Abstract
We prospectively compared visual and refractive outcomes in patients with high myopia and myopic astigmatism after small-incision lenticule extraction (SMILE) and photorefractive keratetctomy (PRK) with mitomycin C. Sixty-six eyes of 33 patients (mean age, 29.7 ± 5.6 years) were included (SMILE: 34 eyes, PRK 32 eyes). Preoperatively, no significant difference was noted in manifest spherical equivalent (p = 0.326), manifest sphere (p = 0.277), and manifest cylinder (p = 0.625) between both groups. At 1 month, there were significant differences in logMAR uncorrected distance visual acuity, efficacy index and manifest refraction spherical equivalent between SMILE and PRK (p ≤ 0.029). At 6 months, the logMAR corrected distance visual acuity (p = 0.594), logMAR uncorrected visual acuity (p = 0.452), efficacy index (p = 0.215) and safety index was (p = 0.537) was comparable between SMILE and PRK. Significant differences were observed in postoperative manifest spherical equivalent (p = 0.044) and manifest cylinder (p = 0.014) between both groups. At the end of 6 months, 100% of the eyes in SMILE group and 69% of the eyes in PRK group were within ±0.50 D of the attempted cylindrical correction. The postoperative difference vector, magnitude of error and absolute angle of error were significantly smaller after SMILE compared to PRK (p ≤ 0.040) implying a trend towards overcorrection of cylindrical correction following PRK.
The traditional management options for correction of high myopia include laser in situ keratomileusis (LASIK) and photorefractive keratetctomy (PRK). The correction of high myopia is challenging owing to the associated risk of treatment regression and corneal ectasia. Although LASIK provides quick visual rehabilitation, PRK has been shown to offer early stabilization of cornea while allowing for correction of higher myopic correction compared to LASIK1. Moreover, corneal biomechanics are affected less by flapless techniques than it is with LASIK2. Small-incision lenticule extraction (SMILE) is a flapless, all femtosecond refractive surgery technique in which a corneal stromal lenticule is fashioned using femtosecond laser and removed through a small peripheral corneal incision3,4. Previous studies have demonstrated that SMILE is safe and effective for management of myopia and myopic astigmatism5,6,7,8. A retrospective study comparing SMILE and laser-assisted subepithelial keratectomy showed that changes in corneal hysteresis and corneal resistance factor induced by SMILE are more predictable than those induced by laser-assisted subepithelial keratectomy9. However, there is a lack of comparative data on visual and refractive outcomes between SMILE and PRK. The current aims to compare the visual and refractive outcomes between SMILE and PRK with mitomycin C for high myopic eyes with low to moderate (<3 D) astigmatism.
Results
Sixty-six eyes of 33 patients with a mean age of 29.7 ± 5.6 years were included (SMILE: 34 eyes, PRK 32 eyes). Preoperatively, there was no significant difference in manifest spherical equivalent (p = 0.326), manifest sphere (p = 0.277), and manifest cylinder (p = 0.625) between both groups (Table 1). Corneas were thinner in eyes receiving PRK (525.1 ± 22.7 μm) compared to SMILE (545.1 ± 20.6 μm) (p = 0.012). All surgeries were uneventful without any intraoperative complications. No postoperative complications, such as wound dehiscence, inflammation and infection were observed in any patient. Mild subepithelial corneal haze (grade 0.5) was seen in all eyes after PRK, but resolved by three months postoperatively.
Table 1. Comparison of visual and refractive outcomes between small-incision lenticular extraction and photorefractive keratectomy.
| SMILE | PRK | P-value | ||
|---|---|---|---|---|
| CDVA (logMAR) | Pre-Op | 0.001 ± 0.008 | −0.018 ± 0.029 | 0.008 |
| Month 1 | 0.027 ± 0.041 | 0.039 ± 0.091 | 0.581 | |
| Month 3 | 0.018 ± 0.029 | 0.011 ± 0.050 | 0.600 | |
| Month 6 | 0.021 ± 0.055 | 0.013 ± 0.049 | 0.594 | |
| UDVA (logMAR) | Month 1 | 0.051 ± 0.069 | 0.133 ± 0.129 | 0.009 |
| Month 3 | 0.061 ± 0.081 | 0.070 ± 0.085 | 0.732 | |
| Month 6 | 0.044 ± 0.058 | 0.060 ± 0.093 | 0.452 | |
| Efficacy Index | Month 1 | 0.903 ± 0.131 | 0.735 ± 0.200 | 0.002 |
| Month 3 | 0.886 ± 0.146 | 0.833 ± 0.171 | 0.313 | |
| Month 6 | 0.915 ± 0.115 | 0.858 ± 0.193 | 0.215 | |
| Safety Index | Month 1 | 0.947 ± 0.087 | 0.892 ± 0.164 | 0.188 |
| Month 3 | 0.965 ± 0.066 | 0.942 ± 0.122 | 0.463 | |
| Month 6 | 0.962 ± 0.099 | 0.940 ± 0.125 | 0.537 | |
| Spherical | Pre-Op | −7.37 ± 0.72 | −7.65 ± 0.99 | 0.326 |
| Equivalent (D) | Month 1 | 0.00 ± 0.28 | −0.43 ± 0.35 | 0.000 |
| Month 3 | −0.05 ± 0.29 | −0.27 ± 0.27 | 0.011 | |
| Month 6 | −0.12 ± 0.26 | −0.28 ± 0.28 | 0.044 | |
| Sphere (D) | Pre-Op | −6.77 ± 0.82 | −7.10 ± 1.01 | 0.277 |
| Month 1 | 0.07 ± 0.32 | −0.09 ± 0.19 | 0.029 | |
| Month 3 | 0.04 ± 0.29 | −0.03 ± 0.12 | 0.228 | |
| Month 6 | −0.01 ± 0.25 | −0.04 ± 0.18 | 0.680 | |
| Cylinder (D) | Pre-Op | −1.20 ± 0.58 | −1.10 ± 0.66 | 0.625 |
| Month 1 | −0.16 ± 0.23 | −0.70 ± 0.59 | 0.001 | |
| Month 3 | −0.19 ± 0.23 | −0.47 ± 0.53 | 0.037 | |
| Month 6 | −0.21 ± 0.21 | −0.48 ± 0.45 | 0.014 |
SMILE = small-incision lenticule extraction, PRK = photorefractive keratectomy, CDVA = corrected distance visual acuity, Pre-Op = preoperative, UDVA = uncorrected distance visual acuity.
