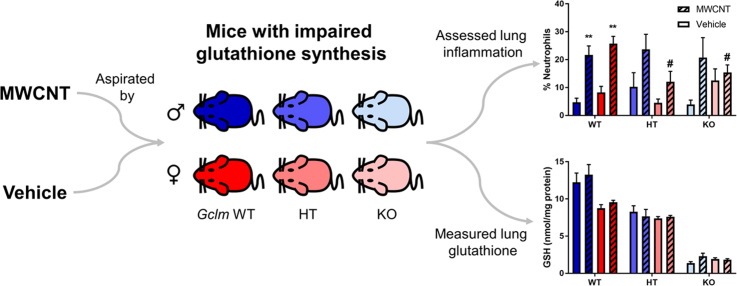
Abstract
Inhalation of multiwalled carbon nanotubes (MWCNTs) during their manufacture or incorporation into various commercial products may cause lung inflammation, fibrosis, and oxidative stress in exposed workers. Some workers may be more susceptible to these effects because of differences in their ability to synthesize the major antioxidant and immune system modulator glutathione (GSH). Accordingly, in this study we examined the influence of GSH synthesis and gender on MWCNT-induced lung inflammation in C57BL/6 mice. GSH synthesis was impaired through genetic manipulation of Gclm, the modifier subunit of glutamate cysteine ligase, the rate-limiting enzyme in GSH synthesis. Twenty-four hours after aspirating 25 µg of MWCNTs, all male mice developed neutrophilia in their lungs, regardless of Gclm genotype. However, female mice with moderate (Gclm heterozygous) and severe (Gclm null) GSH deficiencies developed significantly less neutrophilia. We found no indications of MWCNT-induced oxidative stress as reflected in the GSH content of lung tissue and epithelial lining fluid, 3-nitrotyrosine formation, or altered mRNA or protein expression of several redox-responsive enzymes. Our results indicate that GSH-deficient female mice are rendered uniquely susceptible to an attenuated neutrophil response. If the same effects occur in humans, GSH-deficient women manufacturing MWCNTs may be at greater risk for impaired neutrophil-dependent clearance of MWCNTs from the lung. In contrast, men may have effective neutrophil-dependent clearance, but may be at risk for lung neutrophilia regardless of their GSH levels.
Keywords: Gender differences, Glutathione, Inflammation, Multiwalled carbon nanotubes, Nanotoxicology, Oxidative stress
Graphical abstract
Highlights
-
•
We study how sex and glutathione levels alter carbon nanotube-induced lung toxicity.
-
•
Carbon nanotubes induce neutrophilia in male mice, regardless of glutathione levels.
-
•
Low glutathione levels in female mice reduce carbon nanotube-induced neutrophilia.
-
•
Low-iron carbon nanotubes do not induce oxidative or nitrative stress.
1. Introduction
Multiwalled carbon nanotubes (MWCNTs) are concentric cylinders of graphene under 100 nm in diameter which possess useful electronic, optical, and chemical properties [23], [25], [53]. Following improvements in large-scale manufacturing [34], worldwide carbon nanotube (CNT) manufacturing capacity grew to 4 million kg in 2011 [8], [9]. This growing worker population is at risk for inhaling CNTs during manufacturing and handling [8], [18], [28]. Thus, the National Institute for Occupational Safety and Health recommended an inhalation-based occupational exposure limit of 1 µg/m3 [43]. However, this recommendation was limited by a lack of in vivo data on sensitive subpopulations [43], which may be at greater risk for MWCNT-induced lung inflammation, fibrosis, and oxidative stress [34], [39], [48], [49], [55], [63].
CNT-induced oxidative stress has been observed in exposed workers and rodent models. In workers, exposure was associated with elevated breath condensate levels of oxidative stress markers [29], as well as lung dysfunction and suppression of glutathione peroxidase activity [32]. In mice, exposure was associated with depletion of the major cellular tripeptide antioxidant glutathione (GSH). Among C57BL/6 mice exposed via inhalation (5 mg/m3, 5 h/day, 4 days) to single-walled CNTs (SWCNTs) contaminated with 17.7% iron, exposure was associated with approximately a 50% decrease in the lung's total GSH content [55]. Decreases of 40–50% in lung total GSH were also observed in juvenile BALB/c mice exposed via inhalation (estimated deposition 5 µg/g/day, 7 days) [51]. Similarly, aspiration of 32 µg MWCNT in C57BL/6 mice was associated with a comparable decrease in total GSH, and a significant increase in the ratio of oxidized (GSSG) to reduced GSH [33].
Conversely, elevated GSH levels may protect against lung damage. Mice treated with the GSH precursor N-acetylcysteine had increased GSH levels and were resistant to the fibrosis and neutrophilia induced by exposure to long MWCNTs contaminated with 4.49% nickel [57]. Similarly, N-acetylcysteine co-treatment ameliorated the SWCNT-induced increase in pro-inflammatory cytokines in murine macrophages [3].
Taken together, these reports indicate that GSH deficiency is a potential consequence of CNT exposure, and that GSH supplementation may protect against CNT-induced lung damage. However, there have been no investigations into how pre-existing GSH deficiency may modulate the lung's pathological response to MWCNTs.
In humans, GSH deficiencies can result from inadequate dietary intake of cysteine or methionine, or from chronic diseases (e.g., chronic bronchitis, idiopathic pulmonary fibrosis) [1], [12]. GSH levels can also be affected by polymorphisms in Gclc and Gclm, which respectively encode the catalytic (GCLC) and modifier (GCLM) subunits for glutamate cysteine ligase (GCL), the rate-limiting enzyme in de novo GSH synthesis [11], [60]. Functional genetic polymorphisms in Gclc and Gclm have been reported in 30% and 20% of humans, respectively [41], [56].
GSH deficiency can dramatically alter the lung's response to toxicants. Genetic manipulation of Gclm in a mouse model of GSH deficiency indicated that Gclm heterozygous mice developed significantly greater lung inflammation following diesel exhaust particle exposure [65]. Furthermore, Gclm null mice had a significantly impaired inflammatory response to ozone [24] and quantum dots [36]—underscoring GSH's importance in modulating the immune response, as well as oxidative stress [12].
Intriguingly, gender may modulate the effects of GSH deficiency. Following acetaminophen exposure, female Gclm null mice had twice the liver damage of wild-type mice; in contrast, male Gclm null mice only had 20% more damage than wild-type mice [37]. In humans, gender influences susceptibility to lung diseases: Interstitial lung diseases are approximately 20% more prevalent in men [7], [15], while asthma is 34% more prevalent in women [5].
Together, these observations suggest that both gender and GSH deficiency may affect a worker's susceptibility to MWCNTs. Thus, we examined how GSH deficiencies may modulate MWCNT-induced acute lung inflammation in a gender-dependent manner using male and female Gclm deficient mice. The information from our study could potentially identify subpopulations of workers who may be more vulnerable to MWCNT-induced lung damage based on their gender and/or GSH status.
2. Materials and methods
2.1. Reagents
Except where noted, we obtained all reagents from Sigma-Aldrich (St. Louis, MO).
2.2. MWCNT characterization
When handling multiwalled carbon nanotubes (MWCNTs) for these experiments, we followed the National Research Council's recommendations on engineering controls and personal protective equipment to reduce the risk of inhalation and dermal exposure to MWCNTs [45].
For these experiments, we used MWCNTs manufactured by Cheap Tubes, Inc., via catalytic chemical vapor deposition. The manufacturer reported that the MWCNTs ranged in length from 500 to 2000 nm, and in outer diameter from 10 to 20 nm. The MWCNTs were supplied to us through our participation in the NIEHS Centers for Nanotechnology Health Implications Research Consortium. We used the MWCNTs as provided, and neither chemically modified nor purified the MWCNTs of non-graphene carbon or residual metal catalysts.
Physicochemical characterization of these MWCNTs has been previously reported. Hamilton et al. reported these MWCNTs to have an average length of 1108 nm and an outer diameter of 18 nm, with a surface area of 140.6 m2/g [17]. The Nanotechnology Characterization Laboratory reported that these MWCNTs have an elemental composition of 94.1% carbon, 4.7% oxygen, 0.8% nickel, and 0.4% iron by weight, and were contaminated with 0.06 EU/mg of bacterial endotoxin [42]. Therefore, our dose of 25 μg MWCNTs/mouse imparted a negligible endotoxin dose of 0.0015 EU/mouse.
To image the MWCNTs, we resuspended the dry powder to 0.5 mg/ml (the same concentration used in mouse exposures) in distilled water, sonicated the solution for 19 s in a 40 kHz Branson 2510DTH bath sonicator (Branson Ultrasonics Corp., Danbury, CT), and vortexed the solution for 1 s. The MWCNT solution was then drop-cast onto a holey carbon transmission electron microscopy grid, and the images captured with a helium ion microscope (ZEISS, Oberkochen, Germany).
To characterize the dispersion state of the MWCNTs in solution, we resuspended them to a concentration of 0.5 mg/ml in either distilled water or dispersion medium dosing vehicle [phosphate buffered saline (PBS)+0.6 mg/ml mouse serum albumin+10 μg/ml 1,2-dipalmitoyl-sn-glycero-3-phosphocholine surfactant in ethanol (0.1% v/v)] [2]. Following bath sonication and vortexing as described above, we then measured the resuspended sample's hydrodynamic size and zeta potential using dynamic light scattering over 45 min (Malvern Instruments Ltd., Malvern, UK). The relationship between the size of the resuspended particles and their Brownian motion is described by the Stokes-Einstein equation [10]. A particle's zeta potential influences its electrophoretic mobility, as described by the Henry equation and Smoluchowski approximation [22].
2.3. Gclm mice
We conducted all animal experiments in accordance with the National Institutes of Health Guide for the Use and Care of Laboratory Animals [44], and with the approval of the University of Washington Institutional Animal Care and Use Committee (UW IACUC Protocol 2384-08). We made all efforts to minimize animal distress and suffering.
For these studies, we used male and female Gclm wild-type (Gclm+/+), Gclm heterozygous (Gclm+/−), and Gclm null (Gclm−/−) mice which had been backcrossed onto a C57BL/6 background [37]. The mice were group housed in a modified specific pathogen free vivarium on a 12-hour light/dark cycle with nesting materials and access to water and chow provided ad libitum. At the time of MWCNT exposure, the mice were 3–5 months old with no significant difference in mean age between any of the experimental groups.
