Abstract
Spreading depolarization (SD) is generated in the central nervous systems of both vertebrates and invertebrates. SD manifests as a propagating wave of electrical depression caused by a massive redistribution of ions. Mammalian SD underlies a continuum of human pathologies from migraine to stroke damage, whereas insect SD is associated with environmental stress-induced neural shutdown. The general cellular mechanisms underlying SD seem to be evolutionarily conserved throughout the animal kingdom. In particular, SD in the central nervous system of Locusta migratoria and Drosophila melanogaster has all the hallmarks of mammalian SD. Locust SD is easily induced and monitored within the metathoracic ganglion (MTG) and can be modulated both pharmacologically and by preconditioning treatments. The finding that the fly brain supports repetitive waves of SD is relatively recent but noteworthy, since it provides a genetically tractable model system. Due to the human suffering caused by SD manifestations, elucidating control mechanisms that could ultimately attenuate brain susceptibility is essential. Here we review mechanisms of SD focusing on the similarities between mammalian and insect systems. Additionally we discuss advantages of using invertebrate model systems and propose insect SD as a valuable model for providing new insights to mammalian SD.
Keywords: extracellular potassium, spreading depolarization continuum, sodium ion-potassium ion-adenosinetriphosphatase, protein kinase G, locust, Drosophila
it is a truism that neural function is critically important for the survival of most, if not all, species within the animal kingdom. At the core, optimal neural activity relies on the continued generation of electrical signals, which is dependent on the maintenance of appropriate ionic gradients. Indeed a loss in ionic homeostasis causes neural failure (Money et al. 2009; Rodgers et al. 2007; Rounds 1967; Wu and Fisher 2000), and thus animals invest much energy in regulatory processes to ensure that ion gradients are constantly restored. However, there are times when such mechanisms fail, and, as a result, disruptions in neural communication are observed. For instance, a substantial increase in extracellular potassium ion concentration ([K+]o) can overwhelm the capacity of the Na+-K+-ATPase to restore ion gradients (Anderson and Andrew 2002) and provoke propagating waves of cellular depolarization and suppression of electrical activity, a phenomenon known as spreading depolarization (SD) (Leão 1944; Rodgers et al. 2007; Somjen 2001). SD is an ionic disturbance characterized by a massive redistribution of ions between intracellular and extracellular compartments. A rapid increase in [K+]o and drop in the concentrations of extracellular sodium, chloride, and calcium occur at the onset of SD (Pietrobon and Moskowitz 2014; Somjen 2001). Exactly how all of these ions suddenly become free to run down their concentration gradients is not known, but it is clear that standard voltage- and ligand-gated channels are not involved (Gagolewicz et al. 2016; Muller and Somjen 2000). Membrane repolarization with recovery of neural activity is dependent on the restoration of ionic gradients primarily by the Na+-K+-ATPase and usually occurs within minutes following the eruption of a SD episode (Leão 1944; Marshall 1959; Rodgers et al. 2007; Somjen 2001). It is thought that the onset of the ionic disturbance initiating SD occurs when [K+]o levels exceed a critical threshold and thus waves of SD can be triggered by treatments or conditions that promote the accumulation of extracellular K+. For example, in healthy neural tissue, SD can be experimentally induced by electrical stimulation, high-KCl solutions, or by pharmacological inhibition of the Na+-K+-ATPase (Pietrobon and Moskowitz 2014; Somjen 2001).
SD has been demonstrated in several species, and it can effectively shut down neural function in both the vertebrate and invertebrate central nervous system (CNS). For instance, SD has been implicated in a number of human pathologies and is associated with environmental stress-induced coma in the insect CNS (Pietrobon and Moskowitz 2014; Rodgers et al. 2010). The many similarities that exist between vertebrate and invertebrate SD suggest that the cellular mechanisms underlying the events are the same and so are evolutionarily conserved (Rodgers et al. 2010). The first description of SD occurred over seven decades ago, and, although we understand enough about the ionic mechanisms, and the role of energetics and the Na+-K+-ATPase, to reproduce the main features of SD using mathematical models (Hubel et al. 2014; Hubel et al. 2016; Hubel and Ullah 2016; Wei et al. 2014), it is important to note that there are still many unanswered questions about the phenomenon that arises in complex neural tissue. Given the role that SD plays in human health, elucidating its underlying mechanisms is critically important. Here we review SD mechanisms in both the vertebrate and invertebrate CNS and describe how the use of insect model systems could prove to be a fruitful approach for future investigations.
Occurrence and Relevance of SD in the Vertebrate and Invertebrate CNS
SD was first discovered in the CNS of the rabbit by Leão (1944) who found that electrical stimulation of the cerebral cortex generated a silencing of brain activity that lasted for several minutes. Since the initial report, SD has been demonstrated in a variety of vertebrate species. In the mammalian CNS SD has been described in the mouse, rat, cat, monkey, and human (Fabricius et al. 2006; Gorji et al. 2001; Lauritzen et al. 1982; Van Harreveld A. et al. 1956). Furthermore, it has been shown to occur in the CNS of the frog and turtle and in the retinas of the chicken, toad, and lizard (Guedes et al. 2005; Lauritzen et al. 1988; Martins-Ferreira and de Castro 1966; Streit et al. 1995; Van Harreveld A. 1978). Although SD has been most documented in the vertebrate CNS, its occurrence in the invertebrate nervous system is without question. For instance, SD in the insect CNS was first proposed in the cockroach many years ago (Rounds 1967) and since then has been well characterized in the CNS of Locusta migratoria (Armstrong et al. 2009; Rodgers et al. 2007; Rodgers et al. 2009; Rodgers et al. 2010) and more recently the brain of Drosophila melanogaster (Armstrong et al. 2011; Spong et al. 2016b).
SD occurring in the neocortex of mammals has been extensively studied due to its association with human pathologies such as migraine, stroke, and traumatic brain injury (for reviews, see Dreier 2011; Dreier and Reiffurth 2015; Pietrobon and Moskowitz 2014; Somjen 2001). In the healthy brain SD is benign, but during periods of metabolic compromise the disturbance worsens and, depending on the severity, can lead to irreversible neuronal damage. Recently, an SD continuum of neural pathologies has been described with migraine at the relatively benign end and stroke at the more injurious end of the spectrum (Dreier and Reiffurth 2015). Although benign, waves of SD in the healthy cortex are thought to underlie migraine with aura and thus migraine pain. SD occurring under anoxic conditions [anoxic depolarization (AD)] such as during ischemia in vivo and simulated stroke [oxygen/glucose deprivation (OGD)] in brain slices is often terminal, leading to permanent brain injury (Dreier 2011; Joshi and Andrew 2001; Obeidat and Andrew 1998; Pietrobon and Moskowitz 2014; Somjen 2001). Furthermore, peri-infarct depolarizations (PIDs) are spontaneous and repetitive SD events that originate in the penumbra surrounding the dead tissue resulting from stroke or traumatic brain injury. PIDs are destructive, since they often travel into surrounding well-nourished regions and expand the final infarct volume (Fabricius et al. 2006).
Ischemic (anoxic) SD as studied in rodents is not confined to neocortex, being generated throughout the gray matter of the higher brain (hippocampus, striatum, thalamus, and cerebellar cortex). SD strength, rate of propagation, and onset time in live brain slices is similar across these regions of gray matter exposed to OGD. Likewise, subsequent injury, that is, neuronal swelling with dendritic beading, can arise acutely in each higher region in the wake of SD within tens of seconds. In contrast, impairing the Na+-K+-ATPase using OGD, ouabain, or palytoxin evokes weak and more easily recoverable anoxic SD in the lower brain (hypothalamus and brain stem) of rodents (Brisson et al. 2013; Brisson et al. 2014). Moreover, K+-triggered SD cannot be imaged in hypothalamic nuclei or brain stem with the exception of those nuclei near the dorsal brain stem surface (Andrew et al. 2016). This indicates that SD generation is not a default activity of CNS regions in general and raises the question of with what specific neural function it might be associated.
Invertebrate SD has been best described in the metathoracic ganglion (MTG) of L. migratoria and, over the last several years, characterization of such events demonstrates that invertebrate SD shares many similarities with cortical spreading depression in mammals (see Figs. 1, 2, and 3) (Rodgers et al. 2007, 2010). Due to this similarity and the relatively simple and accessible nature of the insect CNS, locust SD is a useful model for investigations aimed at better understanding mammalian SD (Rodgers et al. 2010). In addition to providing new insights for mammalian SD, insect SD is an interesting and ecologically significant phenomenon in its own right because of its association with stress-induced neural shutdown. For instance, in response to environmentally relevant stressors such as anoxia, hyperthermia, hypothermia, and ATP depletion, many insects enter a reversible coma characterized by immobility and a lack of response to any sensory stimulation. Upon removal of the stress, in most cases there is full recovery of neural and muscular systems (Armstrong et al. 2011, 2012; Dawson-Scully et al. 2010; Haddad 2006; Rodgers et al. 2007). In the locust CNS, it is clear that such stress-induced comas are caused by SD events (for a review, see Rodgers et al. 2010). Furthermore, [K+]o measurements recorded from the brain of D. melanogaster show SD-like increases in [K+]o levels in response to both anoxia and cold temperatures (Armstrong et al. 2011, 2012; Rodriguez and Robertson 2012). Insect SD is apparently a protective mechanism used to conserve energy and cope with severe environmental conditions. Adult Drosophila enter a coma within 30 s in response to anoxia and survive for >8 h in an anoxic environment. In contrast, Drosophila larvae continue to move strongly for 20 min or more when exposed to anoxia but survive less than an hour (Callier et al. 2015). Thus stress-induced comas driven by SD are protective in at least two adult insect species.
