Abstract
Evidence about the clinical effects of entecavir (ETV) for patients with hepatitis B decompensated cirrhosis remain controversial. Therefore, we perform this meta-analysis to assess the treatment outcomes of ETV in participants with hepatitis B decompensated cirrhosis. Relevant studies were identified by searching databases until the March 2016. A random-effects model was used to estimate summary relative risks (RRs) and 95% confidence intervals (CIs). GRADEprofiler3.6 was used to evaluate the quality of the evidence. A total of 26 studies (involving 2040 patients) were included. The quality of the evidence was classified from very low to high by the GRADED approach for all included RCTs. Meta-analysis showed that patients were more likely to experience HBV-DNA loss (RR:1.85, 95%CIs: 1.41 to 2.43, P < 0.0001 at 48 weeks), have normalized alanine aminotransferase levels (ALT) (P = 0.003 at 24 weeks, P = 0.02 at 48 weeks), and have a low mortality rate at 24 weeks (P = 0.003) when treated with ETV. There was no significant different between ETV and the control groups at the total mortality (P = 0.06) and HBeAg seroconversion (P = 0.14). In conclusion, ETV could be the first line therapy for patients with HBV related decompensated cirrhosis, because ETV could reduce the early mortality and move HBV DNA load down.
According to the latest clinical practice guidelines1 made by European association for the study of the liver(EASL), approximately one third of the world’s population has serological evidence of past or present infection with hepatitis B virus (HBV) and HBV-related end stage liver diseases or hepatocellular carcinoma(HCC) are responsible for over 0.5–1 million deaths per year. The guideline of prevention and treatment for chronic hepatitis (2015 version)2 of China points that about 7.18% of the population aged 1 to 59 years old in China are chronic HBV surface antigen (HBsAg) carriers, according to the epidemiological investigate nationwide. Now, there are about 0.093 billion people who are HBsAg carriers based on the epidemiological studies. Longitudinal studies of untreated patients with CHB indicate that, after diagnosis, the 5-year cumulative incidence of developing cirrhosis ranges from 8% to 20%. The 5-year cumulative incidence of hepatic decompensation is approximately 20% for untreated patients with compensated cirrhosis. Untreated patients with decompensated cirrhosis have a poor prognosis with a 14–35% probability of survival at 5 years1. Decompensated cirrhosis3 is characterized by significant abnormalities in liver functions, including raised serum bilirubin levels, a prolonged prothrombin time and/or the occurrence of complications such as ascites, hepatic encephalopathy and variceal bleeding. It is necessary for patients with hepatitis B decompensated liver diseases to be treated. Nucleos(t)ide analogue therapy is an important way to be used for decompensated liver according to the clinical treatment guideline. The patients treated with the deoxyguanosine nucleoside analogue ETV could achieve the HBV DNA suppression, the biochemical improvement and the histological improvement4. The efficacy of ETV for the treatment of patients with chronic hepatitis B was proved5, including patients with compensated liver disease6,7.
There were many studies on ETV used for patients with hepatitis B decompensated liver disease. Liaw et al. proved8 that patients generally had a good tolerance to ETV in the treatment of HBV related decompensated cirrhosis, ETV had a better virus response than adefovir dipivoxil (ADV), and mortality of patients with ETV was similar with lamivudine (LAM) by a randomized, open-label study. Yang J et al.9 thought that the HBV DNA level of ETV group reduced more than ADV group after a treatment of 48 weeks. Keating GM 3showed that patients had a significant liver function improvements from baseline after 12 months treatment of ETV in patients with decompensated cirrhosis.
There are several meta-analysis about ETV for patients with HBV related decompensated cirrhosis. For example, Peng H et al.10 only compared LAM combined with ADV with ETV. Ye X et al.11 just showed the effects of LAM and ETV for hepatitis B decompensated cirrhosis. Singal A. K. et al.12 did not study the difference of patients without anti-viral agents with patients who used ETV for HBV related decompensated liver cirrhosis. All records included in Singal A. K. et al.’s meta-analysis are published before 2010. In our review, 26 studies and 2040 patients are involved. We used the HBV DNA loss, ALT normalization, mortality and HBeAg seroconversion to evaluate the effect of ETV for patients with HBV related decompensated cirrhosis.
Results
Description of the included studies
A total of 26 RCTs, with 2040 patients fulfilled the included criteria (Fig. 1 and Table 1). 930 patients were treated with ETV, 1110 patients were treated by other ways. The number of patients who were treated with other NAs drugs except ETV were 561, 549 patients were not treated by NAs drugs. The dominative outcomes are the HBV DNA loss, the recovery of ALT, the mortality and the HBeAg seroconversion.