2.4. Experimental design
To determine if gender and genetic manipulation of GSH levels modulate the lung's pathological response to MWCNTs, we randomly assigned 3- to 5-month-old mice of all Gclm genotypes to receive dispersion medium dosing vehicle only, or 25 μg MWCNTs/mouse by oropharyngeal aspiration (n=5–6 mice per exposure, gender or genotype, for a total of 71 mice). For purposes of comparison, a 10 μg MWCNT/mouse bolus dose is reported to approximate human deposition after one month of light work in an environment with an ambient MWCNT concentration of 400 μg/m3, a level reported in Korean manufacturing settings [18], [49]. Thirty minutes prior to exposing the mice, we prepared fresh dispersion medium dosing vehicle (described under MWCNT characterization), and used it to resuspend the MWCNTs to 0.5 mg/ml. Both the dispersion vehicle and MWCNT solutions were sonicated for 19 s in a Branson 2510 bath sonicator, and then vortexed for 1 s
Immediately before exposure, we weighed each mouse and anesthetized it with 4% Isoflurane. The mouse was exposed via oropharyngeal aspiration of 50 μl dispersion medium only, or 50 μl of dispersion medium containing 25 μg MWCNTs, as previously described [2]. We then monitored the mice until they had recovered from anesthesia, and for any signs of distress (e.g., weight loss, huddling, unkempt fur) over the next 24 h. Neither treatment was associated with distress or mortality.
Twenty-four h after exposure, we weighed each mouse to determine its post-treatment weight, and humanely euthanized each mouse through CO2 narcosis followed by cervical dislocation. We performed bronchoalveolar lavage (BAL) with two serial lavages of sterile PBS (1.2 ml, 0.6 ml) as previously described [36]. From the first lavage, we aliquoted 100 μl for cytospins/differential staining of the recovered cells, and centrifuged the remainder. This acellular supernatant, or bronchoalveolar lavage fluid (BALF), was saved for measurements of cytokines, GSH content of the epithelial lining fluid (ELF), urea, total protein concentration, and lactate dehydrogenase concentration. In addition to BALF, we collected blood via cardiac puncture for serum isolation using serum separator tubes (Becton, Dickinson and Company, Franklin Lakes, NJ). We also removed a lung tissue sample from the right caudal lobe and immersed it in RNAlater® Stabilization Solution (Ambion via Thermo Fisher Scientific Inc., Waltham, MA) for 24 h at 4 °C, and then froze this stabilized sample at −80 °C for later RNA extraction. The remainder of the right lung was snap-frozen in liquid nitrogen and stored at −80 °C until it could be processed for the measurement of GSH content, total protein, and the levels of oxidative stress responsive proteins via Western immunoblotting.
2.5. Assessment of lung inflammation through cell differentials
To determine the lung's inflammatory responses to MWCNT aspiration, we performed differential scoring of the cells recovered in the first BAL. The cells in a 100-μl aliquot of the first lavage were centrifuged (Cytospin 3, Shandon Life Sciences International Ltd., Cheshire, England) and then differentially stained using the Hema 3™ system (Thermo Fisher Scientific, Waltham, MA), a modification of the Wright-Giemsa method. The percentage of specific inflammatory cell types (eosinophils, lymphocytes, macrophages/monocytes, and neutrophils) was determined by counting at least 500 cells from each mouse. We report the percentage of cell types instead of the total number, because a multi-center study using standardized protocols demonstrated significant interlaboratory variation in the total number of recovered cell types, despite standardization [2].
2.6. Measurement of cytokines in bronchoalveolar lavage fluid
The University of Washington's Center for Ecogenetics and Environmental Health Functional Genomics and Proteomics Core (CEEH FGPC) measured levels of BALF cytokines using the V-PLEX Proinflammatory Panel 1 Mouse Kit (Meso Scale Discovery, Rockville, MD), a multiplex sandwich immunoassay. The BALF samples were analyzed per manufacturer's directions for the levels of CXCL1 (KC), IL-1β, IL-6, and TNF-α. For this assay (lot #K0080292-166134), the dynamic range for KC was 0.408–1670 pg/ml; for IL-1β, 0.339–1390 pg/ml; for IL-6, 1.04–4260 pg/ml; and for TNF-α, 0.127–522 pg/ml. No samples were outside the range for KC and TNF-α analyses. For IL-1β, 69 out of 71 BAL samples (97.2%) were below the limit of detection (LOD), and further analysis was not possible for this cytokine. For IL-6, 14 out of 71 BAL samples (19.7%) were below the LOD. For the samples below the LOD, we replaced these values with the analyte's LOD divided by the square root of 2. This replacement method reportedly minimizes bias when fewer than 50% of all samples have non-detectable levels, and when the number of observations ranges from 20 to 100 [20], [46].
2.7. Measurement of total glutathione in lung tissue and epithelial lining fluid
To assess the effects of MWCNT exposure, gender, and Gclm status, we measured levels of the major cellular antioxidant glutathione in lung tissue and ELF. In lung tissue, we measured the total glutathione (GSH+GSSG) content in clarified lung homogenates as previously described [36], using tris-carboxyethyl phosphine to reduce GSSG to GSH, and derivatizing GSH with napthelene-2,3-dicarboxaldehyde. We then measured the relative fluorescence intensity of the derivatized GSH and calculated the GSH levels by interpolating from a standard curve [0.01–0.75 mM]. The total GSH content was normalized to the total protein content of the lung homogenate, which was determined using a commercial Bradford protein assay (Bio-Rad Laboratories, Inc., Hercules, CA). The total GSH and protein contents were both determined using triplicate samples of each homogenate.
To determine the concentration of total GSH in ELF, we used essentially the same procedure in BALF as described above for clarified lung homogenates, but with interpolation from a less-concentrated standard curve [0.25–5 μM]. To adjust this concentration for the dilution of ELF during lavage, we calculated the dilution factor by measuring urea in BALF and serum using the QuantiChrom™ Urea Assay Kit (BioAssay Systems, Hayward, CA). The use of urea to calculate a dilution factor assumes that urea freely diffuses between blood and ELF, thereby equalizing the urea concentrations between these compartments [13].
2.8. Measurement of mRNA levels for genes associated with early pathological responses through quantitative real-time PCR
The University of Washington's CEEH FGPC quantified the mRNA levels of specific genes using fluorogenic 5′ nuclease-based assays as previously described [36]. In brief, we extracted total RNA from a sample of the caudal lobe of the right lung using the miRNeasy Mini Kit (Qiagen, Valencia, CA), and then generated cDNA from 1 μg of total RNA using the manufacturer's protocol for the SuperScript® III First-Strand Synthesis System (Life Technologies, Carlsbad, CA). The PCR reaction mix consisted of TaqMan Gene Expression Master Mix (Applied Biosystems Inc., Foster City, CA), along with primers and dual-labeled probes for each gene designed using ABI Primer Express v.1.5 software (Applied Biosystems). The targets were then amplified and detected using the ABI PRISM 7900 system (Applied Biosystems) using the following reaction profile: 1 cycle at 95 °C for 10 min; 40 cycles each at 95 °C for 30 s; and then 1 cycle at 62 °C for 1 min. To calculate mRNA expression levels, a linear regression formula was derived from Gapdh amplification plots using serial dilutions of an established reference sample.
The analyzed genes included cystic fibrosis transmembrane regulator protein (Cftr), glutamate cysteine ligase catalytic subunit (Gclc), glutamate cysteine ligase modifier subunit (Gclm), granulocyte-macrophage colony stimulating factor (Gmcsf), heme oxygenase-1 (Hmox1), interleukin-1β (Il1β), Cxcl1/keratinocyte-derived cytokine (Kc), monocyte chemotactic protein-1 (Mcp1), transforming growth factor-β 1 (Tgfβ1), and tumor necrosis factor-α (Tnfα). The expression of these targets was normalized to Gapdh mRNA expression. The primer and probe sequences for these genes have been previously published [36].
2.9. Assessment of bronchoalveolar lavage fluid for lung toxicity
To evaluate MWCNT-induced lung toxicity, we measured the BALF concentrations of acellular lactate dehydrogenase and total protein, which respectively indicate cytotoxicity and disruption of alveolar/capillary barrier integrity [66]. The first lavage was centrifuged (500g, 10 min, 4 °C) and the acellular supernatant decanted to obtain BALF. We determined the concentration of lactate dehydrogenase in BALF using the enzyme activity CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega Corporation, Madison, WI). We determined the total protein concentration in BALF using a commercial Bradford protein assay (Bio-Rad Laboratories, Inc., Hercules, CA).
2.10. Semi-quantitative Western immunoblotting for redox-sensitive protein expression and protein nitration
To evaluate redox-sensitive protein expression and protein nitration in homogenates of the right lung, we used SDS-PAGE and Western immunoblot analyses as previously described [59]. The expression of GCLC and GCLM was detected using rabbit polyclonal anti-GCLC and -GCLM peptide antisera [59]; HMOX1 was detected using a rabbit polyclonal heme oxygenase 1 antibody (Santa Cruz Biotechnology, Dallas, TX); and nitrotyrosine modifications were detected using anti-3-nitrotyrosine antibody [58]. We detected β-actin as the loading control using a rabbit monoclonal β-actin antibody (Cell Signaling Technology, Danvers, MA). To evaluate the extent of protein nitration, on each SDS-PAGE gel we ran a positive control (untreated lung homogenate incubated for 1 min at room temperature in 1.0 mM peroxynitrite [58] and negative control (untreated lung homogenate incubated in an equivalent volume of distilled water). For each Western blot, we detected the bound secondary Goat Anti-Rabbit IgG Antibody, HRP-conjugate (EMD Millipore, Billerica, MA), using an enhanced chemiluminescence system (GE Healthcare UK, Buckinghamshire, UK) with X-ray film exposure. The optical densities of the appropriate-sized bands were then analyzed using NIH Image J software v1.48 (National Institutes of Health, Bethesda, MD). The optical density of each band was adjusted to the density of the β-actin band, and the fold-change expression for each sample was calculated compared to the mean value for the dispersion medium vehicle-exposed control mice.
2.11. Statistical analyses
We analyzed the data using two-way analysis of variance (ANOVA) for genotypes, treatments, and their interactions. For each genotype, we also analyzed the data using two-way ANOVA for gender, treatments, and interactions. When two-way ANOVA results indicated significant associations, we performed post-hoc analyses using unpaired, two-tailed t-tests, and adjusted the resulting p-values with Bonferroni corrections for multiple comparisons. For determining correlations, we calculated Pearson's correlation coefficients and report two-tailed p-values for significance. For all analyses, statistical significance was set with α=0.05.
We managed our data in Excel 2013, part of Microsoft Office Professional Plus 2013 (Microsoft, Redmond, WA), and analyzed our data in Prism 5 for Windows, v.5.02 (GraphPad Software, Inc., La Jolla, CA).
3. Results
3.1. MWCNT characterization
The images we collected through helium ion microscopy confirmed that our MWCNT sample consists of carbon nanotubes of varying lengths and diameters (Fig. 1). After resuspending the MWCNTs in ultrapure water or dispersion medium dosing vehicle, we measured the zeta potentials and particle sizes in triplicate at 15-minute intervals for 45 min (Table 1). Over this time period, the measured size and charge remained relatively constant, suggesting that particle aggregation did not change in water or dispersion medium [6]. The resuspended MWCNTs formed aggregates with equivalent hydrodynamic diameters comparable in size to MWCNT aggregates sampled from manufacturing workplaces, which measured 400–2000 nm in diameter [27].
Fig. 1.
Representative helium ion microscopy micrographs of MWCNT. The images are at (A) 22,860x, (B) 57,150x, and (C) 114,300x magnification.