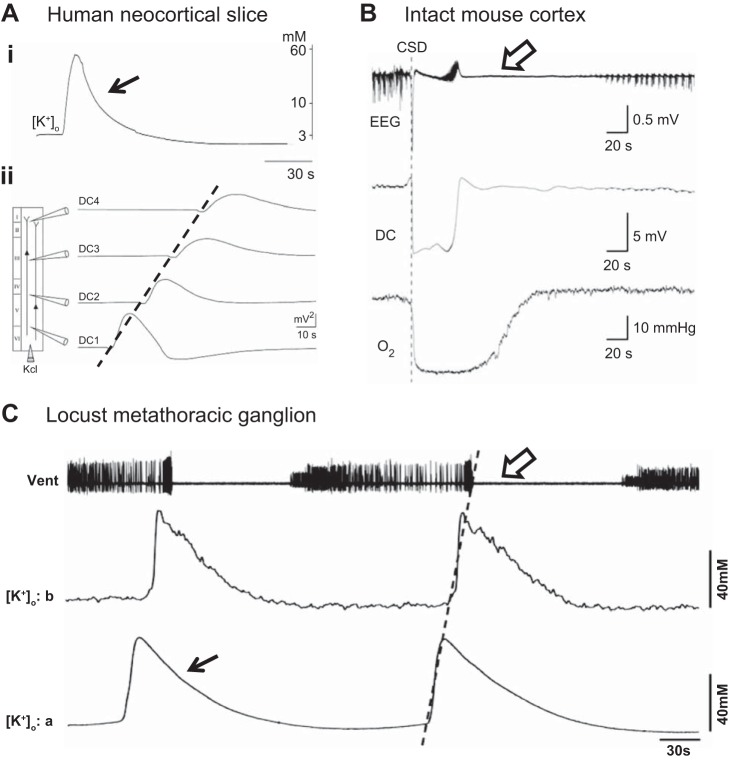
Fig. 1.
A locust model for cortical spreading depression. Ai: changes in extracellular K+ during a spreading depression-like event recorded in a human neocortical slice. Aii: propagating spreading depolarization (SD)-like events evoked by injection of 3 M KCl. Direct current (DC) potential measured at 4 locations in the slice (DC1–DC4). Velocity of propagation was 3.1 mm/min. B: combined measurement of EEG, DC potential, and Po2 during cortical spreading depression (CSD) in intact mouse cortex induced by pressure injection of 1 M KCl at the time of the dotted line. C: spreading depression-like events recorded in the metathoracic ganglion of a locust and induced by injection of 150 mM KCl. Vent, electromyographic recording of ventilatory motor pattern. Velocity of propagation was 1.9 mm/min. Note similarities in extracellular potassium ion concentration ([K+]o) surge (closed arrows), propagation of the event (broken lines), and period of electrical depression (open arrows) in the mammalian and insect preparations. A: adapted from Gorji et al. (2001); B: adapted from Takano et al. (2007); C: from Rodgers et al. (2007), CCBY 4.0, http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0001366.
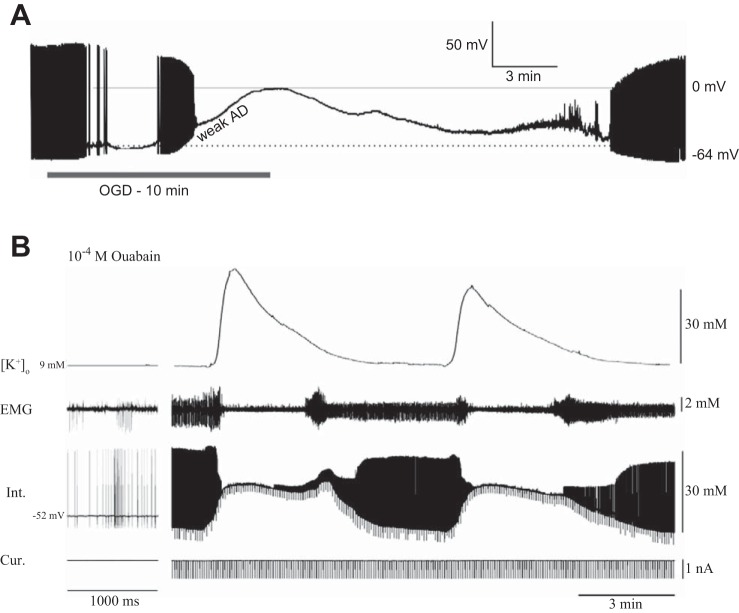
Fig. 2.
A Drosophila model for anoxic depolarization. A: changes in EEG activity, [K+]o, and DC potential in the cortex of a rat following cardiac arrest induced by iv injection of MgCl2 solution (at arrow). B: recording of [K+]o surges in the Drosophila brain during anoxic coma induced by N2 gas. The preparation is shown on the left. An abrupt surge in [K+]o is coincident with a negative DC potential (recorded from the reference electrode). Note the similarities in the recordings from anoxic rat brain and anoxic fly brain (with a difference that the fly brain recovers at the end of the pulse of N2). A: adapted from Hansen (1985); B: adapted from Armstrong et al. (2011), CCBY 4.0, http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0028994.
Fig. 3.
Neuronal responses to SD in rat midbrain and locust ganglion. A: whole cell recording of a midbrain neuron (locus ceruleus) of a rat in response to 10 min of oxygen/glucose deprivation (OGD), which induces a weak anoxic depolarization (weak AD). B: intracellular recording of a metathoracic ventilatory interneuron and [K+]o surges during repetitive SD induced with bath application of 1 mM ouabain. EMG indicates activity of a ventilatory muscle, and the current trace (Cur) indicates repetitive pulses for measurement of neuronal input resistance. Note that the mammalian neuron depolarizes by ∼60 to 0 mV, whereas for 19 insect neurons the membrane potential depolarized by only ∼15 to −35 mV. A: adapted from Brisson et al. (2014), CCBY 4.0, http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0096585; B: adapted from Armstrong et al. (2009).
Mechanisms of Vertebrate and Invertebrate SD
The basic neural tissues and mechanisms of vertebrate and invertebrate nervous systems are equivalent. For instance, both the insect and mammalian CNS are composed of interconnected neurons and glial cells. In both nervous systems, gap junctions are widely expressed, forming direct connections between cell types in addition to providing neuron-neuron and glial-glial cell coupling. Although insect and mammalian gap junctions are formed from different proteins, they are thought to play similar roles in both systems (Baranova et al. 2004; Evans and Martin 2002). Neurons are the main signaling cells; however, glial cells are involved in numerous processes that are critical for healthy neural development and function (Freeman and Doherty 2006; Parpura et al. 2012; Zwarts et al. 2015). Mammalian glial cells have been classified into several subgroups, each associated with different morphology and primary functions (Freeman and Doherty 2006). Insect glial cells are also characterized into subgroups that notably share many morphological and functional traits with their mammalian counterparts (Freeman and Doherty 2006). While the vertebrate and invertebrate CNS is similar in terms of the basic structure and organization, it is no surprise that important differences exist due to fundamental physiological differences related to the generally smaller size of invertebrates. The most obvious distinction is that insects, in contrast to vertebrates, have an open circulatory system and therefore lack a neurovascular component. The absence of a vascular system may be viewed as an advantage, creating a system more accessible than that of mammals; however, it is an important difference to consider, since neural phenomena would occur without the influence of any endothelial modulators. The presence of myelinated axons, which form the white matter of the mammalian CNS, is another distinction. Although myelination is found in some invertebrates, it is absent in insects (Hartline and Colman 2007). In the mammalian brain the majority of excitatory neurotransmission is mediated by ionotropic glutamate receptors, including N-methyl-d-aspartate (NMDA) receptors, a subtype that gates the influx of extracellular Ca2+. NMDA receptor activity has been implicated in both SD initiation and propagation in the mammalian CNS and may be responsible for neuronal death caused by prolonged exposure to anoxic conditions (Aiba and Shuttleworth 2012; Anderson and Andrew 2002; Choi 1992; Iijima et al. 1992; Lauritzen and Hansen 1992; Zhang et al. 2015). In contrast, the main excitatory transmitter in the insect nervous system is thought to be acetylcholine (Oleskevich 1999; Osborne 1996). Nonetheless, NMDA receptors and glutamatergic transmission have been implicated in a number of processes in the insect CNS (Barbara et al. 2005; Robinson et al. 2016; Sombati and Hoyle 1984; Xia et al. 2005), but their relative roles during insect SD have not been investigated. Unlike mammalian neurons, insect neurons can survive for long periods without oxygen supply. A similar anoxia tolerance is exhibited in the western painted turtle (Chrysemys picta), which is dependent on a reduction in NMDA receptor activity (Bickler et al. 2000; Buck and Bickler 1998). Thus, it is reasonable to suggest that differences in anoxia vulnerability, between mammalian and insect neurons, may be due to differences in glutamate transmission and/or NMDA receptor activity.
