Abstract
Objectives
This study assessed the effect of diet habits on lower urinary tract symptoms (LUTS) and sexual function in Chinese men with LUTS/benign prostatic hypertrophy (LUTS/BPH).
Setting
Multicentre study conducted between July 2013 and December 2013 in 11 hospitals in 3 geographic regions in China.
Participants
Overall, participants with LUTS/BPH accounted for 61.4% (2584/4208) of the respondents, whose data were processed in the following statistical analysis.
Primary and secondary outcome measures
LUTS and sexual function were assessed based on the International Prostate Symptom Score (IPSS) and the International Index of Erectile Function 5 (IIEF-5) score. Prostate volume (PV) was determined by ultrasound.
Results
A total of 4208 participants met the inclusion criteria. The average age of the whole participants was 65.8±7.7 years. Overall, participants with LUTS/BPH accounted for 61.4% (2584/4208) of the respondents, whose data were processed in the following statistical analysis. Generally, prostate enlargement was greatest in south China. LUTS and male sexual dysfunction (MSD) were most severe in northwest China. Based on multivariable analysis, PV enlarged as the age (p<0.001), body mass index (BMI; p<0.001) and vegetable intake (p<0.001) increased. Age (p<0.001) and BMI (p<0.05) independently increased the IPSS. A higher level of education (p<0.001) and more frequent meat, fish and egg intake (p<0.05) decreased the IPSS. Age (p<0.001), BMI (p<0.001), low education level (p<0.05), vegetable intake (p=0.001), and milk and dairy product intake (p=0.001) decreased the IIEF-5 score.
Conclusions
In addition to factors including age, obesity and level of education, dietary habits and geographic difference might also play an important role in the variation of PV, LUTS and MSD for Chinese men with LUTS/BPH.
Keywords: diet habit, lower urinary tract symptoms, prostate volume, sexual function, China
Strengths and limitations of this study.
The first strength of the study is its relatively large sample size and multiregional design.
The second strength of the study is attributed to the detection of geographic difference in diet habits and clinical outcomes (the prostate volume, International Prostate Symptom Score (IPSS) and International Index of Erectile Function 5 (IIEF-5) score), which might support the planning and implementation of public health policies.
The interesting associations of prostate volume and IPSS under the influence of geography and diet contribute to another strength of the study.
However, the limitations of the study include the regional sample size diversity, selection bias of participants and broad classification of diet.
Introduction
Benign prostatic hyperplasia (BPH) is very common among ageing men and causes lower urinary tract symptoms (LUTS), which lead to a diminished health-related quality of life.1 Although BPH was rare in some Asian countries in the early years of the 20th century, it has become a common disease in recent decades, the prevalence of which is comparable to the West.2 Accumulating evidence suggests that other than ageing, modifiable factors, such as increasing prostate volume (PV), obesity, diet, dyslipidaemia, hormonal imbalance, hypertension, metabolic syndrome, alcohol and smoking, also contribute to the development of BPH, LUTS or both.1 3–5
The prevalence of erectile dysfunction (ED) in elderly men has been reported to be 23.2% in Australia,6 19.2% in Germany7 and 12% in China.2 An increasing number of studies in recent years have suggested that patients with BPH or LUTS may be more prone to ED, and that LUTS/BPH symptoms often coexist with ED.8 9 Additionally, age, household income, lifestyle, smoking and alcohol consumption, obesity, and physical inactivity have also been reported to be associated with the occurrence and severity of ED.10–12 Recently, epidemiological data suggest the effects of nutritional factors modulating metabolism substantially prior to the development of BPH, LUTS and ED.13–15
In the current study, we used data from a recent outpatient-based screening survey to assess the epidemiological characteristics of prostate health and male sexual function in different regions in China.
Patients and methods
The data were collected between July 2013 and December 2013 in three geographic regions in China (Beijing and Tianjin (north China), Guangzhou (south China) and Xi'an (northwest China)). First, leaflets, printed with health education concerning prostate disease and information about a clinical check-up on the prostate, were handed out in the communities in the above three regions. Then our sample consisted of 5484 native males who attended the clinic for a prostate check-up at medical centres (The First, Second, and Third Affiliated Hospitals of Peking University, Peking Union Medical College Hospital, Beijing Chao-Yang Hospital, Beijing Friendship Hospital, Beijing Hospital in Beijing, The Second Affiliated Hospital of Tianjin Medical University in Tianjin, The Second and Third Affiliated Hospitals of Zhongshan University in Guangzhou, and The First Affiliated Hospitals of Xi’an Jiaotong University in Xi'an). Temporary residents or refugees were not involved in this study. Participants who were <50 years of age were excluded. The exclusion criteria included urinary deformities, urinary injuries, urinary neoplasms, urethral strictures, neurobladders, urinary tract infections, acute prostatitis, treatment for BPH or sexual dysfunction, and treatments with known urinary tract or sexual function side effects. The study was approved by the Institutional Review Board at all participating centres. All participants provided written informed consent.
The research was carried out by trained interviewers at the respective centres. The interviewers were clinical physicians who were trained uniformly to be certified in conducting the survey. The main tasks for the interviewers were to deliver the survey, instruct participants on how to fill out the questionnaire, and collect the participants' information from completed questionnaires and clinical tests.
Peking University Clinical Research Institute was responsible for creating, upgrading, maintaining, backing up and recovering the database according to standard operating procedures, and for checking the data. The questionnaires were entered in the database twice by different individuals, and both versions were compared to ensure the accuracy of the data.
