Changes in the phasic activity of midbrain dopamine neurons are associated with reward processing. The lateral habenula inhibits dopamine neurons indirectly, but there is also evidence for a direct, albeit weak, excitatory influence. Here, we confirm the existence of a habenular projection to midbrain dopamine neurons that is excitatory and more pronounced than previously thought. This input, which appears to be latent under normal conditions, may modulate dopamine neuron activity during exposure to aversive stimuli.
Keywords: rostromedial tegmental nucleus, tail of the ventral tegmental area, dopamine, brain slice, tyrosine hydroxylase, lateral habenula
Abstract
The lateral habenula, a phylogenetically conserved epithalamic structure, is activated by aversive stimuli and reward omission. Excitatory efferents from the lateral habenula predominately inhibit midbrain dopamine neuronal firing through a disynaptic, feedforward inhibitory mechanism involving the rostromedial tegmental nucleus. However, the lateral habenula also directly targets dopamine neurons within the ventral tegmental area, suggesting that opposing actions may result from increased lateral habenula activity. In the present study, we tested the effect of habenular efferent stimulation on dopamine and nondopamine neurons in the ventral tegmental area of Sprague-Dawley rats using a parasagittal brain slice preparation. Single pulse stimulation of the fasciculus retroflexus excited 48% of dopamine neurons and 51% of nondopamine neurons in the ventral tegmental area of rat pups. These proportions were not altered by excision of the rostromedial tegmental nucleus and were evident in both cortical- and striatal-projecting dopamine neurons. Glutamate receptor antagonists blocked this excitation, and fasciculus retroflexus stimulation elicited evoked excitatory postsynaptic potentials with a nearly constant onset latency, indicative of a monosynaptic, glutamatergic connection. Comparison of responses in rat pups and young adults showed no significant difference in the proportion of neurons excited by fasciculus retroflexus stimulation. Our data indicate that the well-known, indirect inhibitory effect of lateral habenula activation on midbrain dopamine neurons is complemented by a significant, direct excitatory effect. This pathway may contribute to the role of midbrain dopamine neurons in processing aversive stimuli and salience.
NEW & NOTEWORTHY
Changes in the phasic activity of midbrain dopamine neurons are associated with reward processing. The lateral habenula inhibits dopamine neurons indirectly, but there is also evidence for a direct, albeit weak, excitatory influence. Here, we confirm the existence of a habenular projection to midbrain dopamine neurons that is excitatory and more pronounced than previously thought. This input, which appears to be latent under normal conditions, may modulate dopamine neuron activity during exposure to aversive stimuli.
aversive events, ranging from noxious stimuli to the loss of anticipated rewards, typically elicit a transient cessation in the spontaneous activity of midbrain dopamine (DA) neurons (Cohen et al. 2012; Roesch et al. 2007; Schultz 1998; Ungless et al. 2004). A number of studies have suggested that this response is mediated by the lateral habenula (LHb) and rostromedial tegmental nucleus (RMTg) (Hong et al. 2011; Jhou et al. 2013; Stamatakis and Stuber 2012; Stopper et al. 2014). Glutamatergic neurons within the LHb, which are generally activated by aversive stimuli (Benabid and Jeaugey 1989; Matsumoto and Hikosaka 2009), project heavily to a cluster of GABAergic neurons within the RMTg, a region that provides an important source of inhibitory input to the ventral tegmental area (VTA) (Brinschwitz et al. 2010; Jhou et al. 2009a, 2009b; Kaufling et al. 2009; Matsui and Williams 2011). Transient activation of the RMTg by LHb efferents is presumably responsible for the feedforward inhibition in DA cell activity elicited by aversive stimuli (Brown and Shepard 2013; Hong et al. 2011; Jhou et al. 2013). These brief cessations in neuronal firing in response to unpleasant events play a central role in error prediction-based models of associative learning (Montague et al. 1996; Roesch et al. 2012; Schultz, 1998).
While the majority of VTA DA neurons are inhibited by aversive stimuli, a minority are excited by these events (Brischoux et al. 2009; Guarraci and Kapp 1999; Matsumoto and Hikosaka 2009; Mirenowicz and Schultz 1996; Wang and Tsien 2011). The circuitry driving this response has yet to be elucidated, although neurons within the medial LHb represent a potential source of excitatory input to aversion-activated VTA DA neurons (Goncalves et al. 2012; Omelchenko et al. 2009). In anesthetized rats, LHb stimulation excites a small proportion of DA neurons, and many cells that are initially inhibited also show a delayed excitation of unknown origin (Christoph et al. 1986; Ji and Shepard 2007). Notably, local blockade of GABAA receptors significantly increases the number of midbrain DA neurons excited by LHb stimulation in vivo (Ji and Shepard 2007), suggesting that the initial inhibitory response could mask an underlying excitation. Ex vivo experiments also suggest that the LHb exerts a direct excitatory effect on VTA DA neurons (Lammel et al. 2012; Matsuda and Fujimura 1992). However, loss of the RMTg during the preparation of coronal slices could have exaggerated the influence of excitatory inputs on VTA activity.
In the present experiment, a parasagittal slice preparation containing the habenular efferent pathway [fasciculus retroflexus (fr)], VTA, and RMTg was used to assess the effects of LHb efferent activation on VTA neuronal firing in neonatal and adult rats. To unambiguously identify DA-containing neurons, all VTA cells recorded via patch clamp were filled with neurobiotin and identified as tyrosine hydroxylase (TH) positive or negative (TH+ or TH−) using immunohistochemical techniques. The role of the RMTg in modulating the response of VTA neurons to LHb input was evaluated using slices in which input from the RMTg was intact (RMTg+) or severed (RMTg−) by knife cut. Our results support a role for the LHb as a significant source of monosynaptic excitatory input to a subpopulation of VTA DA neurons.
MATERIALS AND METHODS
Animals.
Female Sprague-Dawley rats (dam with 5- to 7-day-old pups on arrival, Charles River Laboratories, Wilmington, MA) were delivered to the animal facilities at the Maryland Psychiatric Research Center and maintained on a 12:12-h light-dark cycle with food and water ad libitum. Male and female pups were used for slice recording either as neonates [postnatal days (PND) 7–21] or adults (PND 55–65). This study was conducted in accordance with recommendations in The Guide for the Care and Use of Laboratory Animals (Committee for the Update of the Guide for the Care and Use of Laboratory Animals 2011). All procedures were approved by the University of Maryland-Baltimore School of Medicine Institutional Care and Use Committee.
Slice preparation and patch-clamp electrophysiology.
Rat pups (n = 102) were decapitated, and the brain was removed and placed in ice-cold oxygenated artificial cerebrospinal fluid (aCSF) containing (in mM) 124 NaCl, 4 KCl, 1.25 NaH2PO4, 1.2 MgSO4, 25.7 NaHCO3, 11 glucose, 1 ascorbate, and 2.45 CaCl2. Whole brains were blocked in the coronal plane using a single edged razor blade between the optic chiasm (anterior boundary) and anterior cerebellum (posterior boundary). In the sagittal plane, both hemispheres were trimmed at an angle of ∼15° along the anterior-posterior axis to compensate for the angle made by the fr along the same axis as it descends to the midbrain. The tissue block was mounted sagittally on the stage of a vibrating tissue slicer (Leica VT 1200, Leica Biosystems, Buffalo Grove, IL) using ethyl cyanoacrylate glue. Parasagittal slices (350 μm, 1 slice/brain) containing the fr, VTA, and RMTg were prepared in ice-cold oxygenated aCSF. One-half of the slices collected were notched with a single-edged razor blade at the level of the third cranial nerve to sever RMTg projections to the VTA (RMTg− preparation). The remaining tissue slices were notched posterior to the RMTg (RMTg+ preparation). Slices were transferred to a static incubation chamber and submerged in oxygenated aCSF at room temperature for a minimum of 1 h.
For recording, individual slices were transferred to a submersion-style chamber (RC-22C, Warner Instruments, Hamden, CT) and perfused at 1.5 ml/min with oxygenated aCSF maintained at 30°C using a feedback-controlled bath heater (TC344B, Warner Instruments). Stimulating electrodes were prepared from a twisted pair of 38-gauge insulated tungsten wires, positioned in the fr ∼3 mm from the recording area using a manual micromanipulator, and connected to a constant current stimulator (model 2100, A-M Systems, Carlsborg, WA).
Whole cell patch-clamp recordings were made from VTA neurons visualized with infrared differential interference contrast video microscopy at ×40 using an Olympus BX51WI microscope equipped with a charge-coupled device camera (Center Valley, PA). Patch pipettes were pulled from standard wall borosilicate tubing (1.5-mm outer diameter, WPI, Sarasota, FL) using a horizontal micropipette puller (P-97, Sutter Instruments, Novato, CA) and filled with a solution containing (in mM) 131 K-gluconate, 9 KCl, 20 HEPES, 0.1 EGTA, 5 Mg-ATP, 0.5 GTP Tris, and 15.5 N-(2-aminoethyl)biotinamide hydrochloride (Neurobiotin Tracer, Vector Laboratories, Burlingame, CA). Pipette solutions were adjusted to a final pH of 7.2 and an osmolality of 280–290 mosm. Electrodes (7–15 MΩ) were advanced under positive pressure using a robotic micromanipulator (MP-285, Sutter Instruments), and, on contact with a neuron, high-resistance seals were formed by the application of continuous negative pressure. The membrane was ruptured by suction, and voltage recordings were obtained using an Axoclamp 2B amplifier (Molecular Devices, Sunnyvale, CA) in continuous current-clamp mode. Neurons were initially hyperpolarized by the application of a continuous negative bias current to suppress spontaneous activity and facilitate sealing. Cells were retained for recording only if they showed spontaneous spiking and required <0.05 nA of injected current to maintain a hyperpolarized, nonfiring state. Voltage recordings were digitized at 10 kHz using a laboratory interface (Digidata 1320A, Molecular Devices) and acquired with the Clampex 9.0 software package (Molecular Devices). Timed current pulses were generated using a digital pulse generator (PG4000, Neuro Data Instruments, New York, NY) and applied to the electrode through a balanced bridge circuit of the amplifier. As we were primarily investigating changes in spiking activity and relative changes in membrane potential, we did not correct for the junction potential.
Active and passive electrical properties of all neurons were determined before fr stimulation. The input resistance and membrane time constant (τ) were determined from the electrotonic response to a hyperpolarizing current pulse sufficient to fully charge the membrane capacitance (10–50 pA, 350 ms) and were made during continuous application of a small, hyperpolarizing bias current to prevent spiking. After removal of the bias current, spontaneous activity was measured from spikes collected over a 60-s interval. Action potential characteristics were determined from the mean of 10 single spikes.
