Abstract
Background:
Protecting the upper airway from microbial infection is an important function of the immune system. Proper detection of these pathogens is paramount for sinonasal epithelial cells to be able to prepare a defensive response. Toll-like receptors and, more recently, bitter taste receptors and sweet taste receptors have been implicated as sensors able to detect the presence of these pathogens and certain compounds that they secrete. Activation of these receptors also triggers innate immune responses to prevent or counteract infection, including mucociliary clearance and the production and secretion of antimicrobial compounds (e.g., defensins).
Objective:
To provide an overview of the current knowledge of the role of innate immunity in the upper airway, the mechanisms by which it is carried out, and its clinical relevance.
Methods:
A literature review of the existing knowledge of the role of innate immunity in the human sinonasal cavity was performed.
Results:
Clinical and basic science studies have shown that the physical epithelial cell barrier, mucociliary clearance, and antimicrobial compound secretion play pivotal innate immune roles in defending the sinonasal cavity from infection. Clinical findings have also linked dysfunction of these defense mechanisms with diseases, such as chronic rhinosinusitis and cystic fibrosis. Recent discoveries have elucidated the significance of bitter and sweet taste receptors in modulating immune responses in the upper airway.
Conclusion:
Numerous innate immune mechanisms seem to work in a concerted fashion to keep the sinonasal cavity free of infection. Understanding sinonasal innate immune function and dysfunction in health and disease has important implications for patients with respiratory ailments, such as chronic rhinosinusitis and cystic fibrosis.
Keywords: Cilia, defensin, innate immunity, sinusitis, T2R, taste receptor, TLR
The immune system's ability to recognize and communicate information to the nervous system about the presence of viruses and bacteria has earned it a label as the so-called sixth sense.1–3 Because humans are constantly being exposed to a myriad of potentially pathogenic organisms, it is important for the body to be able to recognize the presence of these organisms so that an adequate counterattack can be mounted against them. As with the other senses, there must be mechanisms by which signal transduction occurs. The purpose of this review was to summarize the current state of knowledge of these innate defenses and the signal transduction mechanisms that control them.
In upper respiratory diseases, e.g., chronic rhinosinusitis (CRS), the inability to properly identify, destroy, and clear the airway of harmful bacteria can lead to recurrent bacterial infection, biofilm formation, and/or persistent inflammation of the paranasal sinuses.4 Although multiple etiologies are associated with CRS, a common pathophysiologic sequela is ineffective sinonasal mucociliary clearance,4 a mechanism by which debris is removed from the airway lumen in the nondiseased state. Symptomatically, CRS is associated with nasal congestion, rhinorrhea, sinus pressure, and a decreased sense of smell that persists for >12 weeks.5 Due to the chronic nature of the disease, CRS requires prolonged treatment and drastically decreases patients' quality of life.4 Although CRS affects ∼1 in 20 adults in the United States6 and accounts for >$8 billion in direct health care costs,7–9 there are less prevalent but nonetheless critically important diseases, e.g., cystic fibrosis, that also cause recurrent upper airway infection and sinonasal inflammation.4,5 To better treat these diseases, a more thorough understanding of the function of innate immunity in the upper airway in the healthy state and its dysfunction in the pathologic state is required.
Confounding our ability to make sense of the innate immune system and its role in defending the sinonasal cavity against pathogens is the fact that, much like the gut, the upper airway has a resident microbiota of commensal nonpathogenic bacteria that maintains a healthy environment. Thus, the cells of the upper airway must be able to differentiate between healthy bacteria and pathogenic bacteria. Although recent studies used molecular diagnostics in an attempt to determine the makeup of the microbiome of the human sinonasal cavity in both healthy patients (i.e., those without CRS) and patients with disease, there has not been consistent evidence to indicate that any specific microorganisms are causative of the diseased condition.5 There, however, is evidence to indicate that there is dysbiosis, or a microbial imbalance, in addition to reduced microbial diversity associated with CRS.5 When local inflammation is the result of bacterial community dysbiosis, this indicates that the natural balance of microorganisms is immunomodulatory.10
METHODS
Existing reviews about sinonasal innate immunity as well as primary publications of which we were aware were used as the initial source of information for this systematic review. In addition to these resources, PubMed was used as the primary data base to conduct keyword searches for relevant publications. Example search terms included “sinonasal innate immunity,” “chronic rhinosinusitis pathogenesis,” and “antimicrobial compounds.” No exclusion criteria for articles were used. Ultimately, 85 publications were selected for inclusion in the systematic review.
