Abstract
Fungal infections have become an important cause of morbidity and mortality in hospitalized children due to many complicating and underlying conditions. We present the case of a newborn infant with fungemia due to Kodamaea ohmeri who had a good outcome of the infection after using the combination of antifungal treatment and central venous catheter removal.
Keywords: Kodamaea ohmeri, Fungemia, Infant, Yeast, Liposomal amphotericin B
1. Introduction
Invasive candidiasis is the most common fungal infection reported in healthcare-associated infections. Depending on the series, Candida albicans is the 3rd or 4th cause of catheter-associated blood stream infections in children. In recent years, non-albicans species have been more frequently isolated, including Candida parapsilosis in neonates and others such as, C. glabrata, C. krusei, C. guillermondi all of these important because of their resistance to azoles [1], [2].
Kodamaea ohmeri, formerly named Pichia ohmeri, corresponds to the telemorphic (sexual) state of Candida guilliermondii var. membranaefaciens belonging to the class Ascomycetae and Saccharomycetaceae family [3]. It is a yeast which was initially isolated in cucumber salts used in the food industry for the fermentation of pickled foods, tree barks and fruits. It has also been isolated from environmental sources such as, pools, sand, floors and sea water [3]. In the past, Kodamaea ohmeri, was considered a contaminant, but at present some species are recognized as emerging opportunistic pathogens which can cause infection in patients with special underlying conditions including prematurity, immunosuppression, prolonged hospitalizations, prosthetic valves, CVL's or other medical devices such as peritoneal catheters [4].
2. Case
A male infant 54 days old, developed fever, tachycardia, leukocytosis, high reactive C protein and thrombocytopenia while he was hospitalized since 21 days ago because diarrhoea, respiratory distress and hypovolemic shock that needed NICU admission, with diagnosis of acute necrotizing enterocolitis (NEC).
He was born at 35 weeks, delivered at home and small for gestational age. During the hospitalization he had required mechanical ventilation and treatment with ampicillin, vancomycin, meropenem and fluconazole for 9 days because suspect of fungemia and had also bacteremia by S. epidermidis, multilobar pneumonia and Cytomegalovirus pneumonitis treated with ganciclovir.
At time of clinical worsening, yeasts were isolated from peripheral and central line blood cultures, caspofungin was started and CVL was removal. Further identification of the yeast by the automatic system Vitek-2 (YST bioMérieux, Marcy-l’Etoile, France) was Kodamaea ohmeri (Pichia ohmeri). Subsequently, it was subcultured in sabouraud dextrose agar in which white, rugged colonies were observed, which did not grow in mycosel culture media. In addition, it was also plated in CHROMagar media for Candida withgrowth of pink colonies that later turned to a blue-green color (Fig. 1, Fig. 2). Finally, API20C was done which confirmed the isolate as Kodamaea ohmeri.
Fig. 1.
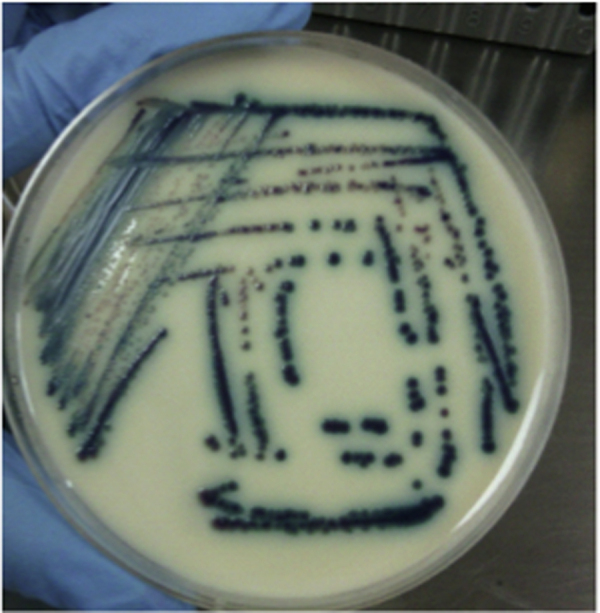
