – |
| Vallerand | 2010 | USA | Family carers of patients with cancer pain receiving care from home care nurses (not hospice nurses) | Cluster RCT | 24 | 22 (UC) |
| Valeberg | 2013 | Norway | Family carers of hospital outpatients with cancer diagnosis and radiographic evidence of bone metastasis; pain ≥2.5 on 1–10 scale; KPS ≥50 | 2-arm RCT | 58 | 54 (APC+ booklet) |
Study design: RCT, randomised controlled trial; SGPP, single group pretest and post-test design.
Control: APC, attention placebo control; UC, usual care.
BPI, Brief Pain Inventory.
The studies included a total of 582 family carer–patient dyads at baseline; 329 dyads were allocated to receive interventions (table 2) and, as far as can be ascertained from the data provided, 326 carers received an intervention with the patient present; none of the studies delivered interventions to carers only. Sample sizes ranged from 10 to 161 dyads at baseline assessment, with 8–109 dyads reported as completing follow-up. However, only three studies provided attrition data: the proportion of carers completing follow-up in intervention (I) and control (C) groups in Keefe et al's29 study at an average of 1 week were I 68%, C 76%; in Ward et al's30 study at 5 weeks I 80%, C 75% and at 9 weeks I 69%, C 67%; and in Capewell et al's31 study 8 of the 10 carers who received the intervention completed follow-up to 1 month. Heterogeneity in patient populations, educational interventions, outcome measures and timing of data collection makes quantitative meta-analysis inappropriate.
Study quality
Total quality scores for the studies ranged from 7 to 20 (out of a possible total of 28 on the Downs and Black27 checklist), with a median of 12. The checklist also creates a profile of the methodological strengths and weaknesses of a paper, assessing quality of reporting; internal validity (bias and confounding); external validity and power. Scores on these dimensions for each study are shown in table 3. Deficiencies in design and methods were found in most studies. The two studies with the highest total scores29 30 achieved the ‘good’ category in Samoocha et al's28 four-level classification; the other six studies had scores at the ‘poor’ level. None were classified as excellent or fair. Low total scores reflect inadequate reporting that often prevented assessment of items relating to risk of bias, but may, in part, be a consequence of the methodological challenges inherent in conducting research on interventions in palliative care.32 Internal validity scores were reduced because the educational interventions in these studies precluded blinding of recipients and those delivering the intervention; in addition, blinding of researchers assessing outcomes was often not achieved or was ambiguously reported. Only one study had a statistical power calculation; some studies were of very small scale, or designated by the authors as ‘preliminary’ or exploratory research. There is no apparent trend towards better reporting or more rigorous methodology, with year or decade of publication.
Table 3.
Study quality: reporting and risk of bias summary (Downs and Black27)
| Study lead author, date | Reporting score n/11 | External validity score n/3 | Internal validity—bias score n/7 | Internal validity—confounding score n/6 | Power score n/1 | Total score n/28 | Quality level* |
|---|---|---|---|---|---|---|---|
| Ferrell, 1995 | 4 | 1 | 2 | 0 | 0 | 7 | Poor |
| Wells, 2003 | 6 | 1 | 4 | 2 | 0 | 13 | Poor |
| Keefe, 2005 | 9 | 3 | 3 | 5 | 0 | 20 | Good |
| Lin, 2006 | 6 | 1 | 3 | 2 | 0 | 12 | Poor |
| Ward, 2009 | 8 | 2 | 5 | 4 | 1 | 20 | Good |
| Capewell, 2010 | 6 | 1 | 2 | 1 | 0 | 10 | Poor |
| Vallerand, 2010 | 5 | 1 | 3 | 2 | 0 | 11 | Poor |
| Valeberg, 2013 | 6 | 1 | 3 | 2 | 0 | 12 | Poor |
*Based on Samoocha et al's28 classification of quality level: excellent (26–28); good (20–25); fair (15–19); poor (≤14).
Intervention characteristics
All eight interventions included between one and three face-to-face education or training sessions, typically supported by written and/or other resources, opportunities for questions and discussion, and follow-up contacts for reinforcement or further coaching (table 4). The interventions were delivered to patients and family carers together by a specially trained clinician (nurse, psychologist) or a researcher in the patient's home (5 studies29 30 33–35), or in hospital outpatient clinics (3 studies31 36 37). None of the studies used health professionals who were providing routine patient care to deliver interventions, although Vallerand et al's34 study included home care nurses, some of whom received pain management education (Power over Pain) independently of the patient-carer dyads they recruited to the trial.
Table 4.