The structured questionnaire elicited information regarding age, level of education (≤9 years or >9 years), height, weight, tobacco and alcohol use (never/ever or current user), weekly common food intake frequency, medical history, recent drug history, LUTS conditions according to the International Prostate Symptom Score (IPSS) questionnaire, and sexual function conditions according to the International Index of Erectile Function-5 (IIEF-5). Body mass index (BMI) was calculated as the weight in kg/height in m2. Dietary intake over the past 1 year period was assessed and sorted into three categories (vegetables (green vegetables, carrot and tomato); meat (pork, beef and chicken), fish and eggs; and milk and dairy products). The monthly consumption of each dietary category was obtained from weekly consumption multiplied by 4, which was then distributed into tertiles based on the distribution of the entire study population and defined as low, moderate and high.16 Additionally, the following clinical examinations were performed: serum prostate-specific antigen (PSA) level; routine urinalysis; prostatic fluid analysis; digital rectal examination (DRE) and transrectal ultrasound (TRUS). The type of TRUS used varied among the participating institutions, but all measurements were made using a 7.5 MHz rectal probe. PV was calculated by substituting the formula for an ellipsoid, as follows: π/6×(height)×(length)×(width), with the height, length and width of the prostate measured by TRUS.
A prostate biopsy was obtained from all participants with a PSA level >4 ng/mL or findings suspicious of prostate cancer on DRE. Those participants who were diagnosed with prostate cancer were excluded from the study.
LUTS/BPH was defined as ≥50 years of age and an IPSS ≥8; LUTS/BPH data were used for the final analysis. SPSS V.16.0 was used for statistical analysis. The means and SDs were used to present normally distributed continuous variables; the median (q1 and q3) was used to present non-normally distributed variables. The Kruskal-Wallis test or one-way analysis of variance (ANOVA) was used to compare the dietary habits, PV, IPSS and IIEF-5 score of participants with LUTS/BPH from the three geographic regions. In the univariate part: the t-test or one-way ANOVA was used for PV and IPSS and the Wilcoxon test or Kruskal-Wallis test was used for the IIEF-5 score in categorical variables (education, smoking, alcohol consumption and region). Univariate linear regression was used for all the three clinical outcomes in continuous variables (age, BMI and diet). Multiple linear regression was performed as a multivariate analysis of the PV, IPSS and IIEF-5 score. The IIEF-5 score was log-changed to conform to a normal distribution. A dummy variable change was performed for the variable of the region to satisfy the multiple linear logistic model. Finally, univariate and multiple linear regression (adjusted for age, level of education, BMI, smoking and alcohol consumption, and diet) were performed to detect the associations of the PV, IPSS and IIEF-5 score in the entire cohort. A p<0.05 was considered statistically significant.
Results
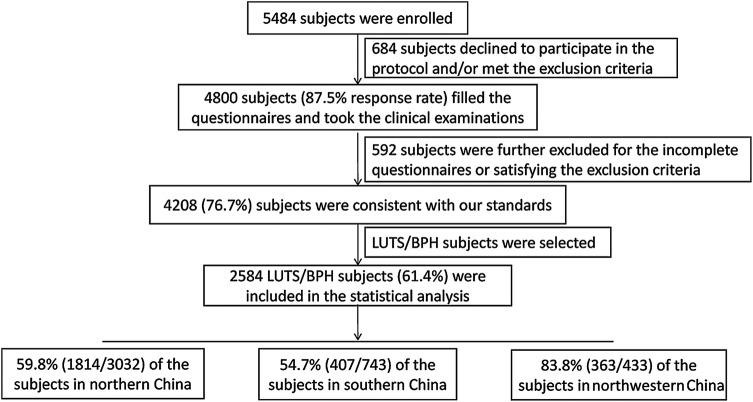
The process of the study design and participant exclusion is presented in figure 1. Of the 5484 enrolled participants, 4800 filled the questionnaire and underwent all of the clinical tests; 684 declined to participate in the protocol and/or met exclusion criteria. Thus, the response rate was 87.5%. Five hundred and ninety-two participants were further excluded for the incomplete questionnaires or satisfying the exclusion criteria. The data from 4208 (76.7%) participants were consistent with our standards. The average age of the 4208 participants was 65.8±7.7 years. The overall prevalence of LUTS/BPH among the study participants was 61.4% (2584/4208); 59.8% (1814/3032), 54.7% (407/743) and 83.8% (363/433) of the participants resided in northern China, southern China and northwestern China, respectively (table 1). Therefore, the data from 2584 participants were included in the statistical analysis.
Figure 1.
Flow diagram for explaining our study design and participants excluded from analysis. LUTS/BPH was defined as an age ≥50 years and an IPSS≥8. IPSS, International Prostate Symptom Score; LUTS/BPH, lower urinary tract symptoms/ benign prostatic hypertrophy.
Table 1.