Kodamaea ohmeri colonies in CHROMagar Candida. Pink colonies that change to blue colonies in 48 h. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
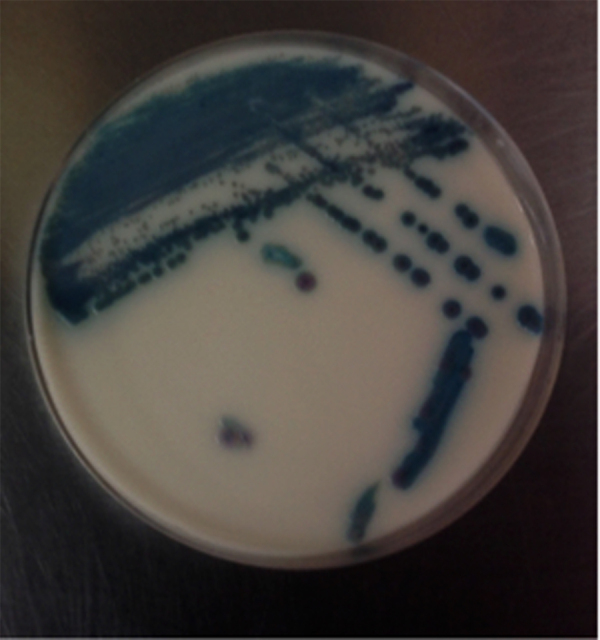
K. ohmeri colonies in CHROMagar Candida. Pink colonies that change to blue colonies in 48 h. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Susceptibility testing revealed resistance to fluconazole with the following minimal inhibitory concentrations (MIC's): Fluconazole=8 μg/dl, Amphotericin B=0.5 μg/mL and voriconazole <=0.12 μg/mL (see Table 1). Treatment with amphotericin B deoxicolate (AMB-Doc) IV at a dose of 1 mg/kg/day was started along with good clinical response. Follow up blood cultures on the 5th day of treatment were negative and antifungal treatment was continued for a total of 14 days since the first negative blood culture. The patient had a full recovery and was discharged at 3 months of age.
Table 1.
Minimal inhibitory concentrations (MIC's) of the K. ohmeri isolate of our patient.
| Antifungal | MIC (μg/mL) | Classification |
|---|---|---|
| Fluconazole | 8 | Resistant |
| Amphotericin B | 0.5 | Susceptible |
| Caspofungin | <=0.12 | Susceptible |
3. Discussion
The first clinical isolate of Kodamaea ohmeri was reported in 1984 from a pleural fluid sample but at that time it was considered a contaminant. That same year, fungemia in a 48 year old diabetic patient with immunosuppression due renal transplantation was reported, who subsequently died from the infection [5]. Since then, more infections with this yeast have been reported considering it a true clinical pathogen, especially in patients with underlying immunosuppression.
The most of those case reports are from Asia (Korea, Turkey, India). There are a few from North America [5], some from Brazil [6] and none from Colombia. The largest series reported in the same site included 38 patients from a tertiary care hospital in North India, with 78.9% of these cases corresponding to neonates in intensive care units. In that series, the attributed mortality from this infection was 31.8%, but there are other reports of mortality as high as 50% in the pediatric population [3], [7], [8].
Of the 5 species recognized in the Kodamaea genus, only K. ohmeri has the capacity to grow at 37 °C and cause human disease. The clinical relevance of the other species of Kodamaea (K. anthrophila, K. kakaduensis, K. laetipori, K. nitidulidarum) has not been established [9].
One of the methods currently available for identification is the API 20C test for carbohydrate fermentation, which is considered the gold standard for identification of yeasts. In this test, K. ohmeri is able to assimilate raphinose but not D-xylose. This method can have false positive results identifying some species of C. haemulonii or C.parapsilosis as K. ohmeri. Another method is the Vitek 2 ID-YST system which identifies with more accuracy this species of Candida, although there have been some missed identifications with C. haemulonii. [7].
The CHROMagar Candida (BBL, Beckton Dickinson, Sparks, MD) method allows species identification according to the color changes of the colonies in the agar, in which K. ohmeri changes from pink to blue in a period of 48 h, as was observed in our case. (Fig. 1).