The Epithelial Cell Barrier as the First Line of Defense
Sinonasal mucosal epithelial cells adhere to one another to form a physical barrier that protects the underlying sinonasal tissue from inhaled pathogens, allergens, and other airborne irritants. Cell junctions, including desmosomes, adherens junctions, and tight junctions, are the intercellular connections responsible for cellular adhesion, which make such a physical barrier possible.5 For patients with CRS, however, there is evidence to indicate that this barrier is not fully functional. A decrease in expression of the tight junction proteins zonula occludens-1 and occludin was found in specimens from patients with CRS with nasal polyps compared with those from healthy controls.11 Air-liquid interface cultures from patients with CRS with nasal polyps also demonstrated decreased transepithelial electrical resistance compared with healthy controls.11 There are conflicting beliefs as to whether epithelial cells in patients with CRS are intrinsically faulty or, instead, have dysfunction due to certain environmental exposures or a response to internal stimuli.5 Pathogenic microbes have also been observed to play a role as external actors that harm the integrity of the sinonasal epithelial cell barrier by producing proteases that can cleave tight junction proteins and/or activate epithelial changes through protease-activated receptors.12–15 This, in turn, could further exacerbate microbial infection, colonization, and biofilm formation.5
Mucociliary Clearance: Mopping Away Bugs and Debris
Although coughing and sneezing serve supplementary reflex functions for the lung and sinonasal cavity, respectively, mucociliary clearance is the primary mechanism by which the airway epithelium removes pathogens and debris from the airway lumen (Fig. 1). It is a specialized function made up of two complementary components: mucus production and mucus transport. The airway surface liquid that sits atop the epithelium consists of two layers. The superficial layer is composed of an antimicrobial-rich mucus “gel,” which is composed of glycosylated mucin proteins produced by the mucous cells of the submucosal exocrine glands16,17 and epithelial goblet cells.18–20 The carbohydrate side chains of the mucins serve as “sticky” binding sites that trap inhaled pathogens and irritants. Below the mucus layer is a less-viscous periciliary fluid layer that permits the submerged cilia of the airway epithelial cells to beat rapidly at a frequency of ∼8–15 Hz. Perpetual, coordinated ciliary beating enables transport of the overlying mucus layer, along with any trapped debris and pathogens, to the oropharynx. The mucus and particulate matter are then swallowed or expectorated.
Figure 1.
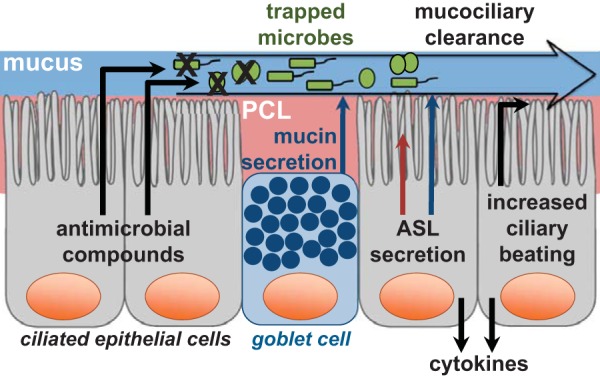
Mechanisms involved in sinonasal epithelial innate immunity. Airway surface liquid (ASL) is made up of two layers: A mucus layer (top) and a periciliary fluid layer (PCL) (bottom). The mucus layer is largely composed of sticky mucin proteins produced by goblet cells and submucosal exocrine glands (not depicted) that trap inhaled pathogens. Ciliated epithelial cells similarly are responsible for regulating ASL secretion into both the mucus layer and the PCL as well as regulating ciliary beating. Ciliary beating is facilitated by the PCL, which surrounds the cilia and allows them to beat rapidly, and drives mucociliary clearance, which removes trapped pathogens from the sinonasal cavity. Ciliated epithelial cells also secrete antimicrobial peptides and reactive oxygen and nitrogen species capable of directly killing pathogenic microbes. In cases of prolonged pathogenic exposure, sinonasal epithelial cells secrete cytokines, which activate an inflammatory response and recruit immune cells.
Small molecule neurotransmitter and neuropeptide receptors are largely responsible for regulating ciliary beating21 and airway surface liquid secretion.22 Other receptors also regulate mucociliary clearance, including, e.g., the bitter taste receptor (T2R) T2R38 (discussed below in Regulation of Antimicrobial Compounds) that binds bacterial metabolites23 and those receptors that respond to mechanical stimulation.24 Studies found that dehydrated mucus, as occurs in patients with cystic fibrosis,22,25,26 and increased mucus production27 can lead to mucociliary clearance dysfunction. Abnormalities that affect epithelial cilia function may also result in impaired mucociliary clearance.28,29
Further, physical obstruction of sinus ostia or mucostasis can cause hypoxic conditions within the cellular environment, which inhibits ion transport30 or induces nasal polypogenesis,31 either of which can lead to decreased mucociliary clearance. In fact, there is evidence that these near-anaerobic conditions may significantly contribute to the pathogenesis of CRS.30,32–34 Regardless of the mechanism by which mucociliary clearance dysfunction arises, the inability to effectively clear the sinonasal cavity of inhaled pathogens and debris via mucociliary clearance is likely to play a role in the development of this chronic condition. For this reason, therapeutic approaches intended to increase ciliary beating or regulate airway surface liquid secretion remain attractive targets for treatment of CRS.