Demographic characteristics of the 4208 participants in the three regions
| Characteristics | Northern China (n=3032) | Southern China (n=743) | Northwestern China (n=433) | Total (n=4208) |
|---|---|---|---|---|
| LUTS/BPH, n (%) | 1814 (59.8) | 407 (54.7) | 363 (83.8) | 2584 (61.4) |
| Age (years) (means±SDs) | 66.1±8.0 | 65.5±8.3 | 65.1±7.7 | 65.8±7.7 |
| BMI (kg/m2) (means±SDs) | 24.6±4.5 | 23.6±3.2 | 24.1±2.5 | 24.3±4.1 |
| Education (years), n (%) | ||||
| ≤9 | 690 (22.8) | 193 (26.0) | 129 (29.8) | 1012 (24.0) |
| >9 | 2342 (77.2) | 550 (74.0) | 304 (70.2) | 3196 (76.0) |
| Smoking, n (%) | ||||
| No | 1835 (60.7) | 399 (53.7) | 257 (59.4) | 2491 (59.2) |
| Yes | 1197 (39.3) | 344 (46.3) | 176 (40.6) | 2717 (40.8) |
| Alcohol consumption, n (%) | ||||
| No | 1910 (63.0) | 459 (61.8) | 279 (64.4) | 2648 (62.9) |
| Yes | 1122 (37.0) | 284 (38.2) | 154 (35.6) | 1560 (37.1) |
BMI, body mass index; BPH, benign prostatic hyperplasia; LUTS, lower urinary tract symptoms.
The dietary and clinical characteristics (the PV, IPSS and IIEF-5 score as a function of different age groups) among the participants with LUTS/BPH residing in the three geographic regions are listed in table 2. Participants in southern China consumed the highest amounts of meat, fish and eggs, while participants in northern China and northwestern China consumed the highest amounts of vegetables and milk and dairy products, respectively. The PV, IPSS and IIEF-5 score followed a deteriorating trend with ageing. PV was significantly different among the participants residing in the three geographic regions for all age groups (p<0.01). It was largest among men residing in southern China and smallest among men residing in northwestern China. Comparisons between any two of the three regions were statistically significant (p<0.001) for all men and men 60–70 years of age. In contrast, IPSS showed an inverse distribution in the three geographic regions. It was highest in northwestern China and lowest in southern China. Comparisons between any two of the three geographic regions were nearly statistically significant with the exception of participants >80 years of age. Participants residing in northern and southern China had higher IIEF-5 scores than those residing in northwestern China (p<0.001 and p<0.01, respectively).
Table 2.
Comparison of dietary (stratified by tertile) and clinical characteristics (stratified by age decades) among LUTS/BPH participants from the three geographic regions
| Characteristics | Northern China (n=1814) | Southern China (n=407) | Northwestern China (n=363) | p Value |
|---|---|---|---|---|
| Dietary (monthly) | ||||
| Meat, fish and eggs, n (%) | ||||
| Low (<16 times/month) | 347 (19.1) | 7 (1.7) | 82 (22.6) | |
| Moderate (16–28 times/month) | 503 (27.7) | 86 (21.1) | 242 (66.7) | |
| High (>28 times/month) | 964 (53.1) | 314 (77.1) | 39 (10.7) | <0.001 |
| Vegetables | ||||
| Low (<24 times/month) | 606 (33.4) | 134 (32.9) | 121 (33.3) | |
| Moderate (24–32 times/month) | 542 (29.9) | 180 (44.2) | 240 (66.1) | |
| High (>32 times/month) | 666 (36.7) | 93 (22.9) | 2 (0.6) | <0.001 |
| Milk and dairy products | ||||
| Low (<4 times/month) | 594 (32.7) | 188 (46.2) | 67 (18.5) | |
| Moderate (4–12 times/month) | 561 (30.9) | 134 (32.9) | 161 (44.4) | |
| High (>12 times/month) | 659 (36.3) | 85 (20.9) | 135 (37.2) | <0.001 |
| Clinical (means±SDs) | ||||
| PV | ||||
| 50–60 years (n=387) | 27.3±13.4 | 30.0±13.7 | 23.1±8.2 | 0.007 |
| 60–70 years (n=977) | 32.6±16.9 | 38.8±19.7 | 27.6±14.5 | <0.001 |
| 70–80 years (n=931) | 35.4±21.4 | 42.2±21.5 | 34.5±27.5 | 0.004 |
| ≥80 years (n=289) | 35.8±20.7 | 56.9±23.4 | 37.3±19.1 | <0.001 |
| All (n=2584) | 33.1±19.0 | 39.6±20.8 | 28.8±19.2 | <0.001 |
| IPSS | ||||
| 50–60 years (n=387) | 14.89±5.91 | 12.68±4.90 | 14.92±44.55 | 0.002 |
| 60–70 years (n=977) | 15.96±6.10 | 13.86±5.43 | 17.90±5.57 | <0.001 |
| 70–80 years (n=931) | 16.80±6.59 | 15.95±6.12 | 21.67±5.46 | <0.001 |
| ≥80 years (n=289) | 18.26±6.39 | 19.32±6.36 | 22.55±8.05 | 0.066 |
| All (n=2584) | 16.44±6.37 | 14.84±5.95 | 18.65±5.96 | <0.001 |
| IIEF-5 | ||||
| 50–60 years (n=387) | 15 (8, 20) | 19 (15, 21) | 13 (11, 15) | <0.001 |
| 60–70 years (n=977) | 12 (5, 17) | 13 (9, 17) | 9 (7, 11) | <0.001 |
| 70–80 years (n=931) | 5 (0, 13) | 3 (0, 8) | 1 (0, 5) | <0.001 |
| ≥80 years (n=289) | 2 (0, 12) | 1 (0, 5) | 0 (0, 1) | 0.003 |
| All (n=2584) | 9 (1, 15) | 10 (3, 16) | 8 (5, 12) | <0.001 |
Means and SDs were used to present normally distributed continuous variables, and medians (q1, q3) were used to present non-normally distributed variables (IIEF-5 score).
