Abstract
Introduction
Low back pain and neck pain are extremely prevalent and are responsible for an enormous burden of disease globally. Strong analgesics, such as opioid analgesics, are recommended by clinical guidelines for people with acute low back pain or neck pain who are slow to recover and require more pain relief. Opioid analgesics are widely and increasingly used, but there are no strong efficacy data supporting the use of opioid analgesics for acute low back pain or neck pain. Concerns regarding opioid use are further heightened by the risks of adverse events, some of which can be serious (eg, dependency, misuse and overdose).
Methods and analysis
OPAL is a randomised, placebo-controlled, triple-blinded trial that will investigate the judicious use of an opioid analgesic in 346 participants with acute low back pain and/or neck pain who are slow to recover. Participants will be recruited from general practice and randomised to receive the opioid analgesic (controlled release oxycodone plus naloxone up to 20 mg per day) or placebo in addition to guideline-based care (eg, reassurance and advice of staying active) for up to 6 weeks. Participants will be followed-up for 3 months for effectiveness outcomes. The primary outcome will be pain severity. Secondary outcomes will include physical functioning and time to recovery. Medication-related adverse events will be assessed and a cost-effectiveness analysis will be conducted. We will additionally assess long-term use and risk of misuse of opioid analgesics for up to 12 months.
Ethics and dissemination
Ethical approval has been obtained. Trial results will be disseminated by publications and conference presentations, and via the media.
Trial registration number
ACTRN12615000775516: Pre-results.
Keywords: Opioid analgesics, low back pain, neck pain
Strengths and limitations of this study.
This is the world's first placebo-controlled trial to investigate whether using a short course of an opioid analgesic, in people who are slow to recover from acute low back pain or neck pain, can effectively reduce pain, improve other outcomes (eg, function), be reasonably tolerated and cost-effective, and have minimal risk of long-term misuse.
The trial will inform the appropriate and judicious use of opioid analgesics.
Misuse data will be collected via self-report and therefore subject to reporting bias.
Introduction
Low back pain and neck pain are among the most burdensome conditions globally.1 The enormous burden of these conditions is associated with their high prevalence: the 1-year prevalence for low back pain and neck pain is 38% and 26%, respectively.2 3 There is also a high economic burden: the latest estimates show that low back pain and neck pain cost A$4.8 billion in healthcare costs and $8 billion in lost productivity per annum.4 5
Clinical guidelines recommend that first-line care for people with acute low back pain or neck pain should consist of advice (patient reassurance, staying active and avoiding bed rest) and, if required, simple analgesics (paracetamol or non-steroidal anti-inflammatory drugs, NSAIDs).6 For people who do not respond to first-line care, guidelines recommend stronger analgesics such as an opioid analgesic, particularly if the pain is severe.6 There is evidence that opioid analgesics are widely and increasingly used by patients and general practitioners for low back pain and neck pain. We found that 12% of participants surveyed reported using an opioid analgesic or opioid analgesic combination (eg, paracetamol plus codeine) as their first-choice medicine when self-managing low back pain.7 This proportion increased to 28% for those with severe pain. We also found that opioid analgesics were prescribed for up to 19% of patients with low back pain or neck pain by general practitioners in Australia, and were second only to NSAIDs as the most prescribed medication.8 Internationally there is a trend of increasing opioid consumption over the past decades.9 10
Despite guideline recommendations and the widespread use of opioid analgesics, there is no rigorous evidence to support their use for acute low back pain and neck pain.10 Although there is evidence suggesting that opioid analgesics may be effective in the short term for persistent low back pain,11 there are no randomised placebo-controlled trials evaluating the use of opioid analgesics for acute low back pain11 or for acute or persistent neck pain.12 Furthermore, evidence from persistent low back pain is not compelling due to methodological issues such as high drop-out rates and the use of enrichment designs where only those participants who responded and tolerated the medicine in the run-in phase were subsequently enrolled.10 11
The lack of efficacy data is alarming given the adverse events associated with opioid analgesics. The most common adverse events include nausea and constipation,11 but there are also concerns of more serious adverse events such as dependency, misuse and overdose.10 13 14 Australian data suggest that hospital separations for opioid poisoning doubled between 2005–2006 and 2006–2007,15 and there has been a multifold increase in drug overdose deaths in the USA in the last 15 years, primarily driven by opioid analgesics.16 Alarmingly, since 2003, more overdose deaths have involved opioid analgesics than heroin and cocaine combined.17 Although some suggest that these serious adverse events are rare,18–20 are primarily associated with long-term use and may have stabilised with respect to rate,14 there is no doubt that the use of opioid analgesics is a contentious issue globally.