Table 1.
Characteristics of multiwalled carbon nanotubes in liquid suspension.
| Characteristic | Measurementa |
|---|---|
| Hydrodynamic diameter (nm) | |
| DMb | 425.4±56.1 |
| Ultrapure water | 322.1±151.9 |
| Zeta potential (mV) | |
| DM | −12.4 |
| Ultrapure water | −44.6 |
The results are means±SD.
DM=dispersion medium vehicle.
3.2. Lung inflammation
To investigate gender- and GSH-dependent susceptibility to acute lung inflammation, we determined the percentage of neutrophils and eosinophils through differential staining of cells recovered through BAL 24 h after MWCNT aspiration. Mice which aspirated an equal volume of dispersion medium served as controls.
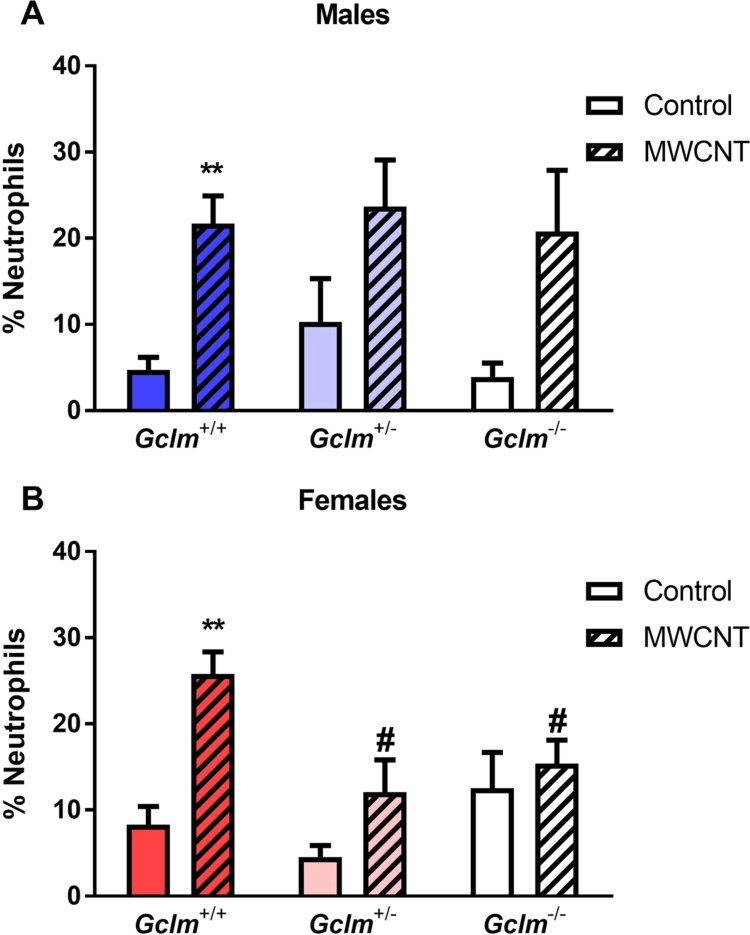
MWCNT exposure significantly increased lung neutrophilia in Gclm+/+ mice of both genders (Fig. 2), but Gclm genotype was significantly associated with neutrophilia only among female mice (two-way ANOVA p=0.0191).
Fig. 2.
Lung neutrophilia. Percentage of neutrophils in cells recovered through bronchoalveolar lavage of (A) male and (B) female Gclm mice, 24 h after aspiration of dispersion medium vehicle (Control) or 25 µg MWCNT. Results are means and SEM (n=5–6 mice/group). **p< 0.01 compared to same-genotype control, # p< 0.05 compared to MWCNT-exposed Gclm+/+. Abbreviation: MWCNT, multiwalled carbon nanotube.
When compared to MWCNT-exposed female Gclm+/+ mice, the MWCNT-exposed female Gclm+/− and Gclm−/− mice had significantly less neutrophilia. In contrast, MWCNT exposed male Gclm+/− and Gclm−/− mice developed neutrophilia to an extent comparable to that of MWCNT exposed male Gclm+/+ mice, although these were statistically non-significant when compared to their respective dispersion medium-alone control groups.
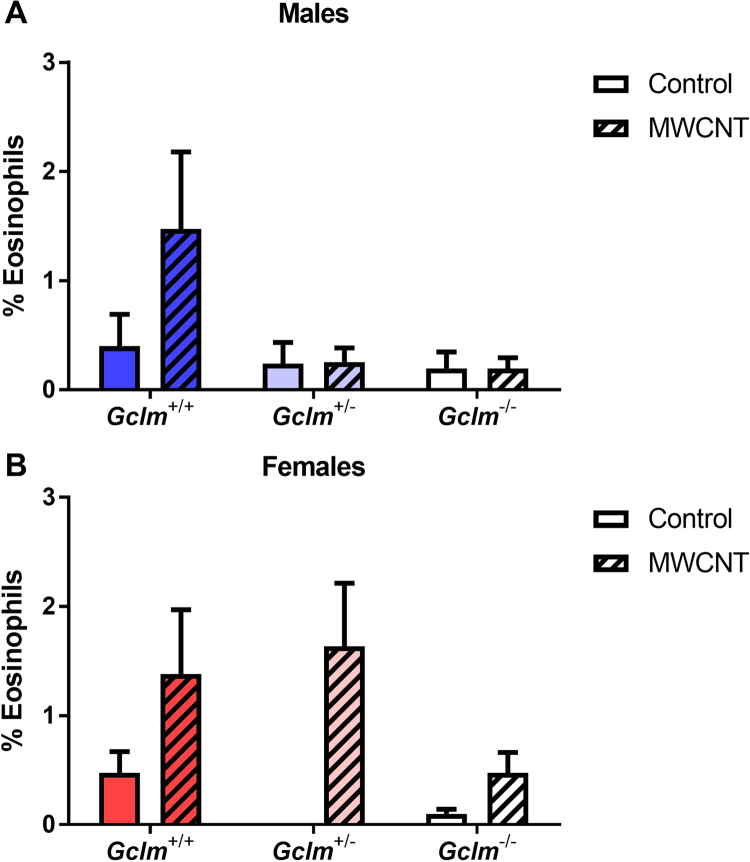
In addition to neutrophilia, MWCNT exposure induced a mild (<2%) but statistically significant eosinophilia among female mice (two-way ANOVA p=0.0022), although none of the post-hoc analyses were significant when comparing exposure groups within each Gclm genotype. In contrast to the genotype-dependent pattern of neutrophilia among female mice, this mild eosinophilia did not significantly vary by Gclm genotype (Fig. 3).
Fig. 3.
Lung eosinophilia. Percentage of eosinophils in cells recovered through bronchoalveolar lavage of (A) male and (B) female Gclm mice, 24 h after aspiration of dispersion medium vehicle (Control) or 25 µg MWCNT. Results are means and SEM (n=5–6 mice/group). Abbreviation: MWCNT, multiwalled carbon nanotube.
Taken together, these results indicate that male and female wild-type mice demonstrate a comparable inflammatory response to MWCNTs. However, moderate and severe GSH deficiencies significantly reduce this neutrophilic response among female, but not male, mice. Furthermore, these results demonstrate that MWCNTs induce a mild but significant eosinophilia in female but not male mice, irrespective of Gclm genotype.
3.3. BALF cytokines
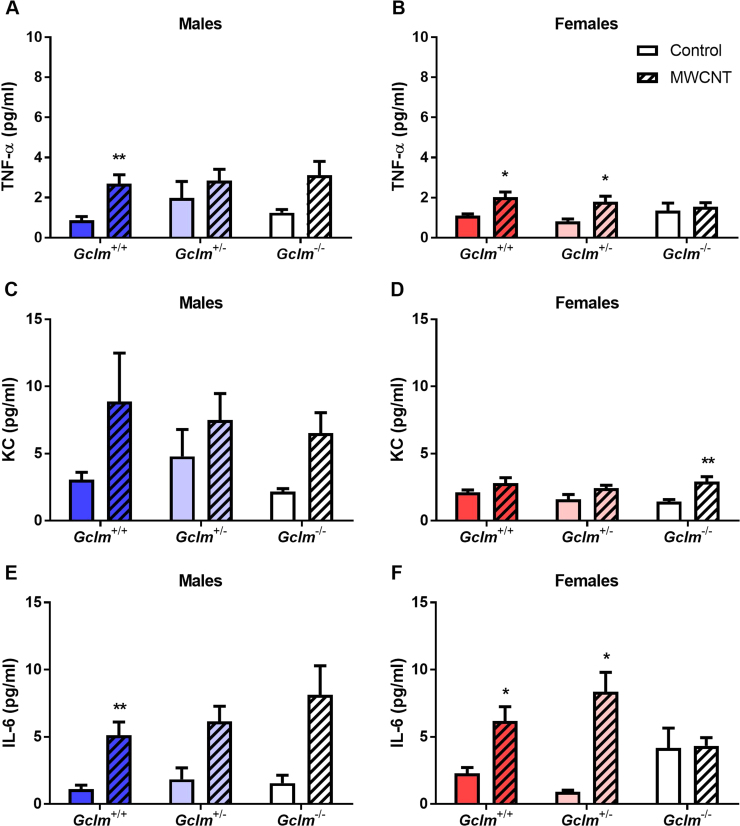
To determine if gender and GSH levels alter immune signaling 24 h after MWCNT aspiration, we measured the concentration in BALF of cytokines involved with pro-inflammatory signaling (TNF-α, IL-6) and neutrophil chemoattraction (KC).
MWCNT aspiration significantly increased BALF levels of TNF-α and IL-6 in both genders, and significantly increased KC levels in females (Fig. 4). However, MWCNT aspiration but not Gclm genotype was significantly associated with higher levels of TNF-α, KC, and IL-6. We did not observe significant differences in cytokine levels between genders.
Fig. 4.
Pro-inflammatory cytokines in lungs. Levels of inflammation-associated cytokines in bronchoalveolar lavage fluid recovered 24 h after aspiration of dispersion medium vehicle (Control) or 25 µg MWCNT by male and female Gclm mice. Cytokines include TNF-α (A, male; B, female), KC (C, male; D, female), and IL-6 (E, male; F, female). Results are means and SEM (n=5–6 mice/group). *p<0.05,**p<0.01 compared to same-genotype control. Abbreviation: MWCNT, multiwalled carbon nanotube.
We found in both genders that BALF cytokine levels correlated significantly with the proportion of neutrophils recovered in BAL. In males, this correlation was significant for TNF-α (Pearson's r=0.6974, p<0.0001) and IL-6 (r=0.6164, p<0.0001), but not for KC. In females, this correlation was significant for TNF-α (r=0.7062, p<0.0001), IL-6 (r=0.5649, p=0.0003), and KC (r=0.4496, p=0.0059).