A p<0.05 by the Kruskal-Wallis test or one-way ANOVA was considered significant.
ANOVA, analysis of variance; BPH, benign prostatic hyperplasia; IIEF-5, International Index of Erectile Function 5; IPSS, International Prostate Symptom Score; LUTS, lower urinary tract symptoms; PV, prostate volume.
Tables 3 and 4 present the univariable and multivariable analyses for the PV, IPSS and IIEF-5 score. Considering the strong differences in diet across regions, the two factors were separated in two multivariate analysis models. First, based on the multivariable linear analysis, PV enlarged as the age, BMI and vegetable intake increased (β=0.44, p<0.001; β=0.42, p<0.001; β=0.80, p<0.001). The association between PV and meat, fish and eggs intake failed to reach a statistical significance (β=0.05, p=0.711). Second, age (β=0.13, p<0.001), BMI (β=0.08, p<0.05) and cigarette smoking (β=0.89, p=0.001) independently increased IPSS. A higher level of education (β=−1.50, p<0.001) and more frequent meat, fish and eggs intake (β=−0.11, p<0.05) decreased IPSS. Third, age (β=−0.03, p<0.001), BMI (β=−0.02, p<0.001), low education level (β=0.10, p=0.025), vegetable intake (β=−0.04, p<0.001), and milk and dairy product intake (β=−0.08, p=0.001) decreased the IIEF-5 score. Meanwhile, regional differences were independently correlated with the PV, IPSS and IIEF-5 score, with the exception of participants in northern and southern China, who had similar IIEF-5 scores.
Table 3.
Univariate analysis for the PV, IPSS and IIEF-5 score
| PV |
IPSS |
IIEF-5 |
||||
|---|---|---|---|---|---|---|
| Variables | Means±SDs | p Value | Means±SDs | p Value | Medians (q1, q3) | p Value |
| Education (years) | ||||||
| ≤9 | 35.3±20.6 | 17.84±6.50 | 8 (1, 14) | |||
| >9 | 32.9±19.1 | 0.007 | 16.05±6.21 | <0.001 | 10 (2, 15) | 0.001 |
| Smoking | ||||||
| No | 33.2±19.4 | 16.14±6.02 | 8 (1, 14) | |||
| Yes | 34.1±19.7 | 0.250 | 17.04±6.49 | <0.001 | 10 (2, 15) | 0.070 |
| Alcohol consumption | ||||||
| No | 33.4±19.3 | 16.32±6.21 | 9 (1, 15) | |||
| Yes | 33.8±19.9 | 0.600 | 16.80±6.54 | 0.066 | 10 (2, 16) | 0.002 |
| Region | ||||||
| Northern China | 33.1±19.0 | 16.44±6.37 | 9 (1, 15) | |||
| Southern China | 39.6±20.8 | 14.84±5.95 | 10 (3, 16) | |||
| Northwestern China | 28.8±19.2 | <0.001 | 18.65±5.96 | <0.001 | 8 (5, 12) | <0.001 |
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Age (years) | 0.44 (0.35 to 0.52) | <0.001 | 0.14 (0.11 to 0.16) | <0.001 | −0.35 (−0.38 to −0.32) | <0.001 |
| BMI (kg/m2) | 0.48 (0.24 to 0.71) | <0.001 | 0.10 (0.03 to 0.18) | 0.008 | −0.20 (−0.28 to −0.12) | <0.001 |
| Meat, fish and eggs (times/week) | −0.01 (−0.25 to 0.26) | 0.961 | −0.13 (−0.21 to −0.05) | 0.002 | 0.01 (−0.01 to 0.02) | 0.269 |
| Vegetables (times/week) | 0.82 (0.47 to 1.16) | <0.001 | 0.09 (−0.02 to 0.21) | 0.098 | −0.04 (−0.06 to −0.02) | <0.001 |
| Milk and dairy products (times/week) | −0.02 (−0.10 to 0.14) | 0.743 | 0.05 (0.01 to 0.09) | 0.010 | −0.01 (−0.20 to −0.01) | <0.001 |
Means and SDs were used to present normally distributed continuous variables, and medians (q1, q3) were used to present non-normally distributed variables (IIEF-5 score).
Categorical variables (education, smoking, alcohol consumption and region): t-test or one-way ANOVA was used for PV and IPSS; Wilcoxon test or Kruskal-Wallis test was used for the IIEF-5 score.
Continuous variables (age, BMI and diet): univariate linear regressions was used for PV, IPSS and the IIEF-5 score.
β: Unstandardised coefficients.
ANOVA, analysis of variance; BMI, body mass index; IIEF-5, International Index of Erectile Function 5; IPSS, International Prostate Symptom Score; PV, prostate volume.
Table 4.