Antimicrobial Peptides and Radicals
Simultaneously carrying out mucociliary transport and functioning as a physical barrier, sinonasal epithelial cells also generate and secrete antimicrobial compounds to directly counteract pathogens (Fig. 1). These compounds have various antibacterial, antifungal, and antiviral effects, and include proteins such as lysozyme, lactoferrin, defensins, and cathelicidins as well as reactive oxygen and nitrogen species, e.g., nitric oxide (NO).35 Although some of these substances are secreted tonically, many demonstrate upregulated expression during active infection.5
Lysozyme is an enzyme present in many tissues and almost all bodily fluids, especially mucus, saliva, tears, gastric juice, and mother's milk.36,37 It catalyzes the hydrolysis of the β-1,4-glycosidic linkages in the peptidoglycan layer of bacterial cell walls.38 For this reason, lysozyme has a higher affinity for gram-positive bacteria due to their thick peptidoglycan; however, effects against gram-negative bacteria39,40 and fungi41 have also been observed. Several studies found that the amount of lysozyme produced by epithelial cells is well correlated with the elimination of pathogens.42 In the study of CRS, there remains some debate as to whether lysozyme levels are increased or decreased.5
Lactoferrin functions to chelate essential iron away from bacteria and fungi, which they use for metabolism.42–44 As a cofactor for oxidation-reduction reactions,43 iron is used by bacteria to catalyze mucin degradation, which aids the bacteria in penetrating the mucosal barrier and colonizing the airway.5 However, lactoferrin also has direct antimicrobial effects. By chelating iron, lactoferrin inhibits the development of biofilms in Pseudomonas aeruginosa, an opportunistic sinonasal pathogen, by stimulating a specialized type of surface motility known as bacterial twitching.45 Lactoferrin also binds bacteria directly via conserved structures known as pathogen-associated molecular patterns, such as lipopolysaccharide in the gram-negative bacteria cell wall and unmethylated 5'–C–phosphate–G–3' dinucleotide sites in bacterial DNA.5 In doing so, lactoferrin may prevent these pathogen-associated molecular patterns from binding to proinflammatory receptors in the airway.46 This anti-inflammatory response can help prevent the harmful effects of chronic inflammation, e.g., tissue damage, which can result in more-extensive pathogenic colonization. Through the binding of lipopolysaccharide, lactoferrin can disrupt the membrane of gram-negative pathogens and work in tandem with lysozyme to kill these bacteria by giving the enzyme access to attack peptidoglycan.42,47 In CRS, there is evidence that lactoferrin levels are decreased, particularly in patients with bacterial biofilms.5,48
Defensins are an antimicrobial peptide family distributed rather ubiquitously in mammalian epithelial cells and phagocytes.49 Members of both defensin subfamilies, α- and β-defensins, are expressed in sinonasal epithelial cells. They have far-reaching antimicrobial properties, which are capable of affecting membrane permeabilization in both bacteria and fungi, and of exhibiting a wide range of antiviral effects, including directly attacking viral envelopes, capsids, and glycoproteins, inhibiting viral entry, and preventing viral replication.5,50–52 Defensin expression is upregulated in the airway epithelium on pathogenic bacterial or viral exposure,53,54 and elevated levels of β-defensins correlate with inflammation in patients with cystic fibrosis.42
Cathelicidins are another major class of antimicrobial peptides, although LL-37 is the only cathelicidin member found in humans. LL-37 is produced in the human nasal mucosa and has extensive antimicrobial properties. In animal models of CRS, LL-37 has been found to exhibit effects against P. aeruginosa biofilms and to diminish their bacterial counts.55 Similarly, it has been shown to restore bacterial killing of P. aeruginosa and Staphylococcus aureus to noncystic fibrosis levels when overexpressed in a cystic fibrosis animal model.56 LL-37 may also have anti-inflammatory properties, which counteract the effects of the gram-negative endotoxin lipopolysaccharide.57 Recent research has focused on the relationship between vitamin D and the regulation of the LL-37 gene in sinonasal epithelial cells58,59 and the implications of this in innate immunity,60 CRS, and allergic rhinitis.61–64
Reactive oxygen and nitrogen species generated by the sinonasal epithelium are also thought to play an essential role in upper airway innate immunity. NO in the upper airway originates predominantly from the paranasal sinuses, particularly the maxillary sinuses.65 NO is formed from arginine in a reaction catalyzed by several isoforms of the enzyme NO synthase, which are expressed in cilia and microvilli of epithelial cells.66 Reactive species such as NO and its derivatives, including peroxynitrite formed readily on reaction of NO with superoxide,67 have been shown to exhibit antimicrobial properties. There is evidence that reactive species can damage bacterial DNA, modify proteins,68,69 and prevent viral replication.70 Although the production of NO has been implicated in some studies as an important mechanism for host defense,71–73 others indicate that it may have damaging effects on the host.74 Perhaps there is a delicate balance between NO levels that provide beneficial protection against pathogenic microbes and those levels that contrastingly enhance microbial growth.