At 1 month, there were significant differences in UDVA, efficacy index and manifest refraction spherical equivalent between SMILE and PRK (p ≤ 0.029) (Table 1). The logMAR CDVA and safety index remained comparable between groups (p ≥ 0.188).
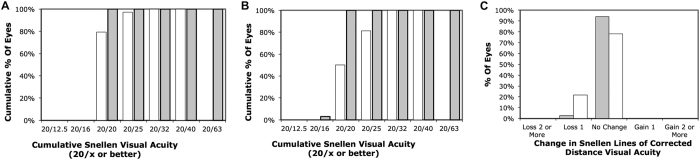
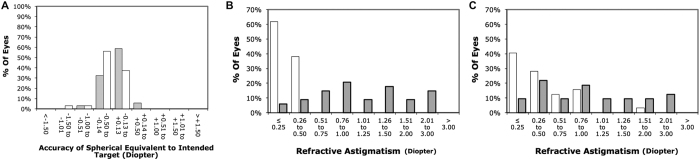
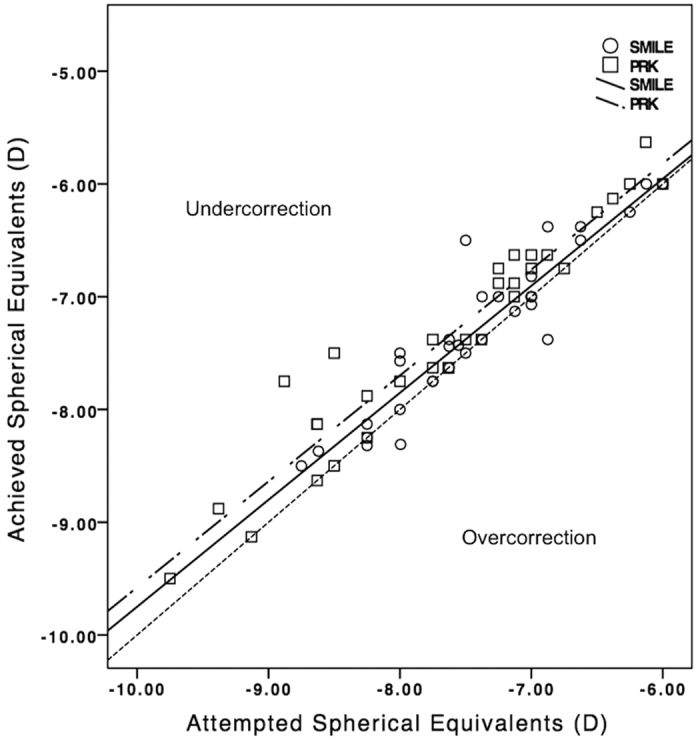
At 6 months, the logMAR CDVA was 0.021 ± 0.055 and 0.013 ± 0.049 following SMILE and PRK, respectively (p = 0.594). The corresponding logMAR UDVA was 0.044 ± 0.058 and 0.060 ± 0.093 (p = 0.452). The efficacy index was 0.92 ± 0.12 after SMILE and 0.86 ± 0.19 after PRK (p = 0.215). The corresponding safety index was 0.96 ± 0.10 and 0.94 ± 0.13 (p = 0.537) (Table 1). The distribution of UDVA and CDVA following SMILE and PRK was presented in Fig. 1. Significant differences were observed in postoperative manifest spherical equivalent (p = 0.044) and manifest cylinder (p = 0.014) between SMILE and PRK (Table 1). At the end of 6 months, 97% (33 eyes) and 94% were within ±0.50 D of the attempted spherical equivalent correction after SMILE and PRK respectively (Fig. 2). As for manifest cylinder, 100% (34 eyes) and 69% (22 eyes) were within ±0.50 D of the attempted cylindrical correction after SMILE and PRK, respectively (Fig. 2). Scatterplots of achieved versus attempted spherical equivalent are shown in Fig. 3.
Figure 1.
Visual outcomes by 6 months: Cumulative percentage of eyes attaining specified cumulative levels of postoperative uncorrected distance visual acuity (UDVA) for small-incision lenticule extraction (A) and photorefractive keratectomy (B) (grey = preoperative, white = postoperative), and change in preoperative and postoperative CDVA (grey = SMILE, white = PRK).
Figure 2.
Refractive outcomes by 6 months: Percentages of eyes within different diopter ranges of the intended correction in spherical equivalent (A) (grey = SMILE, white = PRK), and percentage of eyes attaining specified levels of astigmatism before and after small-incision lenticule extraction (B) and photorefractive keratectomy (C) (grey = preoperative, white = postoperative).