Multivariate analysis for PV, IPSS and the IIEF-5 score
| PV |
IPSS |
IIEF-5 score |
||||
|---|---|---|---|---|---|---|
| Variables | β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value |
| Model 1 (included age, BMI, education, smoking, alcohol consumption and diet (weekly)) | ||||||
| Age (years) | 0.44 (0.35 to 0.53) | <0.001 | 0.13 (0.10 to 0.16) | <0.001 | −0.03 (−0.04 to −0.02) | <0.001 |
| BMI (kg/m2) | 0.42 (0.19 to 0.65) | <0.001 | 0.08 (0.01 to 0.16) | 0.030 | −0.02 (−0.04 to −0.01) | <0.001 |
| Education (≤9/>9 years) | −1.60 (−3.40 to 0.20) | 0.081 | −1.50 (−2.08 to −0.91) | <0.001 | 0.10 (0.01 to 0.18) | 0.025 |
| Smoking (no/yes) | 1.14 (−0.50 to 2.78) | 0.173 | 0.89 (0.36 to 1.42) | 0.001 | −0.01 (−0.08 to 0.08) | 0.979 |
| Alcohol consumption (no/yes) | 0.72 (−0.96 to 2.39) | 0.400 | 0.32 (−0.22 to 0.86) | 0.246 | 0.01 (−0.07 to 0.09) | 0.794 |
| Meat, fish and eggs (times/week) | 0.05 (−0.21 to 0.31) | 0.711 | −0.11 (−0.18 to −0.01) | 0.031 | 0.01 (−0.01 to 0.02) | 0.095 |
| Vegetables (times/week) | 0.80 (0.46 to 1.14) | <0.001 | 0.09 (−0.02 to 0.20) | 0.127 | −0.04 (−0.05 to −0.02) | <0.001 |
| Milk and dairy products (times/week) | −0.07 (−0.19 to 0.05) | 0.272 | 0.06 (0.02 to 0.10) | 0.005 | −0.08 (−0.13 to −0.04) | 0.001 |
| Model 2 (adjusted for age, BMI, education, smoking, alcohol consumption) | ||||||
| Northern China | ref | – | ref | – | ref | – |
| Southern China | 7.52 (5.48 to 9.57) | <0.001 | −1.42 (−2.08 to −0.76) | <0.001 | −0.05 (−0.14 to 0.05) | 0.369 |
| Northwestern China | −2.94 (−5.10 to −0.78) | 0.008 | 2.49 (1.79 to 3.18) | <0.001 | −0.21 (−0.31 to −0.10) | <0.001 |
Log change was performed for non-normally distributed data (IIEF-5); multiple linear regression was performed.
β: Unstandardised coefficients.
BMI, body mass index; IIEF-5, International Index of Erectile Function 5; IPSS, International Prostate Symptom Score; PV, prostate volume.
Additionally, univariable and multivariable linear analyses of the PV, IPSS and IIEF-5 scores were performed in the 2584 participants with LUTS/BPH (see online supplementary table S1). A larger PV (β=−0.03, p<0.001) and a higher IPSS (β=−0.02, p=0.001) decreased the IIEF-5 score, and a larger PV resulted in a higher IPSS (β=0.04, p<0.001).
Univariable and multiple linear analysis among prostate volume, IPSS-score and IIEF-5 score.
bmjopen-2015-010863supp_table.pdf (73.9KB, pdf)
Discussion
This study was the first with a relatively large sample size to investigate the effect of diet habits on LUTS and sexual function in Chinese men with LUTS/BPH from three different geographic regions. Our survey indicated that increased age, obesity and a low level of education are independently associated with an enlarged PV, more severe LUTS and worse male sexual dysfunction (MSD). Moreover, we also found that there were geographic variations with respect to PV, LUTS and MSD, which might partially be attributed to different dietary habits in different geographic regions.
Generally, the term clinical BPH is synonymous with LUTS in the absence of urethral strictures or prostate enlargement as detected on DRE.17 The LUTS metric most often used in epidemiological studies is the self-reported IPSS, which assesses self-reported severity of urinary symptoms and defines a moderate or severe condition as an IPSS≥8.17 Also, the adverse effects of BPH are relatively apparent in men >50 years of age.18 19 According to the results of this study, among Chinese adults with LUTS/BPH, PV and LUTS deteriorated as age increased. Indeed, Shi et al18 and Chokkalingam et al17 selected age ≥50 years as an inclusion criterion for studying BPH. Therefore, we selected LUTS/BPH cases among participants who were ≥50 years of age and had an IPSS≥8, and excluded non-BPH-associated LUTS cases.
The reported prevalence of LUTS/BPH varies widely in different studies due to the use of different measuring instruments, the heterogeneity of different study populations and population sampling methods. Chokkalingam et al's17 population-based study on West Africans reported the prevalence of DRE-detected prostate enlargement to be 62.3%. In total, 22.9% of 106 435 men >45 years of age had moderate-to-severe LUTS in Smith et al's20 population-based study. Also, a prevalence of 38.2% for moderate-to-severe LUTS was reported among residents in China >65 years of age by Wong et al.2 In 2009, Xu et al21 reported a prevalence of 66.9% for BPH among those >50 years of age in Tianjin, China. Of note, the aforementioned studies all involved the general population and the age ranges of the participants were different. In this study, the general prevalence of LUTS/BPH was as high as 61.4% (2584/4208). The discrepancy between our results and others might be attributed to our selection of participants seeking a regular prostate check-up from the outpatient clinic. These participants might be more aware of LUTS or have LUTS due to BPH.