Figure 1. Flowchart of study inclusion protocol.
Of the 2295 studies initially identified from our research, 26 met the inclusion criteria were included in this meta-analysis.
Table 1. Characteristics of included studies.
| Study | Year | Number |
Mean age | Intervention |
Duration | Outcomes | ||
|---|---|---|---|---|---|---|---|---|
| experiment | control | (years) | experiment | control | (weeks) | |||
| (mg/day) | (mg/day) | |||||||
| Yang J et al.29 | 2012 | 30 | 30 | 50.7 | 0.5 | ADV 10 | 48 | A/B/C/D/E |
| 30 | UC | LAM 100 | 48 | A/B/C/D/E | ||||
| 30 | UC | CT | 48 | A/B/C/D/E | ||||
| Lin XS et al.23 | 2011 | 32 | 32 | 49.7 | 0.5 | CT | 72 | A/E/F |
| Ren WX et al.30 | 2014 | 27 | 27 | 48 | 0.5 | CT | 48 | A/B/D/E |
| Luo HB et al.31 | 2009 | 48 | 48 | 54.9 | 0.5 | CT | 48 | A/B |
| Ning ZH et al.32 | 2009 | 37 | 38 | 47.5 | 0.5 | CT | 24 | A/B/F |
| Han ZQ et al.24 | 2009 | 30 | 30 | 56.2 | 0.5 | CT | 24 | A/B/D/E/F |
| Chen FZ et al.16 | 2010 | 26 | 26 | 48.5 | 0.5 | LAM 100 | 48 | A/B/F |
| Zhang FL33 | 2011 | 28 | 26 | 45.6 | 0.5 | CT | 24 | A/B/D |
| Zhang RL34 | 2010 | 18 | 18 | UC | 0.5 | CT | 24 | A/B/E/F |
| Guo YM et al.35 | 2014 | 42 | 42 | UC | 0.5 | CT | 24 | A/B/D/E |
| Yang J et al.9 | 2012 | 40 | 40 | 46.6 | 0.5 | ADV 10 | 48 | A/B/E/F |
| Li H36 | 2009 | 20 | 20 | UC | 0.5 | CT | 24 | A/B/D/E |
| Xu Y22 | 2013 | 43 | 43 | 50.1 | 0.5 | LDT 600 and ADV10 | 48 | A/B/D |
| Feng J et al.37 | 2008 | 22 | 25 | UC | 0.5 | LAM 100 | 48 | A/B/D/F |
| 25 | UC | ADV10 | 48 | A/B/D/F | ||||
| 24 | UC | CT | 48 | A/B/D/F | ||||
| Zhang DH et al.18 | 2011 | 27 | 27 | UC | 0.5 | LAM 100 | 48 | A/B/D/F |
| 27 | UC | ADV 10 | 48 | A/B/D/F | ||||
| 24 | UC | CT | 48 | A/B/D/F | ||||
| Liaw et al.8 | 2011 | 100 | 91 | 51 | 1 | ADV 10 | 96 | A/B/F |
| Liaw yf et al.13 | 2011 | 22 | 45 | 54 | 0.5or1 | TDF 300 | 48 | A/B/F |
| Yang L38 | 2015 | 40 | 40 | UC | 0.5 | LAM 100 | 48 | C/E |
| Gulizire•Maola39 | 2015 | 35 | 35 | UC | 0.5 | CT | 48 | A/B/C/D |
| Hu XM19 | 2014 | 36 | 36 | 46.5 | 0.5 | LAM 100 | 1 year | A/B/C/F |
| Zhang J40 | 2014 | 27 | 27 | UC | 0.5 | CT | 48 | A/B |
| Zhao ZY41 | 2014 | 36 | 36 | UC | 0.5 | CT | 48 | A/B/E |
| Li MX42 | 2014 | 48 | 48 | UC | 0.5 | CT | 48 | A/B/G |
| Zhou XL et al.43 | 2015 | 44 | 44 | UC | 0.5 | CT | 24 | A/B/C/E |
| Bi YL20 | 2014 | 43 | 48 | UC | 0.5 | LAM 100 and ADV 10 | 48 | A/B/C/D |
| Shao JB et al.21 | 2010 | 29 | 28 | 43.6 | 0.5 | LAM 100 | 96 | A/E |
UC: unclear; CT: comprehensive therapy (patients who did not use any NAs); A: HBV DNA; B: Hepatic function; C: Adverse Drug Reaction; D: Mortality; E: Child-pugh; F: HBeAg seroconversion; G: hepatitis B virus mutation rate.