The effects of fr stimulation were initially assessed during the application of a negative bias current sufficient to prevent spiking. Synaptic responses were evoked using both single biphasic pulses (150-μs duration, 20–50 V) and trains composed of three identical pulses at 50 Hz. Voltage responses were averaged across 10 consecutive trials. Among neurons showing evidence of a reliable evoked response, jitter (SD of the onset latency) was calculated using previously described methods (Doyle and Andresen 2001; Shao et al. 2009). After removal of the hyperpolarizing bias current, neurons were again tested for their response to single pulse fr stimulation. No more than three neurons were recorded in each slice. In some experiments, the response of individual neurons to fr stimulation was reexamined in the presence of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-d-aspartate (NMDA) glutamate receptor antagonists [10 μM 6,7-dinitroquinoxaline-2,3-dione (DNQX) and 50 μM (2R)-amino-5-phosphonovaleric acid (APV), respectively]. Recording sessions were of sufficient length to allow neurobiotin to fill recorded cells via passive diffusion (Chitwood et al. 1999; Li et al. 2004). At the conclusion of the experiment, slices were fixed in 10% formalin overnight at 4°C and stored in cryoprotectant (30% sucrose, 30% ethylene glycol, and 1% PVP-40 in PBS at 4°C) for subsequent immunohistochemical processing.
Fluorescent microsphere injections.
A separate cohort of rat pups (PND 7–8, n = 12) was anesthetized with isoflurane (2.0–5.0% in 100% oxygen) and secured to a styrofoam platform anchored in a stereotaxic apparatus, and their scalps were incised (Brady et al. 2008). A 30-gauge needle served as a cannula and was lowered through the skull into either the prefrontal cortex (PFC; 2.0 mm anterior, 0.8 mm lateral, and 2.7 ventral to the bregma) or ventral striatum (VST; 1.0 mm anterior, 1.5 mm lateral, and 5.5 mm ventral to the bregma) using coordinates from the neonatal rat atlas of Sherwood and Timiras (1970). Yellow-green fluorescent microspheres (F8795, Life Technologies, Grand Island, NY) were delivered using a 500-nl syringe (SGE, Victoria, Australia) connected via polyethylene tubing to the cannula needle and driven by a syringe infusion pump (Harvard Apparatus, Holliston, MA) at a rate of 40 nl/min for 5 min. Cannulae remained in place for 5 min after the infusion termination. Wounds were closed with stainless steel clips, and, after recovery on a warming pad, pups were returned to their mothers until testing. Slice preparation and patch recordings in microsphere-injected pups occurred between PND 11 and 19, and the procedures used were identical to those described above with the exception that all experiments were conducted in RMTg+ preparations and only microsphere-filled cells were targeted for patch recording.
Extracellular single unit recording.
Male and female rat pups (PND 9–21, n = 9) and adults (PND 55–65, n = 15) were anesthetized with isoflurane (3.0% in 100% oxygen) to loss of consciousness and decapitated. Brains were removed, sliced as described above with the exception that adult brains were cut in ice-cold oxygenated sucrose-aCSF with HEPES (normal aCSF with the exchange of 200 mM sucrose for 124 mM NaCl and the addition of 20 mM HEPES), and incubated in aCSF with HEPES (normal aCSF with the addition of 20 mM HEPES) to prevent excitotoxicicty (Aghajanian and Rasmussen 1989) and edema (MacGregor et al. 2001; Ting et al. 2014).
Extracellular single unit recordings were obtained using glass pipettes pulled on a vertical puller (PE-2, Narshige, Tokyo, Japan) to an impedance of 3–10 MΩ when filled with 2 M NaCl. Recordings were conducted in a grid pattern of nine tracks with a new pipette used for each track. From the most dorsal point of the interpeduncular fossa, the pipette tip was moved 150 μm perpendicular to the fr in the posterior-dorsal direction to reach the first recording track. Subsequent tracks were made in 150-μm increments posterior and dorsal to the first track to form a 300 × 300-μm grid within the VTA with one side of the grid parallel to the fr and nine recording tracks total. Electrodes were slowly advanced (1–2 μm/s) until a spontaneously active cell could be isolated from background noise. Electrode potentials were amplified, filtered (0.1- to 8-kHz bandpass), and monitored in real time using a digital oscilloscope and audiomonitor. Well-isolated spikes were digitized at 20 kHz using a 16-bit laboratory interface (Micro 1401, CED, Manchester, UK) and stored for offline analysis using Spike 2 software (CED). Once a neuron was isolated, a baseline of 100 s was recorded before being tested for a response to fr stimulation using the same parameters described above for patch recording. In some adult slices, a second stimulating electrode was placed ∼1 mm anterior to the fr in the mediodorsal thalamic nucleus.
TH immunohistochemistry and neurobiotin labeling.
Brain slices in which patch-clamp recordings occurred were processed en bloc at room temperature to visualize TH-containing and neurobiotin-filled neurons. Tissue slices were rinsed in PBS three to six times before each step. To quench endogenous fluorescence, slices were rinsed in 0.1 M glycine in PBS for 10 min followed by 0.05 M NH4Cl for 60 min. To block nonspecific binding, slices were rinsed in 3.0% normal goat serum (NGS) and 0.3% Triton X-100 in PBS for 60 min. This was followed by an incubation in primary antibody solution containing mouse anti-TH monoclonal primary antibody (1:50,000, Immunostar, Hudson, WI), 1.0% NGS, and 0.3% Triton X-100 in PBS overnight. Primary antibody incubation was terminated by a rinse in 0.3% Triton X-100 in PBS for 30 min. Tissue slices were then incubated in secondary antibody solution containing Alexa 594 goat anti-mouse secondary antibody (1:150, Invitrogen, Grand Island, NY) in 1.0% NGS and 0.3% Triton X-100 in PBS for 120 min. Concurrent with this step, slices were incubated with Alexa 488 streptavidin (surgery naïve pups, 1:150, Invitrogen) or Alexa 350 streptavidin (microsphere-injected pups, 1:150, Life Technologies, Grand Island, NY). Slices were given a final rinse in double-distilled H2O, mounted on glass slides, and air dried for 30–60 min before being coverslipped with fluorescent mounting media (Vectashield, Vector Laboratories) and sealed with clear nail polish. Slices were viewed and photomicrographs captured with a fluorescent microscope (Axioplan, Zeiss, Thornwood, NY) equipped with a digital camera and photocapture software (DP 70 and DP controller, Olympus America, Center Valley, PA). Sections were illuminated using a 100-W mercury lamp and each of the following filter sets: exciter 365 nm, splitter 395 nm, and emitter 420 nm (for Alexa 350); exciter 485 nm, splitter 510 nm, and emitter 515–565 nm (for Alexa 488); and exciter 546 nm, splitter 580 nm, and emitter 590 nm (for Alexa 594).
c-Fos immunohistochemistry.
Rat pups (PND 10–20, n = 10) received a single subcutaneous injection of 10 mg/kg (+)-methamphetamine hydrochloride (mAMPH; Sigma-Aldrich, St. Louis, MO) or an equivalent volume (0.1 ml) of 0.9% saline. Ninety minutes after injection, pups were decapitated; the brains were placed in ice-cold oxygenated aCSF and blocked for coronal (150 μm) or parasagittal sectioning (100 μm) as described above. Tissue sections were placed in 10% formalin, fixed overnight at 4°C, and stored in cryoprotectant at 4°C. The procedure used to visualize c-Fos was similar to that used for TH, with the following modifications. Tissue slices were incubated with an additional primary antibody (rabbit anti-c-Fos polyclonal antibody, 1:5,000, EMD Chemicals, San Diego, CA) and secondary antibody (Alexa 488 goat anti-rabbit secondary antibody, 1:150, Invitrogen). As neurobiotin was not used in this experiment, fluorescent streptavidin was omitted from the secondary antibody solution.
Data analysis.
Individual spikes were isolated from background noise and stimulation artifacts offline using the window discriminator function in pClamp or Spike2. Peristimulus time histograms (PSTHs) were compiled over the 200 ms immediately preceding and 800 ms immediately after stimulus presentation and were constructed using 1-ms bin widths. PSTHs composed of fewer than 250 total spikes were excluded from analysis. CUMSUM plots were constructed from PSTHs by adding the contents of each bin to a running sum of all previous events and analyzed as previously described (Ji and Shepard 2007). Baseline activity was determined from the slope of a least-squares fit to a 200-ms sample of prestimulus activity. Stimulation-induced changes in firing probability were determined by comparing the slope of discrete regions of each CUMSUM plot with control values. A response was defined as a change in slope equal to or exceeding 30% of control (Ji and Shepard 2007). The latency to onset and duration of excitatory and inhibitory responses to fr stimulation were determined from the intersection of adjacent regression lines. For comparison between treatment groups, data were expressed as firing rate (in Hz) averaged across 25-ms bins. A mean of the four bins (100 ms) preceding stimulation was used as a baseline. Repeated-measures ANOVA with Fisher post hoc analysis was then conducted on the baseline and five bins (125 ms) after stimulation, for six time points in total.
Categorical data were analyzed using Fisher's exact test with post hoc significance determined by the adjusted standardized residual method with Bonferroni-adjusted α (Sharpe 2015). All other data were analyzed with t-tests or two-way ANOVAs as appropriate with a post hoc Fisher's test. All data are expressed as arithmetic means ± SE.
RESULTS
Active and passive electrophysiological properties of VTA neurons.
