Abstract
Liraglutide, a glucagon-like peptide (GLP-1) receptor agonist, has showed favorable effects in the glycaemic control and weight reduction in patients with type 2 diabetes mellitus (T2DM). The meta-analysis was to compare the efficacy and safety of liraglutide added to metformin with other treatments in patients with T2DM. A systematic literature search on PubMed, Embase, Web of Science and the Cochrane library databases were performed. Eligible studies were randomized controlled trials (RCTs) of patients with T2DM who received the combination treatment of liraglutide and metformin. Pooled estimates were performed using a fixed-effects model or random-effects model. A total of nine RCTs met the inclusion criteria. Compared with control (placebo, sitagliptin, glimepiride, dulaglutide, insulin glargine, and NPH), liraglutide in combination with metformin resulted in significant reductions in HbA1c, bodyweight, FPG, and PPG, and similar reductions in SBP, and DBP. Moreover, liraglutide combined with metformin did not increase the risk of hypoglycemia, but induced a higher incidence of gastrointestinal disorders. In conclusion, this meta-analysis confirmed the use of liraglutide as add-on to metformin appeared to be effective and safe for patients with T2DM. However, considering the potential limitations in this study, more large-scale, well-conducted RCTs are needed to identify our findings.
Type 2 diabetes mellitus (T2DM) is a complex and progressive multi-system disease characterized by declining beta cell function and insulin resistance, which lead to loss of glycemic control and eventual diabetes complications1,2. Lifestyle modifications and metformin have been recommended by the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) as the first-line therapy for T2DM3. For the currently available therapies, because of the un-addressed issue of declining beta cell function and weight or cardiovascular concerns, the glycaemia are not adequately controlled in the long term. Moreover, such therapies often contain complex treatment and titration regimens, and can result in increased risk of hypoglycaemia and undesirable outcomes such as oedema and weight gain4.
Glucagon-like peptide-1 (GLP-1) is a naturally occurring incretin hormone released from neuroendocrine intestinal L-cells5. It could reduce the glucose levels by increasing the secretion of insulin and lowering glucagon, delaying gastric emptying, and decreasing food intake5,6. In animal models, native GLP-1 showed beneficial effects in the stimulation of beta cell proliferation and suppression of apoptosis in vitro7. Moreover,GLP-1 also reduces the risk of hypoglycaemia since its activities in the glucose-lowering are glucose dependent8.However, GLP-1 is rapidly metabolized and inactivated by the protease dipetidyl peptidase-4 (DPP-4)9, which leads to a very short half-life and limits its therapeutic potential in the clinical practice10. The short half life of GLP-1 has prompted efforts to develop novel agents for the therapeutic applications.
Liraglutide is a novel, long-acting GLP-1receptor agonist that is administered once-daily for the treatment of T2DM11. It has been approved by the European Union regulatory agency and United States Food and Drug Administration (FDA). Liraglutide has a half-life of 13 h, which allows for once-daily dosing subcutaneous administration12,13,14. The efficacy and safety of liraglutide have been extensively evaluated in several large-scale clinical trials, and their results suggest its positive effects in the reduction of blood glucose, body weight and systolic blood pressure (SBP)15. Liraglutide also was generally tolerated and was associated with most commonly adverse events, including transient nausea, vomiting, and diarrhea16,17,18,19,20.
In this study we conducted a meta-analysis to compare the efficacy and safety of liraglutide plus metformin with other drugs in patients with T2DM.
Methods and Materials
Search strategy
We conducted this meta-analysis of the current literature according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines21. A comprehensive systematic search of several major electronic databases (PubMed, Embase, Web of Science and the Cochrane library) was conducted before February 3, 2016. The following search terms used were: (“diabetes mellitus, type 2”[MeSH Terms] OR “type 2 diabetes mellitus”[All Fields] OR “type 2 diabetes”[All Fields]) AND (“metformin”[MeSH Terms] OR “metformin”[All Fields]) AND (“liraglutide”[MeSH Terms] OR “liraglutide”[All Fields]). Additional relevant articles were obtained by searching the reference lists of the articles included in this study. No language restriction and publication status were imposed.
Inclusion criteria and study selection
All clinical trials assessing the efficacy and safety of liraglutide plus metformin in the treatment of T2DM were considered eligible for analysis. The predetermined study inclusion criteria were: (1) randomized controlled trials (RCTs); (2) adult patients had T2DM [HbA1c between either 6.5 or 7.0 and either 10.0 or 11.0%, depending on previous treatment]; (3) compared liraglutide in addition to metformin with another antidiabetic therapy or placebo; (4) reported the data on changes from baseline in HbA1c, bodyweight, fasting plasma glucose (FPG), postprandial plasma glucose(PPG), systolic blood pressure (SBP), and diastolic blood pressure (DBP); (5) treat patients more than 12 weeks after randomization.
Data extraction and quality assessment
Two independent investigators used a standardized tool to extract the following data from each study: first author’s name, year of publication, country, number of study patients, baseline patient characteristics (age, sex, race, diabetes duration), mean changes from baseline in HbA1c, bodyweight, FPG, PPG, SBP, and DBP, and incidence of treatment-emergent adverse events (nausea, diarrhea, vomiting, dyspepsia, constipation, and nasopharyngitis).
We used the Jadad scale22 to assess the methodological quality of the included studies. The Jadad scale consists of three items describing randomization (0–2 points), blinding (0–2 points), and dropouts and withdraws (0–1 point) to report the quality of a RCT22. A score of 1 point is given for each of the points described. A further point is obtained when the randomization and/or blinding is described and appropriate. The quality scale ranges from 0 to 5 points, and higher scale suggests better reporting. Any study with a score ≥3 is considered to be of high quality22,23.
Statistical analysis
Data were analyzed using Stata version 12.0 (Stata Corporation, College Station, TX, USA). Before the data were synthesized, we first test the heterogeneity between the studies using Q chi-square test24, in which a P value <0.10 was considered as significant heterogeneity. I2 statistic was used to describe the percentage of the variability that attributed to heterogeneity across the studies rather than the chance. Studies with an I2 statistic of <25%, ~50%, ~75%, ~100% are considered to have no, low, moderate, and high degree of heterogeneity, respectively25. Pooled estimates were calculated using a fixed-effects model (Mantel–Haenszel method)26; otherwise, a random-effects model (DerSimonian–Laird method)27 was applied when significant heterogeneity among the included studies was found. If the heterogeneity was tested, subgroup analysis or sensitivity analysis was performed to explore the potential sources of heterogeneity.
Continuous variables, including mean changes from baseline in HbA1c, bodyweight, FPG, PPG, SBP, and DBP, were expressed as weight mean difference (WMD) with 95% confidence intervals (95%CIs); dichotomous variables, including the incidence of treatment-emerge adverse events, were expressed as relative risk (RR) with 95%CIs. The assessment of publication bias was evaluated by using Egger28 and Begger29 test. A P value less than 0.05 was judged as statistically significant, except where otherwise specified.