Risk of bias in included studies
The summary results of the risk of bias were showed in Fig. 2. All trials were free from baseline imbalance bias and incomplete outcome bias. All trials were random control trials. None of the trials had adequate allocation concealment. One trial13 had adequate blinding. All trials might have academic bias and funding bias.
Figure 2. Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Meta-analysis results
We used risk ratio (RR) as summary measures. We also stated the 95% confidence intervals.
HBV DNA loss
HBV DNA loss at 12 weeks
7 RCTs were included. There were 568 patients in total. The analysis of heterogeneity showed that I2 = 71%. These trials were considered statistically significant heterogeneity. We got RR = 3.52, 95%CI [1.77, 6.99], P = 0.0003. The experiment groups were higher than the control groups. This revealed a statistically significant. The result was showed in Fig. 3.
Figure 3. Comparison of ETV versus other treatments, outcome of HBV DNA loss at 12 weeks (forest plot).
At 12 weeks, 232 patients treated with ETV moved more HBV DNA undetectable than 336 patients with other treatment. RR = 3.52, 95%CI [1.77, 6.99], P = 0.0003.
Subgroups of HBV DNA loss at 12w
There are four subgroups of HBV DNA loss in our research. Firstly, the control groups were patients with other NAs on the basis of CT.4 RCTs were included. There were 302 patients in total. The analysis of heterogeneity showed that I2 = 57%. These trials were considered statistically significant heterogeneity. We got RR = 4.37, 95%CI [1.58, 12.09], P = 0.004. The experiment groups were higher than the control groups. This revealed a statistically significant. Secondly, the control groups were patients with CT without any NAs. 3 RCTs were included. There were 217 patients in total. The analysis of heterogeneity showed that I2 = 31%. We got RR = 39.44, 95%CI [8.54, 182.03], P < 0.00001. The experiment groups were higher than the control groups. This revealed a statistically significant. Thirdly, the control groups were patients with LAM. 3 RCTs were included. There were 171 patients in total. The analysis of heterogeneity showed that I2 = 81%. These trials were considered statistically significant heterogeneity. We got RR = 1.23, 95%CI [0.23, 6.76], P = 0.81. There was no statistically significant between the experiment groups and the control groups. Fourthly, the control groups were patients with ADV only. 3 RCTs were included. There were 181 patients in total. The analysis of heterogeneity showed that I2 = 0%. These trials were no statistically significant heterogeneity. We got RR = 8.01, 95%CI [3.22, 19.94], P < 0.00001. The experiment groups were higher than the control groups. This revealed a statistically significant.
HBV DNA loss at 24 weeks
17 RCTs were included. Total patients were 1324. The analysis of heterogeneity showed that I2 = 93%. These trials were considered statistically significant heterogeneity. We got RR = 4.51, 95%CI [2.51, 8.12], P < 0.00001. The experiment groups were higher than the control groups. This revealed a statistically significant. The result was showed in supplementary information. And the funnel pool was showed in Fig. 4.
Figure 4. Funnel pool of comparison of ETV versus other treatments outcome of HBV DNA loss at 24 weeks.

Subgroups of HBV DNA loss at 24 weeks
There are four subgroups of HBV DNA loss at 24 weeks. Firstly, the control groups were patients with other NAs. 8 RCTs were included. Total patients were 689. The analysis of heterogeneity showed that I2 = 80%. These trials were considered statistically significant heterogeneity. We got RR = 1.64, 95%CI [1.16, 2.32], P = 0.005. There was statistically significant between the experiment groups and the control groups. Secondly, the control groups were patients without NAs drugs. 11 RCTs were included. Total patients were 684. The analysis of heterogeneity showed that I2 = 0%. We got RR = 13.04, 95%CI [7.99, 21.27], P < 0.00001. There was statistically significant between the experiment groups and the control groups. Thirdly, the control groups were patients with LAM. 4 RCTs were included. Total patients were 223. The analysis of heterogeneity showed that I2 = 57%. These trials were considered statistically significant heterogeneity. We got RR = 1.31, 95%CI [0.91, 1.86], P = 0.14. There was no statistically significant between the experiment groups and the control groups. Fourthly, the control groups were patients with ADV. 4 RCTs were included. Total patients were 372. The analysis of heterogeneity showed that I2 = 0%. We got RR = 3.13, 95%CI [2.1, 4.66], P < 0.00001. There was statistically significant between the experiment groups and the control groups.