We have designed the current trial to provide high-quality data to inform the use of opioid analgesics for treating acute low back pain or neck pain. The primary aim of the trial is to investigate whether taking a short course of an opioid analgesic, compared to placebo, will reduce pain severity over the 6-week treatment period in people with acute low back pain or neck pain who are slow to recover. The secondary aim is to investigate whether taking a short course of an opioid analgesic, compared to placebo, will improve other clinical outcomes, be tolerated, be cost-effective and not result in long-term opioid misuse.
Methods and analysis
Design
OPAL—a randomised, placebo-controlled trial of opioid analgesia for the reduction of pain severity in people with acute spinal pain—is a randomised, placebo-controlled, assessor-blinded, clinician-blinded and participant-blinded superiority trial, with two parallel groups randomised at a 1:1 allocation. We have chosen a randomised, placebo-controlled design in order to provide high-level evidence on the efficacy or safety of opioid analgesics in those with acute spinal pain. The trial has been prospectively registered on the Australian New Zealand Clinical Trials Registry (ACTRN12615000775516). The current report describes the detailed trial protocol and follows the SPIRIT 2013 Statement.21
Setting
The OPAL trial will be conducted in primary care. Registered general practitioners in the Sydney metropolitan and surrounding areas in the state of New South Wales of Australia with no regulatory impediment to prescribe opioid analgesics will be invited to participate as trial general practitioners, who will be involved in participant screening and recruitment as well as treatment provision. It is anticipated that up to 200 general practice sites will be required.
Eligibility criteria
Participants who have not recovered from acute low back pain and/or neck pain and present to a trial general practitioner for care will be eligible if they fulfil all of the inclusion criteria and none of the exclusion criteria. Trial general practitioners will determine a participant's eligibility via a history and physical examination.
Inclusion criteria are as follows:
Low back pain (pain between the 12th rib and buttock crease) and/or neck pain (pain in the area below the occiput to the most distal cervical spine) with or without distal radiation to the leg (for low back pain) or arm (for neck pain).
- Current episode of pain is at least 2 weeks but no more than 12 weeks since onset, and preceded by at least a 1 month pain-free period (to screen out those with recurrent pain).
Pain severity is at least moderate (as measured by adaptations of item 7 of the SF-36, ie, how much low back pain or neck pain have you had in the last week? None/very mild/mild/moderate/severe/very severe).
Exclusion criteria are as follows:
Known or suspected serious spinal pathology (eg, cauda equina syndrome, spinal fracture).
Contraindications to opioid analgesics (including previous intolerance, addiction history, allergy, abuse of psychoactive drugs, alcoholism), or scoring ‘high risk’ on the Opioid Risk Tool.24
Have taken a prescription opioid analgesic for the current episode of low back pain and/or neck pain.
Spinal surgery in the preceding 6 months.
Scheduled or being considered for surgery or interventional procedures for low back pain and/or neck pain during the 6-week treatment period.
Less than 18 years of age.
Not having sufficient English to understand trial procedures or complete assessments, or suitable translation is not available.
For female participants: planning conception, or is pregnant or breast feeding.
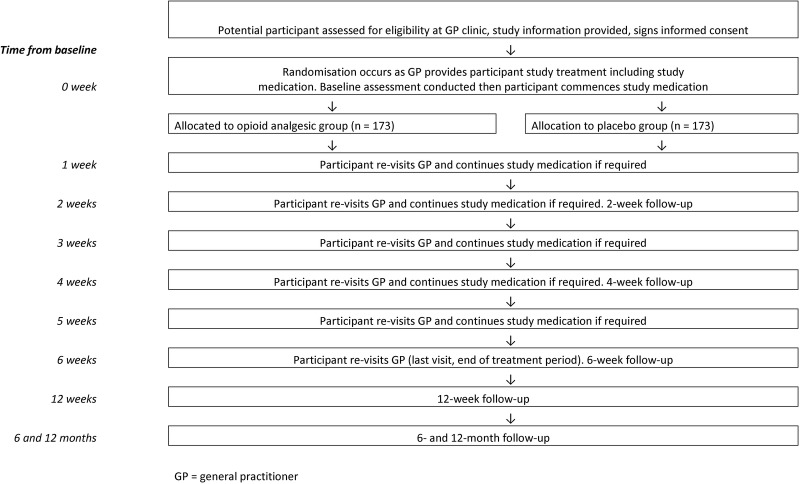
After a participant has given consent and been recruited, the general practitioner will notify the trial team via phone or fax. For a participant to be officially enrolled in the trial, a researcher (blinded) will collect baseline data directly from the participant before the participant starts the trial medication and within 72 hours of visiting the general practitioner. A participant timeline is shown in figure 1.
Figure 1.
Participant timeline. GP, general practitioner.