BMI is regarded as a health indicator, which would be influenced by dietary habits to some extent. Evidence supporting the influence of BMI on PV and LUTS was mixed. According to Schuurman et al,22 an increase in BMI is associated with higher levels of oestrogen and oestradiol, and lower levels of plasma testosterone, which could deteriorate BPH or LUTS; however, according to Gupta et al13 and Joseph et al,23 no relationship between BMI and BPH or LUTS existed. Bhindi et al24 reported that obesity is associated with increased PV, but not with worse LUTS. In this study, BMI was a risk factor for PV (β=0.42, p<0.001) and LUTS (β=0.08, p<0.05), with the exception of fluctuating IPSS in men with a BMI<18.5 kg/m2. The results of this study were consistent with those of Smith et al20 and Hammersten and Hogstedt;14 specifically, an increase in BMI is a risk factor for BPH or LUTS. In addition, we showed that MSD had a deteriorating trend as the BMI increased from 18.5 kg/m2 and at a low level (<18.5 kg/m2), which was consistent with the results of Teles et al10 and Ghalayini et al,11 who reported that the prevalence of ED increases in men who are overweight.
Interestingly, Zhao et al25 also reported that elderly men of southern China (Guangdong) consumed relatively more meat food and less green vegetables than some northern cities (eg, Shandong), which might be attributed to the different dwellers' taste, cuisine areas and economy level in China. One of the clinically important findings of this study was the effect of diet habits on PV and LUTS, under the condition that a larger PV resulted in a higher IPSS (β=0.04, p<0.001) in the 2584 participants with LUTS/BPH (see online supplementary table S1). In other words, the association between the frequency of meat, fish and egg consumption and PV was not statistically significant (p=0.71); the frequency of meat, fish and egg consumption was inversely correlated to IPSS (β=−0.11, p<0.05). First, the reported influence of diet on PV is inconsistent. According to Zhang et al,26 the frequency of animal protein intake is positively correlated with PV. However, according to Shirazi et al,19 no relationship was detected between dietary patterns and PV. Second, the relationship between diet and BPH is mixed. Bravi et al27 and Suzuki et al28 reported that men with high protein and low vegetable intake are at greater risk for developing BPH; however, self-reported measuring instruments were used to judge the status of BPH, and no information involving PV was available. In contrast, the results of Bhindi et al24 indicated that obesity, which is caused by a high-energy diet, is associated with a larger PV, but not worse LUTS. Moreover, Maserejian et al29 claimed that the inverse association between protein intake and LUTS is supported by evidence that high-protein diets suppress sympathetic nervous system activity. The hormonal pathway, in which dietary protein can lower the plasma testosterone concentration or affect the oestradiol level, might also be a conceivable mechanism. Third, with respect to vegetables, we only showed that the frequency of vegetable intake was positively associated with PV (β=0.80, p<0.001). Although most evidence supports the notion that increased vegetable intake can reduce the risk of BPH, no association existed between the frequency of vegetable intake and the risk for BPH according to Lagiou et al.16 Also, we suspect that the difference in the proportion of vegetables consumption between Chinese and Western populations might have influenced the effect of vegetables on LUTS/BPH. The result that the frequency of milk and dairy products was positively associated with IPSS (β=0.06, p<0.05) was consistent with the previous studies.16 Therefore, uniform standards and more large-scale studies are needed to clarify the relationship between dietary habits and LUTS/BPH.
Additionally, Oksuz and Malhan30 reported that men without diet-based lifestyle changes are at higher risk for MSD, and Khoo et al15 found that a reduced-fat diet in obese Chinese men enhances male sexual function. In this study, however, vegetables and milk and dairy products were risk factors for MSD. These findings indicate that a balanced diet might benefit male sexual function. Of note, age (β=−0.03, p<0.001), PV (β=−0.03, p<0.001) and LUTS (β=−0.02, p=0.001) were all independent risk factors for MSD, which is consistent with previous studies.6–8 12 Thus, participants in our research were at high risk for MSD, which might explain the results of our study. More attention by clinicians should be paid to these groups of men to alleviate MSD.
Another clinically meaningful finding of our research was the dissociative trend of PV and LUTS in terms of geographic variation. In 1997, Yu et al31 conducted the survey on prevalence of prostatic hyperplasia in China, mainly relying on PV from DRE. According to the results of Yu et al's32 study, the population in south China had more severe prostate enlargement than that in northwest China, which is consistent with the results of our study. Although PV was positively associated with LUTS for the 2584 participants with LUTS/BPH (see online supplementary table S1), men with LUTS/BPH in south and northwest China had the mildest and most severe LUTS, respectively. First, regional differences in demographic characteristics like diet habits, education level, economic development, natural environment, etc, might contribute to the geographic variation of PV and LUTS to some extent.2 3 Second, morphological differences of prostate due to genetics and races might also act on the geographic distribution of PV and LUTS.32 For instance, compared with the Caucasian population, Korean men have an incidence of LUTS similar to that in men in the Western hemisphere, despite having a smaller average PV.33 34 Third, BPH, especially in the early stage, might be more closely connected with voiding symptoms of LUTS.17 34 Meanwhile, MSD had a similar geographical distribution as LUTS. Therefore, the assignment of public health policies for the authorities, as well as future research on LUTS/BPH and MSD for the epidemiologists, should be further performed.
Limitations affecting our current findings must be considered. First, sample size diversity existed in the three geographic regions. Meanwhile, our participants were native males ≥50 years of age who attended the clinic for a prostate check-up, who represented a more highly educated, higher health literate and higher socioeconomic group than the general population. Therefore, more community-based studies are imperative to reduce the selection bias. Second, the food frequency in our study, which classified the daily foods into three main categories, was designed to study the broad influence of the categories on PV, LUTS and MSD; we did not evaluate the associations between one single food and PV, LUTS and MSD.