HBV DNA loss at 48 weeks
14 RCTs were included. Total patients were 1195. The analysis of heterogeneity showed that I2 = 89%. These trials were considered statistically significant heterogeneity. We got RR = 1.85, 95%CI [1.41, 2.43], P < 0.0001. The experiment groups were higher than the control groups. This revealed a statistically significant. The result was showed in supplementary information.
Subgroups of HBV DNA loss at 48 weeks
There are four subgroups. Firstly, 10 RCTs were included. There were 845 patients. The analysis of heterogeneity showed that I2 = 80%. These trials were considered statistically significant heterogeneity. We got RR = 1.42, 95%CI [1.15, 1.74], P = 0.0009. The experiment groups were higher than the control groups. This revealed a statistically significant. Secondly, 7 RCTs were included. There were 449 patients. The analysis of heterogeneity showed that I2 = 96%. These trials were considered statistically significant heterogeneity. We got RR = 7.58, 95%CI [1.35, 42.59], P = 0.02. The experiment groups were higher than the control groups. This revealed a statistically significant. Thirdly, 5 RCTs were included. There were 280 patients. The analysis of heterogeneity showed that I2 = 68%. These trials were considered statistically significant heterogeneity. We got RR = 1.38, 95%CI [1.03, 1.86], P = 0.03. The experiment groups were higher than the control groups. This revealed a statistically significant. Fourthly, 5 RCTs were included. There were 307 patients. The analysis of heterogeneity showed that I2 = 49%. We got RR = 1.50, 95%CI [1.18, 1.89], P = 0.0007. The experiment groups were higher than the control groups. This revealed a statistically significant.
ALT normalization
ALT normalization at 24 weeks
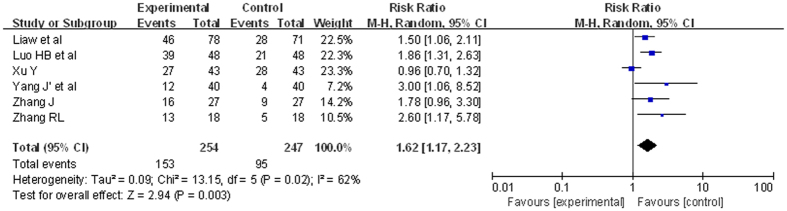
6 RCTs were included. There were 501 patients. The analysis of heterogeneity showed that I2 = 62%. These trials were considered statistically significant heterogeneity. We got RR = 1.62, 95%CI [1.17, 2.23], P = 0.003. The experiment groups were higher than the control groups. This revealed a statistically significant. The result was showed in Fig. 5.
Figure 5. Comparison of ETV versus other treatments outcome of ALT normalization at 24 weeks (forest plot).
At 24 weeks, 254 patients with ETV experienced more ALT normalization than 247 patients with other treatment. RR = 1.62, 95%CI [1.17, 2.23], P = 0.003.
ALT normalization at 48 weeks
7 RCTs were included. Total patients were 622. The analysis of heterogeneity showed that I2 = 77%. These trials were considered statistically significant heterogeneity. We got RR = 1.38, 95%CI [1.06, 1.80], P = 0.02. There was statistically significant between the experiment groups and the control groups.
Mortality
9 RCTs were included. There were 727 patients. The analysis of heterogeneity showed that I2 = 0%. These trials were not considered statistically significant heterogeneity. We got RR = 0.55, 95%CI [0.30, 1.03], P = 0.06. This did not reveal a statistically significant. The result was showed in Fig. 6.
Figure 6. Comparison of ETV versus other treatments’ outcome of mortality.
Subgroups of mortality (forest plot). The mortality of 295 patients treated with ETV did not show a statistically significant to 432 patients with other treatment. RR = 0.55, 95%CI [0.30, 1.03], P = 0.06.
There are two subgroups of mortality. One subgroup was the mortality till 24 weeks.12 RCTs were included. There were 765 patients. The analysis of heterogeneity showed that I2 = 0%. These trials were not considered statistically significant heterogeneity. We got RR = 0.38, 95%CI [0.20, 0.71], P = 0.003. This revealed a statistically significant. The other was mortality till 48 weeks. 9 RCTs were included. There were 627 patients. The analysis of heterogeneity showed that I2 = 0%. These trials were not considered statistically significant heterogeneity. We got RR = 0.58, 95%CI [0.33, 1.03], P = 0.06. This did not reveal a statistically significant.
HBeAg seroconversion
7 RCTs were included. There were 555 patients. The analysis of heterogeneity showed that I2 = 35%. These trials were not considered statistically significant heterogeneity. We got RR = 1.46, 95%CI [0.89, 2.40], P = 0.14. This did not reveal a statistically significant. The result was showed in Fig. 7.