Interventions
General practitioners will be trained on the trial protocol and receive regular monitoring visits from the trial team to ensure adherence to the trial intervention.
Trial medication
General practitioners will prescribe the trial medication to all participants starting at a dose of 5 mg, two times a day and gradually titrated up to the maximum dose of 10 mg, two times a day25 until ‘adequate improvement’ (0–1 of 10 pain for three consecutive days)26 or for a maximum of exposure to oxycodone of 6 weeks. When adequate analgesic improvement is achieved or after a maximum of 5 weeks of treatment, the dose of the trial medication will be titrated down to cessation over 1 week. General practitioners will review participants, between weekly or fortnightly, to titrate the dose based on individual participant progress, tolerability and sedation score (0=wide awake, 1=easy to rouse, 2=easy to rouse but cannot stay awake, 3=difficult to rouse)25 until medication cessation. General practitioners will be asked to keep the sedation score under 2.25
The maximum supply of trial medication prescribed by the general practitioner at each review is 2 weeks, with the cumulative maximum supply per participant being 6 weeks. The opioid analgesic used will be controlled release oxycodone plus naloxone, the latter constituent to counter the tendency to opioid-induced constipation. Participants can be given a laxative (docusate sodium and senna) in addition and advised to use this if required.25
Although opioid dependency is unlikely especially when using opioid analgesics for a limited period of time for acute pain,18 19 we have taken steps to minimise the risk further by excluding people inappropriate for opioid analgesics (history of substance abuse or addiction), implementing regular review by the trial general practitioner and limiting the supply of the trial medication. The general practitioner will also make clear agreements (see online supplementary appendix 1) with participants when starting the treatment regarding dosage, frequency and duration of treatment. We will additionally contact participants up to 12 months to collect information on their spinal pain and use of treatments, including opioid analgesics (see the ‘Outcomes’ section).
bmjopen-2016-011278supp_appendix.pdf (244.4KB, pdf)
Guideline-based care
In addition to the trial medication, general practitioners will deliver guideline-based care to all participants.6 This includes advice (patient reassurance, staying active and avoiding bed rest) delivered by the general practitioners and, if required, other guideline-recommended treatments delivered either by the general practitioners or by referral to other health professionals. For example, spinal manipulative therapy may be offered and simple analgesics or adjuvants may be provided in addition to the trial medication in accordance to the WHO analgesic ladder.6 27 However, no concomitant opioid analgesics should be used. We have not protocolised guideline-based care, other than that all participants will receive advice, to allow the care to be tailored to participants.
This design mimics how opioid analgesics are administered in clinical practice. The use of additional services will be recorded to investigate whether this influences the trial findings, and to inform a comprehensive cost-effectiveness analysis (see the ‘Outcomes’ section).
Outcomes
The outcomes and outcome measures chosen incorporate the core outcomes for pain trials recommended by consensus of international experts.28 The primary outcome, collected at baseline and 2, 4, 6 and 12 weeks, will be pain severity measured by the Pain Severity Score of the Brief Pain Inventory.29 The Pain Severity Score (/10) is calculated by adding the scores from four pain severity questions of the Brief Pain Inventory and dividing by four. The four pain severity questions measure worst pain in the last week, least pain in the last week, pain on average and pain right now, with each question measured by the 0–10 numerical pain rating scale.
Secondary outcomes, collected at baseline and 2, 4, 6 and 12 weeks (unless specified), will be:
Physical functioning (generic) measured by the Pain Interference Score of the Brief Pain Inventory.29
Physical functioning (condition-specific) measured by the Roland-Morris Disability Questionnaire30 (24 items, for participants reporting low back pain only) and Neck Disability Questionnaire31 (for participants reporting neck pain only) at baseline and 6 weeks only.
Time to recovery (average daily pain of 0 or 1 of 10 for the past seven consecutive days)22 measured using a pain diary, with information entered daily until recovery or up to 12 weeks (whichever is sooner).
Quality of life measured by SF-12.32
Participants' rating of global improvement measured by the global perceived effect scale.33
The following data will also be collected:
Adverse events—collected at 2, 4, 6 and 12 weeks by self-report on all adverse events that occur between baseline and 12 weeks. Additionally, we will ask general practitioners to report adverse events (if any) at each participant follow-up visit. Serious adverse events (see the ‘Harms’ section for definition) will be reported to an independent medical monitor and relevant bodies. We will monitor the number and severity of (serious) adverse events and consider tolerability when interpreting the results.
Work absenteeism and use of other treatments or healthcare services will be collected by self-report at 2, 4, 6 and 12 weeks for a cost-effectiveness analysis.
Adherence to trial medication—measured by participants' self-report of daily trial medication intake recorded in a diary and by counting the returned medications. This will be compared against prescription data of trial medication reported by the general practitioner at each participant visit during the 6-week treatment period.