Figure 3. Attempted versus achieved manifest spherical equivalent correction for small-incision lenticule extraction and photorefractive keratectomy by 6 months.
Vector analysis
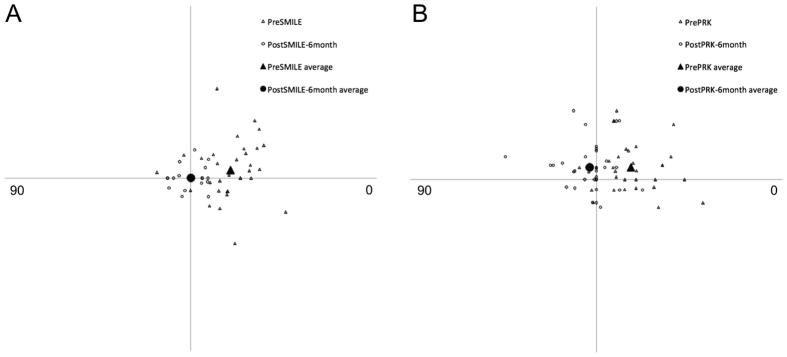
The vector analysis results using the 6-month refractive data are shown in Table 2. There was no significant difference in the arithmetic mean of TIA and SIA between SMILE and PRK (p > 0.235). Scatterplots of SIA versus TIA for both groups are shown in Fig. 4. The postoperative DV, ME and absolute AE were significantly smaller after SMILE compared to PRK (p ≤ 0.040). The spread of absolute AE was higher for increasing TIA in both groups (Fig. 4). The distributions of absolute AE are presented in Fig. 5. Double-angle plots for both procedures are shown in Fig. 6.
Table 2. Vector analysis of astigmatic correction at 6 months after small-incision lenticule extraction and photorefractive keratectomy using Alpins method.
| SMILE | PRK | P-value | ||
|---|---|---|---|---|
| TIA | Arithmetic mean ± SD (D) | 1.05 ± 0.46 | 0.92 ± 0.54 | 0.235 |
| Rangea (D) | 1.02 to 1.07 | 0.89 to 0.96 | ||
| SIA | Arithmetic mean ± SD (D) | 1.08 ± 0.43 | 1.26 ± 0.78 | 0.704 |
| Rangea (D) | 1.05 to 1.10 | 1.22 to 1.31 | ||
| DV | Arithmetic mean ± SD (D) | 0.20 ± 0.20 | 0.48 ± 0.44 | 0.005 |
| Rangea (D) | 0.19 to 0.22 | 0.45 to 0.51 | ||
| ME | Arithmetic mean ± SD (D) | 0.03 ± 0.22 | 0.34 ± 0.47 | 0.001 |
| Rangea (D) | 0.02 to 0.04 | 0.31 to 0.37 | ||
| Absolute AE | Arithmetic mean ± SD (°) | 5.0 ± 9.4 | 9.8 ± 13.6 | 0.040 |
| Rangea (°) | 4.5 to 5.6 | 9.0 to 10.7 | ||
| CI | Geometric mean ± SD | 1.02 ± 0.28 | 1.34 ± 0.86 | / |
| Rangea | 1.00 to 1.04 | 1.29 to 1.40 | ||
| FI | Geometric mean ± SD | 0.78 ± 0.88 | 0.90 ± 1.22 | / |
| Rangea | 0.73 to 0.83 | 0.83 to 0.97 | ||
| IOS | Geometric mean ± SD | 0.13 ± 0.31 | 0.48 ± 0.96 | / |
| Rangea | 0.12 to 0.15 | 0.42 to 0.53 |
SMILE = small-incision lenticule extraction; PRK = photorefractive keratectomy; AE = angle of error; CI = correction index; DV = difference vector; FI = flattening index; IOS = index of success; SIA = surgically induced astigmatism; TIA = target-induced astigmatism.
a95% confidence interval.
Figure 4. Target induced astigmatism vector versus surgically induced astigmatism vector for small-incision lenticule extraction and photorefractive keratectomy by 6 months.
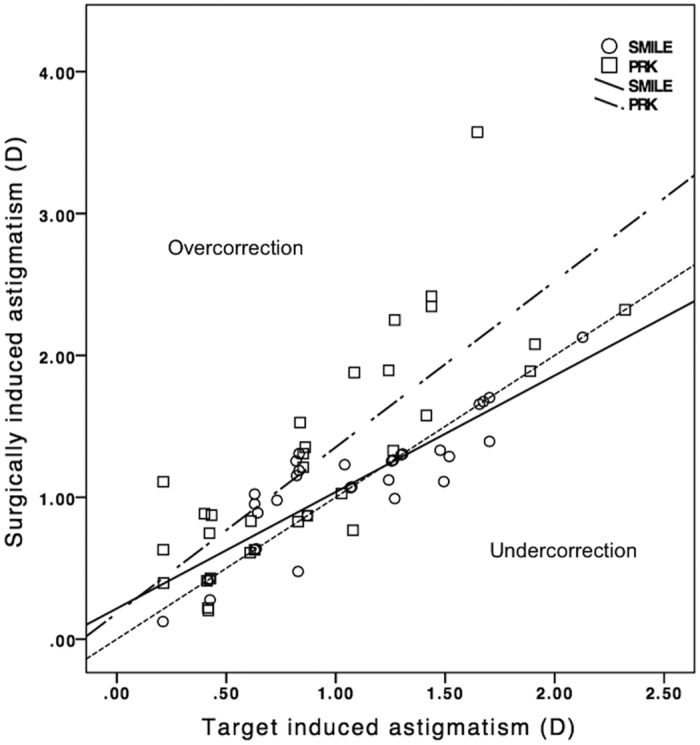
Figure 5. Distribution of absolute angle of error for small-incision lenticule extraction and photorefractive keratectomy by 6 months.