At present, molecular diagnosis is available through the amplification and sequencing of the ITS2 region localized on the rRNA gen of the 5.8S and 28S subunit. It can also be typed through restriction endonucleases by pulsed field gel electrophoresis for analysis of genomic DNA. The latter is one of the most reliable methods for the correct identification of this yeast but only available in research laboratories or for epidemiological purposes [7].
Dominguez et al. [10], reported the identification of K. ohmeri by MALDI-TOF with an accuracy comparable to PCR but better than the one obtained through API20C. These isolates had been misidentified as C. haemuloni by the API20C method [10].
K. ohmeri has been described as causing fungemias, catheter associated infections, phlebitis [10], wound infections, peritonitis [11], endocarditis [12] as well as outbreaks in intensive care units [8] Table 2.
Table 2.
Reported cases of fungemia due to K. ohmeri in children.
| Country, year of report | Number of cases, Ref. | Age/gender | Risk factors | Treatment | Outcome |
|---|---|---|---|---|---|
| Houston, USA 2004 | 1 case Ref. [5] | 14 yrs, M | ALL, chemotherapy, CVL | FCZ, removal of CVL | Survived |
| Turkey, 2005 | 2 cases Ref. [8] | 8 mos-M | Encephalitis, ALL, chemotherapy, neutropenia, CVL | FCZ | Died |
| AMB | Survived | ||||
| 10 yrs-M | |||||
| Qatar 2006 | 1 case, Ref. [13] | Neonate 13 days old | Pretérm 25 wks, 680 gr (quadruples), NEC | AMB+FCZ | Survived |
| South Corea 2007 | 3 cases, Ref. [7] | 11y-M | Burkitt's lymphoma, Neutropenia | FCZ 2 | 2 and 3 died |
| 12yrs- M | Tetralogy of Fallot, | AMB + FCZ 1 | |||
| 4 yrs- F | |||||
| South Corea 2007 | 1 case, Ref. [7] | Neonate | Prematurity, umbilical catheter | Removal of CVL, no antifungal treatment | Survived |
| USA 2007 | 1 case Ref. [4] | 5 mos-M | Short gut | FCZ+ AMB | Survived |
| Hepatic failure CVL | Removal of CVL | ||||
| India 2009 | 1 case Ref. [14] | Neonate-M | Prematurity, 1300 gr, antibiotics, Umbilical catheters | FCZ+ AMB | Died |
| Brazil 2009 | 1 case, Ref. [6] | 3 yrs-F | Ascariasis, peritonitis, Antibiotics CVL | AMB-Liposomal | Survived |
| Kuwait 2011 | 1 case, Ref. [15] | Neonate-F | Preterm, 1280 gr | AMB | Survived |
| India 2013 | 38 cases, Ref. [3] | Neonates Young infants Median age 87 days | Prolonged hospital stay CVL, surgery Orotraqueal intubation Prolonged antibiotic use | FCZ in 23/38 cases Caspofungin in 4/38 Removal of CVL's in all | 19/38 (50%) died |
| China, 2013 | 6 cases Ref. [16] | Young infants, premature | Prolonged antibiotic courses Mechanical ventilation CVL | Caspofungin 5/6 FCZ 1/6 (relapse) Removal of CVL | 100% survived |
| Colombia, 2015 | Present case | Young infant-M | Antibiotics, CVL, TPN prolonged hospital stay | AMB | Survived |
ALL: acute lymphoblastic leukemia, NEC: necrotizing enterocolitis, CVL: central venous line, TPN: total parenteral nutrition, AMB: amphotericin B, FCZ: fluconazole.
Risk factors reported in children are: prematurity, low birth weight, prolonged ICU stay, use of medical devices, prosthetic valves, use of broad spectrum antibiotics, total parenteral nutrition, immunosuppression (leukemias, lymphomas) and neutropenia [17]. Therefore, K. ohmeri can be considered an opportunistic pathogen that can cause severe infections in patients with underlying conditions. To date, there is only one case reported of infection in a previously healthy child with no predisposing conditions except for encephalitis [8].
Other risk factors reported in adults are: diabetes, malignancies, intravenous drug use, cardiac prosthetic valves and chronic renal disorders [17].