3.4. Total glutathione content in lung tissue and epithelial lining fluid
GSH is a major cellular antioxidant involved in regulating the cell's redox state and the immune response. Total GSH content is sensitive to toxicant exposure, in that CNT exposure depletes GSH from lung tissue [51], [55], while cigarette smoke increases glutathione in ELF [14]. Thus, we examined the effect of MWCNT aspiration on total GSH levels in lung tissue and in ELF.
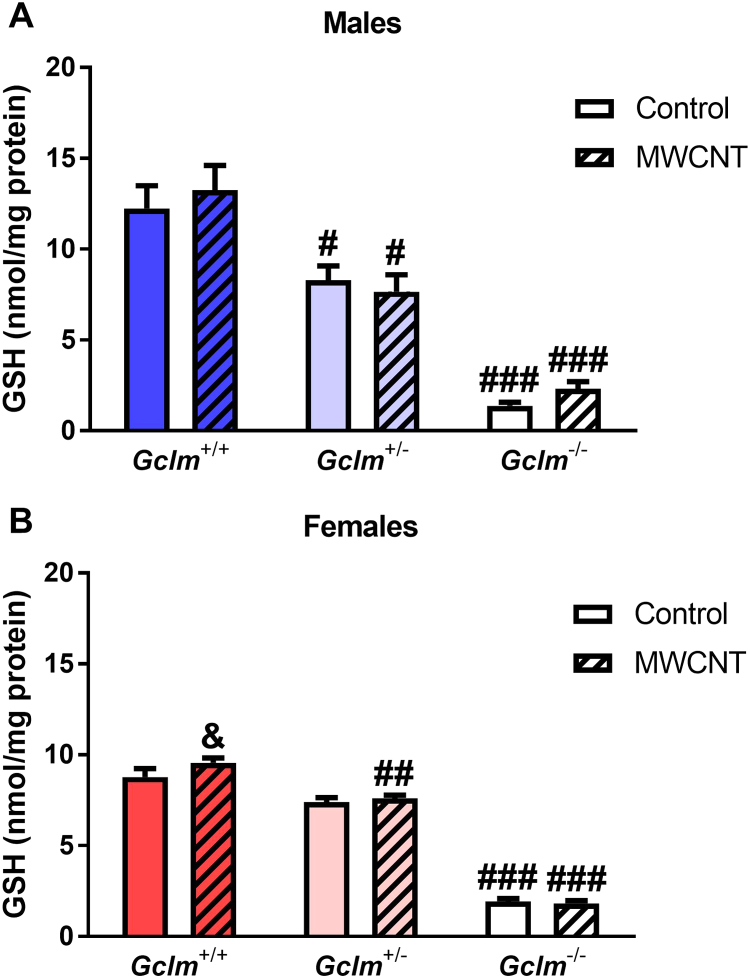
For any Gclm genotype and either gender, MWCNT exposure did not alter the amount of total GSH measured in clarified homogenates of lung tissue (Fig. 5). As expected, genotype was significantly associated with lung GSH for both genders (two-way ANOVA p<0.0001). Among male control mice, the Gclm+/− and Gclm–/− mice had approximately 68% and 11% the levels of Gclm+/+ mice, respectively. Among female control mice, Gclm+/− and Gclm−/− mice had 84% and 22% the levels of Gclm+/+ mice, respectively. While lung GSH levels were comparable between the genders for Gclm+/− and Gclm−/− mice, exposed female Gclm+/+ mice had significantly lower levels (approximately 72%) of lung GSH compared to male Gclm+/+ mice (p=0.0027). Our data on GSH levels in male mice agree with previous reports for this mouse Gclm model; female mice were not examined in these other studies [36], [37], [65].
Fig. 5.
Glutathione levels in lungs. Lung total glutathione content measured 24 h after aspiration of dispersion medium vehicle (Control) or 25 µg MWCNT by (A) male and (B) female Gclm mice. Results are means and SEM (n=5–6 mice/group). # p < 0.05, ## p<0.01, ### p<0.001 compared to same-treatment Gclm+/+, & p<0.05 compared to same-treatment, same-genotype males. Abbreviations: GSH, glutathione; MWCNT, multiwalled carbon nanotube.
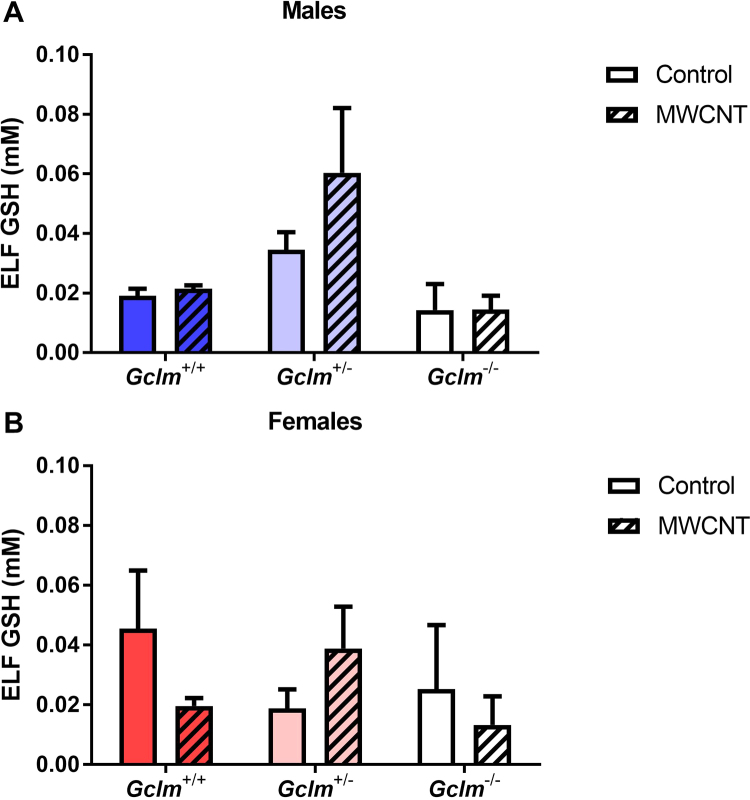
The concentration of GSH in ELF was not associated with MWCNT exposure or Gclm genotype for either gender (Fig. 6).
Fig. 6.
Glutathione levels in epithelial lining fluid. Total glutathione content of lung ELF recovered through bronchoalveolar lavage of (A) male and (B) female Gclm mice, 24 h after aspiration of dispersion medium vehicle (Control) or 25 µg MWCNT. Results are means and SEM (n =5–6 mice/group), corrected for lavage dilution using the urea serum:BALF ratio. Abbreviations: BALF, bronchoalveolar lavage fluid; ELF, epithelial lining fluid; GSH, glutathione; MWCNT, multiwalled carbon nanotube.
Taken together, our data indicate that MWCNT aspiration did not induce oxidative stress sufficient to alter total GSH levels in the lung 24 h after exposure.
3.5. Real-time PCR measurement of inflammation-, oxidative stress-, and fibrosis-associated gene expression
To assess the early pathological responses induced by MWCNT aspiration, we measured at 24 h post-exposure the mRNA levels of genes involved in inflammation, oxidative stress, and fibrosis pathways.
We assessed inflammatory and pro-fibrotic responses by measuring mRNA expression of genes associated with granulocyte production (granulocyte-macrophage colony stimulating factor, Gmcsf), neutrophil chemoattraction (keratinocyte-derived cytokine, Kc), monocyte recruitment (monocyte chemotactic protein-1, Mcp1), inflammasome activation (interleukin-1β, Il1β), pro-inflammatory signaling (tumor necrosis factor-α, Tnfα), and fibrosis (transforming growth factor-β 1, Tgfβ1). MWCNT aspiration was not associated with a change in expression detectable 24 h post-exposure (male, Table 2; female, Table 3), except for increased Gmcsf expression in exposed male Gclm+/+ mice. Intriguingly, gender played a more important role than MWCNT exposure in mRNA expression levels for four of these genes: Female mice of all Gclm genotypes expressed significantly higher levels of Gmcsf, Kc, and Mcp1 than did male mice. Female Gclm+/− and Gclm−/− mice also expressed significantly higher levels of Tgfβ1 than their male counterparts.
Table 2.
Lung mRNA expression levels of inflammation-, oxidative stress-, and fibrosis-associated genes 24 h after aspiration of multiwalled carbon nanotubes by male Gclm mice.
|
Genotype: |
Gclm+/+ |
Gclm+/+ |
Gclm+/− |
Gclm+/− |
Gclm–/− |
Gclm−/− |
|---|---|---|---|---|---|---|
| Treatment: | Control | MWCNT | Control | MWCNT | Control | MWCNT |
| Cftr | 6.38 ± 0.50 | 6.63 ± 0.70 | 6.45 ± 0.56 | 6.86 ± 0.51 | 7.10 ± 0.62 | 6.15 ± 0.87 |
| Gclc | 178.57 ± 7.46 | 203.20 ± 13.88 | 178.45 ± 5.95 | 167.25 ± 9.14 | 199.34 ± 23.48 | 167.45 ± 15.97 |
| Gclm | 45.13 ± 3.07 | 36.89 ± 4.52 | 19.80 ± 1.00### | 20.48 ± 1.15# | 0.02 ± 0.01### | 0.01 ± 0.01### |
| Gmcsf | 0.23 ± 0.05 | 0.39 ± 0.01⁎ | 0.20 ± 0.03 | 0.35 ± 0.07 | 0.25 ± 0.07 | 0.27 ± 0.09 |
| Hmox1 | 55.78 ± 2.54 | 60.33 ± 1.85 | 60.20 ± 3.10 | 57.46 ± 2.88 | 68.03 ± 4.73 | 62.83 ± 5.77 |
| Il1β | 5.52 ± 0.41 | 6.71 ± 1.60 | 6.47 ± 0.93 | 6.24 ± 1.74 | 4.99 ± 1.21 | 3.54 ± 0.83 |
| Kc | 0.33 ± 0.06 | 0.73 ± 0.36 | 0.28 ± 0.05 | 0.28 ± 0.07 | 0.16 ± 0.03 | 0.29 ± 0.12 |
| Mcp1 | 0.27 ± 0.05 | 0.44 ± 0.16 | 0.23 ± 0.02 | 0.22 ± 0.03 | 0.22 ± 0.02 | 0.29 ± 0.04 |
| Tfgβ1 | 38.93 ± 1.24 | 40.52 ± 2.08 | 32.03 ± 2.07# | 34.41 ± 3.29 | 27.71 ± 2.99## | 27.88 ± 4.37 |
| Tnfα | 1.03 ± 0.05 | 1.18 ± 0.10 | 1.01 ± 0.07 | 1.07 ± 0.17 | 0.92 ± 0.09 | 1.16 ± 0.14 |
The results are reported as means±SEM (×10−3), normalized to Gapdh expression. @MWCNT=multiwalled carbon nanotube.
p<0.05 compared to same-genotype control.
p<0.05.
p<0.01.
p< 0.001 compared to similarly treated Gclm+/+ mice.
Table 3.