Conclusion
The results of this study suggest that, in addition to factors including age, obesity and level of education, dietary habits and geographic differences might also play an important role in the variation of PV, LUTS and MSD for Chinese men with LUTS/BPH. Superficially, PV and LUTS might present a dissociative trend under the influence of diet habits and geographic variation.
Acknowledgments
The authors thank Bang Zheng from School of Public Health, Peking University Health Science Center for his assistance with statistics analysis.
Footnotes
Contributors: YC and WY are co-first authors who contributed equally to drafting and revising this manuscript. LZ and SW are the corresponding authors who contributed to designing the whole manuscript. YY, JW, YT, DH, YX, JH, XW, XG, HL, LM, NZ, SZ and XJ contributed equally to collecting and sorting the data.
Funding: This work was supported by Grants from the “The Research Special Fund for Public Welfare Industry of Health” (grant number: 201002010).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The study was approved by the Institutional Review Board of The First, Second, and Third Affiliated Hospitals of Peking University, Peking Union Medical College Hospital, Beijing Chao-Yang Hospital, Beijing Friendship Hospital, Beijing Hospital, The Second Affiliated Hospital of Tianjin Medical University, The Second and Third Affiliated Hospitals of Zhongshan University, and The First Affiliated Hospitals of Xi'an Jiaotong University.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Tewari R, Rajender S, Natu SM et al. Diet, obesity, and prostate health: are we missing the link? J Androl 2012;33:763–76. 10.2164/jandrol.111.015578 [DOI] [PubMed] [Google Scholar]
- 2.Wong SY, Woo J, Hong A et al. Risk factors for lower urinary tract symptoms in southern Chinese men. Urology 2006;68:1009–14. 10.1016/j.urology.2006.05.039 [DOI] [PubMed] [Google Scholar]
- 3.Eckhardt MD, van Venrooij GE, Boon TA. Symptoms, prostate volume, and urodynamic findings in elderly male volunteers without and with LUTS and in patients with LUTS suggestive of benign prostatic hyperplasia. Urology 2001;58:966–71. 10.1016/S0090-4295(01)01413-3 [DOI] [PubMed] [Google Scholar]
- 4.Yang HJ, Doo SW, Yang WJ et al. Which obesity index best correlates with prostate volume, prostate-specific antigen, and lower urinary tract symptoms? Urology 2012;80:187–90. 10.1016/j.urology.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 5.Zucchetto A, Tavani A, Dal Maso L et al. History of weight and obesity through life and risk of benign prostatic hyperplasia. Int J Obes (Lond) 2005;29:798–803. 10.1038/sj.ijo.0802979 [DOI] [PubMed] [Google Scholar]
- 6.Martin SA, Atlantis E, Lange K et al. Predictors of sexual dysfunction incidence and remission in men. J Sex Med 2014;11:1136–47. 10.1111/jsm.12483 [DOI] [PubMed] [Google Scholar]
- 7.Braun M, Wassmer G, Klotz T et al. Epidemiology of erectile dysfunction: results of the “Cologne Male Survey”. Int J Impot Res 2000;12:305–11. [DOI] [PubMed] [Google Scholar]
- 8.Berrada S, Kadri N, Mechakra-Tahiri S et al. Prevalence of erectile dysfunction and its correlates: a population-based study in Morocco. Int J Impot Res 2003;15:3–7. 10.1038/sj.ijir.3900968 [DOI] [PubMed] [Google Scholar]
- 9.Wang GC, Zheng JH, Yang B et al. Impacts of histological prostatitis on sexual function and lower urinary tract symptoms in patients with benign prostatic hyperplasia. Urology 2013;82:1094–7. 10.1016/j.urology.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 10.Teles AG, Carreira M, Alarcão V et al. Prevalence, severity, and risk factors for erectile dysfunction in a representative sample of 3,548 Portuguese men aged 40 to 69 years attending primary healthcare centers: results of the Portuguese erectile dysfunction study. J Sex Med 2008;5:1317–24. 10.1111/j.1743-6109.2007.00745.x [DOI] [PubMed] [Google Scholar]
- 11.Ghalayini IF, Al-Ghazo MA, Al-Azab R et al. Erectile dysfunction in a Mediterranean country: results of an epidemiological survey of a representative sample of men. Int J Impot Res 2010;22:196–203. 10.1038/ijir.2009.65 [DOI] [PubMed] [Google Scholar]
- 12.Fwu CW, Kirkali Z, McVary KT et al. Cross-sectional and longitudinal associations of sexual function with lower urinary tract symptoms in men with benign prostatic hyperplasia. J Urol 2015;193:231–8. 10.1016/j.juro.2014.08.086 [DOI] [PubMed] [Google Scholar]
- 13.Gupta A, Gupta S, Pavuk M et al. Anthropometric and metabolic factors and risk of benign prostatic hyperplasia: a prospective cohort study of Air Force veterans. Urology 2006;68:1198–205. 10.1016/j.urology.2006.09.034 [DOI] [PubMed] [Google Scholar]