Results
Identification of eligible studies
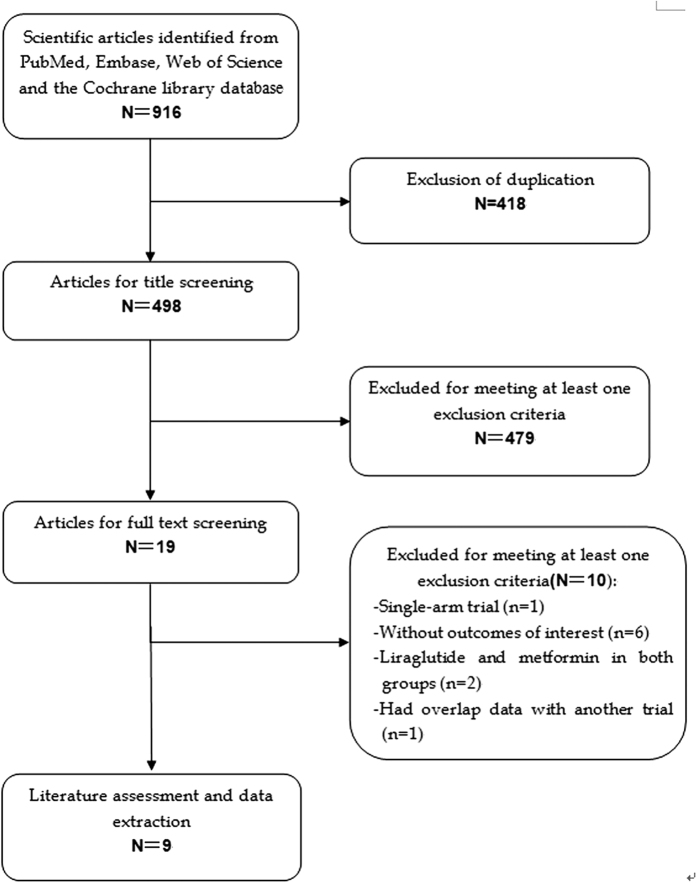
The initial search yielded 916 relevant publications from PubMed, Web of Science, Embase, and the Cochrane library. Of these, 418 were excluded because of duplicate records, and 479 and 9 studies were removed after a review if title/abstract and full-text information, respectively (Fig. 1). Thus, 19 potential studies were identified for the final analysis. However, ten of them were excluded for the following reasons: six studies did not provide outcome of interest or available data30,31,32,33,34,35, two studies had liraglutide and metformin in both groups36,37, one study was a sing-arm design38, and one study39 had overlap data with another trial40. Finally, nine RCTs19,20,40,41,42,43,44,45,46 met the inclusion criteria, and were included in this meta-analysis.
Figure 1. Eligibility of studies for inclusion in meta-analysis.
Characteristics of eligible studies and quality assessment
The main characteristics of the nine included RCTs are presented in Table 1. These studies were published between 2009 and 2015. The total number of included patients was 4,657, ranging from 63 to 1,091 patients per study. The clinical characteristics were well matched for age, sex distribution, duration of diabetes, BMI, waist circumference, HbA1c, FPG, SBP, and DBP in both groups at the beginning of each study. The study durations ranged from 12 to 52 weeks. All these studies provided information regarding the efficacy and safety of combination treatment of liraglutide and metformin in patients with T2DM.
Table 1. Baseline characteristics of patients in the trials included in the meta-analysis.
| Study | Year | Intervention | No. of patients | Age(mean ± SD, y) | Duration of T2DM (mean ± SD, y) | Hb1Ac (mean ± SD, %) | Weight(mean ± SD, kg) | Jadad score |
|---|---|---|---|---|---|---|---|---|
| Nauck M19 | 2009 | 0.6 mg liraglutide | 242 | 56±11 | 7±5 | 8.4±0.9 | NR | 4 |
| 1.2 mgliraglutide | 242 | 57±9 | 7±5 | 8.3±1.0 | NR | |||
| 1.8 mg liraglutide | 242 | 57±9 | 8±5 | 8.4±1.0 | NR | |||
| 4 mg glimepiride | 123 | 57±9 | 8±5 | 8.4±1.0 | NR | |||
| Placebo | 242 | 56±9 | 8±6 | 8.4±1.1 | NR | |||
| Russell-Jones D20 | 2009 | 1.8 mg liraglutide | 232 | 57.6±9.5 | 9.2±5.8 | 8.3±0.9 | 85.5±19.4 | 4 |
| Placebo | 115 | 57.5±9.6 | 9.4±6.2 | 8.3±0.9 | 85.7±16.7 | |||
| 24 IU insulin glargine | 234 | 57.5±10.5 | 9.7±6.4 | 8.2±0.9 | 85±17.9 | |||
| Dungan KM41 | 2014 | 1.8 mg liraglutide | 300 | 56.8±9.9 | 7.3±5.4 | 8.1±0.8 | 94.4±19 | 4 |
| 1.5 mg dulaglutide | 299 | 56.5±9.3 | 7.1±5.4 | 8.1±0.8 | 93.8±18.2 | |||
| Ma ZJ42 | 2015 | 1.8 mg liraglutide | 31 | 51.3±12.5 | NR | 10.1±1.4 | 77.3±2.1 | 3 |
| NPH | 32 | 52.5±11.7 | NR | 9.8±1.8 | 76.7±2.3 | |||
| Davies M43 | 2011 | 1.2 mg liraglutide | 164 | NR | NR | 8.4 | 93.7 | 3 |
| 1.8 mg liraglutide | 171 | NR | NR | 8.4 | 94.6 | |||
| 100 mg sitagliptin | 170 | NR | NR | 8.5 | 93.1 | |||
| Brady EM44 | 2014 | 1.2 mg liraglutide | 47 | 51.5±11.1 | NR | 7.6±1.1 | 86.1±16.9 | 4 |
| 4 mg glimepiride | 52 | 52.2±10.7 | NR | 7.8±1.0 | 79±11.2 | |||
| Yang W45 | 2011 | 0.6 mg liraglutide | 231 | 53.5±9.5 | 7.4±5.4 | 8.5±1.1 | 68.6±11.6 | 3 |
| 1.2 mg liraglutide | 233 | 53.5±9.6 | 7.5±5.3 | 8.6±1.1 | 67.4±11.3 | |||
| 1.8 mg liraglutide | 234 | 52.7±9.1 | 7.2±5.2 | 8.6±1.1 | 68.2±11.9 | |||
| 4 mg glimepiride | 231 | 53.6±9.7 | 7.8±6.1 | 8.5±1.1 | 68.2±11.9 | |||
| Charbonnel B46 | 2013 | 1.2 mgliraglutide | 327 | 67.6±10.8 | 8.2±6.2 | 8.1±0.9 | 92.1±20.4 | 3 |
| 100 mg sitagliptin | 326 | 56.9±10 | 7.6±4.8 | 8.2±1.1 | 91±20.5 | |||
| Pratley R40 | 2011 | 1.2 mgliraglutide | 135 | 55.9±9.6 | 6.0±4.5 | 8.4±0.8 | NR | 3 |
| 1.8 mg liraglutide | 150 | 55.0±9.1 | 6.4±5.4 | 8.4±0.7 | NR | |||
| 100 mg sitagliptin | 151 | 55.0±9.0 | 6.3±5.4 | 8.5±0.7 | NR |
Abbreviation: NR, not reported; SD, standard deviation.
The dosages of liraglutide were fixed in eight trials19,20,40,41,42,43,44,45 with a dose of 0.6, 1.2 or 1.8 mg/day, whereas the other study46 started at a dose of 0.6 mg/day, up-titrated to 1.2 mg/day after 1 week. Placebo was used as control group in two studies19,20, four used 100 mg/day sitagliptin40,43,46, two used 4 mg/day glimepiride19,45,one used 1.5 mg/week dulaglutide41, one used 24 IU/day insulin glargine20, one used NPH42. The median Jadad score of these included studies was 4 (ranged from 3 to 4).