Pain medication management interventions for family carers of patients with advanced cancer
| First author, year, country | Intervention: format*, resources provided to carers, follow-up | Duration and intensity of intervention† | Provider, recipients, site of delivery. | Detail of intervention content theoretical basis |
|---|---|---|---|---|
| Ferrell, 1995, USA | 3 Face-to-face interactive teaching sessions Printed instructions for drug and non-drug treatment Audiotapes of first 2 sessions US$50 for non-drug equipment Follow-up: 2 additional visits (only data collection?) |
3 1 h sessions delivered over 2 weeks. Total duration 3 h Follow-up visits at 1 and 3 weeks after intervention: duration NR |
Teaching sessions delivered by experienced specialist nurse in the patient's home to patient alone or patient and carer together | Pain education program with cognitive/behavioural components
Theoretical basis: NR |
| Wells, 2003, USA | Single interactive education session: 15 min video followed by face-to-face discussion of content and patient's current medication Printed information on analgesics Follow-up: (1) patient given phone number for pain ‘hotline’ (no cost); (2) provider-initiated telephone calls; (3) usual care |
Single session duration 20–30 min. Follow-up: no calls were made to the ‘hotline’. Provider-initiated weekly phone calls for 4 weeks, lasting 5–15 min. Total duration 20–60 min | Education session in hospital outpatient clinic; NR who delivered to patient and carer together. Video featured expert clinicians and patients. Follow-up phone calls: specialist oncology nurse, NR recipient (patient or carer) | Cognitive intervention. Video included information about pain, methods to control pain, opioids, low risk of addiction, side effects of pain meds, and emphasised the importance of communicating pain to providers Follow-up telephone calls: nurse-assessed patients’ understanding of pain medicines, asked about any problems Theoretical basis: NR |
| Keefe, 2005, USA | 3 face-to-face interactive education/training sessions eliciting concerns and providing coaching to develop coping strategies Printed materials (book) Video and audio tapes |
3×45–60 min sessions (average 56 min) delivered over 1–2 weeks (average 14 days, range 8–32 days) Total duration 2.25–3 h |
Education/training sessions delivered by nurse educator in the patient's home to patient and carer together |
Partner-guided pain management training provided
|
| Lin, 2006, Taiwan | Face-to-face session at which content of booklet presented. Questions elicited and answered. Participants encouraged to phone if questions arose Copy of booklet Follow-up: 2 face-to-face sessions, information reiterated and questions answered |
Initial session 30–40 min.Follow-up at 2 and 4 weeks after initial session: duration NR | Intervention delivered by a researcher to patient and carer together in a private room in the hospital outpatient clinic | Cognitive intervention. Culturally specific booklet developed from earlier research into patient barriers to cancer pain management. Information addressed nine major concerns contributing to reluctance to report pain and use analgesics: fatalism, addiction, desire to be good, fear of distracting physicians, disease progression, tolerance, side effects, religious fatalism, and prn meds Theoretical basis: NR |
| Ward, 2009, USA | Single face-to-face interactive education session working through first 6 steps of 7-step intervention Follow-up: 2 telephone calls address 7th step |
Initial session 20–80 min, depending on participant needs Follow-up phone calls at 2 and 4 weeks after initial session, lasting 5–10 min Total duration 30–100 min |
Intervention delivered by specially trained nurse or psychologist at convenient location, usually the patient's home, to the patient alone or patient and carer together Follow-up phone calls: recipient (patient or caregiver) NR |
RIDcancerPAIN+ was designed to elicit and understand patients’ representations of symptoms before providing new information and developing strategies for behaviour change in a 7-step process
|
| Capewell, 2010, UK | 2 education sessions: 6 min video shown, and printed information provided, any questions answered Copies of DVD and printed materials |
Two sessions approximately 1 week apart. Duration NR | Video (featuring palliative care clinicians) shown in hospital outpatient clinic to patient alone or patient and carer together by researcher who answered questions | Brief structured educational intervention focusing on cancer pain and the use of strong opioids, emphasising that cancer pain can often be well controlled, importance of pain assessment and good communication. Addressed ‘common fears’ about opioids found in previous research Theoretical basis: NR |
| Vallerand, 2010, USA |
Patients and carers Initial visit (face-to-face) given printed information; second visit face-to-face education session. Extent of interaction NR Nurses providing home care 2 lecture/discussion sessions Printed resources on pain management and opioid-related side effects |
Patients and carers First contact duration NR. Education 1 week later, duration 1 h Nurses 1st teaching session 4 h 4–6 weeks to apply in practice 2nd teaching session duration NR |
Patients and carers visited at home by PI or researcher; education session delivered by PI to patient alone or patient and carer together Nurses’ teaching delivered in classroom, NR by whom, and size of group |
Power Over Pain: a structured educational intervention on management of pain and side effects in patients with cancer For patients and carers: importance of pain management; misconceptions; analgesics; side-effect management. Based on program for nurses, ‘presented at a level appropriate for the layman’ For nurses, 1st session: misconceptions about analgesics; pharma pain control and side effects; communicating with physicians and patients. 2nd session: ‘more advanced concepts’, ie, dose titration and managing side effects. Role play and assertiveness training to develop communication advocacy skills Theoretical basis: NR |
| Valeberg, 2013, Rustoen; 2012, Norway | 3 face-to-face teaching sessions, interspersed with 3 telephone contacts Printed resources on pain and side effect management Weekly pill box Script to assist communication with physician re pain and medicines |
Contact made every week for 6 weeks Duration of 3 home visits and 3 phone calls NR |
Home visits and phone calls made by a specially trained oncology nurse, who delivered teaching sessions to patient alone, or patient and carer together. Phone calls: NR recipient (patient or caregiver) | Norwegian adaptation of PRO-SELF Pain Control Program, developed in USA. Psychoeducational pain management intervention to increase knowledge and change attitudes to pain management and use of medication. First teaching session addressed knowledge deficits (identified from Family Pain Questionnaire). Subsequent contacts reviewed pain scores and use of medication; reinforced education; and provided further coaching, eg, modifying pain management plans or contacting physicians Theoretical basis: NR |
*Format: face-to-face, telephone calls, video presentation, printed materials.