Figure 7. Comparison of ETV versus other treatments’ outcome of HBeAg seroconversion (forest plot).
There were no significant difference about the rate of HBeAg seroconversion between ETV group and control group. RR = 1.46, 95%CI [0.89, 2.40], P = 0.14.
Evidence quality
The results of the evidence quality were showed in supporting information.
Discussion
ETV an oral deoxyguanosine nucleoside analogue, inhibits serum HBV DNA efficiently, improves the biochemical and histological characters of HBV related diseases4,14. S. Amini-Bavil-Olyaee et al.15 proved ETV was in short term a safe option for HBeAg negative patients. There were several meta-analysis10,11,12 about the oral anti-viral agents for patients with decompensated HBV related liver cirrhosis. However, there were not enough evidence to prove that ETV could be the first line drug for HBV related decompensated liver cirrhosis.
Chen FZ et al.16, Feng J et al.17, Zhang DH et al.18, Hu XM19 and Bi YL20 proved that the patients with ETV could undergo more HBV DNA loss than the patients with LAM at 24 weeks. In the studies of Shao JB et al.21, ETV made more HBV DNA loss than LAM at 12 weeks, but less at 24 weeks. Our data showed that ETV could significantly move viral load down to undetectable levels compared to patients without NAs treatment. The patients with ETV also experienced more HBV DNA loss than patients with ADV therapy. Although, at 12th and 24th weeks there were no significant differences in undetectable viral load between ETV and LAM in patients with Hepatitis B virus-related decompensated liver cirrhosis. ETV efficiently improve the outcome of HBV DNA loss than LAM at 48th week. ETV’s long-term efficacy is superior to LAM at the part of HBV DNA loss.
ETV causes statistically significant sharp decline in ALT level at 24th and 48th week. Although, there were no significant differences between ETV and control groups at 48th week.
Xu Y22 considered the mortality of ETV group was higher than the group with LDT and ADV. In our research, ETV reduces the mortality of patients at 24th week. ETV could reduce early mortality.
Lin XS et al.23, Han ZQ et al.24, Yang J et al.9 and Feng J et al.17 thought ETV could improve the rate of HBeAg seroconversion. Liaw et al.8 thought HBeAg seroconversion was higher with ADV at 24 weeks. In our research, there were no significant different between the ETV group and the control group.
Liaw yf et al.13 thought there were no significant different between ETV and TDF among three treatment regimens(HBV DNA loss, ALT normalization and mortality), TDF was superior to ETV in terms of HBeAg seroconversion. Xu Y22 thought LDT combined with ADV early acting was better than ETV. However, there was only one paper to support their conclusion, more trials were needed.
We used Funnel pool to evaluate the publication bias and found that almost all the related meta-analysis had the publication bias. The results would be affected.
The degree of evidence quality about patients’ mortality (ETV versus other treatments) and HBV DNA loss (ETV versus patients only take CT) at 24 weeks was high. Night outcomes of the degree of evidence quality is moderate. Other results’ degrees were from very low to low. The low and very low quality of the evidence would affect the reliability of the results.
There are still some limits of our research. (i) We only evaluated four outcomes (the HBV DNA loss, the rates of ALT normalization, the mortality, the HBeAg seroconversion). Other results (such as pathological changes of liver tissue, cost-effectiveness issues are not mentioned in our study. (ii) There were no specific descriptions of the lower magnitude to the decline of detectable HBV DNA and the decrease of ALT. (iii) The number of RCTs included in this study is limited and the included samples’ number is insufficient. (iv) The quality of RCTs included in our study is not high. We still need RCTs of multi-center, high qualities and a large of samples to obtain a comprehensive Meta-analysis.
Despite the shortcomings of the studies included in our review, these studies constitute the best level of evidence that is currently available. Overall, the evidence from systematic review and meta-analysis is more trustworthy than observational studies and expert opinions. In our research, ETV could be the first line therapy for patients with HBV related decompensated cirrhosis, because ETV could reduce the early mortality and move HBV DNA load down.
Methods
Search method
A computerized search of The Cochrane Library (CENTRAL, 2016), PubMed (1966-March 2016), Embase (via OVID) (1974-March 2016), China National Knowledge Infrastructure (CNKI) (1978-March 2016), WANFANG (1998-March 2016), China Science and Technology Journal Database(VIP) (1989-March 2016), Chinese BioMedical Literature (CBM) (1978-March 2016) databases was conducted by two authors (WFY and LB) independently. We searched the terms of ETV, decompensated cirrhosis, hepatitis B, and randomized controlled trial. The results were limited by the MeSH terms of these words. Finally, we expanded the search results by the free word retrieval for the newest reports. All the citations of the identified trials were checked. We also checked the citations of published reviews meta-analysis or guidelines. Manual search was made to augment the search strategy.