Change in HbA1c
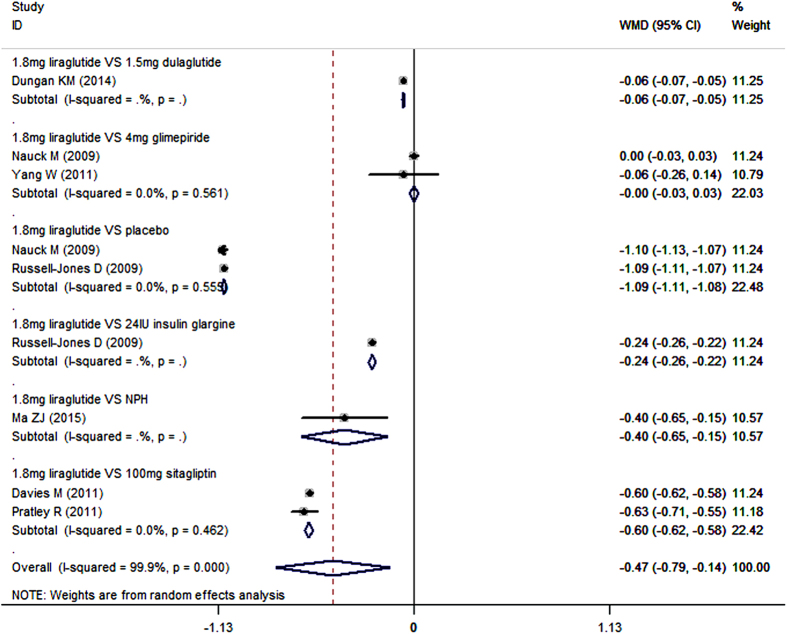
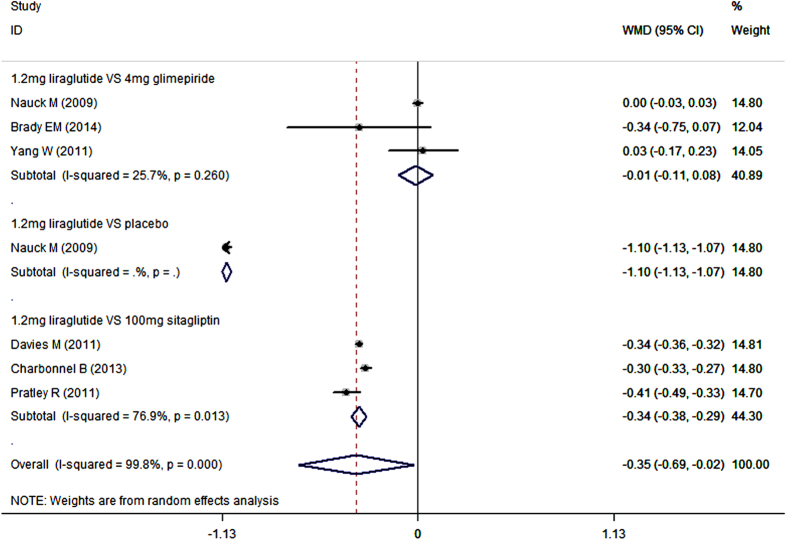
All the studies included reported the data of mean change from baseline in HbA1c19,20,40,41,42,43,44,45,46. When used as add-on to metformin, liraglutide significantly decreased HbA1c compared with control (placebo, sitagliptin, glimepiride, dulaglutide, insulin glargine, and NPH) (WMD = −0.36%, 95%CI: −0.57%, −0.14%; P = 0.001). Subgroup analysis based on the dosage of liraglutide showed that, 1.8 mg/day and 1.2 mg/day liraglutide in combination with metformin notably lowered HbA1c compared with control (for 1.8 mg/day liraglutide: WMD = −0.47%, 95%CI: −0.79%, −0.14%; P = 0.005; for 1.2 mg/day liraglutide: WMD = −0.35%, 95%CI: −0.69%, −0.02%; P = 0.035)(Figs 2 and 3), whereas 0.6 mg/day liraglutide did not (WMD = −0.09%, 95%CI: −0.96%, 0.79%; P = 0.848).
Figure 2. HbA1c: 1.8 mg liraglutide add-on to metformin VS. control.
Figure 3. HbA1c: 1.2 mg liraglutide add-on to metformin VS. control.
Furthermore, we also conducted subgroup-analysis based on the comparators. As an add-on to metformin, 1.8 mg/day liraglutide significantly lowered HbA1c compared with placebo (WMD = −1.09%, 95%CI: −1.11%, −1.08%; P < 0.001), sitagliptin (WMD = −0.60%, 95%CI: −0.62%, −0.58%; P < 0.001), dulaglutide (WMD = −0.06%, 95%CI: −0.07%, −0.05%; P < 0.001), and insulin glargine (WMD = −0.24%, 95%CI: −0.26%, −0.22%; P < 0.001), but not glimepiride (WMD = 0.00%, 95%CI: −0.03%, 0.03%; P = 0.936) (Fig. 2) (Table 2).
Table 2. Effect comparison between liraglutide added to metformin with other therapies in patients with T2DM.
| Pooled changes from baseline between patients treated with combination therapy and other therapies, WMD (95%CI) | ||||||
|---|---|---|---|---|---|---|
| Regimen | Placebo | Sitagliptin | Glimepiride | Dulaglutide | Insulin glargine | NPH |
| HbA1c (%) | ||||||
| 1.8 mg liraglutide | −1.09 (−1.11,−1.08) | −0.60 (−0.62, −0.58) | 0.00(−0.03, 0.03) | −0.06 (−0.07, −0.05) | −0.24 (−0.26, −0.22) | |
| 1.2 mg liraglutide | −1.10 (−1.13,−1.07) | −0.34 (−0.38, −0.29) | −0.01(−0.11, 0.08) | |||
| 0.6 mg liraglutide | −0.80 (−0.83,−0.77) | 0.30(0.27, 0.33) | ||||
| Body weight (kg) | ||||||
| 1.8 mg liraglutide | −1.38 (−1.45, −1.31) | −2.52 (−2.71, −2.33) | −2.50 (−2.84, −2.16) | −0.71 (−0.75, -0.67) | −3.40 (−3.47,−3.33) | −1.90 (−2.89,−0.91) |
| 1.2 mg liraglutide | −2.01(−2.78, −1.25) | −2.42 (−2.73, −2.12) | ||||
| FPG (mmol/L) | ||||||
| 1.8 mg liraglutide | −2.09 (−2.11,−2.07) | −2.43 (−2.49, −2.37) | −0.22 (−0.66, 0.23) | 0.03 (0.01, 0.05) | 0.24 (0.22, 0.26) | |
| 1.2 mg liraglutide | −2.00 (−2.03,−1.97) | −0.91 (−1.32, −0.05) | −0.10 (−0.52, 0.32) | |||
| 0.6 mg liraglutide | −1.50 (−1.53,−1.47) | 0.20 (−0.05, 0.45) | ||||
| PPG (mmol/L) | ||||||
| 1.8 mg liraglutide | −1.91 (−2.07, −1.76) | −0.49 (−1.29, 0.30) | 0.13 (0.12, 0.14) | −0.20 (−0.45, 0.05) | −0.70 (−1.57,0.17) | |
| 1.2 mg liraglutide | −1.70 (−1.73, −1.67) | −0.11 (−0.73, 0.50) | ||||
| 0.6 mg liraglutide | −1.10 (−1.13, −1.07) | 0.47 (−0.25, 1.18) | ||||
| SBP (mm Hg) | ||||||
| 1.8 mg liraglutide | −2.60 (−2.78, −2.42) | −0.42 (−0.88,0.04) | 0.54 (0.43, 0.65) | −4.54 (−4.57, −4.51) | ||
| 1.2 mg liraglutide | −1.08 (−4.46, 2.31) | −3.70 (−9.81, 2.41) | ||||
| DBP (mm Hg) | ||||||
| 1.8 mg liraglutide | 0.60 (0.52, 0.68) | −0.09 (−0.15, −0.03) | ||||
| 1.2 mg liraglutide | 0.27 (−1.04,1.58) | −5.53 (−9.15, −1.91) | ||||
Abbreviation: T2DM, type 2 diabetes mellitus; HbA1c, glycated haemoglobin; FPG, Fasting plasma glucose; PPG, Postprandial plasma glucose; SBP, Systolic blood pressure; DBP, Diastolic blood pressure.
When used combination with metformin, 1.2 mg/day liraglutide notably decreased HbA1c compared with placebo (WMD = −1.10%, 95%CI: −1.13%, −1.07%; P < 0.001), and sitagliptin (WMD = −0.28%, 95%CI: −0.47%, −0.10%; P < 0.001), but not glimepiride (WMD = −0.01%, 95%CI: −0.11%, 0.08%; P = 0.807) (Fig. 3) (Table 2).
When used as an add-on therapy to metformin, 0.6 mg/day liraglutide notably decreased HbA1c compared with placebo (WMD = −0.80%, 95%CI: −0.83%, −0.77%; P < 0.001), but increased HbA1c compared with glimepiride (WMD = 0.30%, 95%CI: 0.27%, 0.33%; P < 0.001) (Table 2).