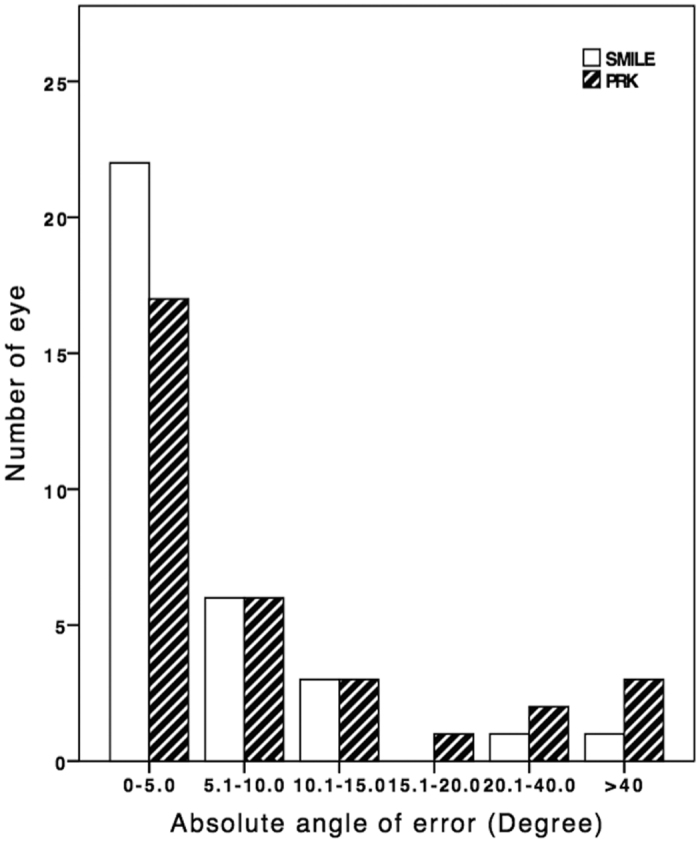
Figure 6.
Double-angle plots for preoperative target induced astigmatism (open triangle) and postoperative difference vector at postoperative 6 months (open circle) after small-incision lenticule extraction (A) and photorefractive keratectomy (B).
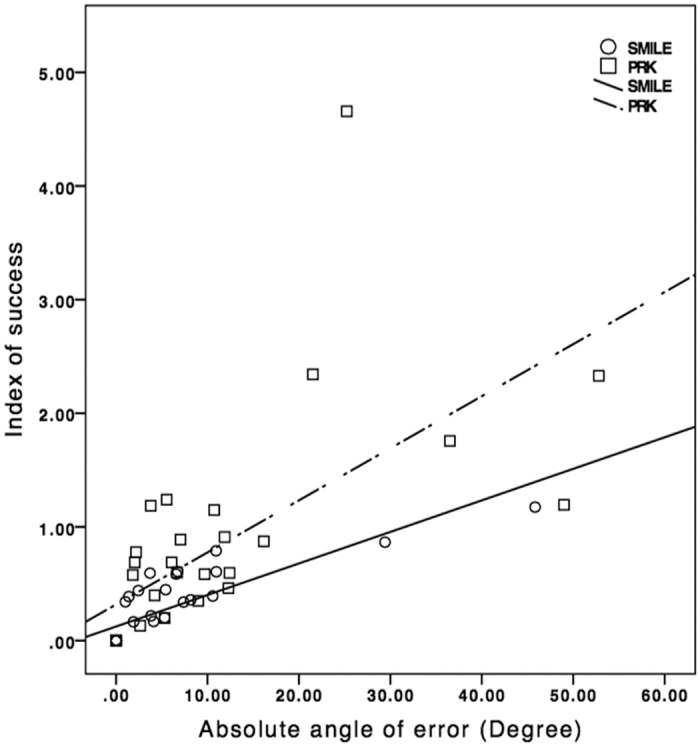
At 6 months, the correction index (CI), defined as the ratio of SIA to TIA, was 1.02 (95% CI: 1.00 to 1.04) in SMILE and 1.34 (95% CI: 1.29 to 1.40) in PRK. Figure 7 shows the scatterplots of CI plotted against TIA in both groups. Index of success (IS), defined as DV divided by TIA, was 0.13 (95% CI: 0.12 to 0.15) and 0.48 (95% CI: 0.42 to 0.53) in SMILE and PRK, respectively. Significant correlation was found between absolute AE and IS for both procedures (r = 0.906 for SMILE, r = 0.827 for PRK; p < 0.001) (Fig. 8). Flattening index (FI), defined as ratio of the amount of astigmatism reduction achieved by the effective proportion of the SIA at the intended meridian to TIA, was 0.78 (95% CI: 0.72 to 0.83) in SMILE and 0.90 (95% CI: 0.83 to 0.97) in PRK (Table 2).
Figure 7. Correction index versus surgically induced astigmatism vector for small-incision lenticule extraction and photorefractive keratectomy by 6 months.