Success of blinding—participants will be asked to guess their group allocation at the 6-week follow-up, as opioid, placebo or do not know.
Long-term outcomes, collected at 3 (12 weeks), 6 and 12 months, will be:
Sample size
Our primary outcome measure is pain severity. A sample size of 173 per group (346 total) will be sufficient to detect a between-group difference of 1 on a 10-point pain scale (Pain Severity Score of the Brief Pain Inventory) at 6 weeks, assuming a SD of 2.5,22 power of 90% and α of 5%, and allowing for 5% dropout (based on our previous study)22 and 10% non-compliance.
A previous study showed that to perceive the effect of NSAIDs as worthwhile, patients need to see a median of 30% more improvement in pain than would occur without intervention.35 For opioid analgesics, no such information is available but we expect patients would need to perceive at least this level of improvement to consider the effect of opioid analgesics as worthwhile. This is estimated to be around 1 point on a 10-point pain scale taking into account the natural recovery of acute low back pain and neck pain.36 37
Strategies for achieving adequate participant enrolment
We plan to recruit 200 general practice (for participant screening, recruitment and provision of trial treatment) and 200 community pharmacy (for trial medication dispensing) sites over the course of the OPAL trial to complete recruitment. Strategies for achieving adequate participant enrolment to reach target sample size will include developing streamlined screening, recruitment and follow-up processes to minimise clinician and participant load, regular site monitoring visits and support from the trial team, reimbursing general practitioners and pharmacists for the time they spend on trial-related tasks, and applying with the relevant professional bodies for trial participation to be credited with continuing education points.
Assignment of interventions: allocation
The allocation sequence will be prepared a priori using a computerised random number generator by an independent statistician not involved in participant recruitment or data collection, in permuted blocks. Trial medication packs will be prepared according to the allocation sequence, sealed and dispatched by an independent manufacturer in small quantities to a trial pharmacy. After a participant has been recruited, the trial general practitioner will provide a prescription to the participant and the next sequentially numbered medication pack will be dispensed by the trial pharmacy, thus randomising the participant to either the opioid analgesic or placebo group in a 1:1 ratio. Active and placebo medicines will have an identical appearance.
Blinding
The randomisation and concealed allocation process ensure blinding of the general practitioner (treatment provider), pharmacist, participant and assessor. The Steering Committee (consisting of authors of this protocol) and data analysts will also be blinded. Blinding will be maintained for the entire duration of the trial until all data have been collected and data analysis and interpretation have been completed and agreed upon. The success of participant blinding will be tested at the 6-week follow-up (end of treatment period) (see the ‘Outcomes’ section).
To maintain the overall quality and rigor of the trial design, non-blinding of the blinded trial personnel would occur only in exceptional circumstances when knowledge of the actual treatment is absolutely essential for further management of the participant. The decision for this will be made in consultation with the clinicians involved in the participant's management (including the trial general practitioner) and the Steering Committee.
If non-blinding is deemed to be necessary, the trial team will facilitate contact between the clinicians involved in the participant's management and the independent statistician who generated the randomisation sequence, and care will be made to ensure that only the trial personnel involved in further management of the individual participant will be non-blinded. Non-blinding should not necessarily be a reason for trial discontinuation.
Data collection methods
Outcomes will be collected by a blinded research assistant via phone or directly completed by the participant online or by email. Research assistants will be trained to ensure data accuracy, consistency and completeness. For participants completing data assessment online, an email reminder will be sent prior to the due date, with a unique link to complete the data online. For participants completing data assessment by email, a blank assessment form will be provided, which can be emailed to the trial team upon completion. Additionally, at each participant visit, general practitioners will be asked to complete data on trial medication prescription and adverse events (if any, excluding the initial visit).
The trial team will make every reasonable effort to contact and complete follow-up of every participant, including those who discontinue the trial treatment prematurely or deviate from the protocol. Attempts will be made to ensure that all outcomes will be collected. At a minimum and only as a last resort, we will attempt to collect the primary outcome. Where possible, we will record the reason for loss to follow-up (eg, consent withdrawn).
Data management
The integrity of data will be closely monitored for omissions and errors. Data collected by phone will be directly entered into a secure, custom-built database by the trial team at the time of data collection, with a prompt for the trial team to double check the accuracy of the primary outcome. Any inconsistencies will be explored and resolved. Data directly completed by participants online will be automatically transcribed into the database. If participants return data by email, the data will be entered into the database with a prompt to check the accuracy of data entry to the primary outcome. Each trial personnel will be able to access the database only with a personal login and password. Range checks and data validation will be built-in to promote data quality.