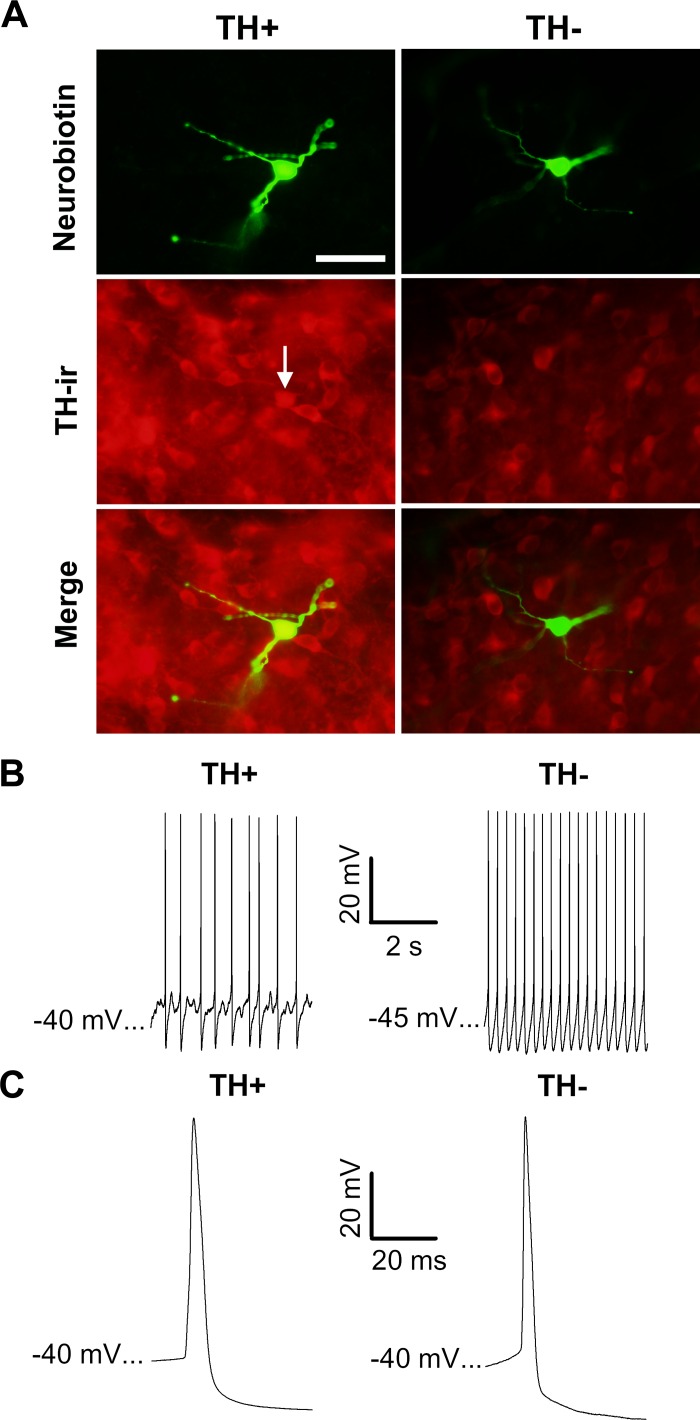
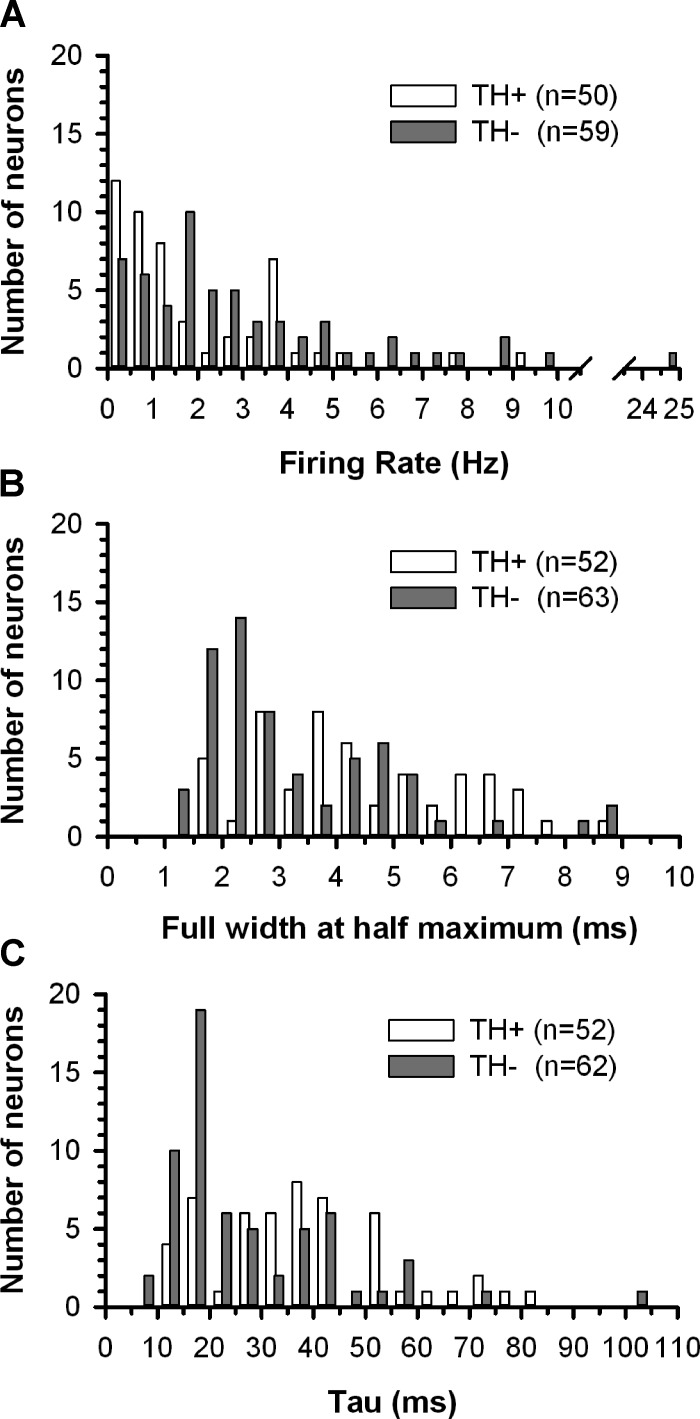
A total of 152 neurons were recorded in brain slices obtained from 102 rats. Clearly demonstrable regions of TH+ immunostaining were used to define the boundaries of the VTA, and cells located outside of these regions were excluded from the analysis. One hundred fifteen neurobiotin-filled neurons were identified within the VTA of 85 slices (44 RMTg+ and 41 RMTg− slices). Of these, 45% (52 of 115) were TH+ neurons (Fig. 1A). Compared with TH− neurons, TH+ cells had significantly slower firing rates [F(1,105) = 6.075, P < 0.05], wider action potentials [F(1,111) = 10.777, P < 0.05], and a slower τ [F(1,111) = 10.453, P < 0.05; Figs. 1, B and C, and 2 and Table 1]. No significant differences were detected between TH− and TH+ neurons with respect to membrane potential at spike peak or input resistance (Table 1). Knife cuts separating the RMTg from the VTA had no significant effect on the active or passive membrane properties of these neurons. Consequently, in Table 1 and all subsequent figures where null results between RMTg+ and RMTg− preparations were obtained, data were collapsed across this variable unless otherwise stated.
Fig. 1.
Electrophysiological properties of immunohistochemically identified ventral tegmental area (VTA) neurons. A: photomicrographs of neurobiotin-filled cells (green) in brain slices processed for tyrosine hydroxylase (TH) immunoreactivity (ir; red). The white arrow indicates the soma of a neurobiotin-filled TH+ cell. Scale bar = 50 μm. B and C: spontaneously firing neurons. TH+ VTA neurons tended to fire more slowly and exhibit wider spikes than TH− VTA neurons.
Fig. 2.
Distribution of spontaneous firing rates (A), spike durations (B), and time constant (tau; C) obtained from TH+ and TH− neurons recorded in the VTA.
Table 1.
Active and passive membrane properties of ventral tegmental area neurons
| Tyrosine Hydroxylase Positive | Tyrosine Hydroxylase Negative | |
|---|---|---|
| Firing rate, Hz* | 1.9 ± 0.3 | 3.4 ± 0.5 |
| Full width at half maximum, ms* | 4.4 ± 0.3 | 3.3 ± 0.2 |
| Time constant, ms* | 38.1 ± 2.4 | 27.3 ± 2.2 |
| Membrance voltage at peak amplitude, mV | 26.6 ± 1.0 | 26.9 ± 1.0 |
| Input resistance, MΩ | 736.0 ± 95.3 | 740.0 ± 81.2 |
Values are means ± SE.
P < 0.05.
Response of spontaneously active VTA neurons to fr stimulation.