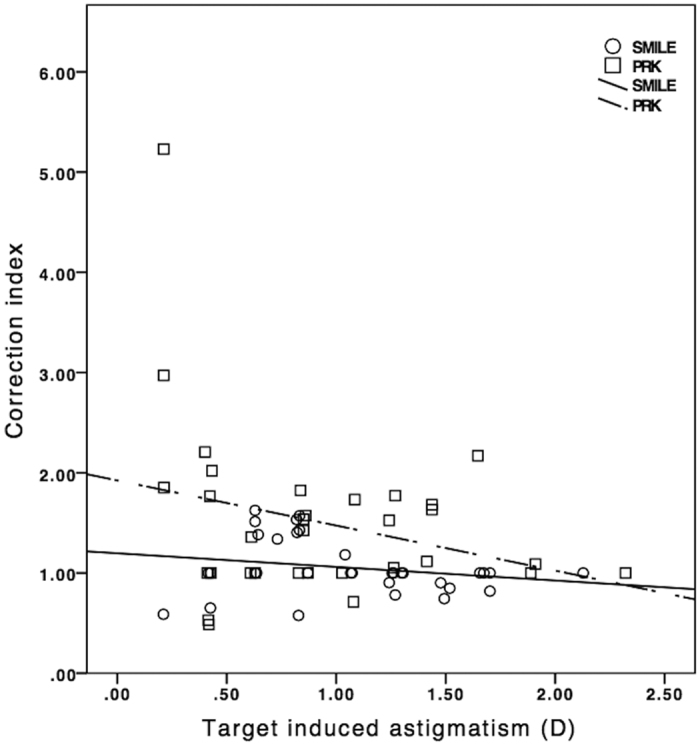
Figure 8. Index of success versus absolute angle of error for small-incision lenticule extraction and photorefractive keratectomy by 6 months.
Discussion
The current study showed that SMILE was more effective in low to moderate astigmatic correction compared to PRK at 6 months. Although both procedures were found to be similar in spherical correction and safety, visual rehabilitation was quicker after SMILE compared to PRK. This could represent a differential healing response between both procedures10. Previous studies investigating astigmatic correction following PRK reported that refractive predictability was worse in PRK for astigmatic treatment compared to spherical correction alone11,12. PRK induced unintentional astigmatism, which increased with the amount of myopic treatment11. The effectiveness was reduced for correction of low astigmatism13,14. In our study, we observed a trend towards overcorrection of cylindrical value despite a slight undercorrection of spherical equivalent following PRK. According to vector analysis, PRK had a CI of 1.34 signifying overcorrection, which appeared to increase with lower TIA. Likewise, Katz et al. reported a CI of 1.25 for PRK in eyes with myopic astigmatism15.
We found that SMILE was more effective than PRK for correction of low (1.0 to 1.5 D) myopic astigmatism. Slight overcorrection was observed in eyes with low astigmatism, while slight undercorrection was observed in eyes with high astigmatism. Ivarsen and Hjortdal reported that undercorrection of astigmatism increased with attempted treatment following SMILE16. Undercorrection of astigmatic error has been reported in previous studies comparing SMILE to other corneal refractive surgeries8,17,18,19. The CI was very close to 1.0 for SMILE in our cohort, representing accurate astigmatic correction. It appeared to remain stable with increasing TIA for low to moderate astigmatic correction. Similar to PRK, we observed a slight undercorrection of spherical equivalent after SMILE. Our results match the findings of a recent large study demonstrating predictable treatment outcomes of up to 3.0 D of astigmatism with SMILE20. In another retrospective study comparing SMILE and laser assisted subepithelial keratectomy (LASEK), overcorrection and undercorrection of astigmatism was noted following SMILE and LASEK, respectively17. At 6 months postoperatively, the reported CI (1.05) for SMILE was similar to our results.
Overall, SMILE demonstrated better astigmatic correction compared to PRK. The residual spherical equivalent and astigmatism postoperatively were lower after SMILE. The observed difference in astigmatic correction between both procedures was likely influenced by the difference in absolute AE, which indicated alignment of treatment. High correlation between absolute AE and IS has been reported as well as demonstrated in the current study21. The absolute AE was significantly lower for SMILE compared to PRK in our cohort. Vector analysis demonstrates that the proportion of flattening effect is halved when treatment is misaligned by 30° 22. This emphasizes the importance of treatment alignment for successful correction of astigmatism, in addition to the treatment magnitude. Correction of low astigmatism is particularly a concern because of a higher tendency towards larger axis deviations upon vector analysis8,19,23. The spread of absolute AE was much wider for correction of low astigmatism for both procedures. Another possible reason for the difference between SMILE and PRK is better centration in SMILE that is maintained via continuous suction during the whole procedure. Comparative analysis between SMILE and LASIK reported less decentration in SMILE24. This may affect the overall alignment of astigmatic treatment.
It is possible that differential healing response between SMILE and PRK could be potential reasons for treatment misalignment. PRK utilizes ultraviolet light from excimer laser to reshape the corneal curvature through direct photoablation. SMILE uses femtosecond laser in near infrared spectrum to achieve the cutting effect within the cornea through photodisruption. The amount of energy delivered to the cornea was much lower with femtosecond laser compared to excimer laser25. The main advantage of femtosecond laser compared to excimer laser is its high cutting precision with minimal collateral damage and associated cellular and structural changes10. The response of corneal epithelium may also be different between SMILE and PRK, which required epithelial removal of the treatment zone. This is important because epithelial hyperplasia has been observed following treatment regression after PRK26,27. Also, stromal tissue deposition correlated with postoperative regression in PRK patients26. Healing of corneal epithelium and stromal remodeling could add uncertainty to refractive outcomes and effectiveness of astigmatism correction. Comparative analysis of epithelial and stromal remodeling between the 2 procedures is warranted to support our hypothesis. Adjunctive mitomycin C has been demonstrated to prevent post-PRK corneal haze, especially for high myopic correction28. Mitomycin C could delay healing and induce apoptosis of anterior stromal cells, modifying the healing response29,30. However, high variance in postoperative spherical equivalent was found after mitomycin C application during PRK compared to PRK alone for low to moderate ablation31. We were unsure if mitomycin C affected predictability of astigmatic correction in PRK in our cohort.