Lung mRNA expression levels of inflammation-, oxidative stress-, and fibrosis-associated genes 24 h after aspiration of multiwalled carbon nanotubes by female Gclm mice.
|
Genotype: |
Gclm+/+ |
Gclm+/+ |
Gclm+/− |
Gclm+/− |
Gclm–/− |
Gclm−/− |
|---|---|---|---|---|---|---|
| Treatment: | Control | MWCNT | Control | MWCNT | Control | MWCNT |
| Cftr | 9.68±0.89& | 11.05±0.70&& | 12.38±1.10 | 8.51±0.96 | 8.81±1.04 | 7.99±1.17 |
| Gclc | 200.61±7.65 | 218.84±14.18 | 221.06±25.17 | 186.26±27.84 | 215.30±19.87 | 204.81±9.04 |
| Gclm | 23.58±2.56&&& | 37.57±8.37 | 15.14±1.07#, && | 13.23±0.57#, &&& | n.d. | n.d. |
| Gmcsf | 1.34±0.16&&& | 1.62±0.18&&& | 1.30±0.08&&& | 1.43±0.11&&& | 1.48±0.09&&& | 1.33±0.13&&& |
| Hmox1 | 55.34±9.04 | 49.67±5.19 | 54.54±2.91 | 41.65±1.75 | 41.21±3.29 | 37.20±1.88 |
| Il1β | 14.81±4.75 | 7.14±1.20 | 7.51±0.69 | 5.55±0.55 | 6.51±1.02 | 8.77±2.77 |
| Kc | 4.97±2.95 | 1.41±0.17 | 1.51±0.17&&& | 1.25±0.26& | 0.98±0.18&& | 1.27±0.54 |
| Mcp1 | 3.99±0.84 | 2.03±0.47 | 2.10±0.24&&& | 1.93±0.21&&& | 1.52±0.30&& | 2.55±1.37 |
| Tfgβ1 | 41.87±4.32 | 45.78±3.85 | 45.39±3.24& | 41.42±4.05 | 57.59±5.26&& | 58.52±5.04&& |
| Tnfα | 3.33±1.51 | 1.20±0.11 | 1.63±0.28 | 1.34±0.21 | 1.26±0.25 | 1.35±0.27 |
The results are reported as means±SEM (×0−3), normalized to Gapdh mRNA expression. Abbreviations: MWCNT, multiwalled carbon nanotube; n.d., not detected.
& p<0.05, && p<0.01, &&& p<0.001 compared to similarly treated males of the same genotype.
p<0.05 compared to similarly treated Gclm+/+ mice.
We analyzed the mRNA levels of oxidative stress genes associated with GSH synthesis (glutamate cysteine ligase catalytic subunit, Gclc; glutamate cysteine ligase modifier subunit, Gclm), oxidative stress (heme oxygenase-1, Hmox1), and GSH transport into lung ELF (cystic fibrosis transmembrane regulator protein, Cftr). For male and female mice of all Gclm genotypes, MWCNT aspiration did not induce oxidative stress sufficient to significantly alter expression of these four mRNAs (male, Table 2; female, Table 3). As expected, Gclm genotype was significantly associated with expression of Gclm mRNA (male and female p<0.001). Gclm genotype was also significantly associated with Hmox1 mRNA expression in female mice (two-way ANOVA p=0.0268). For both genders, Gclm expression significantly correlated with lung GSH levels (male Pearson's r=0.8126, p< 0.0001; female r=0.7700, p<0.0001).
When we compared mRNA expression between genders, we found that Gclm mRNA expression was significantly lower in females for control Gclm+/+, control Gclm+/−, and MWCNT-exposed Gclm+/− mice. Similarly, female Gclm+/- and Gclm−/− mice had significantly lower Hmox1 mRNA expression compared to their male counterparts. In contrast, female mice of all Gclm genotypes had greater Cftr expression than did male mice, although this difference was statistically non-significant. Cftr expression did not significantly correlate with GSH levels in the ELF for either gender.
Collectively, our mRNA expression data indicate that the expression of inflammation-related genes was unaltered 24 h post-MWCNT-exposure, consistent with reports that gene expression of pro-inflammatory cytokines peaks less than 8 h after lung injury [30], [31], [65]. Intriguingly, female mice of all genotypes expressed significantly higher levels of neutrophil and monocyte recruitment cytokines than did male mice—indicating that gender, rather than MWCNT exposure or Gclm genotype, has a significant influence on mRNA expression of these genes. Consistent with our observations on lung GSH levels, we found that MWCNT aspiration did not induce oxidative stress sufficient to change related gene expression at least at 24 h post-exposure.
3.6. Lung toxicity
To assess how MWCNT-induced lung damage might vary between genders and Gclm genotypes, we measured acellular BALF lactate dehydrogenase levels as an indicator of cellular death, and BALF total protein levels as an indication of the integrity of the alveolar/capillary barrier. We found that MWCNT aspiration did not cause sufficient lung damage at 24 h post-exposure to increase lactate dehydrogenase or protein levels (Table 4). Furthermore, these parameters did not significantly differ between genders or Gclm genotypes.
Table 4.
Parameters for lung toxicity and alveolar/capillary barrier integrity 24 h after aspiration of multiwalled carbon nanotubes.
| Group | MWCNT (μg/mouse) | LDH (ng/ml BALF) | Protein (μg/ml BALF) |
|---|---|---|---|
| Males | |||
| Gclm+/+ | 0 | 137.3±6.4 | 241.5±31.5 |
| 25 | 179.3±8.4 | 287.1±38.0 | |
| Gclm+/− | 0 | 147.3±5.9 | 220.0±13.6 |
| 25 | 177.3±18.6 | 288.7±96.7 | |
| Gclm−/− | 0 | 311.5±115.5 | 234.8±42.9 |
| 25 | 179.2±28.9 | 240.4±30.5 | |
| Females | |||
| Gclm+/+ | 0 | 149.3±13.9 | 425.2±145.8 |
| 25 | 155.0±15.8 | 246.2±35.5 | |
| Gclm+/− | 0 | 183.8±47.2 | 630.0±274.9 |
| 25 | 167.7±15.1 | 296.7±38.5 | |
| Gclm−/− | 0 | 175.3±28.7 | 237.8±18.8 |
| 25 | 158.7±15.4 | 249.4±37.7 | |
The results are reported as means±SEM. Abbreviations: BALF, bronchoalveolar lavage fluid; LDH, lactate dehydrogenase; MWCNT, multiwalled carbon nanotube.
These results indicate that neither gender nor GSH deficiency predisposes the lung towards MWCNT-induced cellular damage and alveolar/capillary disruption.
3.7. Western immunoblot analysis
To investigate if MWCNT exposure alters the expression of redox-sensitive proteins (GCLC, GCLM, and HMOX1) in a gender- and/or Gclm genotype-dependent manner, we used semi-quantitative Western immunoblotting to measure each of these proteins in lung homogenates. We adjusted each sample's densitometry value to its β-actin loading control, and then calculated the fold-change by normalizing the adjusted values for the MWCNT-exposed groups to the mean of their respective dispersion medium-only control group.
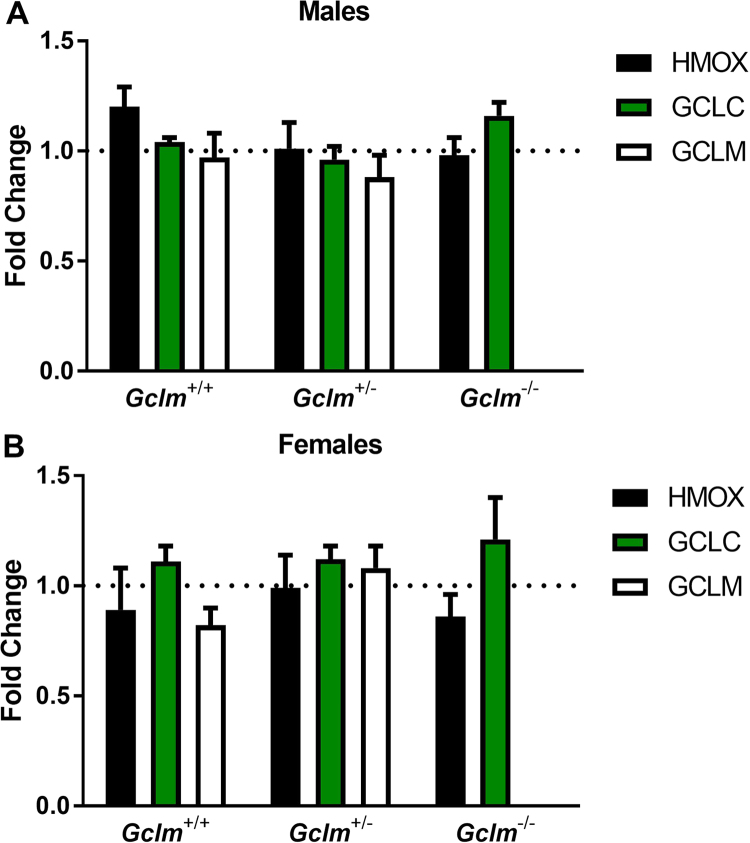
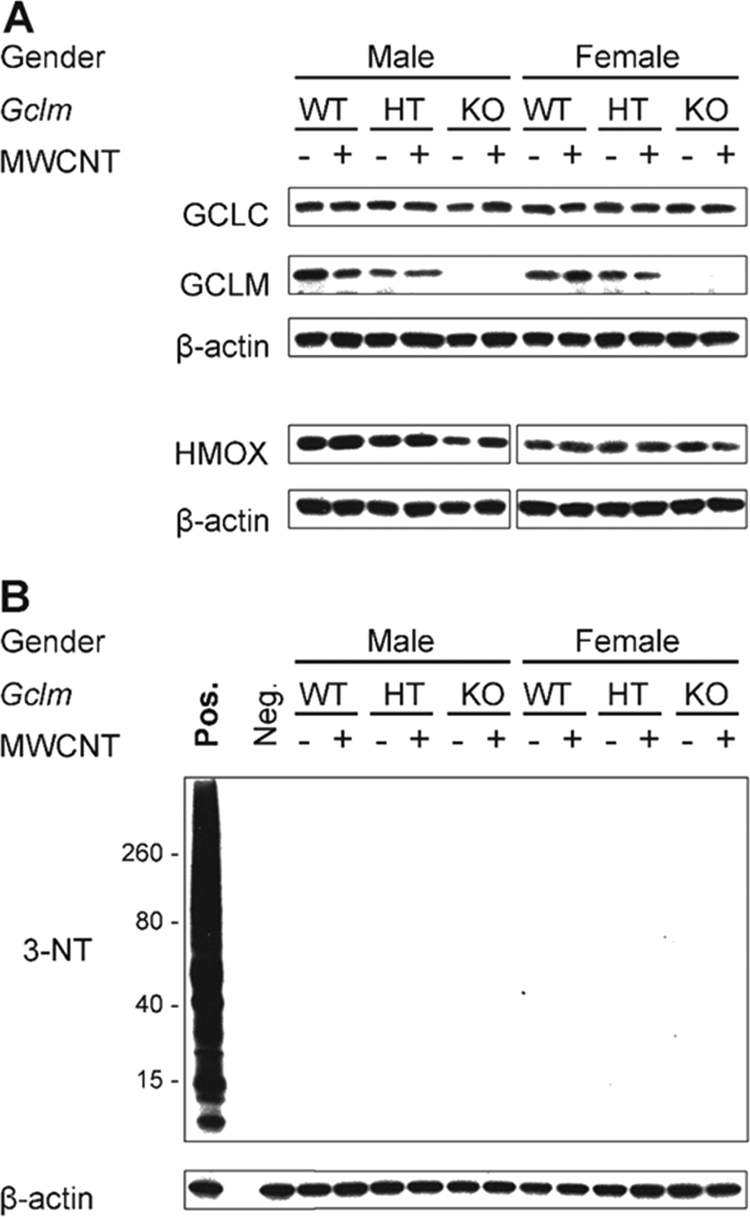
Consistent with our observations for mRNA expression of Gclc, Gclm, and Hmox1, MWCNT aspiration did not significantly alter the expression of these three proteins at 24 h post-exposure (Fig. 7). As expected, we did not detect GCLM protein expression in the lung homogenates prepared from Gclm-/- mice (Fig. 8, A).