Electrophysiological properties.
The rapid increase in [K+]o levels at the onset of SD is accompanied with an abrupt negative shift in the extracellular direct current potential, and both measurements are commonly used by experimenters to identify and monitor SD (Fig. 1). [K+]o in the mammalian CNS is maintained at ∼3 mM under normal conditions; however, during an episode of SD, [K+]o increases to 50–60 mM (Muller and Somjen 2000; Vyskocil et al. 1972). The characteristic drop in extracellular direct current potential has an amplitude in the range of 5–30 mV and is a result of the massive depolarization of brain cells that occurs during SD (Marshall 1959; Somjen 2001). Intracellular recordings from neocortical cells demonstrate that mammalian neurons depolarize almost completely during episodes of SD (Muller and Somjen 2000). Glial cells also experience a substantial change in membrane potential; however, their contribution to the massive depolarization characteristic of SD is less than that of neurons (Muller and Somjen 2000; Somjen 2001). SD in the locust MTG is also associated with abrupt surges in [K+]o that can be conveniently monitored using K+-sensitive microelectrodes (Rodgers et al. 2007). The magnitude of the [K+]o disturbance measured during SD events in the locust CNS (∼50 mM) is similar to what is recorded during mammalian SD (Rodgers et al. 2007). Furthermore, the SD-like increase in [K+]o in the Drosophila brain in response to anoxia occurs simultaneously with an abrupt negative shift in extracellular direct current potential consistent with events in mammalian neural tissue (Fig. 2) (Armstrong et al. 2011). Locust ventilatory neurons depolarize during SD. Although the magnitude of the depolarization is less than that described for mammalian neurons, it is still sufficient to cause inactivation of action potential generation and prevent firing (Fig. 3) (Armstrong et al. 2009). The reason for the smaller depolarization of locust neurons is not clear, but it may be one of the reasons why insects can withstand hours of anoxia without suffering permanent damage (Armstrong et al. 2009). Neuronal recordings from both insect and mammalian cells demonstrate that neurons in both systems exhibit a substantial drop in input resistance during SD, which reflects the opening of voltage-gated ion channels (Armstrong et al. 2009; Czeh et al. 1993; Somjen 2001).
Mechanisms of SD initiation.
Electrical stimulation or the application of high-KCl solutions is commonly used for the induction of experimental SD in the vertebrate CNS (Pietrobon and Moskowitz 2014). Additionally, in both hippocampal and cortical rat brain slices, exposure to ouabain, a Na+-K+-ATPase inhibitor, initiates waves of SD (Balestrino et al. 1999; Brisson et al. 2013). The same methods used to experimentally induce SD in mammalian tissue also induce SD in the insect nervous system. For example, injections of high-KCl saline directly in the locust MTG induces single SD events that propagate throughout the neuropil (Rodgers et al. 2007), and bath application of ouabain induces repetitive SD events for which the rise and fall in [K+]o coincide with the arrest and recovery of electrical activity (Rodgers et al. 2009). Likewise, injecting small volumes of either KCl or ouabain directly in the head capsule of the adult fly (D. melanogaster) reliably induces propagating waves of SD within the brain (Spong et al. 2016b). Notably, ouabain-induced SD in the insect CNS resembles the PIDs that occur in mammalian brain tissue following ischemia (Rodgers et al. 2010) in that they recover to baseline.
Similar models of SD generation have been proposed in both the vertebrate and invertebrate CNS where increases in [K+]o over a critical threshold are thought to be a key event in initiating SD (Armstrong et al. 2009; Cestele et al. 2008; Kager et al. 2002; Pietrobon and Moskowitz 2014; Somjen 2002). The build up of extracellular K+ causes neuronal depolarization that leads to the opening of voltage-dependent ion channels, which have not yet been identified (Gagolewicz et al. 2016; Muller and Somjen 2000), further increasing activity and [K+]o, resulting in additional depolarization (activation of a positive feedback system). One of the core features is the balance between mechanisms of K+ accumulation and those of K+ clearance within the relatively small volume of the extracellular space. Onset occurs when mechanisms of K+ accumulation overwhelm the ability to clear excess ions, which ultimately triggers the positive feedback cycle generating the abrupt surge in [K+]o. Thus, initiating stimuli can predispose toward the generation of SD by activating mechanisms of K+ accumulation or by limiting K+ clearance mechanisms (Armstrong et al. 2009). Recovery occurs once mechanisms of K+ clearance are able to predominate, allowing for the restoration of ionic gradients and repolarization of the membrane potential (Armstrong et al. 2009). In support of this model, increases in neural activity in both mammalian and insect nervous systems predispose tissue toward SD, whereas reductions in neural activity are inhibitory. For instance, increasing the oxygen demand of ischemic cortex, by somatosensory activation, reproducibly triggers PIDs within the stimulated region (von Bornstadt et al. 2015). Furthermore, KCl-induced SD in the rodent brain occurs more frequently during periods of sensory stimulation (visual or whisker stimulation) (Bogdanov et al. 2016). In the locust CNS, increased activity, induced by wind stimulation of thoracic circuits, elicited transient increases in [K+]o levels and decreased the latency to onset of the first SD event (Spong et al. 2016a). Furthermore, the activity-induced increases in [K+]o triggered the first SD event in over half of the preparations, suggesting that local activity can determine the origin of successive events (Spong et al. 2016a). On the other hand, blocking synaptic transmission and thereby reducing neural activity completely abolished ouabain-induced SD in 80% of locust preparations (Spong et al. 2016a). In mammalian systems, disruption of glial cell function inhibits synaptic transmission and delays the onset of SD, a process that depends on the release of adenosine in the extracellular space (Canals et al. 2008). Directly administering endogenous adenosine in the rat hippocampus also reduces SD susceptibility (Kaku et al. 1994). Thus, it seems that adenosine modulates SD susceptibility indirectly by reducing activity levels; however, the role of adenosine during insect SD has not yet been investigated. Nonetheless, it appears that increasing the energy demand of neural tissue initiates SD while reducing the energy demand protects against the development of SD.
Propagation and susceptibility.
Once initiated, waves of SD propagate throughout gray matter at a velocity of about 2–9 mm/min, stopping at regions of white matter (Grafstein 1956; Leão 1944; Somjen 2001; Woitzik et al. 2013). SD events propagate throughout the locust MTG (2.4 mm/min) (Rodgers et al. 2007) and fly brain (∼3 mm/min) (Spong et al. 2016b) at strikingly similar rates. Furthermore, whereas locust SD propagates throughout ganglia, which are equivalent to mammalian gray matter, it does not travel through the connectives, which contain axon tracts similar to mammalian white matter (Rodgers et al. 2007). Thus, the presence of myelin likely does not explain the absence of SD in white matter, since insect axons lack myelin altogether (Hartline and Colman 2007). The mechanisms mediating the propagation of SD have not been clearly described. In the vertebrate CNS, intercellular spread through gap junctions has been implicated as a potential mechanism; however, this has yet to be confirmed (Largo et al. 1997b; Nedergaard et al. 1995). It is thought that the SD wave front must be carried by a chemical substance due to the slow spread of the disturbance. Intercellular spread of Ca2+ and diffusion of interstitial K+ or glutamate have all been proposed as probable mechanisms (Grafstein 1956; Somjen 2001; Van Harreveld A. 1959; Vyskocil et al. 1972). Although it is still a matter of debate, recent evidence suggests that diffusion of extracellular K+ is a leading event during SD in both the mammalian and insect CNS (Enger et al. 2015; Spong et al. 2015). This is supported by the observation that Ca2+ channel and glutamate receptor blockers have no effect on SD initiation or propagation (Gagolewicz et al. 2016).