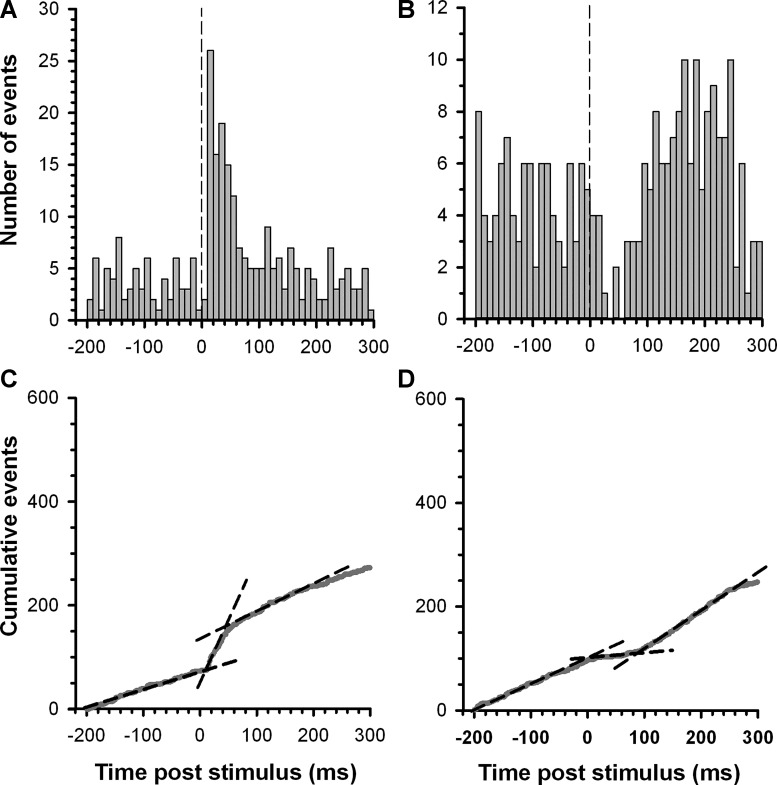
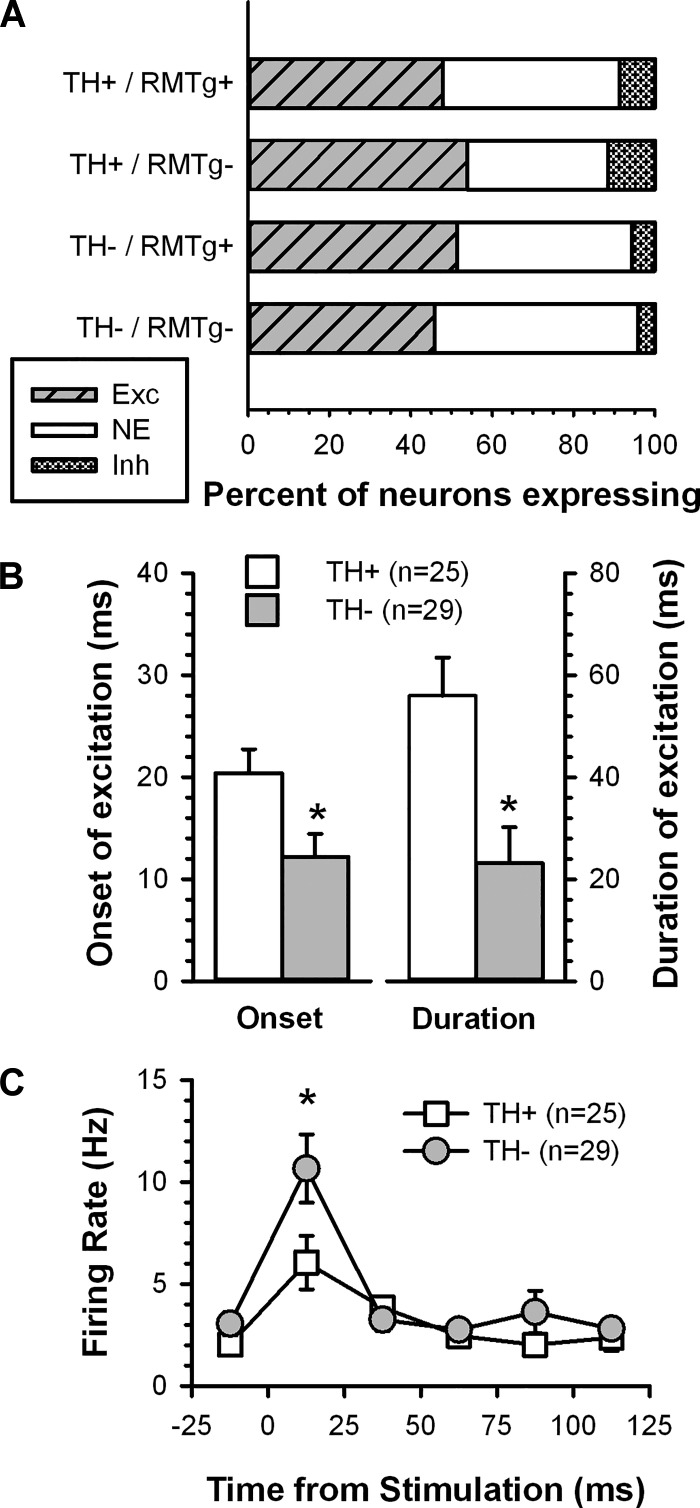
Recordings from 108 VTA neurons in 81 slices (42 from RMTg+ and 39 from RMTg− preparations) were analyzed for changes in firing rate after fr stimulation. Of these, 57% (62 of 108) exhibited an unambiguous response defined as a ≥30% change in slope of a CUMSUM plot compiled before and after fr stimulation (Fig. 3). Given that the parasagittal slice includes the medial mesopontine tegmentum, the region in which the RMTg is located, we expected that TH+ VTA neurons that retained a response to fr stimulation would exhibit an inhibition in firing and, furthermore, that this effect would be less prevalent in RMTg− preparations. However, the majority of cells that responded to fr stimulation exhibited an increase in firing rate [87% (54 of 62)] that was represented nearly equally among TH+ [83.3% (25 of 30)] and TH− [90.1% (29 of 32)] neurons (Fig. 4A). The proportion of cells excited by fr stimulation did not differ between the two slice preparations (RMTg+ vs. RMTg−). Among neurons that responded to fr stimulation with excitation, TH+ neurons had a longer latency to onset [F(1,50) = 6.330, P < 0.05] and duration of excitation [F(1,50) = 10.153, P < 0.05; Fig. 4B] compared with TH− neurons. Neither latency to onset nor duration of excitation differed between the two slice preparations. Combining the RMTg+ and RMTg− groups, an analysis across the first 125 ms after fr stimulation showed a significant effect of time [F(5,260) = 23.375, P < 0.05] and time by TH status interaction [F(5,260) = 3.666, P < 0.05; Fig. 4C]. While both TH+ and TH− neurons showed significantly elevated firing rates at the first poststimulus time point relative their baseline, this increase was greater in TH− neurons (P < 0.05 by Fisher test).
Fig. 3.
Representative examples of the effect of fasciculus retroflexus (fr) stimulation on the activity of TH+ VTA neurons. A and B: peristimulus time histograms (PSTHs) illustrating the response of two VTA dopamine neurons to single pulse stimulation of the fr. The dashed vertical line denotes the onset of the stimulus pulse. C and D: corresponding CUMSUM plots were compiled from a running sum of the bin totals comprising the PSTHs. The dashed lines illustrate the slope of a least-squares regression line fit to each segment of the CUMSUM plot to determine the duration and magnitude of firing rate changes in response to fr stimulation (see materials and methods for details).
Fig. 4.
Summary of the effects of fr stimulation on the activity of VTA neurons. A: bar histogram illustrating the distribution of responses to fr stimulation among TH+ and TH− neurons in slices with and without the rostromedial tegmental nucleus (RMTg+ and RMTg−, respectively). B: the latency to onset (left axis) and duration of fr-induced excitation (right axis) were longer in TH+ neurons than in TH− neurons. C: plot of the time course of spontaneous activity showing the rapid excitation and return to baseline after fr stimulation. *Group difference (P < 0.05) in B and C.
Synaptic responses of VTA neurons to fr stimulation.
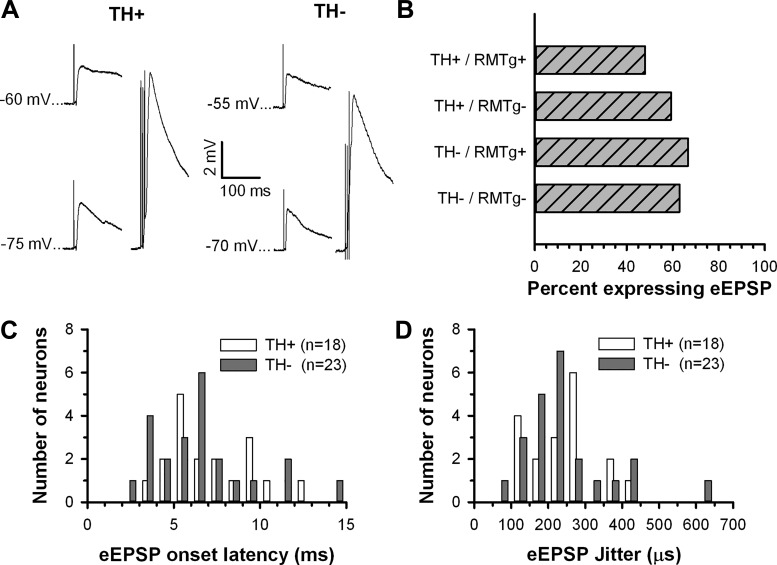
Given that excitation was commonly elicited in VTA neurons, we looked for evidence of evoked excitatory postsynaptic potentials (eEPSPs) in all neurons. More than half of VTA neurons [60.0% (69 of 115)] showed voltage responses consistent with eEPSPs after fr stimulation (Fig. 5A). The proportion of neurons exhibiting eEPSPs was nearly evenly split between TH+ [53.8% (28 of 52)] and TH− [65.1% (41/63)] neurons (Fig. 5B). Neither the age of the animal nor the type of slice preparation (RMTg+ of RMTg−) altered eEPSP expression or characteristics. The mean onset latency of eEPSPs was short (TH+: 7.1 ± 0.5 ms and TH−: 6.6 ± 0.6 ms; Fig. 5C) and exhibited little variation, with most neurons (77.7% of TH+ and 78.3% of TH−) showing a jitter of <300 μs (TH+: 240 ± 20.2 μs and TH−: 243 ± 26.6 μs; Fig. 5D).
Fig. 5.
Summary of the effects of fr stimulation on synaptic potentials recorded in VTA neurons. A: fr stimulation elicited evoked excitatory postsynaptic potentials (eEPSPs) in both TH+ (left) and TH− (right) VTA neurons that increased in amplitude during membrane hyperpolarization (bottom left in each example) and showed evidence of temporal summation (bottom right in each example, 3 pulses at 50 Hz). B: bar histogram illustrating the frequency of occurrence of eEPSPs in TH+ and TH− neurons in slices with and without the RMTg. C and D: neither latency to EPSP onset nor SD of onset latency (jitter) differed between TH+ and TH− VTA neurons.
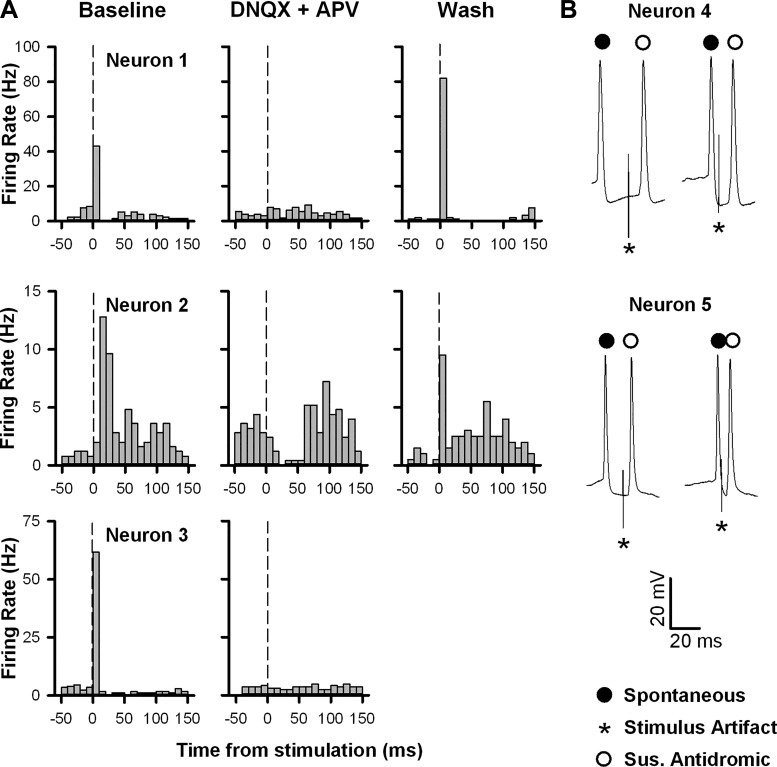
The fact that fr stimulation led to excitation with both rapid onset and low variability suggested that this response was monosynaptic in nature. In three neurons with a particularly robust excitation, bath application of DNQX (10 μM) and APV (50 μM) blocked the induced increase in firing rate, an effect that was reversed upon washout (Fig. 6A). In addition, in two cells showing a prominent response to fr stimulation, spontaneous spikes occurring very close to the onset of the stimulus pulse failed to prevent the elicited spike from occurring, suggesting that the evoked action potentials were not antidromically activated (Fig. 6B). Taken together, these results suggest that fr-induced activation of VTA neurons is monosynaptic and glutamatergic in nature.