Bodyweight
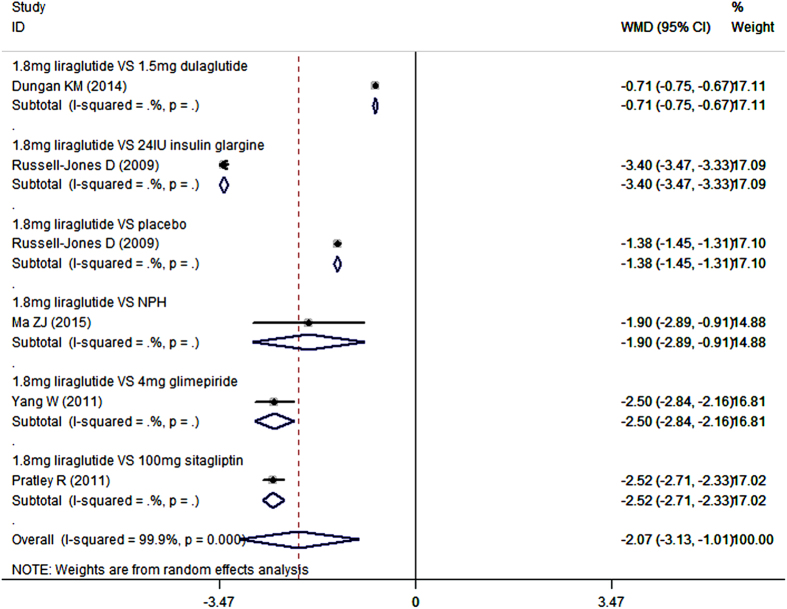
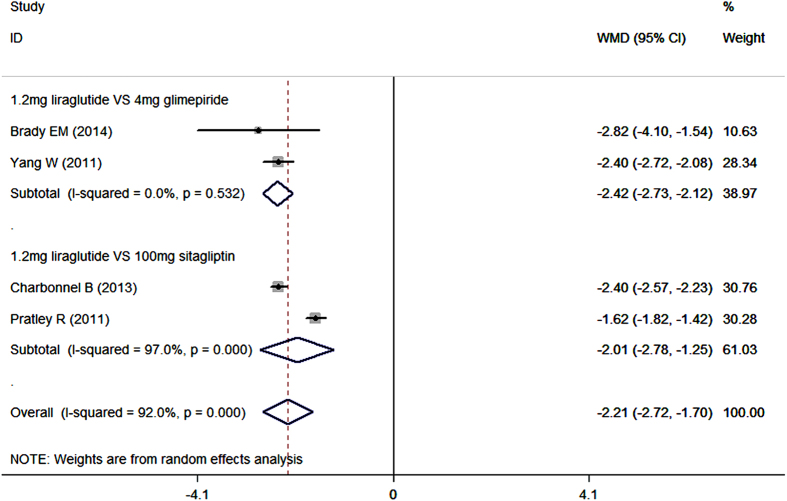
Seven studies reported the data in bodyweight20,40,41,42,44,45,46. As an add-on to metformin, liraglutide lowered bodyweight more than control (placebo, sitagliptin, glimepiride, dulaglutide, insulin glargine, and NPH) (WMD = −2.13 kg, 95%CI: −2.87, −1.38; P < 0.001). Subgroup analysis based on the dosage of liraglutide showed that, liraglutide in all of the three dosages significantly reduced bodyweight more than control when combined with metformin (for 1.8 mg/day: WMD = −2.07 kg, 95%CI: −3.13, −1.01; P < 0.001; for 1.2 mg/day: WMD = −2.21 kg, 95%CI: −2.72, −1.70; P < 0.001; for 0.6 mg/day: WMD = −1.90 kg, 95%CI: −2.19, −1.61; P < 0.001) (Figs 4 and 5).
Figure 4. Bodyweight: 1.8 mg liraglutide add-on to metformin VS. control.
Figure 5. Bodyweight: 1.2mg liraglutide add-on to metformin VS. control.
Furthermore, we also conducted subgroup-analysis based on the comparators. As an add-on to metformin, 1.8 mg/day liraglutide significantly lowered bodyweight compared with placebo (WMD = −1.38 kg, 95%CI: −1.45, −1.31; P < 0.001), sitagliptin (WMD = −2.52 kg, 95%CI: −2.71, −2.33; P < 0.001), glimepiride (WMD = −2.50 kg, 95%CI: −2.84, −2.16; P < 0.001), dulaglutide (WMD = −0.71 kg, 95%CI: −0.75, −0.67; P < 0.001), insulin glargine (WMD = −3.40 kg, 95%CI: −3.47, −3.33; P < 0.001), and NPH (WMD = −1.90 kg, 95%CI: −2.89, −0.91; P < 0.001) (Fig. 4) (Table 2).
When used combination with metformin, 1.2 mg/day liraglutide notably decreased bodyweight compared with sitagliptin (WMD = −2.01 kg, 95%CI: −2.78, −1.25; P < 0.001), and glimepiride (WMD = −2.42 kg, 95%CI: −2.73, −2.12; P < 0.001) (Fig. 5) (Table 2).
Fasting plasma glucose
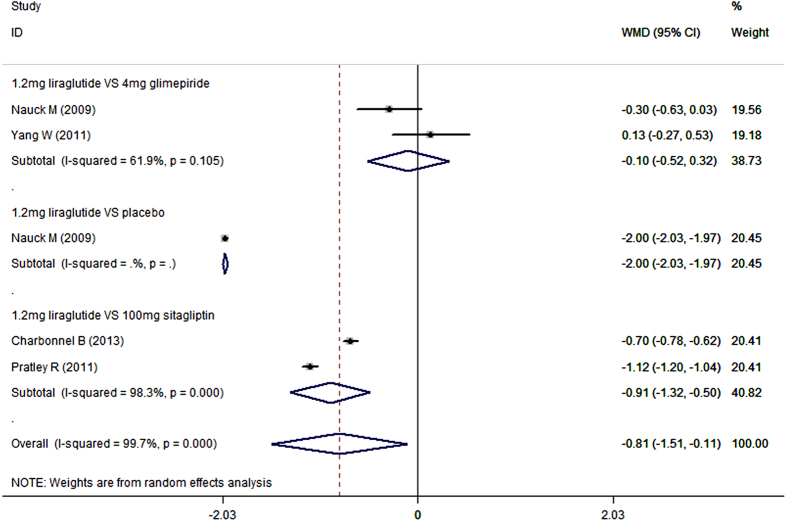
Seven studies reported the data in FPG19,20,40,41,42,45,46. When administered with metformin, liraglutide significantly lowered the FPG concentration compared with control (WMD = −0.72 mmol/L, 95%CI: −1.27, −0.17; P = 0.010). Subgroup analysis based on the dosage of liraglutide showed that, 1.8 mg/day and 1.2 mg/day liraglutide in combination with metformin notably reduced the FPG concentration compared with control (for 1.8 mg/day: WMD = −0.85 mmol/L, 95%CI: −1.69, −0.10; P = 0.046; for 1.2 mg/day: WMD = −0.81 mmol/L, 95%CI: −1.51, −0.11; P = 0.023) (Figs 6 and 7), whereas 0.6 mg/day liraglutide did not (WMD = −0.65 mmol/L, 95%CI: −2.32, 1.01; P = 0.441).
Figure 6. Fasting plasma glucose: 1.8 mg liraglutide add-on to metformin VS. control.
Figure 7. Fasting plasma glucose: 1.2 mg liraglutide add-on to metformin VS. control.