Although vertebrate SD has been demonstrated in almost all gray matter regions of the CNS, the hippocampus and neocortex are areas known to be most susceptible, whereas lower brain regions are more resistant (Brisson et al. 2013; Czeh and Somjen 1990; Somjen 2001). The reason why white matter does not support the propagation of SD or why different areas are more or less susceptible is not fully understood; however, the cytoarchitecture and the number of glial cells in the proximity are likely important factors (Somjen 2001). Moreover, differences in the Na+-K+-ATPase isoforms found in different brain regions may account for region-specific susceptibility, since all isoforms do not pump at the same rate during periods of depolarization (Brisson et al. 2013). Whereas in general neurons express the 1a1 and 1a3 isoforms, astrocytes express 1a2 and 1a4 isoforms (Gottron and Lo 2009). Investigations aimed at studying SD susceptibility are important, since understanding the physiological mechanisms underlying SD resistance could help identify potential targets that may be able to suppress the phenomenon in more vulnerable tissue. Interestingly, susceptibility to SD in the mammalian CNS is also influenced by age. Young rats are more resistant to the development of hypoxic SD compared with mature rats, as evidenced by increased latencies to onset and higher [K+]o threshold levels (Hansen 1977; Isagai et al. 1999; Mares et al. 1976). Furthermore, in newborn rat hippocampal brain slices, hyperthermic SD cannot be reliably induced (Wu and Fisher 2000). A similar age dependence is evident in D. melanogaster. In the presence of ouabain, young flies (4–9 days old) have longer latencies to SD onset, shorter bouts of SD activity, and reduced disturbances in baseline [K+]o levels compared with older individuals (35–39 days old) (Spong et al. 2016b).
Glial mechanisms of K+ homeostasis.
Following a wave of SD, recovery of neural activity is strongly correlated with the restoration of Na+ and K+ gradients (Rodgers et al. 2007). In particular, glial cells are important regulators of the extracellular ionic environment and largely contribute to the maintenance of K+ homeostasis. Mammalian glial cells help restore elevated [K+]o levels following bouts of SD, and disruptions to such mechanisms cause abnormal [K+]o accumulation (D'Ambrosio et al. 1999; Lian and Stringer 2004a). Furthermore, reducing glial cell function in brain slices increases both the susceptibility to SD development and the risk of permanent neuronal damage (Hosoi et al. 2006; Largo et al. 1996, 1997a; Lian and Stringer 2004b). Glial cells are well suited for participation in [K+]o regulation, since they are equipped with a variety of mechanisms allowing for the uptake of excess ions. For example, inwardly rectifying K+ channels found on glial cell membranes increase in conductance when exposed to elevated K+ levels (Newman 1993), and inhibition of this conductance raises [K+]o baseline levels (D'Ambrosio et al. 2002). Glial cells are also equipped with Na+-K+-Cl− cotransporters, which play an important role under ischemic conditions (Chen and Sun 2005; Leis et al. 2005; Walz 1992). Internal mechanisms such as the sequestering of K+ by glial cell mitochondria may also participate in K+ homeostasis (Kozoriz et al. 2010). Furthermore, glial cells are known to be extensively coupled by gap junctions directly connecting the cytoplasm of neighboring cells, creating a spatial buffer for K+ (Orkand et al. 1966). The network allows glia to uptake K+ from areas of elevated [K+]o and quickly redistribute the excess ions to regions of lower [K+]o (Orkand et al. 1966). In mammalian tissue, pharmacological inhibition of gap junctions has been shown to facilitate the initiation of SD and accelerate propagation rates (Tamura et al. 2011). However, opposite trends, whereby gap junction blockade diminished propagation rates and reduced neuronal damage, have also been reported (Frantseva et al. 2002; Nedergaard et al. 1995). This discrepancy could be due to differences in the roles of neuronal vs. glia gap junctions, since the pharmacological agents used are not specific to cell type (Frantseva et al. 2002; Largo et al. 1997b; Theis et al. 2003). Indeed, genetically targeting connexins (proteins that form gap junctions) specifically in astrocytes reduces K+ buffering capabilities and increases rates of SD propagation, suggesting that glial cell gap junctions play a protective role during SD (Theis et al. 2003; Wallraff et al. 2006). In the insect CNS, glial cells also participate in [K+]o regulation. For instance, the insects' blood-brain barrier (BBB) is composed of specialized glial cells (perineurial cells) that are connected to each other and to the deeper layers of glia by gap junctions (Schofield and Treherne 1984; Treherne and Schofield 1981). The BBB is critically involved in regulating the CNS extracellular environment and notably has been shown to actively mediate K+ fluxes (Kocmarek and O'Donnell 2011; Schofield and Treherne 1984; Treherne and Schofield 1981). Furthermore, in response to elevated [K+]o, insect glial cells accumulate K+, demonstrating their ability to clear excess ions from the extracellular space (Coles and Tsacopoulos 1979; Schlue and Wuttke 1983). Consistent with mammalian literature, insect glial cells play a protective role during bouts of SD. For example, pharmacological blockade of gap junctions causes a dramatic impairment in [K+]o regulation and exacerbates ouabain-induced SD in the locust CNS (Spong and Robertson 2013). Moreover, disruption to the locust BBB increases the severity of SD and facilitates propagation rates (Spong et al. 2014).
Extracellular osmolarity and cell swelling.
The extracellular compartment of the CNS is relatively restricted compared with the large intracellular volume, thus providing an environment that is particularly vulnerable to ionic disturbances. Reductions in the size of the extracellular space can heighten the severity of ionic disturbances, since it would facilitate the increase in concentration of extracellular ions due to a reduced volume for dilution (Schwartzkroin et al. 1998). Hypo-osmotic conditions cause acute astrocyte swelling, leading to a reduction in the extracellular space, and prolonged exposure to such conditions can damage cortical brain cells (Andrew et al. 1997, 2007; Risher et al. 2009; Steffensen et al. 2015). Acute hypo-osmotic stress promotes seizures in patients while hyperosmolality reduces cortical excitability (Andrew et al. 1989; Andrew 1991), largely through change in the extracellular volume. However, synaptic strength is also affected (Rosen and Andrew 1990, 1991; Saly and Andrew 1993). Furthermore, hypo-osmotic stress reduces neural recovery following hypoxia (Payne et al. 1996) and can lead to the eruption of spontaneous SDs in rat brain tissue (Chebabo et al. 1995). On the other hand, exposure to hypertonic conditions causes cell shrinkage and is protective against ionic disturbances (Andrew and MacVicar 1994; Balestrino et al. 1999; Huang et al. 1996). For instance, increases in extracellular osmolarity inhibit SDs in rat brain slices (Balestrino et al. 1999; Huang et al. 1996), and both mannitol and hypertonic saline are effective therapies used to treat patients who have suffered from ischemic stroke (Jeon et al. 2014). The volume changes that occur in response to hypo-osmotic and hyperosmotic conditions are likely due to the swelling of astrocytes, since mammalian neurons, in particular cortical pyramidal neurons, do not express functional aquaporin channels (Andrew et al. 2007), and no evidence suggests the contrary for other neuron types. However, during SD, a reduction in the size of the extracellular space is observed, which is largely due to neuronal cell swelling (Zhou et al. 2010), despite the absence of aquaporin channels. Recent evidence suggests that neuronal swelling in metabolically challenged brain tissue involves water influx through Cl−-dependent mechanisms (Hubel and Ullah 2016; Rungta et al. 2015; Steffensen et al. 2015). For example, SD-induced dendritic beading (cell swelling) of pyramidal neurons is reduced by the inhibition of Cl− cotransporters (Steffensen et al. 2015). Computational modeling experiments also suggest that cell volume changes, during SD, are dependent on the opening and closing of anion channels (Hubel and Ullah 2016). The extent of neuronal and glial cell swelling during insect SD has yet to be determined. Nevertheless, changes in cell volume regulation and their effects on SD severity have been investigated. In locusts, hypotonic-induced cell swelling increases susceptibility to ouabain-induced SD, evidenced by a decrease in the latency to onset and period between individual events (Spong et al. 2015). Furthermore, the magnitude of [K+]o disturbance is greater and individual SD events propagate at higher velocities under hypotonic conditions compared with hypertonic conditions (Spong et al. 2015). This exacerbation of SD under hypotonic conditions is consistent with mammalian literature; however, it is important to note that the role and distribution (neuronal vs. glia) of water-permeable channels in the insect CNS is less established.
Insect SD as a Model for Mammalian SD
The use of invertebrate model systems to study human disease is by no means novel. Particularly, both the common fruit fly (D. melanogaster) and the nematode (Caenorhabditis elegans) have emerged as valuable model systems commonly used in the biomedical field (Jeibmann and Paulus 2009; Kaletta and Hengartner 2006; Pandey and Nichols 2011). The use of these model organisms has obvious advantages such as accessibility, their short life span, and genetic tractability. They allow for detailed experimental designs encompassing a wide range of sophisticated techniques. Experiments can be performed on a large scale with high-throughput assays and are cost effective. Such insect model systems have provided insights for both therapeutic drug discovery and for mechanisms underlying a variety of human diseases, including, but not limited to, Alzheimer's disease, Parkinson's disease, cancer, and diabetes (Jeibmann and Paulus 2009; Kaletta and Hengartner 2006; Pandey and Nichols 2011). Clearly, experimentation using insect systems can generate clinically relevant data.