Regulation of Antimicrobial Compounds
To mount an appropriate immune response, there must be mechanisms by which the cells of the sinonasal epithelium can detect and react in a measured manner to potentially harmful threats in the airway. Toll-like receptors (TLR) are one mechanism by which this is accomplished. TLRs recognize pathogen-associated molecular patterns, discussed previously, such as lipoteichoic acid in gram-positive bacteria, lipopolysaccharide in gram-negative bacteria, bacterial DNA, and the bacterial protein flagellin.35 There are ∼10 TLRs expressed in sinonasal epithelial cells, and their levels of expression are altered in patients with CRS.71,75 When stimulated, TLRs upregulate transcription and/or translation of antimicrobial peptides and mucin proteins that make up the mucus layer of airway surface liquid and trap pathogens.42,76 These responses occur over several hours to days. As such, the function of TLRs in this capacity is likely to combat prolonged infection or colonization. TLRs, on stimulation, also trigger the release of cytokines and chemokines, which serve to activate an inflammatory response and recruit immune cells (Fig. 1). There is evidence that patients with CRS, compared with healthy controls, produce less interleukin 8, a chemokine, in response to TLR agonists.5
In addition to TLRs, there is an evolutionarily newer class of receptors that have recently been discovered to play a role in upper airway innate immunity. Recent studies23,76 demonstrated that the mammalian “sixth sense” immune system actually uses components of the traditional sensory signal transduction pathways, viz., chemosensory taste receptors that fall under the superfamily of G-protein-coupled receptors. Although originally identified as “taste” receptors on the tongue, two subfamilies of taste G-protein-coupled receptors have now been found in many other tissues throughout the body.35 In the airway, T2Rs and sweet taste receptors (T1R) have recently been demonstrated to serve a defensive role that guards the airway against infection.77 The discovery of T2Rs in sinonasal epithelial cells was quite interesting to researchers, given the ability of T2Rs to detect the presence and defend against the ingestion of harmful compounds in the oral cavity.78 In humans, there are ∼25 functional isoforms of T2Rs.78
Although multiple T2Rs have been demonstrated to be expressed in the sinonasal cavity, one isoform expressed in ciliated epithelial cells, T2R38, has been demonstrated in vitro to affect an increase in mucociliary clearance and the production of bactericidal amounts of NO on stimulation by physiologic concentrations of acyl-homoserine lactone quorum sensing molecules used by gram-negative bacteria, e.g., P. aeruginosa, to communicate with one another (Fig. 2).23 The gene encoding T2R38, TAS2R38, has two common polymorphisms in white populations, one that encodes a functional form of the receptor and the other encodes a nonfunctional form.78 The in vitro studies that identified T2R38 as the host protein receptor for acyl-homoserine lactone quorum sensing molecules were complemented by subsequent in vivo studies that correlated the nonfunctional form of T2R38 with susceptibility to CRS78–82 and gram-negative upper airway infection.23 T2Rs are also expressed in solitary chemosensory cells, another cell type of the sinonasal epithelium. Activation of solitary chemosensory cells via T2Rs triggers a calcium signal that propagates to other epithelial cells, which causes the rapid secretion of antimicrobial peptides (Fig. 3). As opposed to the much slower action of TLRs, T2Rs seem to constitute a more immediate response mechanism of the sinonasal innate immune system.4
Figure 2.
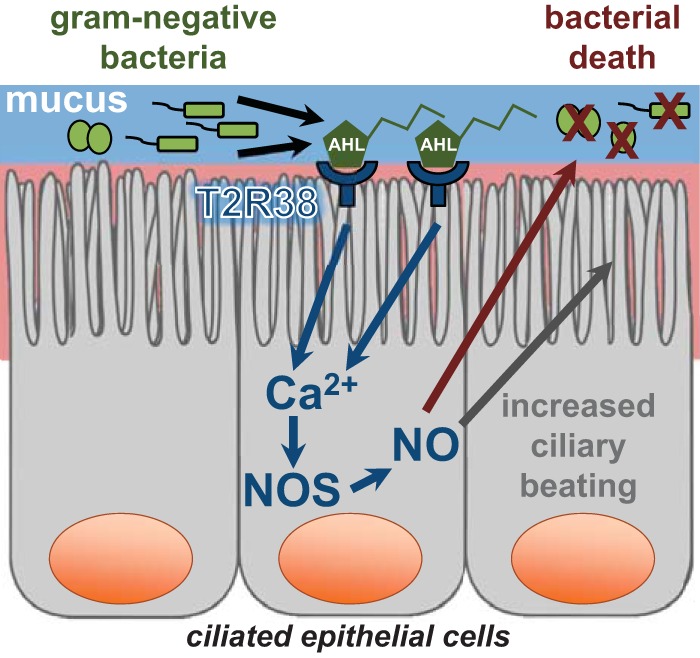
Bitter taste receptor T2R38 innate immune signaling pathway in sinonasal ciliated epithelial cells. Gram-negative bacteria secrete acyl-homoserine lactone quorum sensing molecules (AHL) as a form of microbial communication. These bitter compounds bind to the bitter taste receptor T2R38, expressed in sinonasal ciliated epithelial cells, which activates a biochemical cascade and leads to a calcium (Ca2+) signal that mediates nitric oxide synthase (NOS) dependent nitric oxide (NO) production. NO upregulates ciliary beating, enhances mucociliary clearance of trapped pathogens, and directly kills bacteria after diffusing into the airway surface liquid.