Criteria for considering studies for this review
All the included studies satisfied the following selection criteria: (i) types of studies-we included all randomized clinical trials, which compared the clinical effects of ETV with other nucleos(t)ide analogues (NAs) drugs or without other NAs drugs; (ii) types of participants-patients are older than 16 years who are diagnosed with HBV related decompensated liver cirrhosis according to the Management of chronic hepatitis B virus infection of China (2015); (iii) types of intervention – the experimental group: oral ETV; the control group: oral with/without other NAs drugs on the basis of comprehensive therapy (CT); (iv) types of outcome measures- HBV DNA loss, the level of serum alanine aminotransferase, the mortality, HBeAg seroconversion.
Criteria of excluded studies
(i) repeat reports; (ii) design defect (eg. not a randomized controlled trial); (iii) incomplete data; (iv) co-infection with other viruses (eg. Hepatitis A virus); (v) other decompensated liver disease (eg. autoimmune liver disease).
Assessment of risk of bias
We assessed the risk of bias in the trials by following the instructions given in Cochrane Handbook for Systematic Reviews of Interventions. We assessed the following procedures of each trials because the methodological quality of the trials could have an influence on intervention effects. We assessed the following parts: (i) random sequence generation (ii) allocation concealment (iii) blinding (iv) incomplete outcome data (v) selective outcome reporting (vi) baseline imbalance (vii) academic bias (viii) funding bias. Every domain was evaluated by three degrees which are low risk of bias, unclear risk of bias and high risk of bias.
Subgroup analysis
We planned to perform the following subgroup analyses:
(i) ETV versus other NAs.
(ii) Trials with ETV versus trials only take CT without any antiviral drugs.
(iii) ETV versus LAM.
(iv) ETV versus ADV.
Statistical methods
We use the software package Review Manager 5.3.5 to perform the meta-analysis according to the recommendation of the Cochrane Collaboration25. We use risk ratio (RR) to calculate the 95% confidence interval for our research. In our research, all indices we included were dichotomous variables. We used a random-effects model for all studies. The random-effect model is DerSimonian-Laird. The heterogeneity was explored by chi-squared test with significance set at P value 0.10, and the quantity of heterogeneity26 was measured by I2. I2 < 50% was considered there was heterogeneity of the trials included. Generally, if I2 > 50% was considered statistically significant heterogeneity27.
We performed intention-to-treat analysis for the participants who could not finish the treatment. The patients who did not finish the treatment included patients who died, patients who gave up the treatment, and patients we could not connect with them. We considered these participants as negative results.
Quality of the evidence
We used GRADEprofiler3.6 to evaluate the quality of the evidence according to the guideline of GRADES of Recommendations Assessment Development and Evaluation (GRADE). There are four degrees in GRADE: high, moderate, low and very low. The results of GRADE were showed by evidence profile (EP).
Funnel plot
We intended to use funnel plot to measure the publication bias. Lau J et al.28 thought that at least 10 papers were needed for one funnel plot. In our research, we used funnel plot for studies which involved more than 10 essays.
Additional Information
How to cite this article: Wang, F.Y. et al. Entecavir for Patients with Hepatitis B Decompensated Cirrhosis in China: a meta-analysis. Sci. Rep. 6, 32722; doi: 10.1038/srep32722 (2016).
Supplementary Material
Acknowledgments
We gratefully acknowledge Professor Cheng-yong Qin for his help in guiding and revising the manuscript. We also thank all the study participant. This work was supported in part by the National Natural Science Foundation of China, grant number 81472685, the science and Technology Development Projects of Shandong Province, grant number 2013GSF11852, the project funded by China Postdoctoral Science Foundation, grant number 2013M541926, the Postdoctoral Innovation Project Special Foundation of Shandong Province, grant number 201302031 and the promotive research fund for excellent young and middle-aged scientists of Shandong Province, grant number BS2014YY37.
Footnotes
Author Contributions F.Y.W. and B.L. searched databases, extracted and assessed studies. Y.L. and J.N.Q. helped to draft the manuscript. H.L., W.D.Q. and H.W.X. carried out the statistical analysis. C.Y.Q. participated in the design of the review. All authors read and approved the final.