Vertebrate SD has been extensively studied in a variety of systems since its initial discovery in 1944, largely due to its implication in human pathology (Dreier 2011; Dreier and Reiffurth 2015; Leão 1944; Pietrobon and Moskowitz 2014). Nevertheless, despite vigorous research efforts, there are still many things we do not know. For example, we have a limited understanding of the triggers of SD and the relative roles of glia and neurons in facilitating or limiting the spread of the ionic disturbance. Also, we have very little knowledge of how intrinsic evolutionarily conserved cellular signaling pathways might modulate the susceptibility and vulnerability of the brain to SD. Characterization of SD in the invertebrate CNS, specifically in the MTG of L. migratoria and the brain of D. melanogaster, began relatively recently (less than a decade ago), but it is now clear that insect SD bears all the hallmarks of mammalian SD. As discussed below, both the locust and fly systems possess unique attributes that have helped generate interesting results that are likely relevant to vertebrate neurobiology. We propose that continued use of these insect model systems could help expedite the process of designing therapeutic treatments aimed at mitigating the negative consequences associated with mammalian SD.
L. migratoria.
It has been previously proposed that SD within the locust MTG, particularly ouabain-induced SD (due to the similarity it shares with PIDs), could prove to be a useful model for mammalian SD (Rodgers et al. 2010). Locust dissections can be performed quickly, and the resulting semi-intact preparation exposes the thoracic nervous system, allowing for measurements to be taken from within the MTG while simultaneously monitoring vital neuronal circuits such as the ventilatory central pattern generator (Armstrong et al. 2009; Robertson and Pearson 1982; Rodgers et al. 2007). SD can be reliably induced, and at the same time pharmacological agents, targeting specific processes, can be easily administered to the entire CNS. Not only can SD be conveniently induced and monitored within the locust MTG but it can also be manipulated by pharmacologically targeting evolutionarily conserved pathways, suggesting that similar relationships may exist in other animals, including mammals (Armstrong et al. 2009; Rodgers-Garlick et al. 2011). For example, blockade of the nitric oxide-cyclic guanosine monophosphate-protein kinase G (PKG) pathway suppresses locust SD, whereas activation of the pathway increases the severity of the ionic disturbance (Fig. 4A) (Armstrong et al. 2009). Similarly, ouabain-induced SD is attenuated following pharmacological inhibition of the AMP-activated protein kinase, a sensor of cellular energy status, and is exacerbated by its activation (Rodgers-Garlick et al. 2011). SD events in the locust MTG can also be manipulated using pretreatment methods. Prior exposure of the locust CNS to tetraethylammonium, a blocker of voltage-gated K+ channels, delays the onset of SD, reduces the amplitude of the individual [K+]o events, and decreases propagation rates (Rodgers et al. 2007, 2009; Spong et al. 2015). Treatment with the voltage-gated Na+ channel inhibitor tetrodotoxin also increases the latency to SD-like ionic disturbances but does not affect the magnitude of the associated surges in [K+]o (Rodgers et al. 2007). Inhibition of the Na+-K+-2Cl− cotransporter with bumetanide causes a build up of extracellular K+ and facilitates SD propagation (Spong et al. 2015). Furthermore, subjecting locusts to a prior heat shock treatment (3 h at 45°C) increases both the failure temperature of hyperthermia-induced SD-like events and the rates of K+ clearance compared with control preparations (Rodgers et al. 2007). The increase in K+ clearance is partly due to heat shock-induced trafficking of the Na+-K+-ATPase in neuronal plasma membranes (Hou et al. 2014).
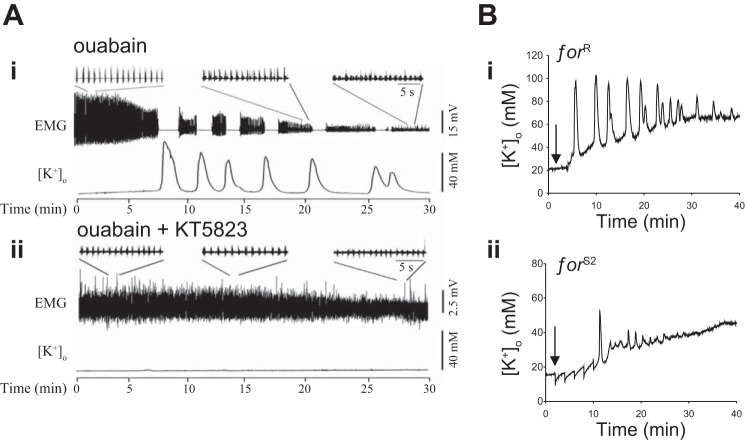
Fig. 4.
Reduction of protein kinase G (PKG) activity mitigates the severity of insect SD. A: pharmacological inhibition of PKG using KT-5823 prevents ouabain-induced SD. Central nervous system (CNS) operation is represented by the EMG monitor of the ventilatory central pattern generator (CPG). [K+]o is monitored in the locust metathoracic ganglion during bath application of ouabain. i, Ouabain-induced SD; ii, inhibition with KT-5823. B: genetic reduction of PKG activity using foraging gene mutants in Drosophila reduces ouabain-induced SD. [K+]o is monitored in the brain. Ouabain is injected at arrow. i, SD in rover flies with high levels of PKG activity; ii, SD in sitter mutant flies in a rover genetic background with low levels of PKG activity. A: adapted from Armstrong et al. (2009); B: adapted from Spong et al. (2016b).
D. melanogaster.
It is without question that pharmacological studies can generate interesting and significant results; indeed, most of our mechanistic understanding of SD today stems from such experiments. However, given the complexity of the phenomenon and the lack of specificity of many pharmacological agents, new experimental approaches to study SD are warranted. The demonstration of SD in the brain of D. melanogaster is noteworthy, since it provides a new model system to genetically dissect mechanisms of SD and their tissue specificity. In the Drosophila brain, SD in response to anoxia (AD) is indistinguishable from events in the mammalian brain induced by cardiac arrest (Fig. 2) (Armstrong et al. 2011; Hansen 1985). Also, the Drosophila brain supports repetitive SD induced by ouabain treatment or by application of KCl (Spong et al. 2016b). Notably, these manifestations described in Drosophila are along the SD continuum described in mammals (Dreier and Reiffurth 2015), confirming that the general phenomenon can now be investigated using the sophisticated molecular genetic toolbox available for Drosophila. In addition to recording SD from the brain of individual flies, whole animal behavioral assays can be used as well, allowing for large-scale and high-throughput screening of genetic strains identifying flies more or less resistant to anoxia. Genetic strains of interest could then be subsequently tested in more detail (electrophysiological experiments). Although investigations using the ouabain model have just begun, importantly, its susceptibility to genetic manipulation has been clearly demonstrated. For instance, flies lacking the white gene (w1118 mutants), which encodes an ATP-binding cassette transporter, are more vulnerable to ouabain-induced SD compared with wild-type (Canton S) flies, evidenced by shorter latencies to SD onset and longer bouts of SD activity (Spong et al. 2016b). Whether or not this difference in ouabain sensitivity is due to the white gene (rather than genetic background) is not clear; however, it is a reasonable suggestion based on recent findings. For example, w1118 mutants take longer to recover from whole animal anoxic comas compared with wild-type individuals, a phenotype shown to be directly caused by the lack of the white gene (Xiao and Robertson 2016). Nonetheless, differences between w1118 and Canton S individuals are important, since they are both commonly used control strains and many transgenic fly lines are created using the w1118 fly background. Differences in SD susceptibility have also been reported between fly strains that share the same genetic background but differ in their levels of PKG activity. Flies with low levels of PKG activity are more resistant to both ouabain-induced SD and the more severe AD compared with flies with higher levels (Fig. 4B) (Spong et al. 2016b). These results are consistent with earlier work showing that pharmacological inhibition of the PKG pathway attenuates ouabain-induced SD in the locust CNS (Armstrong et al. 2009). Together these results indicate that the PKG signaling pathway plays an important role during insect SD, and given the conserved nature of the pathway suggest it may play a similar role during mammalian SD.
Conclusion
Due to the number of humans who suffer from neural pathologies involving the SD continuum, it is critical that we continue to advance our knowledge of the phenomenon. Despite obvious differences in the design of the vertebrate and invertebrate CNS, the general cellular mechanisms underlying SD seem to be conserved. Insect model systems provide a relatively new and alternate approach to study mechanisms of SD and come with many research benefits that higher vertebrate model systems cannot offer. In particular, extending the demonstration of SD to the brain of Drosophila is paramount, since it equips us with a host of new methodologies that will allow us to address specific questions on a tissue-specific level. In addition, these experiments could be accompanied with cell-level recordings, providing novel insights for how SD initiation and propagation exactly take place. In conclusion, we propose that taking advantage of insect model systems will not only help provide answers to unknown questions but may also help identify molecular targets to mitigate brain susceptibility to SD.
GRANTS
This work was funded by the Natural Sciences and Engineering Research Council of Canada (R. M. Robertson) and the Heart and Stroke Foundation of Canada (R. D. Andrew).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.E.S. drafted manuscript; K.E.S., R.D.A., and R.M.R. edited and revised manuscript; K.E.S., R.D.A., and R.M.R. approved final version of manuscript.