Fig. 6.
fr stimulation induced excitation of VTA neurons is glutamate receptor dependent. A: PSTHs illustrating the changes in firing rate elicited by fr stimulation in three separate neurons before (left), during (middle), and after (right) bath application of 6,7-dinitroquinoxaline-2,3-dione (DNQX; 10 μM) and (2R)-amino-5-phosphonovaleric acid (APV; 50 μM). Vertical lines denote the stimulus onset. B: naturally occurring collision tests were evaluated in two neurons with a rapid, high-fidelity response to fr stimulation. Shown for each neuron are spontaneous spikes (closed circles) with a delay to stimulation (*) that was either longer (left trace) or shorter (right trace) than the onset latency to the suspected antidromic spike (open circle). In both neurons, short delays to stimulation failed to produce collisions, demonstrating that the activation was not antidromic. All five neurons in this figure were subsequently shown to be TH−.
Identification of a functional RMTg in neonatal rats.
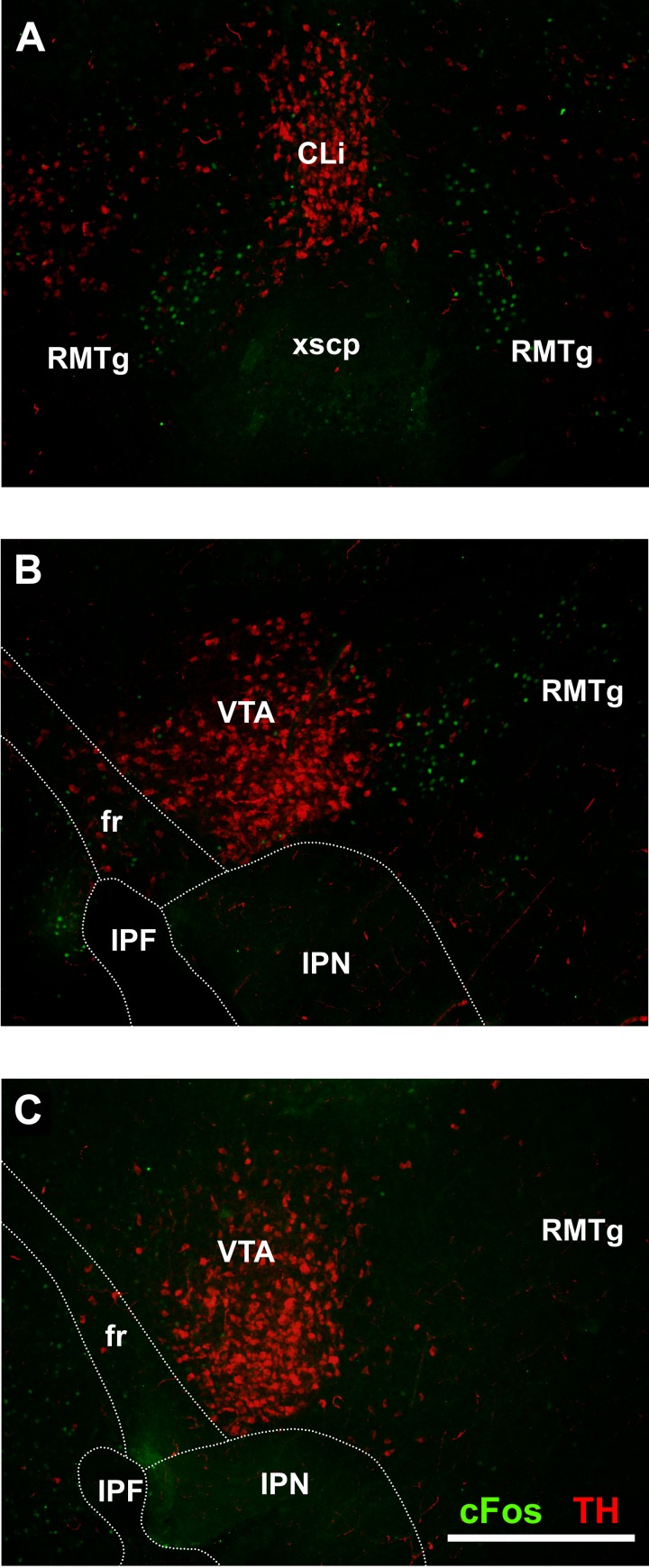
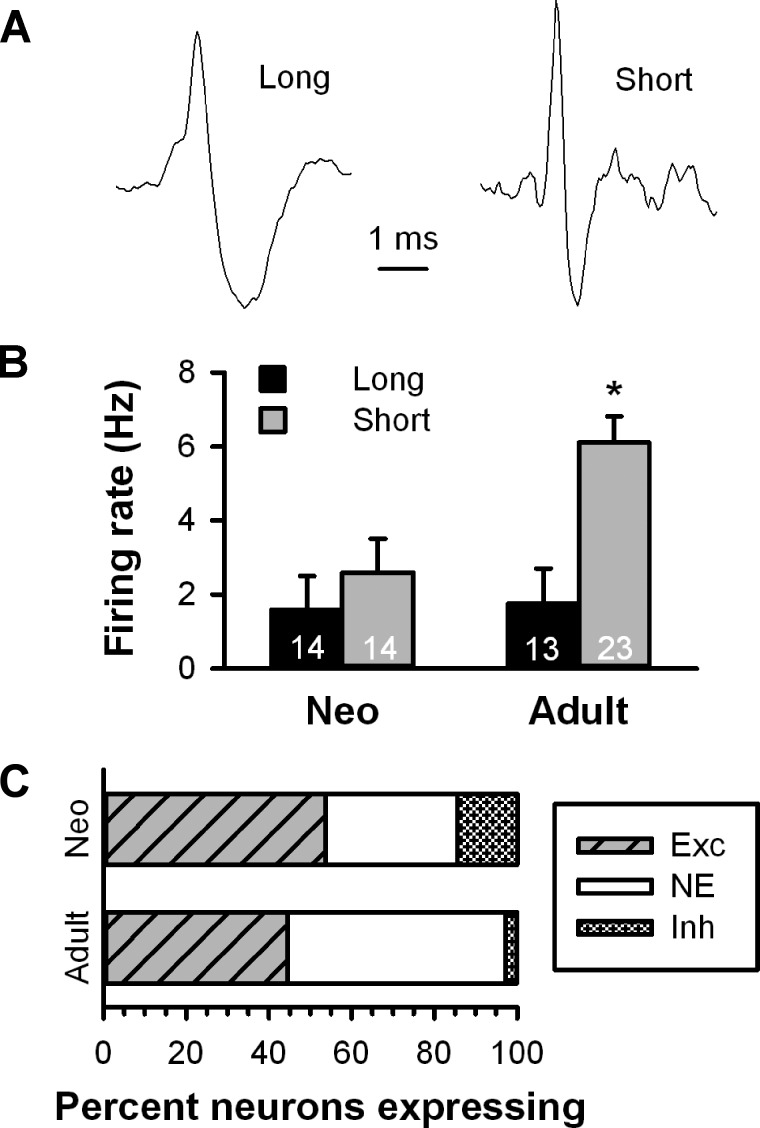
To confirm the presence of a functional RMTg in neonatal rats, a separate group of experiments was conducted to determine whether psychostimulants induced a pattern of c-Fos expression comparable with that seen in adult animals (Jhou et al. 2009a). Coronal sections prepared from neonatal rats 90 min after an injection of mAMPH (10 mg/kg) display c-Fos expression in the area corresponding to the RMTg in adults (Fig. 7A). Sections taken in the same parasagittal plane used in the recording experiments contained c-Fos+ cell bodies directly posterior to TH+ cell bodies in the VTA (Fig. 7B), a region that corresponds directly to the RMTg in adult rats. In contrast, vehicle-treated neonates showed virtually no c-Fos expression in the RMTg (Fig. 7C).
Fig. 7.
Photomicrographs of neonatal rat brain sections immunostained for TH (red) and c-Fos (green) after administration of (+)-methamphetamine hydrochloride. A: coronal section (150 μm) showing c-Fos+ cells ventral and lateral to the TH+ caudal linear nucleus. This c-Fos+ area corresponds to the RMTg in adult rats. B: parasagittal section (100 μm, but otherwise identical to that used in the recording experiments) showing c-Fos+ cells posterior to the TH+ VTA. C: comparable section from a vehicle-treated rat showing TH+ neurons in the VTA but no evidence of c-Fos+ neurons in the RMTg. CLi, caudal linear nucleus; IPF, interpeduncular fossa; IPN, interpeduncular nucleus; xscP, decussation of the superior cerebellar peduncle. Scale bar = 500 μm.
Comparison of the response of PFC- and VST-projecting TH+ neurons to fr stimulation.