Our study results did not include the impact of SMILE and PRK on corneal biomechanics. The SMILE procedure has potential benefit over flap-based surgeries in terms of biomechanical stability. Mathematical models have predicted that the tensile strength after SMILE is better compared to PRK and LASIK2. Dou et al. showed that although both SMILE and Laser-assisted subepithelial keratomileusis alter corneal biomechanical strength, the changes induced by SMILE are more predictable than those induced by LASEK in terms of per unit tissue removed9. Further research is warranted to evaluate the effect of these changes on visual and refractive results after SMILE and PRK.
The current study was limited by a small sample size and non-randomized design. We did not have access to keratometry, epithelial thickness maps and contrast sensitivity values. We also acknowledge the relatively short follow-up duration, which precludes analysis of the long-term stability of our results. However, significant group difference in astigmatism correction was detected between SMILE and PRK in vector analysis during the early postoperative period. In conclusion, both SMILE and PRK were observed to be safe for correction of low to moderate astigmatism in eyes with high myopia. Predictability of refraction was superior in SMILE, which showed a better treatment alignment of astigmatism than PRK in vector analysis. There was a trend towards overcorrection of cylindrical correction following surface ablation.
Methods
This study was a prospective audit of consecutive patients who received SMILE and PRK with mitomycin C at the Hong Kong Laser Eye Center and Chinese University of Hong Kong Eye Centre respectively. Patients were offered either PRK or SMILE in the respective surgery centres. All surgeries were performed between June 2014 and May 2015. The Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee approved the study protocol. An informed consent was obtained from all study participants. The study adhered to the tenets of the Declaration of Helsinki.
Patients with no ocular abnormality except myopia and myopic astigmatism, a corrected distance visual acuity (CDVA) of 20/20 or better, stable refraction for more than 1 year, manifest myopic refraction spherical equivalent of ≥6.0 D and astigmatism between −0.25 D and −2.50 D were included. All eyes had emmetropia as the target refraction. Patients with corneal thickness of <500 μm, suspicion of keratoconus on corneal topography (displacement of the corneal apex, decrease in thinnest-point pachymetry, asymmetric topographic pattern), ocular inflammation, infection were excluded.
Small incision lenticule extraction
SMILE was performed using the 500-kHz VisuMax femtosecond laser (Carl Zeiss Meditec, Jena, Germany) using a standard surgical technique (Sekundo BJO 2011). The following parameters were used: cap thickness, 120 μm; cap diameter, 7.5 mm; lenticule diameter, 6.5 mm with a transition zone of 0.1 mm; cut energy, 1.4 μJ; spot and tracking distance, 2.0–3.0 μm. The back of the intrastromal lenticule was created from periphery to center of cornea. The anterior lamellar cut was subsequently created from center to periphery of cornea, which extended towards the surface to create a 2–3 mm incision, from which the stromal lenticule was extracted. A thin blunt spatula was used to separate the lenticule, which was then grasped with a pair of forceps and removed. The corneal interface was flushed with balanced salt solution.
Photorefractive keratectomy with mitomycin C
Alcohol-assisted corneal epithelial removal was performed over a 9.0-mm optical zone centered over the pupil. Ethanol 20% in distilled water was dropped into a 9.0-mm well and kept in contact with the epithelium for 40 seconds and then absorbed with a dry cellulose sponge. The eye was then washed with Balanced Salt Solution. A blunt spatula was then used to remove the loosened epithelium. Stromal ablation was performed with Allegretto Wave & Eye-Q 400Hz laser (WaveLight Laser Technologie AG, Germany) using a 6.5 mm optical zone. The system has an infrared high-speed camera operating to track the patient’s eye movements that either compensates for changes in eye position or interrupts the treatment if the eye moves outside a preset predetermined range. After the stromal ablation, a circular cellulose sponge soaked with mitomycin C 0.02% (0.2 mg/mL) was placed on the cornea for 60 seconds. The eye was then copiously irrigated with a balanced salt solution to remove the residual mitomycin C. A bandage contact lens was placed over the cornea at the end of the surgery.
Patients were examined preoperatively and 1, 3 and 6 months postoperatively. Severity of corneal haze after PRK was evaluated by slit-lamp biomicroscopy according to Hanna’s scale32. It was graded as follows: 0 = clear cornea, no haze; 0.5 = barely perceptible, seen only by tangential illumination; 1 = trace haze of minimal density seen with difficulty using direct illumination; 2 = moderate haze easily visible with direct slit illumination; 3 = marked haze that partially obscures anterior chamber observation or iris detail; 4 = severe haze that obscures anterior chamber or iris details33. Uncorrected visual acuity (UCVA), corrected distance visual acuity (CDVA) and manifest refraction were analyzed. Efficacy index was calculated as the ratio of postoperative UCVA to preoperative CDVA. Safety index was expressed as the ratio of postoperative CDVA to preoperative CDVA.