- 14.Hammersten J, Hogstedt B. Hyperinsulinaemia as a risk factor for developing benign prostatic hyperplasia. Eur Urol 2001;39:151–8. doi:52430 [DOI] [PubMed] [Google Scholar]
- 15.Khoo J, Ling PS, Tan J et al. Comparing the effects of meal replacements with reduced-fat diet on weight, sexual and endothelial function, testosterone and quality of life in obese Asian men. Int J Impot Res 2014;26:61–6. 10.1038/ijir.2013.36 [DOI] [PubMed] [Google Scholar]
- 16.Lagiou P, Wuu J, Trichopoulou A et al. Diet and benign prostatic hyperplasia: a study in Greece. Urology 1999;54:284–90. 10.1016/S0090-4295(99)00096-5 [DOI] [PubMed] [Google Scholar]
- 17.Chokkalingam AP, Yeboah ED, Demarzo A et al. Prevalence of BPH and lower urinary tract symptoms in West Africans. Prostate Cancer Prostatic Dis 2012;15:170–6. 10.1038/pcan.2011.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi J, Sun Z, Cai T et al. Development and validation of a quality-of-life scale for Chinese patients with benign prostatic hyperplasia. BJU Int 2004;94:837–44. 10.1111/j.1464-410X.2004.05043.x [DOI] [PubMed] [Google Scholar]
- 19.Shirazi M, Ariafar A, Zeyghami S et al. Association of diet with prostate specific antigen and prostate volume. Nephrourol Mon 2014;6:e19411 10.5812/numonthly.19411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith DP, Weber MF, Soga K et al. Relationship between lifestyle and health factors and severe lower urinary tract symptoms (LUTS) in 106,435 middle-aged and older Australian men: population-based study. PLoS ONE 2014;9:e109278 10.1371/journal.pone.0109278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Y, Zhang ZH, Cheng R et al. Prevalence of benign prostate hyperplasia and its relative factors in rural areas of Tianjin in 2008. Chin J Urol 2009;30:761–4. [Google Scholar]
- 22.Schuurman AG, Goldbohm RA, Dorant E et al. Anthropometry in relation to prostate cancer risk in the Netherlands Cohort Study. Am J Epidemiol 2000;151:541–9. 10.1093/oxfordjournals.aje.a010241 [DOI] [PubMed] [Google Scholar]
- 23.Joseph MA, Harlow SD, Wei JT et al. Risk factors for lower urinary tract symptoms in a population-based sample of African-American men. Am J Epidemiol 2003;157:906–14. 10.1093/aje/kwg051 [DOI] [PubMed] [Google Scholar]
- 24.Bhindi B, Margel D, Trottier G et al. Obesity is associated with larger prostate volume but not with worse urinary symptoms: analysis of a large multiethnic cohort. Urology 2014;83:81–7. 10.1016/j.urology.2013.07.039 [DOI] [PubMed] [Google Scholar]
- 25.Zhao W, You Y, Zhang X et al. Study on the food consumption pattern of elderly men in four “cuisine” areas of China. Wei Sheng Yan Jiu 2002;31:34–7. [PubMed] [Google Scholar]
- 26.Zhang SX, Yu B, Guo SL et al. Comparison of incidence of BPH and related factors between urban and rural inhabitants in district of Wannan. Zhonghua Nan Ke Xue 2003;9:45–7. [PubMed] [Google Scholar]
- 27.Bravi F, Bosetti C, Dal Maso L et al. Food groups and risk of benign prostatic hyperplasia. Urology 2006;67:73–9. 10.1016/j.urology.2005.07.030 [DOI] [PubMed] [Google Scholar]
- 28.Suzuki S, Platz EA, Kawachi I et al. Intakes of energy and macronutrients and the risk of benign prostatic hyperplasia. Am J Clin Nutr 2002;75:689–97. [DOI] [PubMed] [Google Scholar]
- 29.Maserejian NN, Giovannucci EL, McKinlay JB. Dietary macronutrients, cholesterol, and sodium and lower urinary tract symptoms in men. Eur Urol 2009;55:1179–89. 10.1016/j.eururo.2008.07.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oksuz E, Malhan S. The prevalence of male sexual dysfunction and potential risk factors in Turkish men: a web-based survey. Int J Impot Res 2005;17:539–45. 10.1038/sj.ijir.3901357 [DOI] [PubMed] [Google Scholar]
- 31.Yu P, Zheng H, Su H et al. Prevalence of prostatic hyperplasia and its relative factors in six cities of China in 1997. Zhonghua Liu Xing Bing Xue Za Zhi 2000;21:276–9. [PubMed] [Google Scholar]
- 32.Yu EY, Siegal JA, Meyer GE et al. Histologic differences in benign prostate hyperplasia between Chinese and American men. Prostate 1997;31:175–9. [DOI] [PubMed] [Google Scholar]
- 33.Lee E, Yoo KY, Kim Y et al. Prevalence of lower urinary tract symptoms in Korean men in a community-based study. Eur Urol 1998;33:17–21. 10.1159/000019529 [DOI] [PubMed] [Google Scholar]
- 34.Lee SH, Chung BH, Kim CS et al. Survey on benign prostatic hyperplasia distribution and treatment patterns for men with lower urinary tract symptoms visiting urologists at general hospitals in Korea: a prospective, noncontrolled, observational cohort study. Urology 2012;79:1379–84. 10.1016/j.urology.2012.02.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Univariable and multiple linear analysis among prostate volume, IPSS-score and IIEF-5 score.
bmjopen-2015-010863supp_table.pdf (73.9KB, pdf)