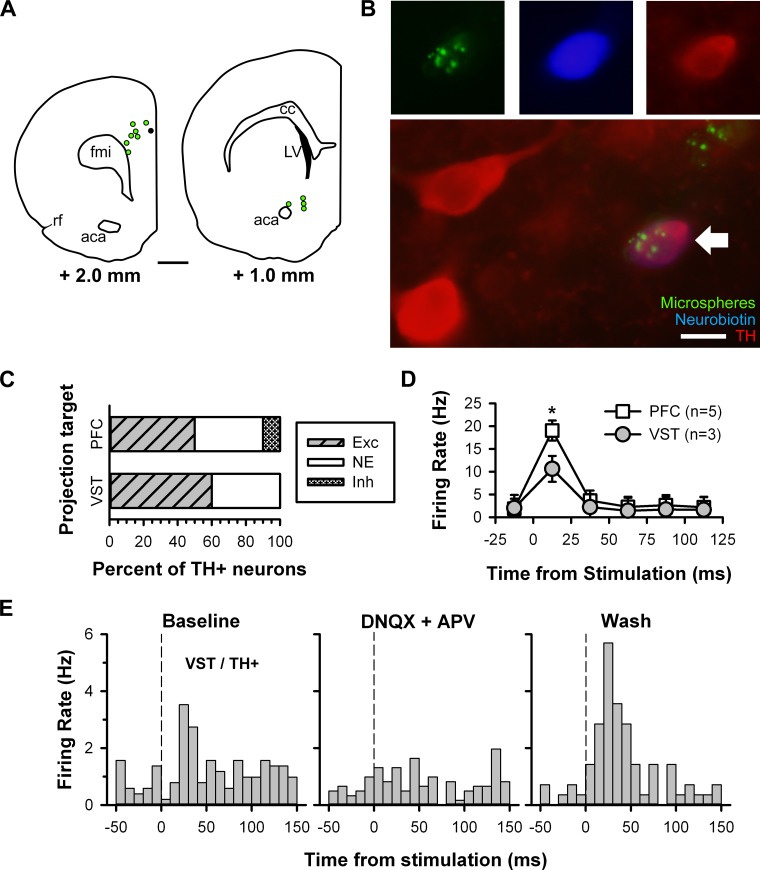
Recent studies have shown that the LHb projects directly to the anteromedial part of the VTA and that DA neurons in this region preferentially target the PFC but not the VST (Lammel et al. 2011, 2012). Given the absence of a robust inhibitory response to fr stimulation in slices containing the RMTg, we considered whether we had sampled DA neurons from a region of the VTA that receives a direct excitatory input from the LHb. To accomplish this, we labeled VTA projection neurons by injecting retrogradely moving fluorescent microspheres in either the PFC or VST of rat pups (Fig. 8A). The beads were used to visualize and record from VTA neurons with a known projection target (Fig. 8B), with the expectation that the majority of TH+ cells excited by fr stimulation would project to the PFC and not the VST.
Fig. 8.
Use of retrograde-traveling fluorescent microspheres to label and record from projection-specific neurons. A: location of microsphere injection sites in the prefrontal cortex (PFC; left) and ventral striatum (VST; right) with approximate distance from the bregma for each. The black circle indicates an injection site ∼0.5 mm posterior to the coronal section shown. Scale bar = 1 mm. aca, Anterior commissure, anterior; cc, corpus collosum; fmi, forceps minor corpus collosum; LV, lateral ventricle; rf, rhinal fissure. B: photomicrographs of a microsphere-filled cell (top left, green) labeled with neurobiotin (top middle, blue) and stained for TH (top right, red). Merge of photomicrographs shows the recorded neuron (bottom, white arrow). Scale bar = 10 μm. C: bar histogram illustrating the distribution of responses to fr stimulation between PFC- and VST-projecting TH+ neurons. D: plot of the time course of spontaneous activity showing the rapid excitation that was stronger in PFC- than VST-projecting neurons. *Group difference (P < 0.05). E: PSTHs illustrating the changes in firing rate elicited by fr stimulation in a VST-projecting TH+ neuron before (left), during (middle), and after (right) bath application of DNQX (10 μM) and APV (50 μM). The vertical lines denote the stimulus onset.
Contrary to expectations, the proportion of neurons excited by fr stimulation was similar between PFC- and VST-projecting TH+ neurons [50% (5 of 10) and 60% (3 of 5), respectively; Fig. 8C], and there were no differences between groups in the latency to onset [t(6) = −0.661, P = 0.53] or duration of excitation [t(6) = −1.114, P = 0.29]. However, fr-induced excitation was greater among PFC- than VST-projecting TH+ neurons [F(5,30) = 9.278, P < 0.05 by Fisher test; Fig. 8D]. In one VST-projecting TH+ neuron tested, the excitation was blocked during bath application of DNQX and APV and subsequently returned during drug washout (Fig. 8E).
Comparison of the response of VTA neurons to fr stimulation in neonatal and adult slices.
The absence of a robust inhibitory response to LHb stimulation might reflect the developmental state of the habenulomesencephalic pathway in that the excitatory response may be uniquely associated with a perinatal preparation. To investigate this possibility, we compared the response of VTA neurons to fr stimulation in slices prepared from neonates and adults (PND 55–65) using extracellular single unit recording techniques.
Action potentials from spontaneously active neurons in both preparations fell into one of two broad categories including cells with a long-duration, triphasic waveform with a shoulder on the rising phase or a short-duration waveform with no shoulder (Fig. 9A). Neurons with long-duration spikes had a lower mean firing rate than neurons with shorter spikes [F(1,60) = 9.414, P < 0.05; Fig. 9B]. Overall, neurons sampled in adult rats had higher basal firing rates than cells recorded in neonates [F(1,60) = 4.422, P < 0.05], and there was a significant cell type by group interaction [F(1,60) = 3.686, P < 0.05], likely driven by the significantly higher firing rate of neurons with short-durations spikes in adults relative to neonates (P < 0.05 by Fisher test).
Fig. 9.
Comparison of extracellular single unit recordings of VTA neurons from neonatal and adult parasagittal slices. A: most spontaneously firing VTA neurons from both neonatal and adult slices presented either a long-duration (left) or short-duration waveform (right). B: bar histogram illustrating the firing rate of long- and short-duration waveform neurons in neonatal and adult VTA neurons. *Group difference within short waveform neurons (P < 0.05). C: bar histogram illustrating the distribution of responses to fr stimulation between neonatal and adult VTA neurons.
Given that the proportion of cells excited by fr stimulation did not differ between TH+ and TH− neurons in our patch recordings and that electrophysiological criteria cannot be used to ascribe a definitive neurochemical phenotype to VTA neurons, we combined all neurons into a single category for comparison between age groups. Although lower, the proportion of neurons excited by fr stimulation in adult rats did not differ significantly from neonates [53.6% (15 of 28) vs. 44.4% (16 of 36), P = 0.1153 by Fisher exact test; Fig. 9C]. Given these group sizes and the proportion of neurons excited within the neonatal group, there was sufficient power to detect a proportion of excited cells in the adult group of <17% (α = 0.05, β = 0.80), which includes the proportion of VTA neurons innervated by LHb neurons (16%) as determined by electron microscopy (Omelchenko et al. 2009). Among neurons that were excited by fr stimulation, the latency to onset was longer in neonates (10.9 ± 2.8 ms) than adults (5.0 ± 0.9 ms), but this effect was short of being statistically significant [t(29) = 2.041, P = 0.0505]. The duration of excitation (neonates: 32.5 ± 7.1 ms and adults: 23.9 ± 9.3 ms) was not significantly different between preparations [t(29) = 0.727, P = 0.47]. Analysis of the first 125 ms showed a significant effect of time [F(5,145) = 22.719, P < 0.05] with no effect of group [F(1,29) = 0.705, P = 0.41].
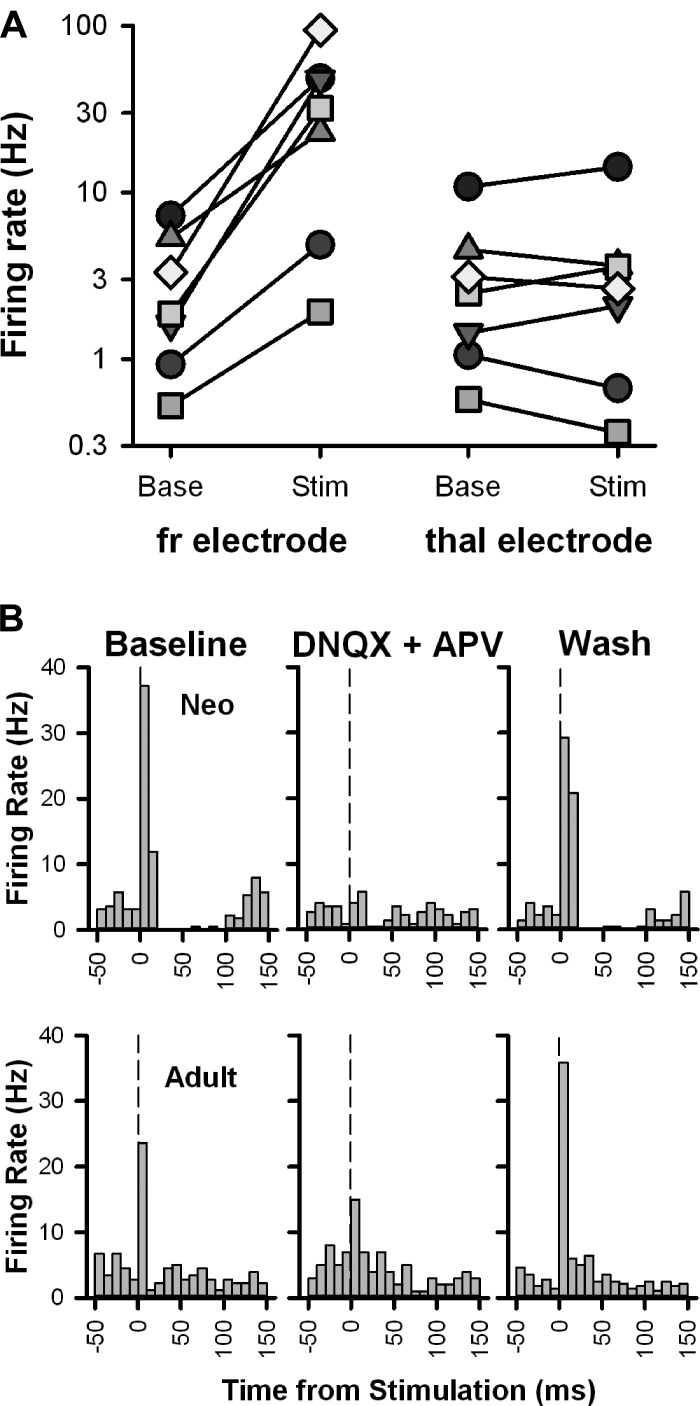
Finally, in an effort to confirm the specificity of the response to fr stimulation, we assessed the response of a subgroup of adult VTA neurons that showed an fr-induced excitatory response to stimulation of the adjacent thalamus (Fig. 10A). Two-way repeated-measures ANOVA (stimulus location by time) showed a significant effect of stimulus location [F(1,6) = 7.486, P < 0.05], time [F(1,6) = 8.161, P < 0.05], and their interaction [F(1,6) = 7.9385, P < 0.05]. Baseline firing rates were similar between tests of stimulus location (fr vs. thalamic stimulation, 3.0 vs. 3.4 Hz, P = 0.96 by Fisher post hoc test). However, there was a significant increase in firing rate after fr stimulation (to 36.1 Hz, P < 0.05 by Fisher post hoc test) that was not seen after thalamic stimulation (to 3.9 Hz, P = 0.96 by Fisher post hoc test), demonstrating that the response to fr stimulation was due to direct activation of fibers within this pathway and not to passive current spread. Finally, bath application of DNQX and APV blocked (in neonates) or strongly attenuated (in adults) fr-induced excitation, which was reversed upon washout (Fig. 10B).