Figure 3.
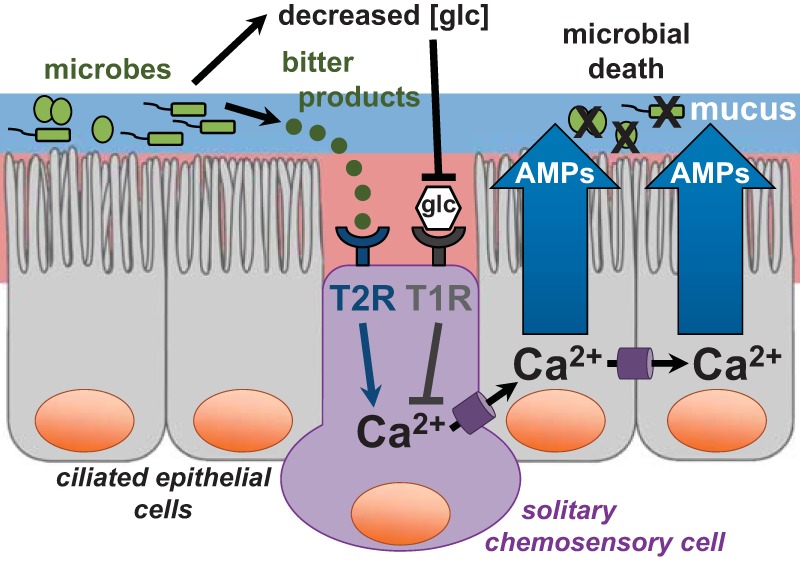
Taste receptors expressed in solitary chemosensory cells regulate an innate immune response. The presence of pathogens in the upper airway has a two-pronged effect that mediates a calcium (Ca2+) response in solitary chemosensory cells. Yet unidentified bitter compounds secreted by microbes bind to and activate bitter taste receptors (T2R). The resulting calcium signal propagates to surrounding epithelial cells, which results in the rapid secretion of antimicrobial peptides (AMP) that directly kill pathogenic microbes. This pathway is attenuated by physiologic glucose levels in the airway surface liquid via sweet taste receptors (T1R2/3), which suppress the calcium response. However, during times of active infection, pathogens consume glucose, which lower levels and facilitates activation of the T2R pathway.
There also are T1R2/3s expressed in solitary chemosensory cells, which evidence suggests may inhibit antimicrobial peptide secretion, in a response, antagonistic to the T2R response, when stimulated by glucose or artificial sweeteners. Even at physiologically low glucose levels in the airway surface liquid, such as in healthy patients (i.e., those without CRS and diabetes mellitus), this T1R pathway likely serves as a method to suppress activation of the T2R pathway. During times of active infection, glucose levels are lowered further due to pathogen consumption, and the T2R pathway can be activated.4 This pathway may be particularly important for patients with CRS and/or diabetes mellitus because they exhibit elevated airway surface liquid glucose levels.76,83–85
CONCLUSION
Although great strides have recently been made in understanding the role of the innate immune system in the upper airway, there remains much to learn. To better substantiate our knowledge of the complex interactions among pathogens, their products, and the human immune response, questions regarding individuals' unique sinonasal microbiomes and combinations of TLR and taste receptor polymorphisms must be answered. The origins and ramifications of epithelial cell barrier malfunction, mucociliary clearance dysfunction, and differential regulation of antimicrobial compound secretion in the diseased state must continue to be studied so that we might have a better understanding of their respective defensive functions when everything is occurring as it should. It is clear that, in healthy individuals, there are numerous mechanisms at work to keep the sinonasal cavity and, as a result, the lower airway, free of microbial infection. Continued research focused on sinonasal innate immunity may lead to beneficial advancements in diagnosing and treating patients with diseases such as CRS and cystic fibrosis.
Footnotes
Presented at the North American Rhinology and Allergy Conference, January 15, 2016, St. Thomas, Virgin Islands
Funding source NIH R01DC013588 and NIH R21DC013886 to N.A.C.