Fig. 7.
Expression of redox-sensitive proteins. Densitometry analyses of Western blots for expression of redox-sensitive proteins GCLC, GCLM, and HMOX1 in lung homogenates from (A) male and (B) female Gclm mice, 24 h after aspiration of dispersion medium vehicle (Control) or 25 µg MWCNT. The results are means and SEM (n=5–6 mice/group), adjusted to the loading control, β-actin, and calculated as the fold-change relative to the mean of each genotype's control.
Fig. 8.
Representative Western blots for redox-sensitive proteins and 3-nitrotyrosine formation. Representative blots for GCLC, GCLM, HMOX1, and 3-nitrotyrosine. The 3-nitrotyrosine film was exposed for 1 h to increase signal sensitivity. Abbreviations: 3-NT, 3-nitrotyrosine; MWCNT, multiwalled carbon nanotube; Neg., negative control (untreated lung homogenate); Pos., positive control (peroxynitrite-treated lung homogenate).
To determine if gender or Gclm genotype confer increased susceptibility to severe nitrative stress, we determined if MWCNT exposure increased the generation of reactive nitrogen species (e.g., peroxynitrite) and consequent nitration of tyrosine residues on proteins in lung homogenates [58]. While Western immunoblotting readily detected 3-nitrotyrosine in our positive control (a peroxynitrite-treated lung homogenate of an unexposed mouse), it did not detect 3-nitrotyrosine residues in the homogenates of any control or exposed mice, even after exposing the film to the immunoblot for 1 h (Fig. 8, B).
These Western immunoblotting data indicate that MWCNT aspiration induces neither subtle alterations in redox-sensitive protein expression, nor substantial nitrative stress, not even in Gclm−/− mice with substantially lower levels of lung GSH.
4. Discussion
In order to identify potential factors modulating MWCNT-induced lung damage, we examined how gender and genetically-induced GSH deficiencies altered lung inflammation and oxidative stress in a mouse model of MWCNT exposure.
Our results indicate that endogenous GSH levels significantly alter the lung's innate immune response to an acute MWCNT exposure in a gender-dependent manner. Twenty-four h after exposing the mice via oropharyngeal aspiration, we found a significant increase in neutrophils recovered from the lungs of Gclm+/+ mice of both genders. While a previous study found that increasing GSH levels through N-acetylcysteine supplementation protected mice against MWCNT-induced inflammation [57], we did not observe increased inflammation in Gclm+/− and Gclm−/− mice compared to Gclm+/+ mice, as would be expected if there was a consistent inverse relationship between GSH levels and inflammation. Our observations suggest that the protective effects observed by Sun et al. may only occur when N-acetylcysteine supplementation significantly increases GSH above the endogenous levels afforded to Gclm+/+ mice.
Gender differences in inflammation emerged when we examined the Gclm+/− and Gclm−/− mice. While male Gclm+/− and Gclm−/− mice demonstrated a MWCNT-induced neutrophilia comparable to their Gclm+/+ counterparts, female Gclm+/− and Gclm–/− mice had significantly less neutrophilia than their Gclm+/+ counterparts. Intriguingly, MWCNT exposure was associated with increased levels of pro-inflammatory cytokines, regardless of Gclm genotype or gender—indicating that the differences in neutrophilia are likely to be independent of altered pro-inflammatory cytokine signaling.
These differences in neutrophilia between male and female mice with GSH deficiencies may relate to steroid hormone regulation of gene expression and the inflammatory response. One possibility is that the male Gclm+/- and Gclm-/- mice may have developed greater neutrophilia because of their higher levels of androgens, which can enhance the lung's inflammatory response. In support of this, lipopolysaccharide was reported to induce significantly less neutrophil influx into the lungs of castrated male mice, compared to intact mice [4]. Another possibility is that the female Gclm+/- and Gclm-/- mice may have been protected against inflammation because of their higher levels of estrogens, which can regulate macrophage-monocyte systems, inhibit the binding of transcription factor NF-ĸB to regulatory regions of pro-inflammatory genes [19], and upregulate expression in vitro of glutathione-S-transferase Pi (GSTp) and Gclc [38]. While we did not observe a significant increase in Gclc mRNA or protein expression, the female Gclm+/− and Gclm−/− mice may have had an estrogen-dependent upregulation of other mechanisms which protected them against MWCNT-induced neutrophilia. Indeed, a previous study found that acute ozone exposure induced significantly greater lung injury (as indicated by BALF total protein levels) in Gclm+/+ mice compared to Gclm-/- mice, which had significantly higher levels of protective enzymes in their lungs (e.g., metallothionein, α-tocopherol transporter protein, sodium-dependent vitamin C transporter) [24].
It is also possible that the reduced neutrophilia among female Gclm+/- and Gclm–/− mice may be due to impairment of a GSH-dependent neutrophil response, with potential repercussions on longer-term pathology. Previously, chemical depletion of GSH in a mouse cecum-puncture model of sepsis was associated with a decrease in neutrophil influx to the infection site, and a concomitant increase in bacterial infection [62]. The lack of a robust neutrophil response among female GSH-deficient mice may similarly impair clearance of MWCNTs from the lung, particularly because of the absence of neutrophil-dependent myeloperoxidase activity. Mice deficient in myeloperoxidase—an enzyme critical to the neutrophil's respiratory burst—were observed to retain CNT significantly longer in their lungs, and to develop significantly more fibrosis [54]. The absence of neutrophils and myeloperoxidase activity may therefore impose similar deleterious effects on GSH-deficient female mice. Moreover, the efficacy of those neutrophils which do influx may be further compromised in GSH-deficient mice, as chemical depletion of GSH in human neutrophils ex vivo reduced the efficacy of neutrophil phagocytosis [47]. If this is the case in humans, women workers who are GSH-deficient because of pre-existing health conditions, nutritional status, or common genetic polymorphisms may be at greater risk of an impaired neutrophil response to CNTs, and may therefore be at greater risk for CNTs persisting in the lung and inducing fibrosis.
In contrast to our data on neutrophilia, we found minimal evidence for MWCNT-induced eosinophilia. While MWCNT exposure was statistically associated with an increased percentage of BAL eosinophils among female mice, regardless of Gclm genotype, this percentage was extremely low (<2%). The lack of eosinophilia is consistent with a previous report, which found that MWCNT exposure was only associated with neutrophilia in male C57BL/6 mice [2].
Intriguingly, we observed gender- but not GSH-dependent differences in inflammation-related gene expression. Regardless of MWCNT exposure or Gclm genotype, female mice had significantly higher expression of the genes Gmcsf, Kc, and Mcp1. The differences may be related to steroid hormones, as these cytokines are produced by numerous cell types (e.g., macrophages, endothelial cells) which possess steroid hormone receptors [16], [52]. Indeed, progesterone pretreatment is reported to increase Kc mRNA expression in female BALB/cCrSlc mice [61]. Similarly, progesterone treatment of murine peritoneal macrophages ex vivo enhanced the expression of MCP-1 and other cytokines following hydrogen peroxide exposure [21]. Differences in Kc/CXCL1 mRNA expression have also been reported among humans, where a study of whole blood from healthy volunteers found that women had a small but significant 1.3- to 1.5-fold increase in levels of Kc/CXCL1 mRNA compared to men [26]. The functional effects associated with higher mRNA levels may be limited in our study, however, as we did not observe significant differences between the genders for cytokine protein levels.
Surprisingly, we did not find evidence for MWCNT-induced oxidative or nitrative stress. Twenty-four h after MWCNT exposure, we found that total GSH levels were significantly associated with Gclm genotype in lung tissue, but not in ELF. However, total GSH levels in either lung tissue or ELF did not differ with MWCNT exposure for either gender or any Gclm genotype. Furthermore, we did not observe any formation of 3-nitrotyrosine residues on proteins in lung tissue homogenate.
There are at least three possible explanations for these observations. First, we may have missed subtle changes in the cell's redox status by measuring total GSH, rather than evaluating the ratio of oxidized to reduced glutathione [64]. However, we did not detect subtle changes in redox status when we measured mRNA or protein expression of the redox-sensitive proteins GCLC, GCLM, and HMOX1 [36]. Furthermore, CNT-induced changes in the ratio of oxidized to reduced glutathione were previously observed to co-occur with significant decreases in total GSH levels [33], which our study would have detected.
Second, CNT-induced GSH depletion may not be detected until more time has elapsed after the initial exposure. In support of this, Shvedova et al. reported that CNT exposure in vivo did not deplete total GSH levels in lung tissue until 7 days post-exposure [55]. Furthermore, repeated exposures over multiple days may be required to overcome the lung's antioxidant reserves. Significant GSH depletion was observed following repeated daily inhalation exposures over 4 days [55] and 7 days [51].
Third, the MWCNT sample used in this study may not induce significant oxidative stress, potentially because it contains low (<1%) metal contamination. CNT contamination with iron may lead to the generation of reactive oxygen species through acellular Fenton reactions [35]. In support of this, Muhlfeld et al. reported that MWCNTs contaminated with 1.76% iron generated significantly more acellular reactive oxygen species than MWCNTs with 0.34%, as measured by electron paramagnetic resonance [39]. Indeed, in vivo studies reporting significant GSH depletion used CNTs with greater iron contamination, ranging from 2.7% [33], [50] to 17.7% [55].
There are several limitations inherent in our study. First, there are many MWCNTs available on the market with physicochemical characteristics different from the sample we examined. Because the pathological effects of MWCNTs have been shown to vary with physicochemical characteristics (e.g., length, width, surface modification, metal contamination) [17], [25], [39], [40], our results may not extrapolate perfectly to other MWCNTs. Second, our results are derived from an artificial exposure method—a single oropharyngeal aspiration of a liquid MWCNT bolus—rather than chronic inhalation of MWCNT, which may better model human occupational exposure. Nonetheless, Shvedova et al. demonstrated in mice that a single aspiration of CNTs recapitulated the pathological response of CNT inhalation, although the response to the aspirated CNTs was less robust than to the inhaled CNTs [55]. Our results indicate that further studies involving sub-chronic or chronic inhalation of MWCNTs would be warranted in Gclm mice of both genders, and would hopefully provide useful information on the effects of GSH and gender on MWCNT clearance and lung fibrosis.