Furthermore, we also conducted subgroup-analysis based on the comparators. As an add-on to metformin, 1.8 mg/day liraglutide decreased FPG concentration more than placebo (WMD = −2.09 mmol/L, 95%CI: −2.11, −2.07; P < 0.001) and sitagliptin (WMD = −2.43 mmol/L, 95%CI: −2.49, −2.37; P < 0.001), and reduced FPG concentration less than dulaglutide (WMD = 0.03 mmol/L, 95%CI: 0.01, 0.05; P = 0.002) and insulin glargine (WMD = 0.24 mmol/L, 95%CI: 0.22, 0.26; P < 0.001), and exhibited a similar reduction in FPG concentration as did glimepiride (WMD = −0.22 mmol/L, 95%CI: −0.66, 0.23; P = 0.340) (Fig. 6) (Table 2).
When used combination with metformin, 1.2 mg/day liraglutide notably decreased FPG concentration more than placebo (WMD = −2.00 mmol/L, 95%CI: −2.03, −1.97; P < 0.001) and sitagliptin (WMD = −0.91 mmol/L, 95%CI: −1.32, −0.05; P < 0.001), and exhibited a similar reduction in FPG concentration as did glimepiride (WMD = −0.10 mmol/L, 95%CI: −0.52, 0.32; P = 0.641) (Fig. 7) (Table 2).
When used as an add-on therapy to metformin, 0.6 mg/day liraglutide notably reduced FPG level compared with placebo (WMD = −1.50 mmol/L, 95%CI: −1.53, −1.47; P < 0.001), and exhibited a similar reduction in FPG concentration as did glimepiride (WMD = 0.20 mmol/L, 95%CI: −0.05, 0.45; P = 0.116) (Table 2).
Postprandial plasma glucose
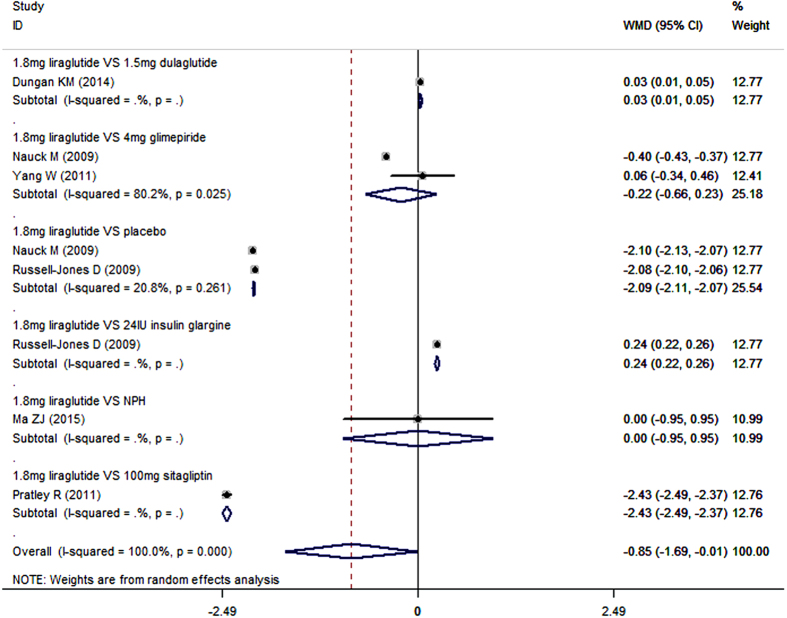
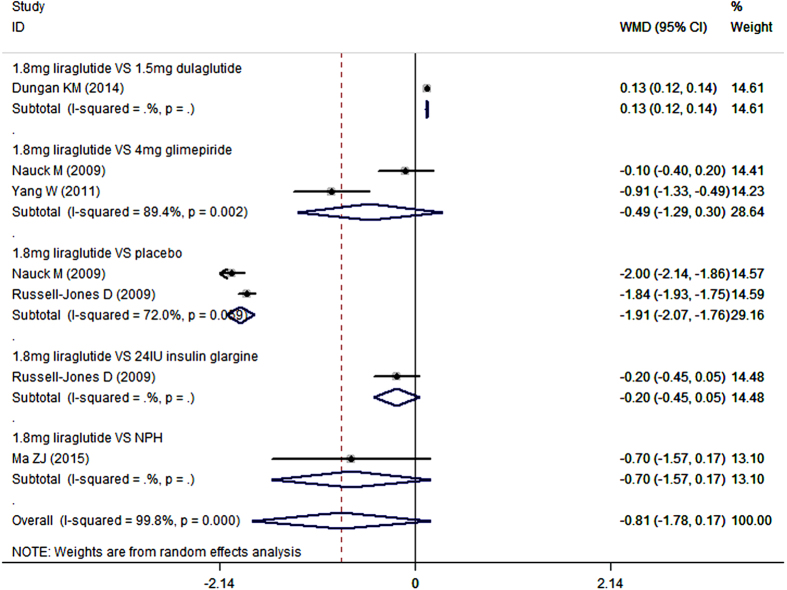
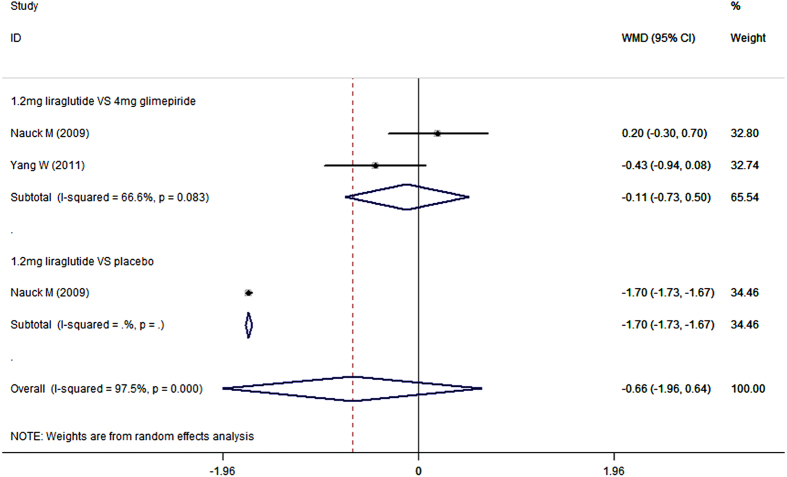
Five studies reported the data in PPG concentration19,20,41,42,45. When administered with metformin, liraglutide showed more reduction in PPG than control (placebo, glimepiride, insulin glarigin, dulaglutide, and NPH) (WMD = −0.60 mmol/L, 95%CI: −1.17, −0.03; P < 0.001). Subgroup analysis based on the dosage of liraglutide showed that, liraglutide in all of the three dosage resulted in similar change in PPG as did control (for 1.8 mg/day: WMD = −0.81 mmol/L, 95%CI: −1.78, 0.17; P = 0.107; for 1.2 mg/day: WMD = −0.66 mmol/L, 95%CI: −1.96, 0.64; P = 0.320; for 0.6 mg/day: WMD = −0.08 mmol/L, 95%CI: −1.60, 1.44; P = 0. 920) (Figs 8 and 9).
Figure 8. Postprandial plasma glucose: 1.8 mg liraglutide add-on to metformin VS. control.
Figure 9. Postprandial plasma glucose: 1.2 mg liraglutide add-on to metformin VS. control.
Furthermore, we also conducted subgroup-analysis based on the comparators. As an add-on to metformin, 1.8 mg/day liraglutide decreased PPG concentration more than placebo (WMD = −1.91 mmol/L, 95%CI: −2.07, −1.76; P < 0.001), less than dulaglutide (WMD = 0.13 mmol/L, 95%CI: 0.12, 0.14; P < 0.001), and similar with glimepiride (WMD = −0.49 mmol/L, 95%CI: −1.29, 0.30; P = 0.224), insulin glarigin (WMD = −0.20 mmol/L, 95%CI: −0.45, 0.05; P = 0.112), and NPH (WMD = −0.70 mmol/L, 95%CI: −1.57, 0.17; P = 0.114) (Fig. 8) (Table 2).
When used combination with metformin, 1.2 mg/day liraglutide notably decreased PPG concentration compared with placebo (WMD = −1.70 mmol/L, 95%CI: −1.73, −1.67; P < 0.001), and resulted in similar change in PPG concentration as did glimepiride (WMD = −0.11 mmol/L, 95%CI: −0.73, 0.50; P = 0.720) (Fig. 9) (Table 2).