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Blalock JE. The immune system as the sixth sense. J Intern Med 257:126–138, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Blalock JE, Smith EM. Conceptual development of the immune system as a sixth sense. Brain Behav Immun 21:23–33, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Bedford FL. The missing sense modality: The immune system. Perception 40:1265–1267, 2011. [DOI] [PubMed] [Google Scholar]
- 4. Lee RJ, Cohen NA. Sinonasal solitary chemosensory cells “taste” the upper respiratory environment to regulate innate immunity. Am J Rhinol Allergy 28:366–373, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Stevens WW, Lee RJ, Schleimer RP, Cohen NA. Chronic rhinosinusitis pathogenesis. J Allergy Clin Immunol 136:1442–1453, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Orlandi RR, Kingdom TT, Hwang PH, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol 6(suppl. 1):S22–S209, 2016. [DOI] [PubMed] [Google Scholar]
- 7. Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl 2012:3 p preceding table of contents, 1–298, 2012. [PubMed] [Google Scholar]
- 8. Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. Ann Otol Rhinol Laryngol 120:423–427, 2011. [DOI] [PubMed] [Google Scholar]
- 9. Bhattacharyya N, Grebner J, Martinson NG. Recurrent acute rhinosinusitis: Epidemiology and health care cost burden. Otolaryngol Head Neck Surg 146:307–312, 2012. [DOI] [PubMed] [Google Scholar]
- 10. Ivanov II, Frutos Rde L, Manel N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4:337–349, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soyka MB, Wawrzyniak P, Eiwegger T, et al. Defective epithelial barrier in chronic rhinosinusitis: The regulation of tight junctions by IFN-gamma and IL-4. J Allergy Clin Immunol 130:1087–1096.e10, 2012. [DOI] [PubMed] [Google Scholar]
- 12. Nomura K, Obata K, Keira T, et al. Pseudomonas aeruginosa elastase causes transient disruption of tight junctions and downregulation of PAR-2 in human nasal epithelial cells. Respir Res 15:21, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malik Z, Roscioli E, Murphy J, et al. Staphylococcus aureus impairs the airway epithelial barrier in vitro. Int Forum Allergy Rhinol 5:551–556, 2015. [DOI] [PubMed] [Google Scholar]
- 14. Rudack C, Steinhoff M, Mooren F, et al. PAR-2 activation regulates IL-8 and GRO-alpha synthesis by NF-kappaB, but not RANTES, IL-6, eotaxin or TARC expression in nasal epithelium. Clin Exp Allergy 37:1009–1022, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev 84:579–621, 2004. [DOI] [PubMed] [Google Scholar]
- 16. Kirkham S, Sheehan JK, Knight D, et al. Heterogeneity of airways mucus: Variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J 361:537–546, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martinez-Anton A, Debolos C, Garrido M, et al. Mucin genes have different expression patterns in healthy and diseased upper airway mucosa. Clin Exp Allergy 36:448–457, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Jackson AD. Airway goblet-cell mucus secretion. Trends Pharmacol Sci 22:39–45, 2001. [DOI] [PubMed] [Google Scholar]
- 19. Groneberg DA, Peiser C, Dinh QT, et al. Distribution of respiratory mucin proteins in human nasal mucosa. Laryngoscope 113:520–524, 2003. [DOI] [PubMed] [Google Scholar]
- 20. Rogers DF. The airway goblet cell. Int J Biochem Cell Biol 35:1–6, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Workman AD, Cohen NA. The effect of drugs and other compounds on the ciliary beat frequency of human respiratory epithelium. Am J Rhinol Allergy 28:454–464, 2014. [DOI] [PubMed] [Google Scholar]
- 22. Lee RJ, Foskett JK. Ca2+ signaling and fluid secretion by secretory cells of the airway epithelium. Cell Calcium 55:325–336, 2014. [DOI] [PubMed] [Google Scholar]
- 23. Lee RJ, Xiong G, Kofonow JM, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest 122:4145–4159, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao KQ, Cowan AT, Lee RJ, et al. Molecular modulation of airway epithelial ciliary response to sneezing. FASEB J 26:3178–3187, 2012. [DOI] [PubMed] [Google Scholar]
- 25. Mall MA, Galietta LJ. Targeting ion channels in cystic fibrosis. J Cyst Fibros 14:561–570, 2015. [DOI] [PubMed] [Google Scholar]
- 26. Illing EA, Woodworth BA. Management of the upper airway in cystic fibrosis. Curr Opin Pulm Med 20:623–631, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seshadri S, Lu X, Purkey MR, et al. Increased expression of the epithelial anion transporter pendrin/SLC26A4 in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol 136:1548–1558.e1–7, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gudis DA, Cohen NA. Cilia dysfunction. Otolaryngol Clin North Am 43:461–472, vii, 2010. [DOI] [PubMed] [Google Scholar]
- 29. Gudis D, Zhao KQ, Cohen NA. Acquired cilia dysfunction in chronic rhinosinusitis. Am J Rhinol Allergy 26:1–6, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blount A, Zhang S, Chestnut M, et al. Transepithelial ion transport is suppressed in hypoxic sinonasal epithelium. Laryngoscope 121:1929–1934, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shin HW, Cho K, Kim DW, et al. Hypoxia-inducible factor 1 mediates nasal polypogenesis by inducing epithelial-to-mesenchymal transition. Am J Respir Crit Care Med 185:944–954, 2012. [DOI] [PubMed] [Google Scholar]