Statistical methods
Data analysis will be blinded, by intention-to-treat and guided by a detailed statistical analysis plan. Analysis and interpretation (also performed blinded) on the primary and key secondary outcomes will be conducted by the trial team and by an independent biostatistician and checked for accuracy. A p value of <0.05 will be considered statistically significant.
Primary analysis
Repeated-measure linear mixed models will be used to assess the effect of treatment group on pain severity over the 6-week treatment period using baseline pain as a covariate. Correlations between repeated measures (weeks 2, 4 and 6 assessments) will be modelled using a repeated effect.38 We will conduct sensitivity analyses with duration of current pain episode and site of pain, that is, low back or neck, as additional covariates. In case >10% of the primary outcome data are missing, multiple imputations will be used to conduct sensitivity analyses for the longitudinal linear mixed model of the primary outcome.
Secondary analysis
For all continuous secondary outcomes measured at weeks 2, 4 and 6, repeated-measure linear mixed models will be used as per the primary analysis. Medium-term effects at week 12 on the primary outcome and all secondary continuous outcomes will be analysed using a linear mixed model with the baseline value included as a covariate. For the secondary outcome of time to recovery, the difference in survival curves will be assessed for the groups using the log-rank statistic. The median days to recovery will be used to express the time to recovery.
Adverse events will be categorised and the proportion of patients with adverse events overall and within each category will be compared between the two groups. Self-reported data will be used as the primary source of data and supported by data reported by the general practitioners.
Cost-effectiveness analysis
Two analyses will be conducted if between-group differences are found in the primary analysis: (1) a cost-effectiveness analysis using pain severity as a measure of effectiveness and (2) a cost-utility analysis where health state utilities (quality-adjusted life-years or QALY) will be based on measures obtained from the SF-12 and transformed into utilities via the SF-6D algorithm. The primary analysis will be conducted from the health sector's perspective to assess the incremental cost per 1-point pain reduction or per QALY gained between the two treatment groups over 12 weeks. To obtain costs, public health services will be valued at published standard rates (eg, the Medical Benefits Scheme standard fees, the Pharmaceutical Benefits Scheme costs). Private non-medical health services (eg, physiotherapy) will be valued at published standard rates, if available, or as reported by participants.
A secondary analysis will entail a societal perspective in which costs associated with the use of community services (eg, exercise classes) and work absenteeism related to low back pain or neck pain will be included. Costs of community services will be based on the self-reported costs. Costs of absenteeism from paid employment will be estimated by the number of days absent from work multiplied by the average wage rate. Sensitivity analyses will test uncertainty in key parameters such as the selection of cost weights and statistical variation in quality-of-life scores.
Analysis of long-term outcomes
Pain severity at 6 and 12 months, and treatment usage and risk of opioid misuse at 3, 6 and 12 months will be reported descriptively. Between-group differences in pain severity and opioid misuse at each time point will be compared using the t-test and χ2 test, respectively.
Data monitoring
An independent Data and Safety Monitoring Board (DSMB) will be convened with the main purpose being to monitor (serious) adverse events, in order to ensure the safety of participants. The frequency of DSMB meetings and the stopping rules will be defined a priori in a charter, in consultation with the DSMB members and Steering Committee. The DSMB will have access to non-blinded data, and data on the primary outcome, adverse events (after coding) and serious adverse events; however, there is no planned interim analysis on efficacy data.
The DSMB will make recommendations to the Steering Committee, who will be responsible for making the final decision on the recommendations. If appropriate, this will be performed in consultation with the ethics committee.
Harms
Harm or safety reporting will follow a standard operating procedure on clinical trial safety reporting established by the trial sponsor, The George Institute for Global Health, which defines serious adverse events as any untoward medical occurrence that
results in death,
is life-threatening,
requires hospitalisation or prolongation of existing hospitalisation,
results in persistent or significant disability or incapacity,
is a congenital anomaly or birth defect and
is a medically significant or important event or reaction.
The relatedness and expectedness of a serious adverse event will be assessed by an independent medical monitor and, if the event occurs during the 6-week treatment period, the general practitioner. If there are differing opinions in relatedness or expectedness, we will take the most conservative opinions. Serious adverse events will be reported to the relevant bodies (eg, ethics committee, regulatory body) within the required timeline.
Auditing
No formal auditing is planned. However if required, independent auditing of core trial processes and documents will be arranged.
Consent or assent
Trial general practitioners will be trained on the informed consent process and will introduce the OPAL trial to potential participants who will also receive an information sheet and consent form. General practitioners, with support from the trial team if required, will also answer any questions that are raised by potential participants, and obtain written consent from those willing to participate in the trial. At any stage, participants can withdraw consent without repercussion.