When used as an add-on therapy to metformin, 0.6 mg/day liraglutide notably reduced PPG concentration compared with placebo (WMD = −1.10 mmol/L, 95%CI: −1.13, −1.07; P < 0.001), and exhibited a similar reduction in PPG concentration as did glimepiride (WMD = 0.47 mmol/L, 95%CI: −0.25, 1.18; P = 0.200) (Table 2).
Systolic blood pressure
Five studies reported the data in SBP20,40,41,44,46. As an add-on to metformin, liraglutide resulted in similar reduction in SBP as did control (placebo, sitagliptin, dulaglutide, insulin glargine, and glimepiride) (WMD = −1.67 mm Hg, 95%CI: −3.67, 0.33; P = 0.102). Subgroup analysis based on the dosage of liraglutide showed that, liraglutide with a dosage of 1.8 mg and 1.2 mg led to similar reduction of SBP compared with control (for 1.8 mg/day: WMD = −1.76 mm Hg, 95%CI: −4.79, 1.28; P = 0.256; for 1.2 mg/day: WMD = −1.49 mm Hg, 95%CI: −4.59, 1.61; P = 0.345).
Furthermore, we also conducted subgroup-analysis based on the comparators. As an add-on to metformin, 1.8 mg/day liraglutide lowered more SBP than placebo (WMD = −2.60 mm Hg, 95%CI: −2.78, −2.42; P < 0.001) and insulin glargine (WMD = −4.54 mm Hg, 95%CI: −4.57, −4.51; P < 0.001), but less SBP than dulaglutide (WMD = 0.54 mm Hg, 95%CI: 0.43, 0.65; P < 0.001), and similar SBP than sitagliptin(WMD = −0.42 mm Hg, 95%CI: −0.88, 0.04; P = 0.074) (Table 2).
When used combination with metformin, 1.2 mg/day liraglutide resulted in similar reduction of SBP compared with glimepiride (WMD = −3.70 mm Hg, 95%CI: −9.81, 2.41; P = 0.235) and sitagliptin (WMD = −1.08 mm Hg, 95%CI: −4.46, 2.31; P = 0.533) (Table 2).
Diastolic blood pressure
Four studies reported the data in DBP40,41,44,46. When used as add-on to metformin, liraglutide exhibited similar DBP change as did control (sitagliptin, glimepiride, and dulaglutide) (WMD = 0.11 mm Hg, 95%CI: −0.53, 0.74; P = 0.744). Subgroup analysis based on the dosage of liraglutide showed that, both dosage of liraglutide resulted in a similar change in DBP as did control (for 1.8 mg/day: WMD = 0.25 mm Hg, 95%CI: −0.42, 0.93; P = 0.460; for 1.2 mg/day: WMD = −0.28 mm Hg, 95%CI: −1.53, 0.97; P = 0.662).
Furthermore, we also conducted subgroup-analysis based on the comparators. As an add-on to metformin, 1.8 mg/day liraglutide decreased more DBP than dulaglutide (WMD = −0.09 mm Hg, 95%CI: −0.15, −0.03; P = 0.006), but less DBP than sitagliptin (WMD = 0.60 mm Hg, 95%CI: 0.52, 0.68; P < 0.001) (Table 2).
When used combination with metformin, 1.2 mg/day liraglutide resulted in greater reduction in DBP than glimepiride (WMD = −5.53 mm Hg, 95%CI: −9.15, −1.91; P = 0.003), and similar reduction than sitagliptin (WMD = 0.27 mm Hg, 95%CI: −1.04, 1.58; P = 0.687) (Table 2).
Treatment-related adverse events
Five studies reported the data in treatment-related adverse events19,20,40,41,46. The most common treatment-related adverse events, including gastrointestinal disorders (nausea, diarrhea, vomiting, dyspepsia, decreased appetite, and constipation), nasopharyngitis, headache, and back pain are listed in Table 3. When used as add-on therapy to metformin, liraglutide significantly increased the risk of gastrointestinal disorders (RR = 1.59, 95%CI: 1.15, 2.19; P = 0.005) compared with control (placebo, dulaglutide, sitagliptin, glimepiride, NPH, and insulin glargine). The incidence of diarrhea was higher in patients treated with combination therapy of metformin and liraglutide than in the control group (RR = 1.98, 95%CI: 1.30, 3.00; P = 0.001).
Table 3. Summary of the risk ration (RR) of treatment-related adverse events between liraglutide added to metformin and other therapies.
| Adverse events | RR | 95%CI | P value |
|---|---|---|---|
| Nausea | 2.26 | 0.98, 5.25 | 0.057 |
| Diarrhea | 1.98 | 1.30, 3.00 | 0.001 |
| Vomiting | 1.62 | 0.94, 2.79 | 0.080 |
| Dyspepsia | 1.31 | 0.69, 2.52 | 0.411 |
| Constipation | 1.17 | 0.80, 1.69 | 0.424 |
| Nasopharyngitis | 0.89 | 0.65, 1.22 | 0.471 |
| Headache | 1.12 | 0.85, 1.47 | 0.419 |
| Back pain | 1.36 | 0.64, 2.91 | 0.430 |
| Deceased appetite | 1.25 | 0.66, 2.36 | 0.499 |
| Influenza | 1.90 | 0.90, 4.03 | 0.092 |
| Hypoglycemia | 0.33 | 0.08, 1.44 | 0.140 |
Abbreviations: CI, confidence interval.
There were 44 out of 1483 (2.97%) patients in metformin plus liraglutide group and 32 out of 397 (8.06%) patients in the control group (placebo, glimepiride, and NPH) that experienced hypoglycemia. Pooled results showed that, compared with control, metformin plus liraglutide did not increase the risk of hypoglycemia (RR = 0.33, 95%CI: 0.08, 1.44; P = 0.140). No cases of pancreatitis were reported in these included studies.
Publication bias
Assessment of publication bias was conducted by using Egger’s and Begg test, and results showed that no publication bias existed among the included studies (Egger’s test: t = −1.33, P = 0.201; Begg test: Z = 0.14, P = 0.887).
Discussion
The addition of GLP-1 receptor agonist is recommended by the ADA and the EASD as a therapeutic option for patients with T2DM whose HbA1c targets are not met or maintained with lifestyle modifications, with consideration of individual patient-related factors47. This meta-analysis investigated the efficacy and safety of liraglutide in combination with metformin, compared to other therapies for patients with T2DM. Overall, the results of our study suggest that compared with other therapies, liraglutide in combination with metformin showed greater reduction in terms of HbA1c levels, body weight, FPG, and PPG, and similar changes in SBP and DBP. In addition, when used as add-on therapy to metformin, liraglutide did not increase the risk of hypoglycemia, but induced a higher incidence of gastrointestinal disorders.
To the bests of our knowledge, this is the first comprehensive meta-analysis to compare the efficacy and safety of liraglutide add-on to metformin with other therapies in patients with T2DM. Our results suggest that, compared with control, combined therapy of liraglutide and metformin reduced the HbA1c significantly by −0.36%. This result is consistent across the subgroup analysis based on dosage of liraglutide, in which 1.8 mg and 1.2 mg liraglutide decreased the HbA1c by −0.47%, and −0.35%, respectively. Furthermore, subgroup analysis based on comparators demonstrated that, liraglutide in combination with metformin was associated with a greater reduction of HbA1c than placebo, sitagliptin, insulin glargin, or dulaglutide, and similar change with glimepiride.