No intrinsic resistance of K. ohmeri to antifungals has been reported to date. There have been several reports of fluconazole resistance, some reports of echinocandin resistance and only one report of an isolate with high MIC's to amphotericin B. Therefore, antifungal treatment should be adjusted according to susceptibility reports of the clinical isolates.
Treatment of infections caused by K. ohmeri is similar to fungemias due to Candida spp. and other yeasts. Removal of CVL's or other medical devices is mandatory, as well as, treatment with parenteral antifungals adjusted to fungal susceptibility reports. K. ohmeri, is frequently resistant to fluconazole, therefore, it should not be used for empirical therapy due to the risk of clinical failure [18]. Amphotericin B is the treatment of choice, with echinocandins as second line therapy. In cases with dose-dependent susceptibility to fluconazole, treatment may be successful using high doses of fluconazole.
Clinical and Laboratory Standards Institute (CLSI) has defined that fungal susceptibility break points for this yeast are the same as those for C. albicans isolates: Fluconazole MIC ≤2 μg/L susceptible, 4 μg/mL dose-dependent susceptibility, and ≥8 μg/L resistant. For Amphotericin B, MIC ≤1 μg/mL susceptible and >1 μg/mL resistant. For caspofungin <=0.25 μg/mL susceptible, 0.5 μg/mL intermediate and >=1 μg/mL resistant, with no category for dose-dependent susceptibility.
In the study reported by Lee et al. [7], all isolates were susceptible to Amphotericin B, voriconazole, micafungin and caspofungin. Eventhough, susceptibility data to equinocandins is scarce, it seems that micafungin and anidulafungin are good treatment options in patients with isolates that are resistant to azoles and in whom treatment with amphotericin B is contraindicated [15]. There are reports of clinical failures with fluconazole, in which case therapy has been continued with amphotericin B or caspofungin [14], [19].
Chakrabarti et al. [3], describes the largest pediatric series to date from a hospital in North India. This series included 38 isolates of K. ohmeri of which 37 were susceptible to azoles with MIC's between 0.5 and 32 (according to break points available at that time), and only one resistant isolate with an MIC of 64 mg/mL. All the isolates were also susceptible to caspofungin with MIC's between 0.12 and 1 mg/mL. Interestingly, 86.8% of the isolates had high MIC's to amphotericin B (MIC 1 mg/mL), in contrast to most of the other cases reported in the literature to date.
4. Conclusion
Catheter- associated fungemias are an important cause of morbidity and mortality in neonates and critically –ill children. Kodamaea ohmeri is a yeast that belongs to the species of Candida guillermondi which is an infrequent cause of infections in children, but the mortality associated with these infections can be as high as 50%. High rates of resistance to azoles has been reported, therefore, empirical treatment with amphotericin B or echinocandins should be started and adjusted according to antifungal susceptibility tests. Removal of central venous catheters or medical devices must be considered in all cases.
Declaration of interest
The authors declare that they have no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Ethical aproval
This case report was aproval for Ethical Committee fo the Hospital.
Acknowledgements
We thank Pablo Tobon Uribe hospital and laboratory services that enabled this publication.