Fig. 10.
Stimulation-induced excitation of VTA neurons is fr specific and glutamate receptor dependent. A: seven neurons (each represented by a different symbol) that responded with excitation to fr stimulation (left) were also tested for their response to thalamic stimulation (right). While there was a strong increase in firing rate from baseline after fr stimulation in all cells, there was no consistent change in firing rate after thalamic stimulation. Note that the ordinate is a log scale to make changes in neurons with slow firing rates more visible. B: PSTHs illustrating the changes in firing rate elicited by fr stimulation in neurons from a neonate and adult rat before (left), during (middle), and after (right) bath application of DNQX (10 μM) and APV (50 μM). The vertical lines denote the stimulus onset.
DISCUSSION
The results of the present study demonstrate that, in a sagittal brain slice preparation, stimulation of the fr, a pathway comprised primarily of efferent projections from the habenula to the ventral midbrain (Herkenham and Nauta 1979; Sutherland 1982), exerts a predominantly excitatory effect on VTA neurons. Similar findings have been previously reported by Matsuda and Fujimura (1992) using a transverse coronal slice preparation in which LHb stimulation evoked depolarizing potentials in 81% of responsive neurons. However, in contrast to these earlier results, which were obtained using sharp electrode recording techniques, in the present study, EPSPs elicited by LHb stimulation were able to bring DA neurons to spike threshold without concomitant application of depolarizing bias current. This discrepancy is likely due to the presence of the Ca2+-chelating agent EGTA in our patch solution, which would have a depolarizing effect on DA neurons by attenuation of the activity of Ca2+-activated K+ channels (Canavier et al. 2007; Shepard and Bunney 1991).
Earlier studies also relied on the electrophysiological properties of VTA neurons to infer their neurochemical identity, an approach that has recently been called into question (Ford et al. 2006; Lammel et al. 2008; Margolis et al. 2006; Wolfart et al. 2001). In an effort to unambiguously determine the neurochemical identity of the cells recorded in the present study, all patch-recorded neurons were filled with neurobiotin and processed for TH immunoreactivity. Consistent with a previous report (Margolis et al. 2006), neither the active (spike width and firing pattern) nor passive (τ and input resistance) electrophysiological properties of VTA neurons, because of their broad distribution overlap, proved to be a definitive indicator of DA phenotype as determined by TH immunoreactivity. Given that DA neurons in the more medial aspects of the VTA, a region included in our slice preparation, exhibit lower levels of DA-related phenotypic markers (Blanchard et al. 1994; Lammel et al. 2008; Li et al. 2013; Yamaguchi et al. 2015), we cannot exclude the possibility that some of the neurons identified as TH− were, in fact, DA-containing cells. Nevertheless, in agreement with an earlier report (Matsuda and Fujimura 1992), the proportion of VTA cells excited by fr stimulation was equally distributed between TH+ and TH− neurons. These observations are in agreement with anatomic results supporting the existence of a direct excitatory projection from the LHb to the VTA (Geisler et al. 2007; Goncalves et al. 2012; Omelchenko et al. 2009) and ultrastructural findings showing that vesicular glutamate transporter 2+ LHb axons innervate VTA DA and non-DA neurons in equal proportion (Omelchenko et al. 2009).
The predominant excitatory effect of fr stimulation on VTA neurons could reflect the unique population of midbrain DA neurons sampled in the present study. To maintain the integrity of the fr, our slices included only the medial aspects of the VTA. Neurons in this region, particularly those in proximity to the fr, show increased firing rates in response to cues predicting aversive events (Guarraci and Kapp 1999) and increased AMPA-to-NMDA ratios after aversive stimuli (Lammel et al. 2011). Recent studies have shown that the LHb, an area known to be excited by aversive stimuli (Benabid and Jeaugey 1989; Brown et al. 1996; Mirrione et al. 2014), innervates anteromedial VTA DA neurons that preferentially project to the PFC (Lammel et al. 2011; Lammel et al. 2012), an area that, in turn, shows DA-dependent neuronal activation in response to aversive stimuli (Deutch and Roth 1990; Thierry et al. 1976). We explored the possibility that the topography of our slice preparation increased the likelihood of recording from PFC-projecting VTA DA neurons that receive a strong and direct excitatory input from the LHb. However, we determined that VTA DA neurons excited by fr stimulation projected to the VST as well as the PFC. The presence of fr-induced excitation of VST-projecting neurons contrasts with previous results in mice showing that habenular efferents preferentially synapse on VTA DA neurons projecting to the PFC (Lammel et al. 2012). Aside from the potential for species differences, the small number of VST-projecting VTA neurons sampled both here and in Lammel et al. (2012) does not enable us to know how prevalent this projection is. In any case, that projections via the fr synapse on some VST-projecting DA neurons is clear, although their significance remains unknown at this point.
Given that the DA system undergoes considerable changes in firing rate (McCutcheon et al. 2013), afferent input (Yetnikoff et al. 2014), and terminal field structure (O'Donnell 2010) during maturation, we also considered the possibility that fr-induced excitation was uniquely associated with younger rats. However, this proved not to be the case as the proportion of VTA neurons showing excitation was similar in adult rats. Given that these results were obtained using extracellular single unit recording techniques the possibility that the excitatory response was related to nonspecific neuronal changes induced by patching onto a cell can be rejected. We can also exclude the possibility of excitation by passive current spread as stimulation of the adjacent thalamus failed to produce excitation. From this series of experiments, combined with the finding that fr-induced excitation was reduced by glutamatergic blockade, we conclude that the excitation reported is indicative of a direct habenula-VTA pathway.
The proportion of neurons excited by fr stimulation is considerably higher than what might have been predicted based on estimates that only 16% of LHb efferents make direct synaptic connections with VTA neurons (Omelchenko et al. 2009). The comparatively high proportion of VTA neurons excited by LHb stimulation relative to the small number of efferents that form conventional synaptic connections imply that a single LHb fiber is likely to innervate a large number of VTA neurons. As discussed above, it is also possible that the region of the VTA in which we conducted our recordings receives a disproportionate input from habenular efferents. While it has been suggested that LHb axons could make en passant connections with their VTA targets (Omelchenko et al. 2009), the majority of neurons we recorded exhibited discrete eEPSPs. In addition, spikes elicited by fr stimulation exhibited minimal jitter, a latency to onset consistent with the conduction velocity of habenular axons (Garland and Mogenson 1983; Park 1987), and could be blocked in neonates or strongly attenuated in adults by bath application of DNQX and APV. Taken together, these data strongly suggest that the excitatory effects of fr stimulation on VTA neurons are mediated by a monosynaptic, glutamatergic connection with LHb projection neurons.
The results of the present study, while comparable to experiments done in slices (Lammel et al. 2012; Matsuda and Fujimura 1992), are orthogonal to results obtained in vivo. In anesthetized rats, single pulse stimulation of the LHb has been repeatedly shown to transiently inhibit the activity of 90–95% of putative DA-containing neurons in the VTA and SN (Christoph et al. 1986; Gao et al. 1990; Ji and Shepard 2007), effects that are substantially blocked by excitotoxic lesions of the habenula (Christoph et al. 1986) or electrolytic lesions of the fr (Ji and Shepard 2007). The absence of habenular GABAergic projection neurons (Brinschwitz et al. 2010) indicates that the inhibition observed in vivo is mediated by a disynaptic pathway and a feedforward inhibitory mechanism (Balcita-Pedicino et al. 2011). While GABAergic neurons within the VTA are targeted by glutamatergic LHb efferents (Omelchenko et al. 2009) and could potentially account for this feedforward inhibition (Tan et al. 2012; van Zessen et al. 2012), the scarcity of that innervation (Omelchenko et al. 2009) and absence of a robust inhibition of DA VTA neurons in the present study and previous studies (Lammel et al. 2012; Matsuda and Fujimura 1992) do not support a role for these neurons in mediating the population level inhibition in DA cell firing typically observed in vivo. Rather, recent evidence favors the involvement of a cluster of GABAergic neurons distributed within the RMTg in mediating LHb-induced inhibition of midbrain DA neurons. RMTg neurons, which are positioned caudally to the VTA, receive a strong projection from lateral aspects of the LHb and, in turn, project massively to the ventral midbrain (Jhou et al. 2009b; Kaufling et al. 2009). Findings that selective stimulation of LHb efferents to the RMTg in vitro (Lammel et al. 2012) or the RMTg in vivo (Hong et al. 2011; Lecca et al. 2012) inhibits the activity of VTA DA neurons is evidence in support of an LHb-RMTg-VTA inhibitory circuit.
If the RMTg provides the principal source of feedforward inhibitory input to the VTA from the LHb, we reasoned that sagittal slices, which preserved the integrity of the connections between these structures, would mimic the effects of LHb stimulation in vivo. However, the responses of VTA neurons in our slices, which included the RMTg, were nearly identical to those obtained in transverse coronal sections, which did not (Lammel et al. 2012; Matsuda and Fujimura 1992). Inhibitory responses were rare, and knife cuts separating the RMTg from the VTA failed to alter the response of VTA neurons to fr stimulation. Hypothesizing that the RMTg may not be present or functionally expressed in the neonatal rats that served as subjects in our first set of experiments, we tested the ability of mAMPH to increase c-Fos expression in this structure. In agreement with results obtained in adult animals (Jhou et al. 2009a; Kaufling et al. 2009), a single injection of the psychostimulant, but not vehicle, increased c-Fos expression in the region immediately caudal to the VTA, suggesting that the RMTg is functionally expressed in the neonatal slice preparation. However, the mere presence of neurons in the mesotegmentum that express c-Fos in response to mAMPH (i.e., RMTg neurons) does not necessarily indicate that the pathway between this region and the VTA is fully functional in the neonatal slice. The prevailing excitatory response of VTA neurons to LHb stimulation indicates that the projection from the LHb to the VTA remained at least partially intact; however, projections from the LHb to the RMTg or from the RMTg to the VTA could have been damaged or severed in the process of preparing these slices. Given the thickness of the slice as well as the magnitude and topography of the LHb projection to the RMTg and ascending projections from the RMTg to the VTA (Jhou et al. 2009b; Kaufling et al. 2009), it is difficult to explain the near total absence of an inhibitory response to LHb stimulation in slices that appear to include a functional RMTg. More so, since previous experiments have shown functional connectivity between the RMTg and VTA DA neurons in the sagittal rat brain slice preparations (Matsui and Williams 2011), the complete loss of this particular connection in our preparation appears to be unlikely.