The HbA1c reduction from baseline with the combination therapy of liraglutide and metformin in this meta-analysis was in line with a previously published study. In the trial conducted by Pratley RE, et al.39, 1.2 mg and 1.8 mg liraglutide decreased the HbA1c from baseline by −1.24% and −1.50%, respectively, whereas sitagliptin lowered the HbA1c by −0.90%. The estimated treatment differences (ETD) for 1.2 mg, 1.8 mg liraglutide versus sitagliptin were −0.34% (95%CI: −0.51, −0.61) and −0.60% (95%CI: −0.77, −0.43), respectively39, which indicated that combination therapy of liraglutide and metformin resulted a more reduction of HbA1c than sitagliptin. The greater HbA1c reduction of combination therapy versus sitagliptin probably can be explained by the pharmacological concentrations of free liraglutide, whereas physiological concentrations of GLP-1 and glucose dependent insulinotropic polypeptide (GIP) are achieved with sitagliptin. Although the active GLP-1 concentrations are increased by two or three times with dipeptidyl peptidase-4 (DPP-4) inhibitors48, the stimulation of GLP-1 receptor activity by liraglutideis estimated to be several times higher than with DPP-4 inhibitors14. Moreover, liraglutide has a long half-life (about 13 h), which may be another reason for the increased efficacy49. Despite sitagliptin has a similar pharmacokinetic half-life as liraglutide50, its effect on the increase of endogenous GLP-1 concentration occurs mainly after meals. Thus, the fasting concentrations of active GLP-1 remain considerably low overnight, so the FPG concentrations with sitagliptin reduced less than liraglutide50.
In this meta-analysis, liraglutide used as an add-on to metformin, reduced bodyweight more than other therapies did. This result can be attributed to the increased stimulation of GLP-1 receptor by liraglutide. T2DM increases morbidity and mortality mainly because of the cardiovascular and cerebrovascular disease. Obesity is one of the specific risk factors for the development of diabetes, and cardiovascular disease51. Thus, antidiabetic drugs should have favorable effects in the treatment of cardiovascular and cerebrovascular disease, as well as reduce the blood glucose. Among the controls (placebo, dulaglutide, insulin glargine, NPH, glimepiride, and sitagliptin) of the included studies, weight gain was observed in patients treated with insulin glargine (1.6 kg increase), and glimepiride (0.25 kg increase). However, when liraglutide is added to metformin, it significantly reduced the bodyweight (by 2.13 kg) compared with control. This result is in contrast to the data from a phase 3 trail, in which 1.8 mg once-daily liraglutide monotherapy was compared with 10 μg twice-daily exenatide16. And liraglutide did not show a greater reduction in bodyweight than exenatide (liraglutide: −3.24 kg VS exenatide: −2.87 kg; ETD, −0.38 kg, 95%: −0.99, 0.23, P = 0.2235)16.
With regard to the FPG, our results showed that combined therapy of liraglutide and metformin was associated with a significant decrease on this clinical outcome compared with control (placebo, sitagliptin, glimepiride, dulaglutide, insulin glargine, and NPH). However, this significant FPG reduction was not observed in the subgroup analysis between liraglutide and glimepiride. Compared with glimepiride, 0.6 mg,1.2 mg, and 1.8 mg liraglutide reduced the FPG by 0.20 mmol/L, −0.10 mmol/L, and −0.22 mmol/L, respectively, though the difference was not significant. These results are in accordance with the findings from the LEAD-2 trial19. In that trial, liraglutide was found to have similar efficacy in FPG reduction compared to glimepiride. The decrease in FPG from baseline was 1.1 mmol/L, −1.6 mmol/L, and −1.7 mmol/L for the 0.6 mg, 1.2 mg, and 1.8 mgliraglutide, respectively, whereas the corresponding value for glimepiride was −1.3 mmol/L19.
For the PPG, we found the pooled results remained confusing. Our finding showed that liraglutide in combination with metformin was associated with a significantly greater reduction in PPG than control (placebo, glimepiride, dulaglutide, insulin glargine, and NPH) (−0.60 mmol/L). However, when it was compared with active comparators, no significant difference was found between them. Compared with glimepiride, 0.6 mg, 1.2 mg, and 1.8 mgliraglutide reduced PPG by 0.47 mmol/L, −0.11 mmol/L, and −0.49 mmol/L, respectively; however, the difference between them was not significant. These findings are in line with what has been observed in the LEAD trial19. In the LEAD trial, it has been found that the decreases from baseline in PPG were −1.7 mmol/L, −2.3 mmol/L, and −2.6 mmol/L for the 0.6 mg, 1.2 mg, and 1.8 mg liraglutide respectively, and −2.5 mmol/L for glimepiride. The corresponding ETD for 0.6 mg, 1.2 mg, and 1.8 mg liraglutide versus glimepiride was 0.8 mmol/L, 0.2 mmol/L, and −0.1 mmol/L, respectively. Similar results were also found in the comparison between liraglutide and dulaglutide41, insulin glargine20, or NPH42, which showed a comparable effect in PPG between them. We speculated that due to the strong response in the placebo group, liraglutide showed beneficial effects in PPG as compared with control.
Hypoglycemia is a challenge and obstacle in the treatment of T2DM. In this meta-analysis, combination treatment of liraglutide and metformin did not increase the risk of hypoglycemia (2.97% VS 8.06%) compared with other therapies (placebo, glimepiride, and NPH). Our results were consistent with the findings of a recently published meta-analysis by Zhang L, et al.52. In that study, another GLP-1 receptor agonist dulaglutide was compared with other antidiabetic drugs for T2DM. Their results indicated that, as a monotherapy, dulaglutide did not increase the risk of hypoglycemia compared with control (placebo, metformin and liraglutide) (7.8% VS 10.6, respectively); as in the combination with OAM and lispro, dulaglutide resulted in a similar incidence of hypoglycemia (24.5% VS 24.5%) compared with control (placebo, sitagliptin, exenatide and liraglutide)52.
In this meta-analysis, although the liraglutide in combination with metformin induced a higher incidence of gastrointestinal disorders than control, these adverse events were mainly mild to moderate. The incidence of diarrhea was higher in patients treated with liraglutide than in the control group, which is consistent with results from previous trials19,20,39,46. No cases of pancreatitis were reported in these included studies, thus we did not assess the potential association between incretin and pancreatitis. However, the recent epidemiological studies indicated that, incretin-based therapies did not increase the risk of pancreatitis as compared with other diabetes treatments, and general population with T2DM was not a risk factor for the development of pancreatitis53.
We admit that there are several potential limitations in this meta-analysis. First, our meta-analysis was conducted based on nine RCTs, and two of them had a relatively small sample size (less than 100). Although all of these included studies were of high-quality (Jadad ≥3), our findings might be overestimated by these small trials since trials with small sample size were more likely to overestimate the treatment effect compared with those larger trials. Second, considerable heterogeneity existed among these included studies. However, it should not be surprising when considering the inclusion criteria for patients, dosage of liraglutide, duration of study, and different comparators. To explore the potential sources of heterogeneity, we conducted subgroup analysis based on the dosage of liraglutide, and comparators. And the issue of heterogeneity was resolved when the data analysis was performed according to the subgroup analysis. Third, our exploration of the effect comparison between liraglutide with active comparators was insufficient because of sparse reporting among the included studies. Therefore, physicians should interpret our findings with caution when applying them into the clinical practice.
In conclusion, our meta-analysis indicated that, liraglutideas added-on to metformin showed greater reduction in HbA1c levels, body weight, FPG, and PPG, and similar change in SBP and DBP compared with other therapies. However, considering the potential limitations in this study, more large-scale, well-conducted RCTs are needed to identify our findings.
Additional Information
How to cite this article: Gu, J. et al. The efficacy and safety of liraglutide added to metformin in patients with diabetes: a meta-analysis of randomized controlled trials. Sci. Rep. 6, 32714; doi: 10.1038/srep32714 (2016).
Acknowledgments
This study was supported by the Science and Technology projects of Liaoning Province (grant no. 2015225024) and the Science and Technology projects of Shenyang City (grant no. F13-221-9-02).
Footnotes
Author Contributions D.W., J.G. and X.M. mainly participated in literature search, study design, writing and critical revision. Y.G., L.W., H.Z., Y.L. and B.W. mainly participated in data collection, data analysis and data interpretation. All authors read and approved the final manuscript.