In conclusion, our study shows that, regardless of Gclm status, male mice are consistently vulnerable to acute MWCNT-induced neutrophilia, while female mice develop an impaired neutrophilic response when heterozygous or null for Gclm. These results indicate that male workers may be consistently at risk for lung inflammation, while women workers who are GSH-deficient may be at risk of impaired neutrophil-based clearance of CNT from the lung, which may put them at greater risk of developing lung fibrosis. Given that GSH levels can be influenced by pre-existing health conditions, nutritional status, and genetic polymorphisms present in 20–30% of humans [41], [56], a significant proportion of the female CNT workforce may therefore be more sensitive to CNT-induced lung pathology. Future risk assessments to set occupational exposure limits should account for both male vulnerability as well as this potentially sensitive subpopulation of female workers, to ensure optimal protection of the growing CNT workforce against CNT-induced lung inflammation, fibrosis, and oxidative stress.
Funding Information
This work was supported by NIH/NIEHS Grants U19ES019544, U19ES019545, P30ES007033 and RC2ES018810; and NSF Grants CBET-0932885 and DGE-0718124.
Acknowledgements
We thank the staff of the University of Washington's Department of Comparative Medicine for their diligent care of our laboratory animals. Helium ion microscopy images were captured using equipment at the Environmental Molecular Sciences Laboratory, a DOE Office of Science User Facility sponsored by the Office of Biological and Environmental Research. EMSL is operated under Contract No. DE-AC05-76RL01830.
References
- 1.Biswas S.K., Rahman I. Environmental toxicity, redox signaling and lung inflammation: the role of glutathione. Mol. Asp. Med. 2009;30:60–76. doi: 10.1016/j.mam.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonner J.C., Silva R.M., Taylor A.J., Brown J.M., Hilderbrand S.C., Castranova V., Porter D., Elder A., Oberdorster G., Harkema J.R., Bramble L.A., Kavanagh T.J., Botta D., Nel A., Pinkerton K.E. Interlaboratory evaluation of rodent pulmonary responses to engineered nanomaterials: the NIEHS Nano GO Consortium. Environ. Health Perspect. 2013;121:676–682. doi: 10.1289/ehp.1205693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bussy C., Pinault M., Cambedouzou J., Landry M.J., Jegou P., Mayne-L′hermite M., Launois P., Boczkowski J., Lanone S. Critical role of surface chemical modifications induced by length shortening on multi-walled carbon nanotubes-induced toxicity. Part. Fibre Toxicol. 2012;9:46. doi: 10.1186/1743-8977-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Card J.W., Carey M.A., Bradbury J.A., DeGraff L.M., Morgan D.L., Moorman M.P., Flake G.P., Zeldin D.C. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J. Immunol. 2006;177:621–630. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC, 2013. Asthma Surveillance Data. Centers for Disease Control and Prevention, Atlanta, GA, pp.
- 6.Corredor C., Hou W.-C., Klein S.A., Moghadam B.Y., Goryll M., Doudrick K., Westerhoff P., Posner J.D. Disruption of model cell membranes by carbon nanotubes. Carbon. 2013;60:67–75. doi: 10.1016/j.carbon.2013.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coultas D.B., Zumwalt R.E., Black W.C., Sobonya R.E. The epidemiology of interstitial lung diseases. Am. J. Respir. Crit. Care Med. 1994;150:967–972. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- 8.Dahm M.M., Evans D.E., Schubauer-Berigan M.K., Birch M.E., Fernback J.E. Occupational exposure assessment in carbon nanotube and nanofiber primary and secondary manufacturers. Ann. Occup. Hyg. 2012;56:542–556. doi: 10.1093/annhyg/mer110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Volder M.F., Tawfick S.H., Baughman R.H., Hart A.J. Carbon nanotubes: present and future commercial applications. Science. 2013;339:535–539. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- 10.Edward J.T. Molecular volumes and the Stokes-Einstein equation. J. Chem. Educ. 1970;47:261. [Google Scholar]
- 11.Franklin C.C., Backos D.S., Mohar I., White C.C., Forman H.J., Kavanagh T.J. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol. Asp. Med. 2009;30:86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghezzi P. Role of glutathione in immunity and inflammation in the lung. Int J. Gen. Med. 2011;4:105–113. doi: 10.2147/IJGM.S15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gould N.S., Min E., Gauthier S., Chu H.W., Martin R., Day B.J. Aging adversely affects the cigarette smoke-induced glutathione adaptive response in the lung. Am. J. Respir. Crit. Care Med. 2010;182:1114–1122. doi: 10.1164/rccm.201003-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould N.S., Min E., Martin R.J., Day B.J. CFTR is the primary known apical glutathione transporter involved in cigarette smoke-induced adaptive responses in the lung. Free Radic. Biol. Med. 2012;52:1201–1206. doi: 10.1016/j.freeradbiomed.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gribbin J., Hubbard R.B., Le Jeune I., Smith C.J., West J., Tata L.J. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006;61:980–985. doi: 10.1136/thx.2006.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta V., Singh S.M. Gender dimorphism of macrophage response to GMCSF and IL-4 for differentiation into dendritic cells. Am. J. Reprod. Immunol. 2008;60:43–54. doi: 10.1111/j.1600-0897.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton R.F., Jr., Wu Z., Mitra S., Shaw P.K., Holian A. Effect of MWCNT size, carboxylation, and purification on in vitro and in vivo toxicity, inflammation and lung pathology. Part. Fibre Toxicol. 2013;10:57. doi: 10.1186/1743-8977-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han J.H., Lee E.J., Lee J.H., So K.P., Lee Y.H., Bae G.N., Lee S.B., Ji J.H., Cho M.H., Yu I.J. Monitoring multiwalled carbon nanotube exposure in carbon nanotube research facility. Inhal. Toxicol. 2008;20:741–749. doi: 10.1080/08958370801942238. [DOI] [PubMed] [Google Scholar]
- 19.Harkonen P.L., Vaananen H.K. Monocyte-macrophage system as a target for estrogen and selective estrogen receptor modulators. Ann. N. Y. Acad. Sci. 2006;1089:218–227. doi: 10.1196/annals.1386.045. [DOI] [PubMed] [Google Scholar]
- 20.Hewett P., Ganser G.H. A comparison of several methods for analyzing censored data. Ann. Occup. Hyg. 2007;51:611–632. doi: 10.1093/annhyg/mem045. [DOI] [PubMed] [Google Scholar]
- 21.Huang H., He J., Yuan Y., Aoyagi E., Takenaka H., Itagaki T., Sannomiya K., Tamaki K., Harada N., Shono M., Shimizu I., Takayama T. Opposing effects of estradiol and progesterone on the oxidative stress-induced production of chemokine and proinflammatory cytokines in murine peritoneal macrophages. J. Med. Investig. 2008;55:133–141. doi: 10.2152/jmi.55.133. [DOI] [PubMed] [Google Scholar]
- 22.Hunter R.J., Ottewill R.H., Rowell R.L. Academic Press Inc; San Diego, CA: 1981. Zeta Potential in Colloid Science: Principles and Applications. [Google Scholar]
- 23.Jain K.K. Advances in use of functionalized carbon nanotubes for drug design and discovery. Expert Opin. Drug Discov. 2012;7:1029–1037. doi: 10.1517/17460441.2012.722078. [DOI] [PubMed] [Google Scholar]
- 24.Johansson E., Wesselkamper S.C., Shertzer H.G., Leikauf G.D., Dalton T.P., Chen Y. Glutathione deficient C57BL/6J mice are not sensitized to ozone-induced lung injury. Biochem. Biophys. Res. Commun. 2010;396:407–412. doi: 10.1016/j.bbrc.2010.04.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston H.J., Hutchison G.R., Christensen F.M., Peters S., Hankin S., Aschberger K., Stone V. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: the contribution of physico-chemical characteristics. Nanotoxicology. 2010;4:207–246. doi: 10.3109/17435390903569639. [DOI] [PubMed] [Google Scholar]
- 26.Karlovich C., Duchateau-Nguyen G., Johnson A., McLoughlin P., Navarro M., Fleurbaey C., Steiner L., Tessier M., Nguyen T., Wilhelm-Seiler M., Caulfield J.P. A longitudinal study of gene expression in healthy individuals. BMC Med. Genom. 2009;2:33. doi: 10.1186/1755-8794-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam C.W., James J.T., McCluskey R., Arepalli S., Hunter R.L. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol. 2006;36:189–217. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- 28.Lee J.H., Lee S.B., Bae G.N., Jeon K.S., Yoon J.U., Ji J.H., Sung J.H., Lee B.G., Yang J.S., Kim H.Y., Kang C.S., Yu I.J. Exposure assessment of carbon nanotube manufacturing workplaces. Inhal. Toxicol. 2010;22:369–381. doi: 10.3109/08958370903367359. [DOI] [PubMed] [Google Scholar]
- 29.Lee J.S., Choi Y.C., Shin J.H., Lee J.H., Lee Y., Park S.Y., Baek J.E., Park J.D., Ahn K., Yu I.J. Health surveillance study of workers who manufacture multi-walled carbon nanotubes. Nanotoxicology. 2015;9:802–811. doi: 10.3109/17435390.2014.978404. [DOI] [PubMed] [Google Scholar]
- 30.Lee V., McMahan R.S., Hu X., Gao X., Faustman E.M., Griffith W.C., Kavanagh T.J., Eaton D.L., McGuire J.K., Parks W.C. Amphiphilic polymer-coated CdSe/ZnS quantum dots induce pro-inflammatory cytokine expression in mouse lung epithelial cells and macrophages. Nanotoxicology. 2015;9:336–343. doi: 10.3109/17435390.2014.930532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lesur I., Textoris J., Loriod B., Courbon C., Garcia S., Leone M., Nguyen C. Gene expression profiles characterize inflammation stages in the acute lung injury in mice. PLoS One. 2010;5:e11485. doi: 10.1371/journal.pone.0011485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao H.Y., Chung Y.T., Lai C.H., Wang S.L., Chiang H.C., Li L.A., Tsou T.C., Li W.F., Lee H.L., Wu W.T., Lin M.H., Hsu J.H., Ho J.J., Chen C.J., Shih T.S., Lin C.C., Liou S.H. Six-month follow-up study of health markers of nanomaterials among workers handling engineered nanomaterials. Nanotoxicology. 2014;8(Suppl. 1):S100–S110. doi: 10.3109/17435390.2013.858793. [DOI] [PubMed] [Google Scholar]