†Duration and intensity: number and duration of sessions/phone calls; total duration of intervention and over what time period.
NR, not reported.
The duration and intensity of interventions, and the period of time over which they were delivered varied greatly between studies. Some authors provided insufficient information to precisely quantify intensity of intervention, and the boundaries of interventions were not always clearly defined: for example, specifying whether ‘follow-up’ contacts with participants were for educational or assessment purposes. However, we were able to classify interventions as higher or lower intensity, based on Cummings et al's18 definition of intensive education: more than four education sessions, or a cumulative total duration of more than 2 h in one setting. None of the interventions in this review involved more than three education sessions. Two studies reported interventions with a total duration of 2 or more hours in the same setting.29 33 Both these interventions had a model of three face-to-face education sessions with a total duration of 2–3 h, delivered over a maximum of 2 weeks, which is similar to that described by Valeberg et al,35 who did not report the length of sessions. Our interpretation of the information supplied in the other five articles is that the initial education session plus follow-up did not exceed a maximum duration of 100 min (mean durations quoted were briefer), thus, these interventions were categorised as lower intensity.30 31 34 36 37
All the interventions focused on managing pain and pain medication, addressing widely recognised ‘barriers’ to pain management: knowledge about how analgesics work; beliefs about addiction and tolerance; fears about side effects and overdose; excessive stoicism; and poor communication between patients, family carers and health professionals.9 They included cognitive and behavioural components, providing a mixture of information and teaching or coaching to develop practical and coping skills, solve problems and/or improve communication. Some interventions had additional components, such as providing information or training in non-drug pain management, for example, relaxation, massage and imagery;29 33 creating plans to maintain coping;29 change behaviour or anticipate problems, which could be reviewed and revised during follow-up.30 35 Structured and tailored elements were present in all interventions, but they differed in the emphasis placed on eliciting and responding to the particular needs and circumstances of a patient–carer dyad. Those with minimal tailoring delivered standardised content and required participants to raise questions or concerns.31 37 A higher degree of tailoring was achieved by initially identifying participants’ gaps in knowledge, misconceptions, concerns or needs (eg, by structured interaction29 30 or questionnaire35), and the information was then used to provide individualised education. Some interventions also assessed learning and/or provided further coaching to modify pain management plans.30 35
Only one study referenced a theoretical framework underpinning the intervention. Ward et al's30 RIDcancerPAIN+ was based on the ‘representational approach to patient education’ which emphasises discovering an individual's understanding of their illness or treatment before providing information to address any gaps or misconceptions.38 None of the interventions was reported to have been informed by psychosocial theory about the nature of the patient–carer relationship or the caring role.
The majority of studies appear to have delivered the same education to patients and family carers.30 31 34 36 37 However, Keefe et al's29 ‘partner-guided pain management training’ explicitly positioned family carers in the role of coach ‘guiding and encouraging the patient’ to use coping skills taught by the nurse educator, who also gave carers feedback on their performance as a coach. None of the studies reported involving patients, family carers or healthcare professionals in the design or development of the intervention.
Fidelity and quality of intervention delivery was addressed to some degree in four studies. Those delivering interventions used written manuals, booklets or guides;29 30 37 completed checklists during education sessions30 audio-taped sessions for later review and/or rating;29 or their practice was observed during training.33 Only one study reported a treatment fidelity score (82%): all sessions were audio-taped, and 58% assessed against the intervention manual for adherence.29
Impact of interventions
The studies specified a variety of family carer outcomes, most selecting a combination of medicines-specific or pain-specific and more global psychological measures, and there was wide variation in how many and when outcome measurements were recorded, with one to six time points for follow-up, ranging from immediately after the intervention to 6 months, although most studies measured outcomes within 1 month. Further details of study design and outcomes are provided in online supplementary appendix 1.2. Family carers’ knowledge and beliefs about pain and medications were measured in seven studies, using the knowledge subscale of the Family Pain Questionnaire (FPQ)31 33–36 and/or the Barriers Questionnaire (BQ).30 34 37 Five studies reported improvements in family carers’ knowledge and beliefs: four studies used the FPQ knowledge subscale31 33 35 36 and one study used the BQ.37 Three studies demonstrated statistically significant treatment effects: two using the FPQ35 36 and one using the BQ.37 Two of these delivered lower intensity, minimally tailored interventions,36 37 and the third a higher intensity, more tailored intervention.35 Improved FPQ knowledge scores were reported in two non-randomised studies, but neither provided data.31 33 Two RCTs, including Ward et al's30 well-conducted study of a lower intensity, tailored intervention, found no effect of interventions on FPQ knowledge subscale and/or BQ scores.34
Three studies reported on the FPQ experience subscale (six items which mainly assess family carer perceptions of the patient's pain and distress, with one question about the carer's perceived ability to control the patient's pain). Two studies found no statistically significant change at 4 weeks;31 34 and one study reported ‘improvements’ at 1 week, although no data were provided.33
Family carers’ self-efficacy, or perceived control over pain, was measured in two studies. Keefe et al's29 well-conducted RCT evaluated a higher intensity, more tailored intervention, and found a statistically significant improvement in self-efficacy scores for the treatment group, at a mean of 7 days (range 0–31) postintervention. Vallerand et al's34 cluster RCT of two interventions (for nurses and patient–carer dyads) found no effect of the dyad intervention on family carers’ perceived control over pain, although family carers whose nurses had received Power over Pain education showed a statistically significant improvement in perceived control over pain at 1 month compared with controls. The intervention for patient–carer dyads was lower intensity (extent of tailoring unclear); nurse education involved two group teaching sessions with a total duration of more than 4 h.