Confidentiality
All data will be stored securely in either locked filing cabinets (paper files) or electronically (electronic database files) with access granted only to the trial team. Where required, general practitioners will have access to data collected from the participants they are responsible for, only after consent from the participants. After the completion of the trial, data will be archived for a minimum of 15 years.39
Access to data
The Steering Committee will have access to the final data set, which may be provided to a statistician and/or a postgraduate student to assist with data analysis if required. To ensure confidentiality, the final data set will contain de-identified information only.
Ancillary and post-trial care
During the 6-week treatment period, general practitioners may refer participants to other guideline-based treatments in addition to receiving the trial medication. The cost of such treatments will not be borne by the trial. Any post-trial care, including continuation or re-initiation of an opioid analgesic, will be determined by the participants and their clinician; whether they are trial general practitioners or other qualified clinicians.
If non-negligent harm associated with the protocol occurs, participants will be covered by professional indemnity and clinical trials insurance of the trial. This will include cover for additional healthcare, compensation or damages.
Dissemination policy
The main results will be submitted for publication in a scientific journal, and presented at relevant professional conferences. The results will also be disseminated to the media and general public. Authorship eligibility guidelines of publications arising from the OPAL trial will align with those outlined by the International Committee of Medical Journal Editors (http://www.icmje.org/). There are no plans to use professional writers. There are currently no plans to grant public access to the raw data.
Discussion
Low back pain and neck pain are among the most burdensome conditions globally, in terms of disease burden to the patient and economic burden to society. Despite the widespread and increasing use of opioid analgesics and guidelines endorsing their use, there remains a lack of reliable evidence on the efficacy of opioid analgesics in acute low back pain or neck pain. Concerns are also being raised because of the risks of adverse events associated with opioid analgesics and, with persistent use, more serious consequences such as overdose.
OPAL will be the world's first placebo-controlled trial of opioid analgesics in people with acute low back pain or neck pain and will provide rigorous evidence to inform the appropriate and judicious use of this medicine. We will establish whether using a short course of an opioid analgesic in people who are slow to recover from acute low back pain or neck pain can effectively reduce pain, improve other outcomes (eg, function), be reasonably tolerated and cost-effective, and have minimal risk of long-term misuse.
The strengths of the OPAL trial include that it is adequately powered and incorporates features that reduce bias, such as concealed allocation and blinding. Since the trial is delivered at the point of care, the results can be directly translated to clinical practice and inform clinical guidelines. The choice of modified release oxycodone plus naloxone as the opioid analgesic for the OPAL trial relates to the ease of dosing (two times per day) compared to the immediate release opioid preparations that need to be taken more frequently. The combination with naloxone is known to reduce the frequency and severity of opioid-induced constipation, which will be important for protecting the blinding of the OPAL trial. A weakness is that although we will monitor opioid misuse, we rely on self-report that can be prone to reporting bias. There are alternative sources, such as government prescription data or urine or blood samples, which may provide more objective data. However, after considering trial resources, the burden on participants, the recruitment of an acute population with a short-term prescription of opioid analgesics and the exclusion of those with a history or at high risk of addiction, drug abuse or alcoholism, we have decided to use self-report to capture such information.
The inclusion and exclusion criteria have been kept as broad as possible, so the results can be applicable to the heterogeneous population that present to primary care with acute, non-specific spinal pain. OPAL is specifically designed to establish the effects of opioid analgesics in acute spinal pain. If the results show that opioid analgesics are indeed efficacious and well tolerated, future research can further investigate if factors such as patient prognostic characteristics, treatment expectation, medication dosage and the type of opioid analgesics used will influence treatment responsiveness.
We have chosen outcomes that are patient-oriented, clinically relevant and recommended by international consensus.28 Since the trial intervention will be implemented over a short period of time for an acute condition where patients in general make an initial, rapid improvement,36 40 we will collect outcomes frequently (every 2 weeks) during the 6-week treatment period to assess treatment effects. We will also collect outcomes at 12 weeks to assess whether treatment effects, if any, can be sustained in the medium term. While we do not expect that a short-term treatment will influence long-term clinical outcomes, we will collect our primary outcome at 6 and 12 months, as well as long-term opioid use and misuse data, to inform our results. We anticipate that participant recruitment will start early 2016.
Footnotes
Contributors: All authors conceived and refined the trial design and procured funding. C-WCL was responsible for the design of the cost-effectiveness analysis. AJM provided expertise in pharmacy. ROD provided expertise in medicine and pharmacology. LB provided statistical expertise. C-WCL drafted the manuscript; all authors critically contributed to the writing and approved the final manuscript for publication.