References
- 1.Weber D.J., Rutala W.A. Central line associated bloodstream infections: prevention and management. Infect. Dis. Clin. North Am. 2011;25:77–102. doi: 10.1016/j.idc.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Hidron A.I., Edwards J.R., Patela J., Horana T.C., Sieverta D.M., Pollocka D.A. Antimicrobial-resistant pathogens associated with healthcare-associated infections. Infect. Control Hosp. Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 3.Chakrabarti A. Epidemiological study of a large cluster of fungaemia cases due to Kodamaea ohmeri in an Indian tertiary care centre. Clin. Microbiol. Infect. 2014;20:O83–O89. doi: 10.1111/1469-0691.12337. [DOI] [PubMed] [Google Scholar]
- 4.Ezeanolue E.E., Piggott K. Pichia ohmeri infection in a pediatric patient: a case report. J. Invest. Med. 2007;55:S146. [Google Scholar]
- 5.Han X.Y., Tarrand J.J., Escudero E. Eur. J. Clin. Microbiol. Infect. Dis. 2004;23:127–130. doi: 10.1007/s10096-003-1067-3. [DOI] [PubMed] [Google Scholar]
- 6.Duque de Barros J. Kodamaea (Pichia) ohmeri fungemia in a pediatric patient admitted in a public hospital. Med. Mycol. 2009;47:775–779. doi: 10.3109/13693780902980467. [DOI] [PubMed] [Google Scholar]
- 7.Lee J.S., Shin J.G., Kim Mi-Na. J. Clin. Microbiol. 2007:1005–1010. doi: 10.1128/JCM.02264-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otag F., Kuyucu N., Erturan Z., Sen S., Emekdas G., Sugita T. An outbreak of Pichia ohmeri infection in the paediatric intensive care unit: case reports and review of the literature. Mycoses. 2005;48:265–269. doi: 10.1111/j.1439-0507.2005.01126.x. [DOI] [PubMed] [Google Scholar]
- 9.Kurtzman C.P., Fell J.W., Boekhout T. The Yeasts: A taxonomic Study. 5th edn. Elsevier; Amsterdam: 2011. [Google Scholar]
- 10.Dominguez Muro M., de Araújo Motta F., Burger M., de Azevedo Melo A.S., Dalla-Costa L.M. Echinocandin resistance in two Candida haemulonii isolates from pediatric patients. J. Clin. Microbiol. 2012;50(11):3783–3785. doi: 10.1128/JCM.01136-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.N.A. Bokhary, I.B. Hussain, Kodamaea (Pichia) ohmeri peritonitis in a nine-year-old child in Saudi Arabia treated with Caspofungin, 2015, J. Taibah Univ. Med. Sci., pp. 1–4.
- 12.Xiao Y. Kodamaea ohmeri as an emerging pathogen in Mainland China: 3 case reports and literature review. Lab. Med. Spring Suppl. 2013 e1–e8. [Google Scholar]
- 13.Taj-Aldeen S.J., Doiphode S.H., Han X.Y. Kodamaea (Pichia) ohmeri fungaemia in a premature neonate. J. Med. Microbiol. 2006;55:237–239. doi: 10.1099/jmm.0.46216-0. [DOI] [PubMed] [Google Scholar]
- 14.Poojary A., Sapre G. Kodamaea ohmeri Infection in a Neonate. Indian Pediatr. 2009:46. [PubMed] [Google Scholar]
- 15.Sweih N.A. Kodamaea ohmeri as an emerging pathogen: a case report and review of the literature. Med. Mycol. 2011;49:766–770. doi: 10.3109/13693786.2011.572300. [DOI] [PubMed] [Google Scholar]
- 16.Liu C.X., Yang J.H., Dong L., Mai J.Y., Zhang L., Zhu J.H. Clinical features and homological analysis of Pichia ohmeri-caused hospital-acquired fungemia in premature infants. Zhonghua Yi Xue Za Zhi. 2013;93(4):285–288. [PubMed] [Google Scholar]
- 17.Shang S.T., Lin J.S., Ho S.J., Yang Y.S., Chang F.Y., Wang N.C. The emerging life-threatening opportunistic fungal pathogen Kodamaea ohmeri: optimal treatment and literature review. J. Microbiol. Immunol. Infect. 2010;43(3):200–206. doi: 10.1016/S1684-1182(10)60032-1. [DOI] [PubMed] [Google Scholar]
- 18.Bing-Heng Yang, Ming-Yieh Peng, Shu-Jin Hou, Jun-Ren Sun, Shih-Yi Lee, Jang-Jih Lu. Fluconazole-resistant Kodamaea ohmeri fungemia associated with cellulitis: case report and review of the literature. Int. J. Infect. Dis. 2009;13:e493–e497. doi: 10.1016/j.ijid.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Chiu C.H., Wang Y.C., Shang S.T., Chang F.Y. Kodamaea ohmeri fungaemia successfully treated with caspofungin. Int. J. Antimicrob. Agents. 2010;35:98–99. doi: 10.1016/j.ijantimicag.2009.09.010. [DOI] [PubMed] [Google Scholar]