Adherence to medication was measured in two studies: an RCT found a statistically significant effect of the intervention on family carers’ self-reported scores at 2 and 4 weeks;37 a small single-group pilot study did not report carer data.31 Other, more global, family carer outcomes were measured in three studies. Keefe et al's29 well-conducted RCT reported improved Caregiver Strain Index scores for the intervention group, a trend that did not reach statistical significance. This study found no effect on family carer mood,29 and another study reported measuring carers’ quality of life but provided no data.33
Patient pain outcomes, measured using the Brief Pain Inventory (BPI), were reported in six articles: four studies found no statistically significant effect of interventions on patients’ BPI scores,29 30 34 36 and two reported statistically significant reductions in pain.31 37 The remaining two articles33 35 did not report patient outcomes from the study but cited publications that provided this information, which were accessed: one study measured pain outcomes;39 and the other assessed patient knowledge, but not pain.40 Ferrell et al39 found trends in mean and median scores for pain intensity, pain distress and pain relief recorded by patients in a ‘self-care log’ over the 5 weeks of the study, which indicated improvements over time, although no statistical tests were applied.
None of the studies assessed adverse effects of interventions on family carers or patients, or discussed possible harms. The review included exploratory and feasibility studies, but there was little, if any, discussion of acceptability of interventions to family carers and patients, and not all authors accounted fully for attrition rates. None of the studies included qualitative data gathered from family carers.
Discussion
Effects of interventions
The results indicate that educational interventions with structured and tailored elements delivered in one or more face-to-face sessions, supported by written and/or other resources, and/or including further contact for reinforcement and review, have the potential to improve carers’ knowledge and self-efficacy for pain medicines management, and change misconceptions about cancer pain and medications. Seven of the eight studies included in the review evaluated family carers’ knowledge about pain and medications: five studies reported improvements, of which three demonstrated statistically significant treatment effects, although these studies were all categorised as poor quality. Two RCTs found statistically significant improvements in carers’ self-efficacy or perceived control over pain. One was a well-conducted study that also showed a positive effect on caregiver strain, a trend that just failed to reach statistical significance. None of the studies provided evidence to suggest that pain management interventions had adverse effects or could harm family carers or patients.
The studies provide equivocal evidence for the impact of family carer interventions on patients’ experience of pain: four studies found no effect on pain, and three studies reported positive effects. Neither of the two well-conducted RCTs included in the review found statistically significant treatment effects on patient pain. In addition, no significant changes in more global measures of family carer psychological state were observed. Plausible explanations can be put forward for non-significant outcomes, such as assessment too soon after the intervention, and low levels of pain in the patient sample so that floor effects were encountered. Additionally, patient pain is a distal indicator of the effect of an educational intervention influenced by other factors, such as disease progression, changes in treatment and psychosocial variables, which make it difficult to attribute observed effects (or lack of them) to the intervention. Authors of other reviews of family carer interventions have suggested that outcome measures should be chosen to reflect the impacts that interventions can realistically be expected to accomplish in a limited time.21
Quality of research studies
Only eight studies met the criteria for inclusion in the review and all were conducted in developed countries, the majority in the USA, limiting the ability to generalise results to other health systems. Quality assessment identified methodological limitations and deficiencies in reporting, and comparability of results was compromised by heterogeneity of patient populations; diversity of interventions; use of different outcome measures; and variation in when outcomes were assessed. This suggests that we should be cautious about interpreting findings and generalising from this small collection of published reports, whose results are at best equivocal.
The studies’ lack of assessment of potential adverse intervention effects on family carers or patients is an important omission in research on pain management in palliative care because of the relationship between pain, anxiety and coping, and the potential risk of negative impacts if information content or timing of delivery is misjudged. In addition, the studies reported only quantitative outcomes: none, even those designated as feasibility studies, included qualitative methods to explore intervention effects, positive or negative, on family carers and patients, or to evaluate research processes. This limits the usefulness of the studies for informing future research and palliative care practice.