It remains to be determined how and why the pronounced excitatory influence of habenular efferents on VTA neurons is so strongly attenuated in the intact animal. Several lines of evidence favor the interpretation that much of the available excitatory input to the VTA arising from the LHb is ordinarily masked or attenuated by a pronounced inhibitory input, especially from the RMTg. Excitatory inputs from the LHb often target smaller, presumably more distal, dendrites of VTA neurons (Omelchenko et al. 2009), while RMTg axons are directed toward larger, presumably more proximal, sites (Balcita-Pedicino et al. 2011). In vivo, local application of GABA receptor blockers dramatically increase the proportion of DA neurons excited by LHb stimulation (Ji and Shepard, 2007). Nearly identical results have recently been observed in response to excitotoxic lesions of the RMTg (Shepard et al. 2015, Abstract from Society for Neuroscience Annual Meeting). Such latent excitatory drive from the LHb to the ventral midbrain suggests that changes in the tonic activity of RMTg neurons may cause the emergence of this excitation and consequently alter the response valence of midbrain DA neurons to aversive or salient stimuli.
GRANTS
This work was supported by a National Association for Research on Schizophrenia and Depression Independent Investigator Award (to P. D. Shepard), National Institute of Mental Health Grants R01-MH-094489 (to P. D. Shepard) and T32-MH-067533, and a Ruth L. Kirschstein National Research Service Award F31-DA-030893 from the National Institute on Drug Abuse (to P. L. Brown).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.L.B. and P.D.S. conception and design of research; P.L.B. performed experiments; P.L.B. and P.D.S. analyzed data; P.L.B. and P.D.S. interpreted results of experiments; P.L.B. and P.D.S. prepared figures; P.L.B. and P.D.S. drafted manuscript; P.L.B. and P.D.S. edited and revised manuscript; P.L.B. and P.D.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors extend thanks to Dr. Anne Marie Brady for demonstrating the neonatal surgical techniques used here.
This work was submitted to the University of Maryland-Baltimore in partial fulfillment of the degree requirements for the Doctor of Philosophy of P. L. Brown.
REFERENCES
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new methd for obtaining viable motoneurons in adult rat brain slices. Synapse 3: 331–338, 1989. [DOI] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: Ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol 519: 1143–1164, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid AL, Jeaugey L. Cells of the rat lateral habenula respond to high-threshold somatosensory inputs. Neurosci Lett 96: 289–294, 1989. [DOI] [PubMed] [Google Scholar]
- Blanchard V, Raisman-Vozari R, Vyas S, Michel PP, Javoy-Agid F, Uhl G, Agid Y. Differential expression of tyrosine hydroxylase and membrane dopamine transporter genes in subpopulations of dopaminergic neurons of the rat mesencephalon. Brain Res Mol Brain Res 22: 29–38, 1994. [DOI] [PubMed] [Google Scholar]
- Brinschwitz K, Dittgen A, Madai VI, Lommel R, Geisler S, Veh RW. Glutamatergic axons from the lateral habenula mainly terminate on GABAergic neurons of the ventral midbrain. Neuroscience 168: 463–476, 2010. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci USA 106: 4894–4899, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AM, McCallum SE, Glick SD, O'Donnell P. Enhanced methamphetamine self-administration in a neurodevelopmental rat model of schizophrenia. Psychopharmacology 200: 205–215, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LL, Siegel H, Etgen AM. Global sex differences in stress-induced activation of cerebral metabolism revealed by 2-deoxyglucose autoradiography. Horm Behav 30: 611–617, 1996. [DOI] [PubMed] [Google Scholar]
- Brown PL, Shepard PD. Lesions of the fasciculus retroflexus alter footshock-induced cFos expression in the mesopontine rostromedial tegmental area of rats. PLos One 8: e60678, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavier CC, Oprisan SA, Callaway JC, Ji H, Shepard PD. Computational model predicts a role for ERG current in repolarizing plateau potentials in dopamine neurons: implications for modulation of neuronal activity. J Neurophysiol 98: 3006–3022, 2007. [DOI] [PubMed] [Google Scholar]
- Chitwood RA, Hubbard A, Jaffe DB. Passive electrotonic properties of rat hippocampal CA3 interneurones. J Physiol 515: 743–756, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci 6: 613–619, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482: 85–88, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals (8th ed). Washington, DC: National Academic Press, 2011. [Google Scholar]
- Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res 85: 363–402, 1990. [DOI] [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic sensory transmission in brain stem neurons in vitro. J Neurophysiol 85: 2213–2223, 2001. [DOI] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci 26: 2788–2797, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao DM, Jeaugey L, Pollak P, Benabid AL. Intensity-dependent nociceptive responses from presumed dopaminergic neurons of the substantia nigra, pars compacta in the rat and their modification by lateral habenula inputs. Brain Res 529: 315–319, 1990. [DOI] [PubMed] [Google Scholar]
- Garland JC, Mogenson GJ. An electrophysiological study of convrgence of entopeduncular and lateral preoptic inputs on lateral habenular neurons projecting to the midbrain. Brain Res 263: 33–41, 1983. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci 27: 5730–5743, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves L, Sego C, Metzger M. Differential projections from the lateral habenula to the rostromedial tegmental nucleus and ventral tegmental area in the rat. J Comp Neurol 520: 1278–1300, 2012. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Kapp BS. An electrophysiological characterization of ventral tegmental area dopaminergic neurons during differential pavlovian fear conditioning in the awake rabbit. Behav Brain Res 99: 169–179, 1999. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol 187: 19–47, 1979. [DOI] [PubMed] [Google Scholar]
- Hong S, Jhou T, Smith M, Saleem K, Hikosaka O. Negative reward signals from lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci 31: 11457–11471, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 61: 786–800, 2009a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol 513: 566–596, 2009b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, Hoffman AF, Lupica CR, Ikemoto S. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci 33: 7501–7512, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABAA receptor-mediated mechanism. J Neurosci 27: 6923–6930, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol 513: 597–621, 2009. [DOI] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57: 760–773, 2008. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 70: 855–862, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491: 212–217, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M. Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology 37: 1164–1176, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Soffe SR, Roberts A. Glutamate and acetylcholine corelease at developing synapses. Proc Natl Acad Sci USA 101: 15488–15493, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Qi J, Yamaguchi T, Wang HL, Morales M. Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain Struct Funct 218: 1159–1176, 2013. [DOI] [PubMed] [Google Scholar]
- MacGregor DG, Chesler M, Rice ME. HEPES prevents edema in rat brain slices. Neurosci Lett 303: 141–144, 2001. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol 577: 907–924, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Fujimura K. Action of habenular efferents on ventral tegmental area neurons studied in vitro. Brain Res Bull 28: 743–749, 1992. [DOI] [PubMed] [Google Scholar]
- Matsui A, Williams JT. Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J Neurosci 31: 17729–17735, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci 12: 77–84, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Conrad KL, Carr SB, Ford KA, McGehee DS, Marinelli M. Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. J Neurophysiol 108: 1620–1630, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature 379: 449–451, 1996. [DOI] [PubMed] [Google Scholar]
- Mirrione MM, Schulz D, Lapidus KA, Zhang S, Goodman W, Henn FA. Increased metabolic activity in the septum and habenula during stress is linked to subsequent expression of learned helplessness behavior. Front Hum Neurosci 8: 29, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci 16: 1936–1947, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P. Adolescent maturation of cortical dopamine. Neurotoxicity Res 18: 306–312, 2010. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci 30: 1239–1250, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MR. Monosynaptic inhibitory postsynaptic potentials from lateral habenula recorded in dorsal raphe neurons. Brain Res Bull 19: 581–586, 1987. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Calu DJ, Schoenbaum G. Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards. Nat Neurosci 10: 1615–1624, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Esber GR, Li J, Daw ND, Schoenbaum G. Surprise! Neural correlates of Pearce-Hall and Rescorla-Wagner coexist within the brain. Eur J Neurosci 35: 1190–1200, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol 80: 1–27, 1998. [DOI] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Kiyokage E, Szabo G, Shipley MT. Two GABAergic intraglomerular circuits differentially regulate tonic and phasic presynaptic inhibition of olfactory nerve terminals. J Neurophysiol 101: 1988–2001, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe D. Your chi-square test is statistically significant: now what? Pract Assess Res Eval 20: 2–10, 2015. [Google Scholar]
- Shepard PD, Bunney BS. Repetitive firing properties of putative dopamine-containing neurons in vitro: regulation by an apamin-sensitive Ca2+-activated K+ conductance. Exp Brain Res 86: 141–150, 1991. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Timiras PS. A Stereotaxic Atlas of the Developing Rat Brain. Berkley, CA: Univ. of California Press, 1970. [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci 15: 1105–1107, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Tse MT, Montes DR, Wiedman CR, Floresco SB. Overriding phasic dopamine signals redirects action selection during risk/reward decision making. Neuron 84: 177–189, 2014. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev 6: 1–13, 1982. [DOI] [PubMed] [Google Scholar]
- Tan KR, Yvon D, Turiault M, Mirzabekhov JJ, Doehner J, Labouebe G, Deisseroth K, Tye KM, Luscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron 73: 1173–1183, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of mesocortical DA system by stress. Nature 263: 242–244, 1976. [DOI] [PubMed] [Google Scholar]
- Ting JT, Daigle TL, Chen Q, Feng G. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol Biol 1183: 221–242, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 303: 2040–2042, 2004. [DOI] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron 73: 1184–1194, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DV, Tsien JZ. Convergent processing of both positive and negative motivational signals by the VTA dopamine neuronal populations. PLos One 6: e17047, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfart J, Neuhoff H, Franz O, Roeper J. Differential expression of the small-conductance, calcium-activated potassium channel SK3 is critical for pacemaker control in dopaminergic midbrain neurons. J Neurosci 21: 3443–3456, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Qi J, Wang HL, Zhang S, Morales M. Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area. Eur J Neurosci 41: 760–772, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetnikoff L, Reichard RA, Schwartz ZM, Parsely KP, Zahm DS. Protracted maturation of forebrain afferent connections of the ventral tegmental area in the rat. J Comp Neurol 522: 1031–1047, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]