Vector analysis
Refractive astigmatism at the spectacle plane was converted to corneal plane using vertex distance of 12 mm. It was then analyzed using vector analysis, with consideration of change in the astigmatic axis, measuring 3 vectors and relationship among them (Alpins JCRS 2001). The target induced astigmatism vector (TIA) was defined as the astigmatic change which the surgery was intended to induce; the surgically induced astigmatism vector (SIA) was defined as the astigmatic change which the surgery actually induced; and the difference vector (DV) was defined as the induced astigmatic change that would enable the initial surgery to achieve its intended target or the postoperative astigmatism. Magnitude of error (ME) is the arithmetic difference between the SIA and TIA. Angle of error (AE) is the angle between the axis of the SIA and TIA.
Statistical Analysis
Statistical analysis was performed using R 2.15.3 (R Foundation, Vienna, Austria). Visual and refractive outcomes, efficacy and safety index were evaluated at different time points. Linear mixed effect models were used to adjust for correlation between fellow eyes. Comparisons of mean values were performed using repeated measures analysis of variance (ANOVA) at each time point. A p-value < 0.05 was considered as statistically significant. Bonferroni adjustments were considered for multiple testing.
Additional Information
How to cite this article: Chan, T. C. Y. et al. Early outcomes after small incision lenticule extraction and photorefractive keratectomy for correction of high myopia. Sci. Rep. 6, 32820; doi: 10.1038/srep32820 (2016).
Footnotes
Author Contributions concept and design (T.C. and V.J.); analysis and interpretation (T.C., M.Y., Z.W. and V.J.); writing the article (T.C., A.N. and V.J.); critical revision of the article (T.C., M.Y., A.N., Z.W., G.C. and V.J.); final approval of the article (T.C., M.Y., A.N., Z.W., G.C. and V.J.); data collection (G.C.); provision of materials, patients or resources (G.C. and V.J.); statistical expertise (T.C. and M.Y.); literature research (T.C., M.Y., A.N., Z.W., G.C. and V.J.); and administrative, technical or logistic support (Z.W., G.C. and V.J.).
References
- Chan T. C., Liu D., Yu M. & Jhanji V. Longitudinal evaluation of posterior corneal elevation after laser refractive surgery using swept-source optical coherence tomography. Ophthalmology 122, 687–692, doi: 10.1016/j.ophtha.2014.10.011 (2015). [DOI] [PubMed] [Google Scholar]
- Reinstein D. Z., Archer T. J. & Randleman J. B. Mathematical model to compare the relative tensile strength of the cornea after PRK, LASIK, and small incision lenticule extraction. J Refract Surg 29, 454–460, doi: 10.3928/1081597X-20130617-03 (2013). [DOI] [PubMed] [Google Scholar]
- Sekundo W., Kunert K. S. & Blum M. Small incision corneal refractive surgery using the small incision lenticule extraction (SMILE) procedure for the correction of myopia and myopic astigmatism: results of a 6 month prospective study. Br J Ophthalmol 95, 335–339, doi: 10.1136/bjo.2009.174284 (2011). [DOI] [PubMed] [Google Scholar]
- Ivarsen A., Asp S. & Hjortdal J. Safety and complications of more than 1500 small-incision lenticule extraction procedures. Ophthalmology 121, 822–828, doi: 10.1016/j.ophtha.2013.11.006 (2014). [DOI] [PubMed] [Google Scholar]
- Reinstein D. Z., Carp G. I., Archer T. J. & Gobbe M. Outcomes of small incision lenticule extraction (SMILE) in low myopia. J Refract Surg 30, 812–818, doi: 10.3928/1081597X-20141113-07 (2014). [DOI] [PubMed] [Google Scholar]
- Vestergaard A. H., Grauslund J., Ivarsen A. R. & Hjortdal J. O. Efficacy, safety, predictability, contrast sensitivity, and aberrations after femtosecond laser lenticule extraction. J Cataract Refract Surg 40, 403–411, doi: 10.1016/j.jcrs.2013.07.053 (2014). [DOI] [PubMed] [Google Scholar]
- Ganesh S. & Gupta R. Comparison of visual and refractive outcomes following femtosecond laser- assisted lasik with smile in patients with myopia or myopic astigmatism. J Refract Surg 30, 590–596 (2014). [DOI] [PubMed] [Google Scholar]
- Chan T. C. et al. Vector analysis of astigmatic correction after small-incision lenticule extraction and femtosecond-assisted LASIK for low to moderate myopic astigmatism. Br J Ophthalmol 100, 553–559, doi: 10.1136/bjophthalmol-2015-307238 (2016). [DOI] [PubMed] [Google Scholar]
- Dou R. et al. Comparison of Corneal Biomechanical Characteristics After Surface Ablation Refractive Surgery and Novel Lamellar Refractive Surgery. Cornea 34, 1441–1446, doi: 10.1097/ICO.0000000000000556 (2015). [DOI] [PubMed] [Google Scholar]
- Wei S. et al. Ultrastructural Changes and Corneal Wound Healing After SMILE and PRK Procedures. Curr Eye Res, 1–10, doi: 10.3109/02713683.2015.1114653 (2016). [DOI] [PubMed] [Google Scholar]