Our findings suggest that educational interventions have the potential to help family carers manage cancer pain and associated medications, but the mechanisms that bring about positive effects remain unclear. A theoretical framework was described for only one intervention.30 and none was informed by behaviour change theories. However, all the studies appear to have been influenced by the literature on ‘barriers’ to the effective use of pain medication, and most interventions incorporated elements to mitigate culturally specific barriers, and/or elicit and address individual beliefs known to inhibit effective use of pain medicines. Implicit assumptions about how ‘barriers’ can be overcome were stated by Cagle et al41 as: ‘increasing knowledge and improving attitudes about pain management (the immediate effect of the intervention) would lead to changes in behaviour such as better administration and adherence to pain medications (a secondary effect), which will in turn lead to improved pain relief for patients (the primary distal effect)’. This proposed mechanism is helpful for specifying appropriate outcome measures, but it does not distinguish between patients, health professionals and family carers. It does not take account of patient–carer interdependency, the unique nature of the family carer role, or the complex social and emotional context of providing palliative care in the home. Lack of theoretical underpinning for interventions including family carers has been identified in a broader review of cancer symptom management interventions.23 Clarity about the theories or assumptions on which interventions are based, and specification of appropriate and sensitive outcome measures, could greatly strengthen future studies.
Unpacking interventions: what works best?
The interventions included in the review have been categorised broadly as educational, but comparisons across studies proved difficult because of heterogeneity in intervention characteristics, such as format, content, duration and method of delivery. Owing to this diversity and the small number and limited quality of the studies, we were unable to discern any clear pattern of association between particular intervention characteristics and measured effects on family carer outcomes. There was no indication that outcomes were improved by providing multiple face-to-face education sessions, or by spending more time with family carers. Reviews of cancer pain self-management interventions have reported similar findings,17 19 although Cummings et al18 described a relationship between higher educational ‘dose’ patient interventions and improved outcomes in pain management knowledge, skills and attitudes, and pain control. Another feature of educational interventions that has been linked to efficacy is the ability to ‘tailor’ delivery to address individual gaps in knowledge, needs and circumstances.18 We found interventions varied in the emphasis placed on individualising information and education, but there was no discernible pattern in effects, a finding that is consistent with Koller et al's19 review of cancer pain self-management interventions.
It is worth noting that intervention duration and tailoring are connected, since tailoring is likely to result in education sessions of variable duration, as demonstrated in the study by Ward et al.30 This raises questions about assessing ‘dose’ or ‘intensity’ based only on time, or attempting to determine an optimal intervention dose or duration. Bennett et al17 concluded that duration may be less important than other aspects of pain management interventions, yet to be elucidated by research. However, we have doubts about the value of trying to isolate discrete ‘active ingredients’ of complex interventions, whose properties are better understood as emergent and contingent on contextual factors,42 which may contribute substantially to effectiveness.43 44 Understanding the context in which interventions are delivered, and the influence of contextual factors on outcomes merit more attention in pain and medication management research.45
When is the best time to intervene?
A frequently asked question is ‘when might be the most appropriate time, in relation to the course of the patient's illness, treatment or experience of pain, to provide pain and medication information and education for family carers?’ It is especially salient in end-of-life cancer research since ‘there is a small window of opportunity to recruit carers when they move into the caregiving role but are not overwhelmed by it’,45 and pain medicines may include opioids, which have negative associations that influence family carers’ readiness to learn, thus calling into question the feasibility of early intervention. Most of the studies in this review did not consider timing of intervention delivery, although participating patients were at various stages of illness and treatment. One study focused specifically on patients near the end of life: Keefe et al's29 well-conducted RCT recruited patients experiencing pain, and who were eligible for US hospice care, that is, with <6 months life expectancy, and demonstrated statistically significant effects on family carer self-efficacy but not patients’ pain. A recent US study by Cagle et al41 recruited a similar population and found that a brief, tailored pain management intervention produced statistically significant improvements in family carers’ knowledge, attitudes and assessment of patient pain at 2 weeks. We suggest that these positive effects may be due, in part, to delivering an appropriate intervention to a well-defined group for whom pain management is a pressing concern. In addition, transition to hospice care in the USA (and palliative care in the UK) is a significant milestone for patients and family carers, when carers may recognise the need to take on more responsibility for managing pain medication, and so be receptive to help. Targeting interventions at specific groups, possibly identified at a key point in the palliative care pathway, in addition to individualising content in response to individual needs, should be considered in future studies.
Recruiting patients at a particular point of contact with services is convenient for research purposes, but clearly this will not achieve optimal timing of intervention for all participants. Stajduhar et al46 described wide variation in family carers’ preferences for when and how they received or accessed information and support, which indicates that a standardised approach may be inappropriate. In Healy et al's47 study of introducing a subcutaneous medication management intervention for family carers, palliative care nurses rejected the idea of defining an objective, fixed ‘right time’ based on the patient's palliative care trajectory, in favour of making the decision with each family carer individually. We suggest that for pain and medication management interventions it is more meaningful to ask not about timing, per se, but how to provide timely help for family carers, conceptualising ‘timeliness’ as a subjective and context-dependent aspect of an intervention.
Implications for research
This review confirms that further good-quality research is needed to identify how best to help family carers of patients with advanced cancer to manage pain and pain medicines. Some authors have argued that research with patients receiving palliative care, and their family carers, would be strengthened by taking a more pragmatic approach, in particular, by evaluating interventions that are brief and easily delivered in the context of routine care.17 48 The studies reviewed here offer little indication of what might be achievable by healthcare professionals in routine practice, although recently published studies by Healy et al47 and Cagle et al41 describe interventions integrated with regular nursing care. In Cagle et al's41 US study, hospice nurses delivered a brief, tailored pain management intervention to newly registered patients and their family carers, with positive effects reported by carers.