- 32. Baroody FM. Mucociliary transport in chronic rhinosinusitis. Clin Allergy Immunol 20:103–119, 2007. [PubMed] [Google Scholar]
- 33. Pahl A, Szelenyi S, Brune K. Hypoxia induced chemokine expression in nasal epithelial cells: Development of an in vitro model for chronic rhinosinusitis. ALTEX 23:59–63, 2006. [PubMed] [Google Scholar]
- 34. Steinke JW, Woodard CR, Borish L. Role of hypoxia in inflammatory upper airway disease. Curr Opin Allergy Clin Immunol 8:16–20, 2008. [DOI] [PubMed] [Google Scholar]
- 35. Lee RJ, Cohen NA. Taste receptors in innate immunity. Cell Mol Life Sci 72:217–236, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hankiewicz J, Swierczek E. Lysozyme in human body fluids. Clin Chim Acta 57:205–209, 1974. [DOI] [PubMed] [Google Scholar]
- 37. Klockars M, Reitamo S. Tissue distribution of lysozyme in man. J Histochem Cytochem 23:932–940, 1975. [DOI] [PubMed] [Google Scholar]
- 38. Yoshimura K, Toibana A, Nakahama K. Human lysozyme: Sequencing of a cDNA, and expression and secretion by Saccharomyces cerevisiae. Biochem Biophys Res Commun 150:794–801, 1988. [DOI] [PubMed] [Google Scholar]
- 39. Nash JA, Ballard TN, Weaver TE, Akinbi HT. The peptidoglycan-degrading property of lysozyme is not required for bactericidal activity in vivo. J Immunol 177:519–526, 2006. [DOI] [PubMed] [Google Scholar]
- 40. Prokhorenko IR, Zubova SV, Ivanov AY, Grachev SV. Interaction of Gram-negative bacteria with cationic proteins: Dependence on the surface characteristics of the bacterial cell. Int J Gen Med 2:33–38, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Woods CM, Hooper DN, Ooi EH, et al. Human lysozyme has fungicidal activity against nasal fungi. Am J Rhinol Allergy 25:236–240, 2011. [DOI] [PubMed] [Google Scholar]
- 42. Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol 45:189–201, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Howard DH. Acquisition, transport, and storage of iron by pathogenic fungi. Clin Microbiol Rev 12:394–404, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Philpott CC. Iron uptake in fungi: A system for every source. Biochim Biophys Acta 1763:636–645, 2006. [DOI] [PubMed] [Google Scholar]
- 45. Singh PK, Parsek MR, Greenberg EP, Welsh MJ. A component of innate immunity prevents bacterial biofilm development. Nature 417:552–555, 2002. [DOI] [PubMed] [Google Scholar]
- 46. Legrand D. Lactoferrin, a key molecule in immune and inflammatory processes. Biochem Cell Biol 90:252–268, 2012. [DOI] [PubMed] [Google Scholar]
- 47. Ellison RT, III, Giehl TJ. Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest 88:1080–1091, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Psaltis AJ, Wormald PJ, Ha KR, Tan LW. Reduced levels of lactoferrin in biofilm-associated chronic rhinosinusitis. Laryngoscope 118:895–901, 2008. [DOI] [PubMed] [Google Scholar]
- 49. Ganz T. Defensins: Antimicrobial peptides of innate immunity. Nat Rev Immunol 3:710–720, 2003. [DOI] [PubMed] [Google Scholar]
- 50. Wilson SS, Wiens ME, Smith JG. Antiviral mechanisms of human defensins. J Mol Biol 425:4965–4980, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Feng Z, Jiang B, Chandra J, et al. Human beta-defensins: differential activity against candidal species and regulation by Candida albicans. J Dent Res 84:445–450, 2005. [DOI] [PubMed] [Google Scholar]
- 52. Bai X, Tian T, Wang P, et al. Potential roles of placental human beta-defensin-3 and apolipoprotein B mRNA-editing enzyme catalytic polypeptide 3G in prevention of intrauterine transmission of hepatitis B virus. J Med Virol 87:375–379, 2015. [DOI] [PubMed] [Google Scholar]
- 53. Proud D, Sanders SP, Wiehler S. Human rhinovirus infection induces airway epithelial cell production of human beta-defensin 2 both in vitro and in vivo. J Immunol 172:4637–4645, 2004. [DOI] [PubMed] [Google Scholar]
- 54. Harder J, Meyer-Hoffert U, Teran LM, et al. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol 22:714–721, 2000. [DOI] [PubMed] [Google Scholar]
- 55. Chennupati SK, Chiu AG, Tamashiro E, et al. Effects of an LL-37-derived antimicrobial peptide in an animal model of biofilm Pseudomonas sinusitis. Am J Rhinol Allergy 23:46–51, 2009. [DOI] [PubMed] [Google Scholar]
- 56. Bals R, Weiner DJ, Meegalla RL, Wilson JM. Transfer of a cathelicidin peptide antibiotic gene restores bacterial killing in a cystic fibrosis xenograft model. J Clin Invest 103:1113–1117, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Golec M. Cathelicidin LL-37: LPS-neutralizing, pleiotropic peptide. Ann Agric Environ Med 14:1–4, 2007. [PubMed] [Google Scholar]
- 58. Yim S, Dhawan P, Ragunath C, et al. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3). J Cyst Fibros 6:403–410, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sultan B, Ramanathan M, Jr, Lee J, et al. Sinonasal epithelial cells synthesize active vitamin D, augmenting host innate immune function. Int Forum Allergy Rhinol 3:26–30, 2013. [DOI] [PubMed] [Google Scholar]