REFERENCES
- Aiba I, Shuttleworth CW. Sustained NMDA receptor activation by spreading depolarizations can initiate excitotoxic injury in metabolically compromised neurons. J Physiol 590: 5877–5893, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TR, Andrew RD. Spreading depression: imaging and blockade in the rat neocortical brain slice. J Neurophysiol 88: 2713–2725, 2002. [DOI] [PubMed] [Google Scholar]
- Andrew RD. Seizure and acute osmotic change: clinical and neurophysiological aspects. J Neurol Sci 101: 7–18, 1991. [DOI] [PubMed] [Google Scholar]
- Andrew RD, Fagan M, Ballyk BA, Rosen AS. Seizure susceptibility and the osmotic state. Brain Res 498: 175–180, 2989. [DOI] [PubMed] [Google Scholar]
- Andrew RD, Hsieh YT, Brisson CD. Spreading depolarization triggered by elevated potassium is weak or absent in the rodent lower brain. J Cereb Blood Flow Metab In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew RD, Labron MW, Boehnke SE, Carnduff L, Kirov SA. Physiological evidence that pyramidal neurons lack functional water channels. Cereb Cortex 17: 787–802, 2007. [DOI] [PubMed] [Google Scholar]
- Andrew RD, Lobinowich ME, Osehobo EP. Evidence against volume regulation by cortical brain cells during acute osmotic stress. Exp Neurol 143: 300–312, 1997. [DOI] [PubMed] [Google Scholar]
- Andrew RD, MacVicar BA. Imaging cell volume changes and neuronal excitation in the hippocampal slice. Neuroscience 62: 371–383, 1994. [DOI] [PubMed] [Google Scholar]
- Armstrong GA, Rodgers CI, Money TG, Robertson RM. Suppression of spreading depression-like events in locusts by inhibition of the NO/cGMP/PKG pathway. J Neurosci 29: 8225–8235, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong GA, Rodriguez EC, Meldrum RR. Cold hardening modulates K+ homeostasis in the brain of Drosophila melanogaster during chill coma. J Insect Physiol 58: 1511–1516, 2012. [DOI] [PubMed] [Google Scholar]
- Armstrong GA, Xiao C, Krill JL, Seroude L, Dawson-Scully K, Robertson RM. Glial Hsp70 protects K+ homeostasis in the Drosophila brain during repetitive anoxic depolarization. PLoS One 6: e28994, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrino M, Young J, Aitken P. Block of (Na+,K+)ATPase with ouabain induces spreading depression-like depolarization in hippocampal slices. Brain Res 838: 37–44, 1999. [DOI] [PubMed] [Google Scholar]
- Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 83: 706–716, 2004. [DOI] [PubMed] [Google Scholar]
- Barbara GS, Zube C, Rybak J, Gauthier M, Grunewald B. Acetylcholine, GABA and glutamate induce ionic currents in cultured antennal lobe neurons of the honeybee, Apis mellifera. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 191: 823–836, 2005. [DOI] [PubMed] [Google Scholar]
- Bickler PE, Donohoe PH, Buck LT. Hypoxia-induced silencing of NMDA receptors in turtle neurons. J Neurosci 20: 3522–3528, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov VB, Middleton NA, Theriot JJ, Parker PD, Abdullah OM, Ju YS, Hartings JA, Brennan KC. Susceptibility of primary sensory cortex to spreading depolarizations. J Neurosci 36: 4733–4743, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson CD, Hsieh YT, Kim D, Jin AY, Andrew RD. Brainstem neurons survive the identical ischemic stress that kills higher neurons: insight to the persistent vegetative state. PLoS One 9: e96585, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson CD, Lukewich MK, Andrew RD. A distinct boundary between the higher brain's susceptibility to ischemia and the lower brain's resistance. PLoS One 8: e79589, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck LT, Bickler PE. Adenosine and anoxia reduce N-methyl-d-aspartate receptor open probability in turtle cerebrocortex. J Exp Biol 201: 289–297, 1998. [DOI] [PubMed] [Google Scholar]
- Callier V, Hand SC, Campbell JB, Biddulph T, Harrison JF. Developmental changes in hypoxic exposure and responses to anoxia in Drosophila melanogaster. J Exp Biol 218: 2927–2934, 2015. [DOI] [PubMed] [Google Scholar]
- Canals S, Larrosa B, Pintor J, Mena MA, Herreras O. Metabolic challenge to glia activates an adenosine-mediated safety mechanism that promotes neuronal survival by delaying the onset of spreading depression waves. J Cereb Blood Flow Metab 28: 1835–1844, 2008. [DOI] [PubMed] [Google Scholar]
- Cestele S, Scalmani P, Rusconi R, Terragni B, Franceschetti S, Mantegazza M. Self-limited hyperexcitability: functional effect of a familial hemiplegic migraine mutation of the Nav1.1 (SCN1A) Na+ channel. J Neurosci 28: 7273–7283, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebabo SR, Hester MA, Aitken PG, Somjen GG. Hypotonic exposure enhances synaptic transmission and triggers spreading depression in rat hippocampal tissue slices. Brain Res 695: 203–216, 1995. [DOI] [PubMed] [Google Scholar]
- Chen H, Sun D. The role of Na-K-Cl co-transporter in cerebral ischemia. Neurol Res 27: 280–286, 2005. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol 23: 1261–1276, 1992. [DOI] [PubMed] [Google Scholar]
- Coles JA, Tsacopoulos M. Potassium activity in photoreceptors, glial cells and extracellular space in the drone retina: changes during photostimulation. J Physiol 290: 525–549, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh G, Aitken PG, Somjen GG. Membrane currents in CA1 pyramidal cells during spreading depression (SD) and SD-like hypoxic depolarization. Brain Res 632: 195–208, 1993. [DOI] [PubMed] [Google Scholar]
- Czeh G, Somjen GG. Hypoxic failure of synaptic transmission in the isolated spinal cord, and the effects of divalent cations. Brain Res 527: 224–233, 1990. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R, Gordon DS, Winn HR. Differential role of KIR channel and Na+/K+-pump in the regulation of extracellular K+ in rat hippocampus. J Neurophysiol 87: 87–102, 2002. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Impaired K+ homeostasis and altered electrophysiological properties of post-traumatic hippocampal glia. J Neurosci 19: 8152–8162, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson-Scully K, Bukvic D, Chakaborty-Chatterjee M, Ferreira R, Milton SL, Sokolowski MB. Controlling anoxic tolerance in adult Drosophila via the cGMP-PKG pathway. J Exp Biol 213: 2410–2416, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 17: 439–447, 2011. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Reiffurth C. The stroke-migraine depolarization continuum. Neuron 86: 902–922, 2015. [DOI] [PubMed] [Google Scholar]
- Enger R, Tang W, Vindedal GF, Jensen V, Johannes HP, Sprengel R, Looger LL, Nagelhus EA. Dynamics of ionic shifts in cortical spreading depression. Cereb Cortex 25: 4469–4476, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WH, Martin PE. Gap junctions: structure and function (Review). Mol Membr Biol 19: 121–136, 2002. [DOI] [PubMed] [Google Scholar]
- Fabricius M, Fuhr S, Bhatia R, Boutelle M, Hashemi P, Strong AJ, Lauritzen M. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain 129: 778–790, 2006. [DOI] [PubMed] [Google Scholar]
- Frantseva MV, Kokarovtseva L, Perez Velazquez JL. Ischemia-induced brain damage depends on specific gap-junctional coupling. J Cereb Blood Flow Metab 22: 453–462, 2002. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Doherty J. Glial cell biology in Drosophila and vertebrates. Trends Neurosci 29: 82–90, 2006. [DOI] [PubMed] [Google Scholar]
- Gagolewicz P, Tresidder K, Andrew RD. Still unidentified: The channel driving spreading depolarization during ischemia. Soc Neurosci Ann Meet 2016. [Google Scholar]
- Gorji A, Scheller D, Straub H, Tegtmeier F, Kohling R, Hohling JM, Tuxhorn I, Ebner A, Wolf P, Werner PH, Oppel F, Speckmann EJ. Spreading depression in human neocortical slices. Brain Res 906: 74–83, 2001. [DOI] [PubMed] [Google Scholar]
- Gottron MA, Lo DC. The Na+/K+-ATPase as a drug target for ischemic stroke. In: New Strategies in Stroke Intervention, edited by Annunziato L. New York, NY: Humana, 2009, p. 129–151. [Google Scholar]
- Grafstein B. Mechanism of spreading cortical depression. J Neurophysiol 19: 154–171, 1956. [DOI] [PubMed] [Google Scholar]
- Guedes RC, Tsurudome K, Matsumoto N. Spreading depression in vivo potentiates electrically-driven responses in frog optic tectum. Brain Res 1036: 109–114, 2005. [DOI] [PubMed] [Google Scholar]
- Haddad GG. Tolerance to low O2: lessons from invertebrate genetic models. Exp Physiol 91: 277–282, 2006. [DOI] [PubMed] [Google Scholar]