None of the studies in the review reported following recommended research practices by involving family carers and healthcare professionals in developing intervention studies.49 Future research would be strengthened by engaging family carers to ensure that interventions meet their needs and preferences, and in involving healthcare professionals to design interventions that can be assimilated into clinical practice and service delivery. In particular, participation by family carers and clinicians could facilitate finding solutions to the issue of timeliness discussed earlier, by identifying timings and settings for intervention compatible with local practice in when and how patients and family carers are introduced to pain medicines. Our review also indicates that more attention should be given to the process of developing interventions informed by theory that are likely to be effective in pragmatic trials. We suggest that these recommendations for future research could be addressed by adopting a complex interventions framework for the development and testing of future interventions for family carers.50
We found an absence of qualitative research in the studies reviewed, despite widespread acceptance of its value in process evaluation, and as a complement to quantifiable outcomes.44 Pragmatic studies of pain medication management interventions would be enhanced by using qualitative methods to illuminate the influence of contextual factors on intervention delivery and to investigate how clinicians, family carers and patients experience pain management education, including identifying adverse and unanticipated effects.
Implications for clinical practice
Research has established that patients benefit from education about managing cancer pain,17 and it is recognised that nurses have a central role in meeting patients’ information needs and providing advice on analgesia. However, family carers consistently identify unmet needs for appropriate and timely information and support to help them manage pain and medications, especially as patients approach the end of life. Current evidence suggests that there is potential for health professionals to improve family carers’ knowledge and self-efficacy in managing cancer pain medicines by including them with patients during face-to-face education, supported by written or other materials, and appropriate follow-up, an approach that has not been linked with any obvious or serious harms. Currently, there is insufficient evidence on which to base more specific directions about interventions likely to be effective in clinical practice, or how, where and when they should be delivered.
Strengths and limitations of the review
As far as we are aware, this is the most comprehensive systematic review of pain medication management interventions for family carers of patients with advanced cancer. The review has some limitations: articles published in languages other than English were not included, and restricting the study population to family carers of patients with advanced cancer reduced the number of eligible studies. While there are strong arguments for maintaining a narrow focus, the review may have benefited from a broader definition of the study population, for example, including family carers of patients with cancer at all stages, or patients with non-cancer diagnoses experiencing pain near the end of life, which may have yielded more studies, a wider range of interventions, and allowed consideration of generalisability of results. The review could also have been extended to include interventions to help family carers manage not only pain but also other symptoms of advanced cancer and the medicines associated with these symptoms.
Conclusions
There is scant good-quality research to inform answers to the question of how family carers of patients who have advanced cancer can be helped to manage pain medicines. Evidence from the eight studies reviewed suggests that educational interventions delivered face-to-face, supported by written and/or other resources and appropriate follow-up, have the potential to improve family carers’ knowledge and self-efficacy for pain management, and reduce attitudinal barriers. No adverse effects of interventions were reported. There were no discernible patterns of association between particular intervention characteristics, for example, time spent in interaction or providing individualised information, and effects on family carer outcomes. Future intervention research would be strengthened by addressing methodological issues and giving more attention to the development of interventions that address family carers’ needs and concerns and are informed by theory, appropriately targeted, and compatible with local clinical practice and service delivery.
Supplementary Material
Acknowledgments
The authors would like to thank Andrew Sibley, University of Southampton, for help with the initial search.
Footnotes
Contributors: SL, JBH and AR were involved in the study conception and obtained funding. EL formulated the search strategy and performed the searches. SL, EL, JBH and JAH identified eligible studies. DE and JAH appraised study quality. SL, JAH, JBH and AR extracted data, contributed to interpretation and drafted results. All authors read and approved the final manuscript. SL is the guarantor.
Funding: This study was supported by Dimbleby Marie Curie Research Fund grant DCMC-RF-12-05.