References
- European Association for the Study of the Liver. et al. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 57, 167-185, doi: 10.1016/j.jhep.2012.02.010 (2012). [DOI] [PubMed]
- Hou J. et al. The guideline of prevention and treatment for chronic hepatitis B: a 2015 update. Zhonghua gan zang bing za zhi 23, 888–905, doi: 10.3760/cma.j.issn.1007-3418.2015.12.002 (2015). [DOI] [PubMed] [Google Scholar]
- Keating G. M. Entecavir: a review of its use in the treatment of chronic hepatitis B in patients with decompensated liver disease. Drugs 71, 2511–2529, doi: 10.2165/ 11208510-000000000-00000 (2011). [DOI] [PubMed] [Google Scholar]
- Chang T.-T. et al. A Comparison of Entecavir and Lamivudine for HBeAg-Positive Chronic Hepatitis B. New England Journal of Medicine 354, 1001–1010, doi: doi: 10.1056/ NEJMoa051285 (2006). [DOI] [PubMed] [Google Scholar]
- Suzuki F. et al. Efficacy and safety of entecavir in lamivudine-refractory patients with chronic hepatitis B: randomized controlled trial in Japanese patients. Journal of gastroenterology and hepatology 23, 1320–1326, doi: 10.1111/j.1440-1746.2008.05455.x (2008). [DOI] [PubMed] [Google Scholar]
- Schiff E. et al. Efficacy and safety of entecavir in patients with chronic hepatitis B and advanced hepatic fibrosis or cirrhosis. The American journal of gastroenterology 103, 2776–2783, doi: 10.1111/j.1572-0241.2008.02086.x (2008). [DOI] [PubMed] [Google Scholar]
- Schiff E. R. et al. Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 9, 274–276, doi: 10.1016/j.cgh.2010.11.040 (2011). [DOI] [PubMed] [Google Scholar]
- Liaw Y. F. et al. Efficacy and safety of entecavir versus adefovir in chronic hepatitis B patients with hepatic decompensation: a randomized, open-label study. Hepatology (Baltimore, Md.) 54, 91–100, doi: 10.1002/hep.24361 (2011). [DOI] [PubMed] [Google Scholar]
- Yang J., Z, X. Wang H. Antiviral effects of entecavir in patients with hepatitis B virus-induced decompensated liver cirrhosis. Jiangxi Medical Journal, 477–480 (2012). [Google Scholar]
- Peng H. et al. Efficacy of lamivudine combined with adefovir dipivoxil versus entecavir monotherapy in patients with hepatitis B-associated decompensated cirrhosis: A meta-analysis. Journal of clinical pharmacology 54, 189–200, doi: 10.1002/jcph.181 (2014). [DOI] [PubMed] [Google Scholar]
- Ye X. G. & Su Q. M. Effects of entecavir and lamivudine for hepatitis B decompensated cirrhosis: meta-analysis. World journal of gastroenterology 19, 6665–6678, doi: 10.3748/wjg.v19.i39.6665 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal A. K. & Fontana R. J. Meta-analysis: oral anti-viral agents in adults with decompensated hepatitis B virus cirrhosis. Aliment Pharmacol Ther 35, 674–689, doi: 10.1111/j.1365-2036.2011.04990.x (2012). [DOI] [PubMed] [Google Scholar]
- Liaw Y.-F. et al. Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology (Baltimore, Md.) 53, 62–72, doi: 10.1002/hep.23952 (2011). [DOI] [PubMed] [Google Scholar]
- Lai C.-L. et al. Entecavir for HbeAgnegative chronic hepatitis B. A randomized comparison of entecavir to lamivudine for treatment of HBeAg-negative chronic hepatitis B in nucleoside-naive patients. N Engl J Med, 1011–1020 (2006). [DOI] [PubMed] [Google Scholar]
- Amini-Bavil-Olyaee S., Herbers U., Luedde T., Trautwein C. & Tacke F. Impact of hepatitis B e antigen-suppressing mutations on the replication efficiency of entecavir-resistant hepatitis B virus strains. Journal of viral hepatitis 18, 804–814, doi: 10.1111/j.1365-2893.2010.01378.x (2011). [DOI] [PubMed] [Google Scholar]
- Chen F. Z. et al. Efficacy of entecavir for patients with HBV related decompensated cirrhosis. J Clini Hepatol 26, 608–609,612 (2010). [Google Scholar]
- Feng J. et al. Effect of an antiretroviral therapy to hepatitis B cirrhosis. China Prac Med, 13–14 (2008). [Google Scholar]
- Zhang D. H. et al. Effect of antiretroviral therapy to hepatitis B cirrhosis. Modern Preventive Medicine 751–753 (2011). [Google Scholar]