- 33.Luyts K., Smulders S., Napierska D., Van Kerckhoven S., Poels K., Scheers H., Hemmeryckx B., Nemery B., Hoylaerts M.F., Hoet P.H. Pulmonary and hemostatic toxicity of multi-walled carbon nanotubes and zinc oxide nanoparticles after pulmonary exposure in Bmal1 knockout mice. Part. Fibre Toxicol. 2014;11:61. doi: 10.1186/s12989-014-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma-Hock L., Treumann S., Strauss V., Brill S., Luizi F., Mertler M., Wiench K., Gamer A.O., van Ravenzwaay B., Landsiedel R. Inhalation toxicity of multiwall carbon nanotubes in rats exposed for 3 months. Toxicol. Sci. 2009;112:468–481. doi: 10.1093/toxsci/kfp146. [DOI] [PubMed] [Google Scholar]
- 35.Madl A.K., Plummer L.E., Carosino C., Pinkerton K.E. Nanoparticles, lung injury, and the role of oxidant stress. Annu Rev. Physiol. 2014;76:447–465. doi: 10.1146/annurev-physiol-030212-183735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McConnachie L.A., Botta D., White C.C., Weldy C.S., Wilkerson H.W., Yu J., Dills R., Yu X., Griffith W.C., Faustman E.M., Farin F.M., Gill S.E., Parks W.C., Hu X., Gao X., Eaton D.L., Kavanagh T.J. The glutathione synthesis gene Gclm modulates amphiphilic polymer-coated CdSe/ZnS quantum dot-induced lung inflammation in mice. PLoS One. 2013;8:e64165. doi: 10.1371/journal.pone.0064165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McConnachie L.A., Mohar I., Hudson F.N., Ware C.B., Ladiges W.C., Fernandez C., Chatterton-Kirchmeier S., White C.C., Pierce R.H., Kavanagh T.J. Glutamate cysteine ligase modifier subunit deficiency and gender as determinants of acetaminophen-induced hepatotoxicity in mice. Toxicol. Sci. 2007;99:628–636. doi: 10.1093/toxsci/kfm165. [DOI] [PubMed] [Google Scholar]
- 38.Montano M.M., Deng H., Liu M., Sun X., Singal R. Transcriptional regulation by the estrogen receptor of antioxidative stress enzymes and its functional implications. Oncogene. 2004;23:2442–2453. doi: 10.1038/sj.onc.1207358. [DOI] [PubMed] [Google Scholar]
- 39.Muhlfeld C., Poland C.A., Duffin R., Brandenberger C., Murphy F.A., Rothen-Rutishauser B., Gehr P., Donaldson K. Differential effects of long and short carbon nanotubes on the gas-exchange region of the mouse lung. Nanotoxicology. 2012;6:867–879. doi: 10.3109/17435390.2011.626533. [DOI] [PubMed] [Google Scholar]
- 40.Muller J., Huaux F., Moreau N., Misson P., Heilier J.F., Delos M., Arras M., Fonseca A., Nagy J.B., Lison D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol. Appl Pharm. 2005;207:221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura S., Kugiyama K., Sugiyama S., Miyamoto S., Koide S., Fukushima H., Honda O., Yoshimura M., Ogawa H. Polymorphism in the 5′-flanking region of human glutamate-cysteine ligase modifier subunit gene is associated with myocardial infarction. Circulation. 2002;105:2968–2973. doi: 10.1161/01.cir.0000019739.66514.1e. [DOI] [PubMed] [Google Scholar]
- 42.NCI, 2012. Characterization Data for Multi-walled Carbon Nanotubes.
- 43.NIOSH, 2013. Current Intelligence Bulletin 65 Occupational Exposure to Carbon Nanotubes and Nanofibers.
- 44.NRC . National Academies Press; Washington, D.C.: 2011. Guide for the Care and Use of Laboratory Animals. [PubMed] [Google Scholar]
- 45.NRC . National Academies Press; 2011. Working with Nanoparticles, Prudent Practices in the Laboratory: Handling and Management of Chemical Hazards: Updated Version; pp. 141–146. [PubMed] [Google Scholar]
- 46.Ogden T.L. Handling results below the level of detection. Ann. Occup. Hyg. 2010;54:255–256. doi: 10.1093/annhyg/mep099. [DOI] [PubMed] [Google Scholar]
- 47.Oliver J.M., Albertini D.F., Berlin R.D. Effects of glutathione-oxidizing agents on microtubule assembly and microtubule-dependent surface properties of human neutrophils. J. Cell Biol. 1976;71:921–932. doi: 10.1083/jcb.71.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pauluhn J. Subchronic 13-week inhalation exposure of rats to multiwalled carbon nanotubes: toxic effects are determined by density of agglomerate structures, not fibrillar structures. Toxicol. Sci. 2010;113:226–242. doi: 10.1093/toxsci/kfp247. [DOI] [PubMed] [Google Scholar]
- 49.Porter D.W., Hubbs A.F., Mercer R.R., Wu N., Wolfarth M.G., Sriram K., Leonard S., Battelli L., Schwegler-Berry D., Friend S., Andrew M., Chen B.T., Tsuruoka S., Endo M., Castranova V. Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology. 2010;269:136–147. doi: 10.1016/j.tox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Pothmann D., Simar S., Schuler D., Dony E., Gaering S., Le Net J.L., Okazaki Y., Chabagno J.M., Bessibes C., Beausoleil J., Nesslany F., Regnier J.F. Lung inflammation and lack of genotoxicity in the comet and micronucleus assays of industrial multiwalled carbon nanotubes Graphistrength((c)) C100 after a 90-day nose-only inhalation exposure of rats. Part. Fibre Toxicol. 2015;12:21. doi: 10.1186/s12989-015-0096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravichandran P., Baluchamy S., Gopikrishnan R., Biradar S., Ramesh V., Goornavar V., Thomas R., Wilson B.L., Jeffers R., Hall J.C., Ramesh G.T. Pulmonary biocompatibility assessment of inhaled single-wall and multiwall carbon nanotubes in BALB/c mice. J. Biol. Chem. 2011;286:29725–29733. doi: 10.1074/jbc.M111.251884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi Y., Liu C.H., Roberts A.I., Das J., Xu G., Ren G., Zhang Y., Zhang L., Yuan Z.R., Tan H.S., Das G., Devadas S. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don't know. Cell Res. 2006;16:126–133. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 53.Shulaker M.M., Hills G., Patil N., Wei H., Chen H.Y., Wong H.S., Mitra S. Carbon nanotube computer. Nature. 2013;501:526–530. doi: 10.1038/nature12502. [DOI] [PubMed] [Google Scholar]
- 54.Shvedova A.A., Kapralov A.A., Feng W.H., Kisin E.R., Murray A.R., Mercer R.R., St Croix C.M., Lang M.A., Watkins S.C., Konduru N.V., Allen B.L., Conroy J., Kotchey G.P., Mohamed B.M., Meade A.D., Volkov Y., Star A., Fadeel B., Kagan V.E. Impaired clearance and enhanced pulmonary inflammatory/fibrotic response to carbon nanotubes in myeloperoxidase-deficient mice. PLoS One. 2012;7:e30923. doi: 10.1371/journal.pone.0030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shvedova A.A., Kisin E., Murray A.R., Johnson V.J., Gorelik O., Arepalli S., Hubbs A.F., Mercer R.R., Keohavong P., Sussman N., Jin J., Yin J., Stone S., Chen B.T., Deye G., Maynard A., Castranova V., Baron P.A., Kagan V.E. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress, and mutagenesis. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;295:L552–L565. doi: 10.1152/ajplung.90287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siedlinski M., Postma D.S., van Diemen C.C., Blokstra A., Smit H.A., Boezen H.M. Lung function loss, smoking, vitamin C intake, and polymorphisms of the glutamate-cysteine ligase genes. Am. J. Respir. Crit. Care Med. 2008;178:13–19. doi: 10.1164/rccm.200711-1749OC. [DOI] [PubMed] [Google Scholar]
- 57.Sun B., Wang X., Ji Z., Wang M., Liao Y.P., Chang C.H., Li R., Zhang H., Nel A.E., Xia T. NADPH oxidase-dependent NLRP3 inflammasome activation and its important role in lung fibrosis by multiwalled carbon nanotubes. Small. 2015;11:2087–2097. doi: 10.1002/smll.201402859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takakusa H., Mohar I., Kavanagh T.J., Kelly E.J., Kaspera R., Nelson S.D. Protein tyrosine nitration of mitochondrial carbamoyl phosphate synthetase 1 and its functional consequences. Biochem. Biophys. Res. Commun. 2012;420:54–60. doi: 10.1016/j.bbrc.2012.02.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson S.A., White C.C., Krejsa C.M., Diaz D., Woods J.S., Eaton D.L., Kavanagh T.J. Induction of glutamate-cysteine ligase (gamma-glutamylcysteine synthetase) in the brains of adult female mice subchronically exposed to methylmercury. Toxicol. Lett. 1999;110:1–9. doi: 10.1016/s0378-4274(99)00133-2. [DOI] [PubMed] [Google Scholar]
- 60.Townsend D.M., Tew K.D., Tapiero H. The importance of glutathione in human disease. Biomed. Pharm. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toyoda Y., Endo S., Tsuneyama K., Miyashita T., Yano A., Fukami T., Nakajima M., Yokoi T. Mechanism of exacerbative effect of progesterone on drug-induced liver injury. Toxicol. Sci. 2012;126:16–27. doi: 10.1093/toxsci/kfr326. [DOI] [PubMed] [Google Scholar]
- 62.Villa P., Saccani A., Sica A., Ghezzi P. Glutathione protects mice from lethal sepsis by limiting inflammation and potentiating host defense. J. Infect. Dis. 2002;185:1115–1120. doi: 10.1086/340042. [DOI] [PubMed] [Google Scholar]
- 63.Wang X., Katwa P., Podila R., Chen P., Ke P.C., Rao A.M., Walters D.M., Wingard C.J., Brown J.M. Multi-walled carbon nanotube instillation impairs pulmonary function in C57BL/6 mice. Part. Fibre Toxicol. 2011;8:24. doi: 10.1186/1743-8977-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weldy C.S., Luttrell I.P., White C.C., Morgan-Stevenson V., Bammler T.K., Beyer R.P., Afsharinejad Z., Kim F., Chitaley K., Kavanagh T.J. Glutathione (GSH) and the GSH synthesis gene Gclm modulate vascular reactivity in mice. Free Radic. Biol. Med. 2012;53:1264–1278. doi: 10.1016/j.freeradbiomed.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weldy C.S., White C.C., Wilkerson H.W., Larson T.V., Stewart J.A., Gill S.E., Parks W.C., Kavanagh T.J. Heterozygosity in the glutathione synthesis gene Gclm increases sensitivity to diesel exhaust particulate induced lung inflammation in mice. Inhal. Toxicol. 2011;23:724–735. doi: 10.3109/08958378.2011.608095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wesselkamper S.C., Chen L.C., Gordon T. Development of pulmonary tolerance in mice exposed to zinc oxide fumes. Toxicol. Sci. 2001;60:144–151. doi: 10.1093/toxsci/60.1.144. [DOI] [PubMed] [Google Scholar]