Competing interests: JBH is a member of the Scientific Board, Cachexia Hub, Helsinn Healthcare.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Morris SM, King C, Turner M, et al. Family carers providing support to a person dying in the home setting: a narrative literature review. Palliat Med 2015;29:487–95. 10.1177/0269216314565706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes B, Higginson IJ. Factors influencing death at home in terminally ill patients with cancer: systematic review. BMJ 2006;332:515–21. 10.1136/bmj.38740.614954.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teunissen S, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manag 2007;34:94–104. [DOI] [PubMed] [Google Scholar]
- 4.Schumacher KL, Plano Clark VL, West CM, et al. Pain medication management processes used by oncology outpatients and family caregivers part II home and lifestyle contexts. J Pain Symptom Manag 2014;48:784–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne S, Turner M, Seamark D, et al. Managing end of life medications at home—accounts of bereaved family carers: a qualitative interview study. BMJ Support Palliat Care 2015;5:181–8. 10.1136/bmjspcare-2014-000658 [DOI] [PubMed] [Google Scholar]
- 6.van Ryn M, Sanders S, Kahn K, et al. Objective burden, resources, and other stressors among informal cancer caregivers: a hidden quality issue? Psychooncology 2011;20:44–52. 10.1002/pon.1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kazanowski M. Family caregivers’ medication management of symptoms in patients with cancer near death. J Hosp Palliat Nurs 2005;7:174–81. [Google Scholar]
- 8.Lin CC. Barriers to the analgesic management of cancer pain: a comparison of attitudes of Taiwanese patients and their family caregivers. Pain 2000;88:7–14. [DOI] [PubMed] [Google Scholar]
- 9.Letizia M, Creech S, Norton E, et al. Barriers to caregiver administration of pain medication in hospice care. J Pain Symptom Manag 2004;27:114–24. [DOI] [PubMed] [Google Scholar]
- 10.Oldham L, Kristjansen LJ. Development of a pain management programme for family carers of advanced cancer patients. Int J Palliat Nurs 2004;10:91–9. 10.12968/ijpn.2004.10.2.12455 [DOI] [PubMed] [Google Scholar]
- 11.Bee P, Barnes P, Luker K. A systematic review of informal caregivers’ needs in providing home-based end-of-life care to people with cancer. J Clin Nurs 2008;18:1379–93. [DOI] [PubMed] [Google Scholar]
- 12.Harrop E, Byrne A, Nelson A. “It's alright to ask for help”: findings from a qualitative study exploring the information and support needs of family carers at the end of life. BMC Palliat Care 2014;13:22 http://www.biomedcentral.com/1472–684X/13/22 (accessed 20 May 2015). 10.1186/1472-684X-13-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schumacher K, Koresawa S, West C, et al. Putting cancer pain regimens into practice at home. J Pain Symptom Manag 2002;23:369–82. [DOI] [PubMed] [Google Scholar]
- 14.Trask P, Teno J, Nash J. Transitions of care and changes in distressing pain. J Pain Symptom Manag 2006;32:104–9. [DOI] [PubMed] [Google Scholar]
- 15.Kelley M, Demiris G, Nguyen H, et al. Informal hospice caregiver pain management concerns: a qualitative study. Palliat Med 2013;27:673–82. 10.1177/0269216313483660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta A, Chan LS, Cohen SR. Flying blind: sources of distress for family caregivers of palliative cancer patients managing pain at home. J Psychosoc Oncol 2014;32:94–111. 10.1080/07347332.2013.856057 [DOI] [PubMed] [Google Scholar]
- 17.Bennett MI, Bagnall AM, José Closs S. How effective are patient-based educational interventions in the management of cancer pain? Systematic review and meta-analysis. Pain 2009;143:192–9. 10.1016/j.pain.2009.01.016 [DOI] [PubMed] [Google Scholar]
- 18.Cummings GG, Olivo SA, Biondo PD, et al. Effectiveness of knowledge translation interventions to improve cancer pain management. J Pain Symptom Manag 2011;41:915–39. [DOI] [PubMed] [Google Scholar]
- 19.Koller A, Miaskowski C, De Geest S, et al. A systematic evaluation of content, structure, and efficacy of interventions to improve patients self management of cancer pain. J Pain Symptom Manage 2012;44:264–84. 10.1016/j.jpainsymman.2011.08.015 [DOI] [PubMed] [Google Scholar]
- 20.Meeker MA, Finnell D, Othman AK. Family caregivers and cancer pain management: a review. J Fam Nurs 2011;17:29–60. 10.1177/1074840710396091 [DOI] [PubMed] [Google Scholar]
- 21.Harding R, Higginson IJ. What is the best way to help caregivers in cancer and palliative care? A systematic literature review of interventions and their effectiveness. Palliat Med 2003;17:63–74. [DOI] [PubMed] [Google Scholar]
- 22.Harding R, List S, Epiphaniou E, et al. How can informal caregivers in cancer and palliative care be supported? An updated systematic literature review of interventions and their effectiveness. Palliat Med 2012;26:7–22. 10.1177/0269216311409613 [DOI] [PubMed] [Google Scholar]
- 23.Hopkinson J, Brown J, Okamoto I, et al. The effectiveness of patient-family carer (couple) interventions for the management of symptoms and other health-related problems in people in people affected by cancer. A systematic literature search and narrative review. J Pain Symptom Manag 2012;43:111–41. [DOI] [PubMed] [Google Scholar]
- 24.Hudson PL, Remedios C, Thomas K. A systematic review of psychosocial interventions for family carers of palliative care patients. BMC Palliat Care 2010;9:17 http://www.biomedcentral.com/1472-684X/9/17 (accessed 20 May 2015). 10.1186/1472-684X-9-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stajduhar K, Funk L, Toye C, et al. Part 1: Home-based family-based caregiving at the end of life: a comprehensive review of published quantitative research (1998–2008). Palliat Med 2010;24:573–93. 10.1177/0269216310371412 [DOI] [PubMed] [Google Scholar]