- XM H. Clinical efficacy of entecavir for 36 patients with HBV related decompensated cirrhosis. China Health Care & Nutrition 03, 1 (2014). [Google Scholar]
- YL B. Comparison of the efficacies of lamivudine combined with adefovir dipivoxil de novo and entecavir alone in patients with hepatitis B virus-related decompensated cirrhosi. Da Lian medical university (2014). [Google Scholar]
- JB S. et al. Clinical Observation of Entecavir in the Treatment of Decompensated Cirrhoti Patient with HBV for 96 weeks. Guide of China Medicine 11–13 (2010). [Google Scholar]
- Y X. Comparison on effect between telbivudine combined with adefovir and entecavir in treatment of patient with hepatitis B virus-related decompensated cirrhosis. Journal of Hubei University of Science and Technology(Medical Sciences) 387–390 (2013). [Google Scholar]
- Lin X. S., X F. & Wang X. Y. Efficacy of Entecavir therapy in decompensated HBV cirrhosis. Clin Med J Metal Indus, 257–258 (2011). [Google Scholar]
- Han Z. Q. et al. The effect of entecavir for HBV related decompensated cirrhosis. J Clin Hepatol, 54–56 (2009). [Google Scholar]
- Higgins J. P. T. G. S. 9.4 Summarizing effects across studies [updated March 2011]. www.handbook.cochrane.org. (Date of acess: 27/7/2016) (2011).
- Higgins JPT T. S. Quantifying heterogeneity in a Meta analysis. Statistics in Medicine 21, 1539–1558 (2002). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. et al. Measuring inconsistency in Meta analyses. BMJ 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J. et al. The case of the misleading funnel plot. BMJ 333, 597–600 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- J Y. & HZ F. Comparison of efficacy of nucleot(s)ide analogues in patients with hepatitis B virus-induced decompensated cirrhosis. Medical Innovation of China, 1–2 (2012). [Google Scholar]
- Ren W. X., J J. & Liu M. Curative effect of entecavir treatment for decompensated cirrhosis resulting from chronic hepatitis B(48 weeks report). Chinese Journal of Coal Industry Medicine, 176–178 (2014). [Google Scholar]
- Luo H. B., H Z. & Guo J. W. Study on 48-week treatment of entecavir in patients with decomensated hepatitis B-induced cirrhosis. J Clin Hepatol, 121–123 (2009). [Google Scholar]
- Ning Z. H. et al. he effect of entecavir for HBV DNA positive decompensated cirrhosis. Clin Medical Herald, 88–89 (2009). [Google Scholar]
- FL Z. The effects of entecavir for HBV related decompensated cirrhosis. J Med Theor&Prac, 2454–2455 (2011). [Google Scholar]
- RL Z. Clinical observation of entecavir for HBV related decompesnsated cirrhosis. Journal of Clinical Medicine in Practice, 59–60 (2010). [Google Scholar]
- Guo Y. M. & S Z. Guo CY. Entecavir for the treatment of hepatitis B with decompensated cirrhosis. Med J NDFNC, 14–15 (2014). [Google Scholar]
- H L. Entecavir for hepatitis B decompensated liver cirrhosis. China Prac Med 159–160 (2009). [Google Scholar]
- Feng J. et al. Effect of an antiretroviral therapy to hepatitis B cirrhosis. China Prac Med, 13–14 (2008). [Google Scholar]
- L Y. Efficacy and safety of entecavir for patients with HBV related decompensated cirrhosis. Med Theor&Prac, 909–910 (2015). [Google Scholar]
- Gulizire•Maola. Analysis of the clinical effect of entecavir treatment of hepatitis B decompensated liver cirrhosis. China&Foreign Medical Treatment, 131-133 (2015).
- J Z. Efficacy of entecvir for patients with HBV related decompensated liver cirrhosis. Chin J Mod Drug Appl, 71–72 (2014). [Google Scholar]
- ZY Z. Efficacy and safety of entecavir for patients with HBV-related decompensated liver cirrhosis. Zhongguo shiyong xiangcun yisheng zazhi 21, 2 (2014). [Google Scholar]
- MX L. Efficacy of entecavir for patients with HBV related decompensated liver cirrhosis. Mod Diagn Treat, 2717–2718 (2014). [Google Scholar]
- XL Z., CX, & CY L. Observation of clinical effective of entecavir in treatment of patients with hepatitis B decompensated cirrhosis. Jilin Medicine, 3242–3243 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.