Funding: This work is supported by the National Health and Medical Research Council (NHMRC) of Australia, grant number APP1082480. C-WCL is funded by a Career Development Fellowship from the NHMRC (APP1061400). CGM is funded by a Principal Research Fellowship from the NHMRC (APP1103022). The funding source has no role in the trial design and will have no role in the trial conduct, data analysis and interpretation, or writing or reporting.
Competing interests: C-WCL has received trial medicines from Pfizer (Australia) for an investigator-initiated trial funded by the National Health and Medical Research Council (NHMRC) of Australia, to evaluate drug treatment of low back pain. AJM has received supplementary funding and/or trial medicines from GlaxoSmithKline and Pfizer (Australia) for investigator-initiated NHMRC-funded trials evaluating drug treatment of low back pain. He is also the Program Director of the NHMRC Centre for Research Excellence in Medicines and Ageing. JL has received supplementary funding and/or trial medicines from GlaxoSmithKline and Pfizer (Australia) for investigator-initiated NHMRC-funded trials evaluating drug treatment of back pain. She has received funding from Baxter Biosciences for research evaluating musculoskeletal outcomes in children with haemophilia. ROD has received supplementary funding and/or trial medicines from GlaxoSmithKline and Pfizer (Australia) for investigator-initiated NHMRC-funded trials evaluating drug treatment of back pain. ROD has been a member of an advisory board about paracetamol for GlaxoSmithKline and ibuprofen for Reckitt Benckiser (Australia). Payments went to an audited hospital account for teaching and research purposes. CGM has received supplementary funding and/or trial medicines from GlaxoSmithKline and Pfizer (Australia) for investigator-initiated NHMRC-funded trials evaluating drug treatment of low back pain.
Ethics approval: Ethics approval has been granted from the Human Research Ethics Committee, The University of Sydney (Project No. 2015-004).
Ethics and dissemination: Any modifications to the protocol which may affect the trial design and conduct, or the potential benefits or harms to the participants, will require a formal amendment to the protocol. Such amendments will be agreed upon by the Steering Committee and approved by the ethics committee prior to implementation.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743–800. 10.1016/S0140-6736(15)60692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoy D, Bain C, Williams G et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum 2012;64:2028–37. 10.1002/art.34347 [DOI] [PubMed] [Google Scholar]
- 3.Hoy DG, Protani M, De R et al. The epidemiology of neck pain. Best Pract Res Clin Rheumatol 2010;24:783–92. 10.1016/j.berh.2011.01.019 [DOI] [PubMed] [Google Scholar]
- 4.Arthritis and Osteoporosis Victoria. A problem worth solving. Elsternwick: Arthritis and Osteoporosis Victoria, 2013. [Google Scholar]
- 5.Walker BF, Muller R, Grant WD. Low back pain in Australian adults: the economic burden. Asia Pac J Public Health 2003;15:79–87. 10.1177/101053950301500202 [DOI] [PubMed] [Google Scholar]
- 6.Koes B, van Tulder M, Lin C et al. An updated overview of clinical guidelines for the management of nonspecific low back pain in primary care. Eur Spine J 2010;19:2075–94. 10.1007/s00586-010-1502-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilk V, Palmer HD, Stosic RG et al. Evidence and practice in the self-management of low back pain: findings from an Australian internet-based survey. Clin J Pain 2010;26:533–40. 10.1097/AJP.0b013e3181dc7abb [DOI] [PubMed] [Google Scholar]
- 8.Michaleff ZA, Harrison C, Britt H et al. Ten year survey reveals differences in GP management of back and neck pain. Eur Spine J 2012;21:1283–9. 10.1007/s00586-011-2135-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mafi JN, McCarthy EP, Davis RB et al. Worsening trends in the management and treatment of back pain. JAMA Intern Med 2013;173:1573–781. 10.1001/jamainternmed.2013.8992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ 2015;350:g6380 10.1136/bmj.g6380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaparro LE, Furlan AD, Deshpande A et al. Opioids compared to placebo or other treatments for chronic low-back pain. Cochrane Database Syst Rev 2013;CD004959 10.1002/14651858.CD004959.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peloso P, Gross A, Haines T et al. Medicinal and injection therapies for mechanical neck disorders. Cochrane Database Syst Rev 2007;CD000319. [DOI] [PubMed] [Google Scholar]
- 13.Chou R, Turner JA, Devine EB et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med 2015;162:276–86. 10.7326/M14-2559 [DOI] [PubMed] [Google Scholar]