- 26.Latter S, Hopkinson J, Lowson E, et al. Study protocol for a feasibility trial of Cancer Carer Medicines Management (CCMM): an educational intervention for carer management of pain medication in cancer patients at the end of life. Working Pap Health Sci 2014;1:8 http://www.southampton.ac.uk/wphs/previous_issues/2014/summer.page (accessed 20 May 2015). [Google Scholar]
- 27.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samoocha D, Bruinvels DJ, Elbers NA, et al. Effectiveness of web-based interventions on patient empowerment: a systematic review and meta-analysis. J Med Internet Res 2010;12:e23 http://www.jmir.org/2010/2/e23/ (accessed 2 Jun 2015). 10.2196/jmir.1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keefe F, Ahles T, Sutton L, et al. Partner-guided cancer pain management at the end of life: a preliminary study. J Pain Symptom Manag 2005;29:263–72. [DOI] [PubMed] [Google Scholar]
- 30.Ward SE, Serlin RC, Donovan HS, et al. A randomised trial of a representational intervention for cancer pain: does targeting \the dyad make a difference? Health Psychol 2009;28:588–97. 10.1037/a0015216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capewell C, Gregory W, Closs S, et al. Brief DVD-based educational intervention for patients with cancer pain: feasibility study. Palliat Med 2010;24:616–22. 10.1177/0269216310371704 [DOI] [PubMed] [Google Scholar]
- 32.Aoun S, Nekolaichuk C. Improving the evidence base in palliative care to inform practice and policy: thinking outside the box. J Pain Symptom Manag 2014;46:1222–35. [DOI] [PubMed] [Google Scholar]
- 33.Ferrell BR, Grant M, Chan J, et al. The impact of cancer pain education on family caregivers of elderly patients. Oncol Nurs Forum 1995;22:1211–8. [PubMed] [Google Scholar]
- 34.Vallerand A, Hasenau S, Templin T. Improving cancer management in the home. J Pain Manag 2010;3:41–52. [Google Scholar]
- 35.Valeberg BT, Kolstad E, Småstuen MC, et al. The PRO-SELF pain control program improves family caregivers knowledge of cancer pain management. Cancer Nurs 2013;36:429–35. 10.1097/NCC.0b013e3182747bcf [DOI] [PubMed] [Google Scholar]
- 36.Wells N, Hepworth J, Murphy B, et al. Improving cancer pain management through patient and family education. J Pain Symptom Manag 2003;25:344–56. [DOI] [PubMed] [Google Scholar]
- 37.Lin CC, Chou PL, Wu SL, et al. Long-term effectiveness of a patient and family pain education program on overcoming barriers to management of cancer pain. Pain 2006;122:271–81. 10.1016/j.pain.2006.01.039 [DOI] [PubMed] [Google Scholar]
- 38.Donovan HS, Ward SE, Song MK, et al. An update on the representational approach to patient education. J Nurs Scholarsh 2007;39:259–65. 10.1111/j.1547-5069.2007.00178.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrell BR, Ferrell BA, Ahn C, et al. Pain management for elderly patients with cancer at home. Cancer 1994;74:2139–46. [DOI] [PubMed] [Google Scholar]
- 40.Rustoen T, Valeberg B, Kolstad E, et al. The PRO-SELF Pain Control Program improves patients’ knowledge of cancer pain management. J Pain Symptom Manag 2012;44:321–30. [DOI] [PubMed] [Google Scholar]
- 41.Cagle J, Zimmerman S, Cohen L, et al. EMPOWER: an intervention to address barriers to pain management in hospice. J Pain Symptom Manag 2015;49:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohn S, Clinch M, Bunn C, et al. Entangled complexity: why complex interventions are just not complicated enough. J Health Serv Res Policy 2013;18:40–3. 10.1258/jhsrp.2012.012036 [DOI] [PubMed] [Google Scholar]
- 43.Clarke DJ, Hawkins R, Sadler E, et al. Introducing structured caregiver training in stroke care: findings from the TRACS process evaluation study. BMJ Open 2014;4:e004473 http://bmjopen.bmj.com/content/4/4/e004473 (accessed 20 May 2015). 10.1136/bmjopen-2013-004473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oakley A, Strange V, Bonell C, et al. Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332:413–16. 10.1136/bmj.332.7538.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grande G, Stajduhar K, Aoun S, et al. Supporting lay carers in end of life care: current gaps and future priorities. Palliat Med 2009;23:339–44. 10.1177/0269216309104875 [DOI] [PubMed] [Google Scholar]
- 46.Stajduhar KI, Funk L, Outcalt L. Family caregiver learning—how family caregivers learn to provide care at the end of life: a qualitative secondary analysis of four datasets. Palliat Med 2013;27:657–64. 10.1177/0269216313487765 [DOI] [PubMed] [Google Scholar]
- 47.Healy S, Israel F, Charles MA, et al. An educational package that supports laycarers to safely manage breakthrough subcutaneous injections for home-based palliative care patients: Development and evaluation of a service quality improvement. Palliat Med 2013;27:562–70. 10.1177/0269216312464262 [DOI] [PubMed] [Google Scholar]
- 48.Hudson P. Improving support for family carers: key implications for research, policy and practice. Palliat Med 2013;27:581–2. 10.1177/0269216313488855 [DOI] [PubMed] [Google Scholar]
- 49.Higginson IJ, Evans CJ, Grande G, et al. , MORECare. Evaluating complex interventions in end of life care: the MORECare Statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Med 2013;11:111 http://www.biomedcentral.com/1741-7015/11/111 (accessed 20 May 2015). 10.1186/1741-7015-11-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: new guidance. BMJ 2008;337:a1655 http://www.mrc.ac.uk/complexinterventionsguidance (accessed 20 May 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.