- Hansen AJ. Extracellular potassium concentration in juvenile and adult rat brain cortex during anoxia. Acta Physiol Scand 99: 412–420, 1977. [DOI] [PubMed] [Google Scholar]
- Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev 65: 101–148, 1985. [DOI] [PubMed] [Google Scholar]
- Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol 17: R29–R35, 2007. [DOI] [PubMed] [Google Scholar]
- Hosoi R, Kashiwagi Y, Hatazawa J, Gee A, Inoue O. Glial metabolic dysfunction caused neural damage by short-term ischemia in brain. Ann Nucl Med 20: 377–380, 2006. [DOI] [PubMed] [Google Scholar]
- Hou N, Armstrong GA, Chakraborty-Chatterjee M, Sokolowski MB, Robertson RM. Na+-K+-ATPase trafficking induced by heat shock pretreatment correlates with increased resistance to anoxia in locusts. J Neurophysiol 112: 814–823, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Aitken PG, Somjen GG. Hypertonic environment prevents depolarization and improves functional recovery from hypoxia in hippocampal slices. J Cereb Blood Flow Metab 16: 462–467, 1996. [DOI] [PubMed] [Google Scholar]
- Hubel N, Andrew RD, Ullah G. Large extracellular space leads to neuronal susceptibility to ischemic injury in a Na+/K+ pumps-dependent manner. J Comput Neurosci 40: 177–192, 2016. [DOI] [PubMed] [Google Scholar]
- Hubel N, Scholl E, Dahlem MA. Bistable dynamics underlying excitability of ion homeostasis in neuron models. PLoS Comput Biol 10: e1003551, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel N, Ullah G. Anions govern cell volume: a case study of relative astrocytic and neuronal swelling in spreading depolarization. PLoS One 11: e0147060, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima T, Mies G, Hossmann KA. Repeated negative DC deflections in rat cortex following middle cerebral artery occlusion are abolished by MK-801: effect on volume of ischemic injury. J Cereb Blood Flow Metab 12: 727–733, 1992. [DOI] [PubMed] [Google Scholar]
- Isagai T, Fujimura N, Tanaka E, Yamamoto S, Higashi H. Membrane dysfunction induced by in vitro ischemia in immature rat hippocampal CA1 neurons. J Neurophysiol 81: 1866–1871, 1999. [DOI] [PubMed] [Google Scholar]
- Jeibmann A, Paulus W. Drosophila melanogaster as a model organism of brain diseases. Int J Mol Sci 10: 407–440, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SB, Koh Y, Choi HA, Lee K. Critical care for patients with massive ischemic stroke. J Stroke 16: 146–160, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi I, Andrew RD. Imaging anoxic depolarization during ischemia-like conditions in the mouse hemi-brain slice. J Neurophysiol 85: 414–424, 2001. [DOI] [PubMed] [Google Scholar]
- Kager H, Wadman WJ, Somjen GG. Conditions for the triggering of spreading depression studied with computer simulations. J Neurophysiol 88: 2700–2712, 2012. [DOI] [PubMed] [Google Scholar]
- Kaku T, Hada J, Hayashi Y. Endogenous adenosine exerts inhibitory effects upon the development of spreading depression and glutamate release induced by microdialysis with high K+ in rat hippocampus. Brain Res 658: 39–48, 1994. [DOI] [PubMed] [Google Scholar]
- Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov 5: 387–398, 2006. [DOI] [PubMed] [Google Scholar]
- Kocmarek AL, O'Donnell MJ. Potassium fluxes across the blood brain barrier of the cockroach, Periplaneta americana. J Insect Physiol 57: 127–135, 2011. [DOI] [PubMed] [Google Scholar]
- Kozoriz MG, Church J, Ozog MA, Naus CC, Krebs C. Temporary sequestration of potassium by mitochondria in astrocytes. J Biol Chem 285: 31107–31119, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largo C, Cuevas P, Somjen GG, Martin del RR, Herreras O. The effect of depressing glial function in rat brain in situ on ion homeostasis, synaptic transmission, and neuron survival. J Neurosci 16: 1219–1229, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largo C, Ibarz JM, Herreras O. Effects of the gliotoxin fluorocitrate on spreading depression and glial membrane potential in rat brain in situ. J Neurophysiol 78: 295–307, 1997a. [DOI] [PubMed] [Google Scholar]
- Largo C, Tombaugh GC, Aitken PG, Herreras O, Somjen GG. Heptanol but not fluoroacetate prevents the propagation of spreading depression in rat hippocampal slices. J Neurophysiol 77: 9–16, 1997b. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Hansen AJ. The effect of glutamate receptor blockade on anoxic depolarization and cortical spreading depression. J Cereb Blood Flow Metab 12: 223–229, 1992. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Jorgensen MB, Diemer NH, Gjedde A, Hansen AJ. Persistent oligemia of rat cerebral cortex in the wake of spreading depression. Ann Neurol 12: 469–474, 1982. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Rice ME, Okada Y, Nicholson C. Quisqualate, kainate and NMDA can initiate spreading depression in the turtle cerebellum. Brain Res 475: 317–327, 1988. [DOI] [PubMed] [Google Scholar]
- Leão AA. Spreading depression of activity in the cerebral cortex. J Neurophysiol 7: 359–390, 1944. [DOI] [PubMed] [Google Scholar]
- Leis JA, Bekar LK, Walz W. Potassium homeostasis in the ischemic brain. Glia 50: 407–416, 2005. [DOI] [PubMed] [Google Scholar]
- Lian XY, Stringer JL. Astrocytes contribute to regulation of extracellular calcium and potassium in the rat cerebral cortex during spreading depression. Brain Res 1012: 177–184, 2004a. [DOI] [PubMed] [Google Scholar]
- Lian XY, Stringer JL. Energy failure in astrocytes increases the vulnerability of neurons to spreading depression. Eur J Neurosci 19: 2446–2454, 2004b. [DOI] [PubMed] [Google Scholar]
- Mares P, Kriz N, Brozek G, Bures J. Anoxic changes of extracellular potassium concentration in the cerebral cortex of young rats. Exp Neurol 53: 12–20, 1976. [DOI] [PubMed] [Google Scholar]
- Marshall WH. Spreading cortical depression of Leão. Physiol Rev 39: 239–279, 1959. [DOI] [PubMed] [Google Scholar]
- Martins-Ferreira H, de Castro GO. Light-scattering changes accompanying spreading depression in isolated retina. J Neurophysiol 29: 715–726, 1966. [DOI] [PubMed] [Google Scholar]
- Money TG, Rodgers CI, McGregor SM, Robertson RM. Loss of potassium homeostasis underlies hyperthermic conduction failure in control and preconditioned locusts. J Neurophysiol 102: 285–293, 2009. [DOI] [PubMed] [Google Scholar]
- Muller M, Somjen GG. Na+ and K+ concentrations, extra- and intracellular voltages, and the effect of TTX in hypoxic rat hippocampal slices. J Neurophysiol 83: 735–745, 2000. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Cooper AJL, Goldman SA. Gap junctions are required for the propagation of spreading depression. J Neurobiol 28: 433–444, 1995. [DOI] [PubMed] [Google Scholar]
- Newman EA. Inward-rectifying potassium channels in retinal glial (Muller) cells. J Neurosci 13: 3333–3345, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeidat AS, Andrew RD. Spreading depression determines acute cellular damage in the hippocampal slice during oxygen/glucose deprivation. Eur J Neurosci 10: 3451–3461, 1998. [DOI] [PubMed] [Google Scholar]
- Oleskevich S. Cholinergic synaptic transmission in insect mushroom bodies in vitro. J Neurophysiol 82: 1091–1096, 1999. [DOI] [PubMed] [Google Scholar]
- Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol 29: 788–806, 1966. [DOI] [PubMed] [Google Scholar]
- Osborne RH. Insect neurotransmission: neurotransmitters and their receptors. Pharmacol Ther 69: 117–142, 1996. [DOI] [PubMed] [Google Scholar]
- Pandey UB, Nichols CD. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol Rev 63: 411–436, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A. Glial cells in (patho)physiology. J Neurochem 121: 4–27, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RS, Schurr A, Rigor BM. Cell swelling exacerbates hypoxic neuronal damage in rat hippocampal slices. Brain Res 723: 210–213, 1996. [DOI] [PubMed] [Google Scholar]
- Pietrobon D, Moskowitz MA. Chaos and commotion in the wake of cortical spreading depression and spreading depolarizations. Nat Rev Neurosci 15: 379–393, 2014. [DOI] [PubMed] [Google Scholar]
- Risher WC, Andrew RD, Kirov SA. Real-time passive volume responses of astrocytes to acute osmotic and ischemic stress in cortical slices and in vivo revealed by two-photon microscopy. Glia 57: 207–221, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RM, Pearson KG. A preparation for the intracellular analysis of neuronal activity during flight in the locust. J Comp Physiol 146: 311–320, 1982. [Google Scholar]