- 14.Dart RC, Surratt HL, Cicero TJ et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med 2015;372:241–8. 10.1056/NEJMsa1406143 [DOI] [PubMed] [Google Scholar]
- 15.Roxburgh A, Bruno R, Larance B et al. Prescription of opioid analgesics and related harms in Australia. Med J Aust 2011;195:280–4. 10.5694/mja10.11450 [DOI] [PubMed] [Google Scholar]
- 16.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths. JAMA 2013;309:657–9. 10.1001/jama.2013.272 [DOI] [PubMed] [Google Scholar]
- 17.Paulozzi LJ, Baldwin G, Franklin G et al. CDC grand rounds: prescription drug overdoses—a US. epidemic. Morb Mortal Wkly Rep 2012;61:10–13. [PubMed] [Google Scholar]
- 18.Minozzi S, Amato L, Davoli M. Development of dependence following treatment with opioid analgesics for pain relief: a systematic review. Addiction 2013;108:688–98. 10.1111/j.1360-0443.2012.04005.x [DOI] [PubMed] [Google Scholar]
- 19.Noble M, Treadwell JR, Tregear SJ et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev 2010;CD006605 10.1002/14651858.CD006605.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belcher J, Nielsen S, Campbell G et al. Diversion of prescribed opioids by people living with chronic pain: results from an Australian community sample. Drug Alcohol Rev 2014;33:27–32. 10.1111/dar.12084 [DOI] [PubMed] [Google Scholar]
- 21.Chan A-W, Tetzlaff JM, Gøtzsche PC et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams CM, Maher CG, Latimer J et al. Efficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trial. Lancet 2014;384:1586–96. 10.1016/S0140-6736(14)60805-9 [DOI] [PubMed] [Google Scholar]
- 23.Koes BW, van Tulder MW, Thomas S. Diagnosis and treatment of low back pain. BMJ 2006;332:1430–4. 10.1136/bmj.332.7555.1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med 2005;6:432–42. 10.1111/j.1526-4637.2005.00072.x [DOI] [PubMed] [Google Scholar]
- 25.Buckley N, ed. Australian medicines handbook 2014. 15 edn Adelaide: Australian Medicines Handbook, 2014. [Google Scholar]
- 26.Mathieson S, Maher C, McLachlan A et al. PRECISE—pregabalin in addition to usual care for sciatica: study protocol for a randomised controlled trial. Trials 2013;14:213 10.1186/1745-6215-14-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organisation. Cancer pain relief. Geneva: WHO, 1996. [Google Scholar]
- 28.Dworkin RH, Turk DC, Farrar JT et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9–19. 10.1016/j.pain.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 29.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap 1994;23:129–38. [PubMed] [Google Scholar]
- 30.Roland M, Fairbank J. The Roland-Morris disability questionnaire and the Oswestry disability questionnaire. Spine 2000;25:3115–24. 10.1097/00007632-200012150-00006 [DOI] [PubMed] [Google Scholar]
- 31.Cleland JA, Childs JD, Whitman JM. Psychometric properties of the Neck Disability Index and Numeric Pain Rating Scale in patients with mechanical neck pain. Arch Phys Med Rehabil 2008;89:69–74. 10.1016/j.apmr.2007.08.126 [DOI] [PubMed] [Google Scholar]
- 32.Ware J Jr, Kosinski M, Keller SD. A 12-item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33. [DOI] [PubMed] [Google Scholar]
- 33.Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J Man Manip Ther 2009;17:163–79. 10.1179/jmt.2009.17.3.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butler SF, Budman SH, Fanciullo GJ et al. Cross validation of the current opioid misuse measure to monitor chronic pain patients on opioid therapy. Clin J Pain 2010;26:770–6. 10.1097/AJP.0b013e3181f195ba [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreira ML, Herbert RD, Ferreira PH et al. The smallest worthwhile effect of nonsteroidal anti-inflammatory drugs and physiotherapy for chronic low back pain: a benefit-harm trade-off study. J Clin Epidemiol 2013;66:1397–404. 10.1016/j.jclinepi.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 36.Costa LdC, Maher CG, Hancock MJ et al. The prognosis of acute and persistent low-back pain: a meta-analysis. CMAJ 2012;184:E613–24. 10.1503/cmaj.111271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hush JM, Kamper SJ, Stanton TR et al. Standardized measurement of recovery from nonspecific back pain. Arch Phys Med Rehabil 2012;93:849–55. 10.1016/j.apmr.2011.11.035 [DOI] [PubMed] [Google Scholar]
- 38.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22. 10.1093/biomet/73.1.13 [DOI] [Google Scholar]
- 39.Australian Government. Australian code for responsible conduct of research. Canberra: (ACT: ): Australian Government, 2007. [Google Scholar]
- 40.Hush JM, Lin C-WC, Michaleff ZA et al. Prognosis of acute idiopathic neck pain is poor. A systematic review and meta-analysis. Arch Phys Med Rehabil 2011;92:824–9. 10.1016/j.apmr.2010.12.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-011278supp_appendix.pdf (244.4KB, pdf)