- 60. Hansdottir S, Monick MM. Vitamin D effects on lung immunity and respiratory diseases. Vitam Horm 86:217–237, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Akbar NA, Zacharek MA. Vitamin D: Immunomodulation of asthma, allergic rhinitis, and chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg 19:224–228, 2011. [DOI] [PubMed] [Google Scholar]
- 62. Mulligan JK, Bleier BS, O'Connell B, et al. Vitamin D3 correlates inversely with systemic dendritic cell numbers and bone erosion in chronic rhinosinusitis with nasal polyps and allergic fungal rhinosinusitis. Clin Exp Immunol 164:312–320, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mulligan JK, White DR, Wang EW, et al. Vitamin D3 deficiency increases sinus mucosa dendritic cells in pediatric chronic rhinosinusitis with nasal polyps. Otolaryngol Head Neck Surg 147:773–781, 2012. [DOI] [PubMed] [Google Scholar]
- 64. Bacchetta J, Salusky IB, Hewison M. Beyond mineral metabolism, is there an interplay between FGF23 and vitamin D in innate immunity? Pediatr Nephrol 28:577–582, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maniscalco M, Sofia M, Pelaia G. Nitric oxide in upper airways inflammatory diseases. Inflamm Res 56:58–69, 2007. [DOI] [PubMed] [Google Scholar]
- 66. Degano B, Valmary S, Serrano E, et al. Expression of nitric oxide synthases in primary ciliary dyskinesia. Hum Pathol 42:1855–1861, 2011. [DOI] [PubMed] [Google Scholar]
- 67. Knight JA. Review: Free radicals, antioxidants, and the immune system. Ann Clin Lab Sci 30:145–158, 2000. [PubMed] [Google Scholar]
- 68. Yoon SS, Coakley R, Lau GW, et al. Anaerobic killing of mucoid Pseudomonas aeruginosa by acidified nitrite derivatives under cystic fibrosis airway conditions. J Clin Invest 116:436–446, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Major TA, Panmanee W, Mortensen JE, et al. Sodium nitrite-mediated killing of the major cystic fibrosis pathogens Pseudomonas aeruginosa, Staphylococcus aureus, and Burkholderia cepacia under anaerobic planktonic and biofilm conditions. Antimicrob Agents Chemother 54:4671–4677, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vareille M, Kieninger E, Edwards MR, Regamey N. The airway epithelium: Soldier in the fight against respiratory viruses. Clin Microbiol Rev 24:210–229, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. MacMicking JD, Nathan C, Hom G, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81:641–650, 1995. [DOI] [PubMed] [Google Scholar]
- 72. Fang FC. Perspectives series: Host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest 99:2818–2825, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Grasemann H, Ratjen F. Nitric oxide and L-arginine deficiency in cystic fibrosis. Curr Pharm Des 18:726–736, 2012. [DOI] [PubMed] [Google Scholar]
- 74. Nathan C. Inducible nitric oxide synthase: What difference does it make? J Clin Invest 100:2417–2423, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Detwiller KY, Smith TL, Alt JA, et al. Differential expression of innate immunity genes in chronic rhinosinusitis. Am J Rhinol Allergy 28:374–377, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lee RJ, Kofonow JM, Rosen PL, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest 124:1393–1405, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lee RJ, Cohen NA. Bitter and sweet taste receptors in the respiratory epithelium in health and disease. J Mol Med (Berl) 92:1235–1244, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lee RJ, Cohen NA. The emerging role of the bitter taste receptor T2R38 in upper respiratory infection and chronic rhinosinusitis. Am J Rhinol Allergy 27:283–286, 2013. [DOI] [PubMed] [Google Scholar]
- 79. Farquhar DR, Kovatch KJ, Palmer JN, et al. Phenylthiocarbamide taste sensitivity is associated with sinonasal symptoms in healthy adults. Int Forum Allergy Rhinol 5:111–118, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Adappa ND, Zhang Z, Palmer JN, et al. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int Forum Allergy Rhinol 4:3–7, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Adappa ND, Howland TJ, Palmer JN, et al. Genetics of the taste receptor T2R38 correlates with chronic rhinosinusitis necessitating surgical intervention. Int Forum Allergy Rhinol 3:184–187, 2013. [DOI] [PubMed] [Google Scholar]
- 82. Mfuna Endam L, Filali-Mouhim A, Boisvert P, et al. Genetic variations in taste receptors are associated with chronic rhinosinusitis: A replication study. Int Forum Allergy Rhinol 4:200–206, 2014. [DOI] [PubMed] [Google Scholar]
- 83. Pezzulo AA, Gutierrez J, Duschner KS, et al. Glucose depletion in the airway surface liquid is essential for sterility of the airways. PLoS One 6:e16166, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hatten KM, Palmer JN, Lee RJ, et al. Corticosteroid use does not alter nasal mucus glucose in chronic rhinosinusitis. Otolaryngol Head Neck Surg 152:1140–1144, 2015. [DOI] [PubMed] [Google Scholar]
- 85. Zhang Z, Adappa ND, Lautenbach E, et al. The effect of diabetes mellitus on chronic rhinosinusitis and sinus surgery outcome. Int Forum Allergy Rhinol 4:315–320, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