References
- DeFronzo R. A. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37, 667–687 (1988). [DOI] [PubMed] [Google Scholar]
- Stumvoll M., Goldstein B. J. & van Haeften. T. W. Type 2 diabetes: principles of pathogenesis and therapy. Lancet 365, 1333–1346 (2005). [DOI] [PubMed] [Google Scholar]
- Inzucchi S. E. et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35, 1364–1379 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. C., Cull C. A., Frighi V. & Holman. R. R. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. Jama 281, 2005–2012 (1999). [DOI] [PubMed] [Google Scholar]
- Holst J. J., Vilsboll T. & Deacon. C. F. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol. 297, 127–136 (2009). [DOI] [PubMed] [Google Scholar]
- Edwards K. L., Stapleton M., Weis J. & Irons. B. K. An update in incretin-based therapy: a focus on glucagon-like peptide-1 receptor agonists. Diabetes Technol Ther. 14, 951–967 (2012). [DOI] [PubMed] [Google Scholar]
- Holst J. J. The physiology of glucagon-like peptide 1. Physiol Rev. 87, 1409–1439 (2007). [DOI] [PubMed] [Google Scholar]
- Nauck M. A., Meier J. J. & Creutzfeldt. W. Incretins and their analogues as new antidiabetic drugs. Drug News Perspect. 16, 413–422 (2003). [DOI] [PubMed] [Google Scholar]
- Li N., Lu J. & Willars. G. B. Allosteric modulation of the activity of the glucagon-like peptide-1 (GLP-1) metabolite GLP-1 9-36 amide at the GLP-1 receptor. PLoS One 7, e47936 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst J. J. Therapy of type 2 diabetes mellitus based on the actions of glucagon-like peptide-1. Diabetes Metab Res Rev. 18, 430–441 (2002). [DOI] [PubMed] [Google Scholar]
- Knudsen L. B. et al. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem. 43, 1664–1669 (2000). [DOI] [PubMed] [Google Scholar]
- Elbrond B. et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of a single-dose of NN2211, a long-acting glucagon-like peptide 1 derivative, in healthy male subjects. Diabetes Care 25, 1398–1404 (2002). [DOI] [PubMed] [Google Scholar]
- Agerso H. et al. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia 45, 195–202 (2002). [DOI] [PubMed] [Google Scholar]
- Degn K. B. et al. One week’s treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes 53, 1187–1194 (2004). [DOI] [PubMed] [Google Scholar]
- Vilsboll T. et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care 30, 1608–1610 (2007). [DOI] [PubMed] [Google Scholar]
- Buse J. B. et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 374, 39–47 (2009). [DOI] [PubMed] [Google Scholar]
- Garber A. et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 373, 473–481 (2009). [DOI] [PubMed] [Google Scholar]
- Marre M. et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med. 26, 268–278 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck M. et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 32, 84–90 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell-Jones D. et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): a randomised controlled trial. Diabetologia. 52, 2046–2055 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Altman D. G.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 339, b2535 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadad A. R. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 17, 1–12 (1996). [DOI] [PubMed] [Google Scholar]
- Moher D. et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 352, 609–613 (1998). [DOI] [PubMed] [Google Scholar]
- Cochran W. The combination of estimates from different experiments. Biometrics. 10, (1954). [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman. D. G. Measuring inconsistency in meta-analyses. Bmj. 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel N. & Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 22, 719–748 (1959). [PubMed] [Google Scholar]
- DerSimonian R. & Laird. N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–188 (1986). [DOI] [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder. C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar. M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994). [PubMed] [Google Scholar]
- Jendle J. et al. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab. 11, 1163–1172 (2009). [DOI] [PubMed] [Google Scholar]
- Hermansen K. et al. Patient-reported outcomes in patients with type 2 diabetes treated with liraglutide or glimepiride, both as add-on to metformin. Prim Care Diabetes 4, 113–117 (2010). [DOI] [PubMed] [Google Scholar]
- Schmidt W. E. et al. Patient-reported outcomes are superior in patients with Type 2 diabetes treated with liraglutide as compared with exenatide, when added to metformin, sulphonylurea or both: results from a randomized, open-label study. Diabet Med. 28, 715–723 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitza C. et al. Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled on metformin. Diabetes Obes Metab. 15, 642–649 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinman B. et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met + TZD). Diabetes Care 32, 1224–1230 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J. J. et al. Contrasting Effects of Lixisenatide and Liraglutide on Postprandial Glycemic Control, Gastric Emptying, and Safety Parameters in Patients With Type 2 Diabetes on Optimized Insulin Glargine With or Without Metformin: A Randomized, Open-Label Trial. Diabetes Care 38, 1263–1273 (2015). [DOI] [PubMed] [Google Scholar]
- Rosenstock J. et al. One-year sustained glycemic control and weight reduction in type 2 diabetes after addition of liraglutide to metformin followed by insulin detemir according to HbA1c target. J Diabetes Complications 27, 492–500 (2013). [DOI] [PubMed] [Google Scholar]
- Pratley R. E. et al. Efficacy and safety of switching from the DPP-4 inhibitor sitagliptin to the human GLP-1 analog liraglutide after 52 weeks in metformin-treated patients with type 2 diabetes: a randomized, open-label trial. Diabetes Care 35, 1986–1993 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. et al. Efficacy and safety of liraglutide monotherapy compared with metformin in Japanese overweight/obese patients with type 2 diabetes. Endocr J. 62, 399–409 (2015). [DOI] [PubMed] [Google Scholar]
- Pratley R. E. et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 375, 1447–1456 (2010). [DOI] [PubMed] [Google Scholar]
- Pratley R. et al. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract. 65, 397–407 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungan K. M. et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet 384, 1349–1357 (2014). [DOI] [PubMed] [Google Scholar]
- Ma Z. et al. Effect of liraglutide vs. NPH in combination with metformin on blood glucose fluctuations assessed using continuous glucose monitoring in patients with newly diagnosed type 2 diabetes. Int J Clin Pharmacol Ther. 53, 933–939 (2015). [DOI] [PubMed] [Google Scholar]
- Davies M. et al. Liraglutide improves treatment satisfaction in people with Type 2 diabetes compared with sitagliptin, each as an add on to metformin. Diabet Med. 28, 333–337 (2011). [DOI] [PubMed] [Google Scholar]
- Brady E. M. et al. A randomized controlled trial comparing the GLP-1 receptor agonist liraglutide to a sulphonylurea as add on to metformin in patients with established type 2 diabetes during Ramadan: the Treat 4 Ramadan Trial. Diabetes Obes Metab. 16, 527–536 (2014). [DOI] [PubMed] [Google Scholar]
- Yang W. et al. Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: a 16-week, randomized, double-blind, active control trial(*). Diabetes Obes Metab. 13, 81–88 (2011). [DOI] [PubMed] [Google Scholar]
- Charbonnel B. et al. Efficacy and safety over 26 weeks of an oral treatment strategy including sitagliptin compared with an injectable treatment strategy with liraglutide in patients with type 2 diabetes mellitus inadequately controlled on metformin: a randomised clinical trial. Diabetologia 56, 1503–1511 (2013). [DOI] [PubMed] [Google Scholar]
- Inzucchi S. E. et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 38, 140–149 (2015). [DOI] [PubMed] [Google Scholar]
- Aschner P. et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 29, 2632–2637 (2006). [DOI] [PubMed] [Google Scholar]
- McGill J. B. Insights from the Liraglutide Clinical Development Program–the Liraglutide Effect and Action in Diabetes (LEAD) studies. Postgrad Med. 121, 16–25 (2009). [DOI] [PubMed] [Google Scholar]
- Herman G. A. et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther. 78, 675–688 (2005). [DOI] [PubMed] [Google Scholar]
- Eckel R. H., Barouch W. W. & Ershow A. G.. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on the pathophysiology of obesity-associated cardiovascular disease. Circulation 105, 2923–2928 (2002). [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang M., Zhang Y. & Tong N.. Efficacy and safety of dulaglutide in patients with type 2 diabetes: a meta-analysis and systematic review. Sci Rep. 6, 18904 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel R. A., Braun D. K., Patterson R. E. & Bloomgren. G. L. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care. 32, 834–838 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]