- Robinson JE, Paluch J, Dickman DK, Joiner WJ. ADAR-mediated RNA editing suppresses sleep by acting as a brake on glutamatergic synaptic plasticity. Nat Commun 7: 10512, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers CI, Armstrong GA, Robertson RM. Coma in response to environmental stress in the locust: a model for cortical spreading depression. J Insect Physiol 56: 980–990, 2010. [DOI] [PubMed] [Google Scholar]
- Rodgers CI, Armstrong GA, Shoemaker KL, Labrie JD, Moyes CD, Robertson RM. Stress preconditioning of spreading depression in the locust CNS. PLoS One 2: e1366, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers CI, Labrie JD, Robertson RM. K+ homeostasis and central pattern generation in the metathoracic ganglion of the locust. J Insect Physiol 55: 599–607, 2009. [DOI] [PubMed] [Google Scholar]
- Rodgers-Garlick CI, Armstrong GA, Robertson RM. Metabolic stress modulates motor patterning via AMP-activated protein kinase. J Neurosci 31: 3207–3216, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez EC, Robertson RM. Protective effect of hypothermia on brain potassium homeostasis during repetitive anoxia in Drosophila melanogaster. J Exp Biol 215: 4157–4165, 2012. [DOI] [PubMed] [Google Scholar]
- Rosen AS, Andrew RD. Osmotic effects upon excitability in rat neocortical slices. Neuroscience 38: 579–590, 1990. [DOI] [PubMed] [Google Scholar]
- Rosen AS, Andrew RD. Glucose concentration inversely alters neocortical slice excitability through an osmotic effect. Brain Res 555: 58–64, 1991. [DOI] [PubMed] [Google Scholar]
- Rounds HD. KCl-induced ‘spreading depression’ in the cockroach. J Insect Physiol 13: 869–872, 1967. [DOI] [PubMed] [Google Scholar]
- Rungta RL, Choi HB, Tyson JR, Malik A, Dissing-Olesen L, Lin PJ, Cain SM, Cullis PR, Snutch TP, MacVicar BA. The cellular mechanisms of neuronal swelling underlying cytotoxic edema. Cell 161: 610–621, 2015. [DOI] [PubMed] [Google Scholar]
- Saly V, Andrew RD. CA3 neuron excitation and epileptiform discharge are sensitive to osmolality. J Neurophysiol 69: 2200–2208, 1993. [DOI] [PubMed] [Google Scholar]
- Schlue WR, Wuttke W. Potassium activity in leech neuropile glial cells changes with external potassium concentration. Brain Res 270: 368–372, 1983. [DOI] [PubMed] [Google Scholar]
- Schofield PK, Treherne JE. Localization of the blood-brain barrier of an insect: Electrical model and analysis. J Exp Biol 109: 319–331, 1984. [Google Scholar]
- Schwartzkroin PA, Baraban SC, Hochman DW. Osmolarity, ionic flux, and changes in brain excitability. Epilepsy Res 32: 275–285, 1998. [DOI] [PubMed] [Google Scholar]
- Sombati S, Hoyle G. Glutamatergic central nervous transmission in locusts. J Neurobiol 15: 507–516, 1984. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev 81: 1065–1096, 2001. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Ion regulation in the brain: implications for pathophysiology. Neuroscientist 8: 254–267, 2002. [DOI] [PubMed] [Google Scholar]
- Spong KE, Chin B, Witiuk KL, Robertson RM. Cell swelling increases the severity of spreading depression in Locusta migratoria. J Neurophysiol 114: 3111–3120, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spong KE, Mazzetti TR, Robertson RM. Activity dependence of spreading depression in the locust CNS. J Exp Biol 219: 626–630, 2016a. [DOI] [PubMed] [Google Scholar]
- Spong KE, Robertson RM. Pharmacological blockade of gap junctions induces repetitive surging of extracellular potassium within the locust CNS. J Insect Physiol 59: 1031–1040, 2013. [DOI] [PubMed] [Google Scholar]
- Spong KE, Rochon-Terry G, Money TG, Robertson RM. Disruption of the blood-brain barrier exacerbates spreading depression in the locust CNS. J Insect Physiol 66: 1–9, 2014. [DOI] [PubMed] [Google Scholar]
- Spong KE, Rodriguez EC, Robertson RM. Spreading depolarization in the brain of Drosophila is induced by inhibition of the Na+/K+-ATPase. J Neurophysiol doi: 10.1152/jn.00353.2016, 2016b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen AB, Sword J, Croom D, Kirov SA, MacAulay N. Chloride cotransporters as a molecular mechanism underlying spreading depolarization-induced dendritic beading. J Neurosci 35: 12172–12187, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit DS, Ferreira Filho CR, Martins-Ferreira H. Spreading depression in isolated spinal cord. J Neurophysiol 74: 888–890, 1995. [DOI] [PubMed] [Google Scholar]
- Tamura K, Alessandri B, Heimann A, Kempski O. The effect of a gap-junction blocker, carbenoxolone, on ischemic brain injury and cortical spreading depression. Neuroscience 194: 262–271, 2011. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Lovatt D, Hansen AJ, Kasischke KA, Nedergaard M. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci 10: 754–762, 2007. [DOI] [PubMed] [Google Scholar]
- Theis M, Jauch R, Zhuo L, Speidel D, Wallraff A, Doring B, Frisch C, Sohl G, Teubner B, Euwens C, Huston J, Steinhauser C, Messing A, Heinemann U, Willecke K. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J Neurosci 23: 766–776, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treherne JE, Schofield PK. Mechanisms of ionic homeostasis in the central nervous system of an insect. J Exp Biol 95: 61–73, 1981. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A. Compounds in brain extracts causing spreading depression of cerebral cortical activity and contraction of crustacean muscle. J Neurochem 3: 300–315, 1959. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A. Two mechanisms for spreading depression in the chicken retina. J Neurobiol 9: 419–431, 1978. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A, Stamm JS, Christensen E. Spreading depression in rabbit, cat and monkey. Am J Physiol 184: 312–320, 1956. [DOI] [PubMed] [Google Scholar]
- von Bornstadt D, Houben T, Seidel JL, Zheng Y, Dilekoz E, Qin T, Sandow N, Kura S, Eikermann-Haerter K, Endres M, Boas DA, Moskowitz MA, Lo EH, Dreier JP, Woitzik J, Sakadzic S, Ayata C. Supply-demand mismatch transients in susceptible peri-infarct hot zones explain the origins of spreading injury depolarizations. Neuron 85: 1117–1131, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyskocil F, Kritz N, Bures J. Potassium-selective microelectrodes used for measuring the extracellular brain potassium during spreading depression and anoxic depolarization in rats. Brain Res 39: 255–259, 1972. [DOI] [PubMed] [Google Scholar]
- Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, Steinhauser C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci 26: 5438–5447, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W. Role of Na/K/Cl cotransport in astrocytes. Can J Physiol Pharmacol Suppl, 70: S260–S262, 1992. [DOI] [PubMed] [Google Scholar]
- Wei Y, Ullah G, Schiff SJ. Unification of neuronal spikes, seizures, and spreading depression. J Neurosci 34: 11733–11743, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woitzik J, Hecht N, Pinczolits A, Sandow N, Major S, Winkler MK, Weber-Carstens S, Dohmen C, Graf R, Strong AJ, Dreier JP, Vajkoczy P. Propagation of cortical spreading depolarization in the human cortex after malignant stroke. Neurology 80: 1095–1102, 2013. [DOI] [PubMed] [Google Scholar]
- Wu J, Fisher RS. Hyperthermic spreading depressions in the immature rat hippocampal slice. J Neurophysiol 84: 1355–1360, 2000. [DOI] [PubMed] [Google Scholar]
- Xia S, Miyashita T, Fu TF, Lin WY, Wu CL, Pyzocha L, Lin IR, Saitoe M, Tully T, Chiang AS. NMDA receptors mediate olfactory learning and memory in Drosophila. Curr Biol 15: 603–615, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Robertson RM. Timing of locomotor recovery from anoxia modulated by the white gene in Drosophila. Genetics 203: 787–797, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XL, Shuttleworth CW, Moskal JR, Stanton PK. Suppression of spreading depolarization and stabilization of dendritic spines by GLYX-13, an NMDA receptor glycine-site functional partial agonist. Exp Neurol 273: 312–321, 2015. [DOI] [PubMed] [Google Scholar]
- Zhou N, Gordon GR, Feighan D, MacVicar BA. Transient swelling, acidification, and mitochondrial depolarization occurs in neurons but not astrocytes during spreading depression. Cereb Cortex 20: 2614–2624, 2010. [DOI] [PubMed] [Google Scholar]
- Zwarts L, Van EF, Callaerts P. Glia in Drosophila behavior. J Comp Physiol A 201: 879–893, 2015. [DOI] [PubMed] [Google Scholar]