- Tabin G. C., Alpins N., Aldred G. F., McCarty C. A. & Taylor H. R. Astigmatic change 1 year after excimer laser treatment of myopia and myopic astigmatism. Melbourne Excimer Laser Group. J Cataract Refract Surg 22, 924–930 (1996). [DOI] [PubMed] [Google Scholar]
- Shen E. P., Yang C. N. & Hu F. R. Corneal astigmatic change after photorefractive keratectomy and photoastigmatic refractive keratectomy. J Cataract Refract Surg 28, 491–498 (2002). [DOI] [PubMed] [Google Scholar]
- Kremer I., Gabbay U. & Blumenthal M. One-year follow-up results of photorefractive keratectomy for low, moderate, and high primary astigmatism. Ophthalmology 103, 741–748 (1996). [DOI] [PubMed] [Google Scholar]
- Zadok D. et al. Excimer laser photoastigmatic refractive keratectomy: eighteen-month follow-up. Ophthalmology 105, 620–623, doi: 10.1016/S0161-6420(98)94014-3 (1998). [DOI] [PubMed] [Google Scholar]
- Katz T. et al. LASIK versus photorefractive keratectomy for high myopic (>3 diopter) astigmatism. J Refract Surg 29, 824–831, doi: 10.3928/1081597X-20131029-03 (2013). [DOI] [PubMed] [Google Scholar]
- Ivarsen A. & Hjortdal J. Correction of myopic astigmatism with small incision lenticule extraction. J Refract Surg 30, 240–247, doi: 10.3928/1081597X-20140320-02 (2014). [DOI] [PubMed] [Google Scholar]
- Qian Y., Huang J., Zhou X. & Wang Y. Comparison of femtosecond laser small-incision lenticule extraction and laser-assisted subepithelial keratectomy to correct myopic astigmatism. J Cataract Refract Surg 41, 2476–2486, doi: 10.1016/j.jcrs.2015.05.043 (2015). [DOI] [PubMed] [Google Scholar]
- Kobashi H. et al. Comparison of astigmatic correction after femtosecond lenticule extraction and small-incision lenticule extraction for myopic astigmatism. PLoS One 10, e0123408, doi: 10.1371/journal.pone.0123408 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. et al. Vector analysis of low to moderate astigmatism with small incision lenticule extraction (SMILE): results of a 1-year follow-up. BMC Ophthalmol 15, 8, doi: 10.1186/1471-2415-15-8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen R. S., Lyhne N., Grauslund J. & Vestergaard A. H. Small-incision lenticule extraction (SMILE): outcomes of 722 eyes treated for myopia and myopic astigmatism. Graefes Arch Clin Exp Ophthalmol 254, 399–405, doi: 10.1007/s00417-015-3226-5 (2016). [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang Y. & Chen X. Comparison of Moderate- to High-Astigmatism Corrections Using WaveFront-Guided Laser In Situ Keratomileusis and Small-Incision Lenticule Extraction. Cornea 35, 523–530, doi: 10.1097/ICO.0000000000000782 (2016). [DOI] [PubMed] [Google Scholar]
- Alpins N. A. Vector analysis of astigmatism changes by flattening, steepening, and torque. J Cataract Refract Surg 23, 1503–1514 (1997). [DOI] [PubMed] [Google Scholar]
- Frings A., Katz T., Richard G., Druchkiv V. & Linke S. J. Efficacy and predictability of laser in situ keratomileusis for low astigmatism of 0.75 diopter or less. J Cataract Refract Surg 39, 366–377, doi: 10.1016/j.jcrs.2012.09.024 (2013). [DOI] [PubMed] [Google Scholar]
- Lazaridis A., Droutsas K. & Sekundo W. Topographic analysis of the centration of the treatment zone after SMILE for myopia and comparison to FS-LASIK: subjective versus objective alignment. J Refract Surg 30, 680–686, doi: 10.3928/1081597X-20140903-04 (2014). [DOI] [PubMed] [Google Scholar]
- Riau A. K. et al. Early corneal wound healing and inflammatory responses after refractive lenticule extraction (ReLEx). Invest Ophthalmol Vis Sci 52, 6213–6221, doi: 10.1167/iovs.11-7439 (2011). [DOI] [PubMed] [Google Scholar]
- Ivarsen A., Fledelius W. & Hjortdal J. O. Three-year changes in epithelial and stromal thickness after PRK or LASIK for high myopia. Invest Ophthalmol Vis Sci 50, 2061–2066, doi: 10.1167/iovs.08-2853 (2009). [DOI] [PubMed] [Google Scholar]
- Reinstein D. Z., Ameline B., Puech M., Montefiore G. & Laroche L. VHF digital ultrasound three-dimensional scanning in the diagnosis of myopic regression after corneal refractive surgery. J Refract Surg 21, 480–484 (2005). [DOI] [PubMed] [Google Scholar]
- Majmudar P. A. et al. Mitomycin-C in corneal surface excimer laser ablation techniques: a report by the American Academy of Ophthalmology. Ophthalmology 122, 1085–1095, doi: 10.1016/j.ophtha.2015.01.019 (2015). [DOI] [PubMed] [Google Scholar]
- Kim T. I. et al. Apoptosis in keratocytes caused by mitomycin C. Invest Ophthalmol Vis Sci 44, 1912–1917 (2003). [DOI] [PubMed] [Google Scholar]
- Kremer I., Ehrenberg M. & Levinger S. Delayed epithelial healing following photorefractive keratectomy with mitomycin C treatment. Acta Ophthalmol 90, 271–276, doi: 10.1111/j.1755-3768.2010.01894.x (2012). [DOI] [PubMed] [Google Scholar]
- Sy M. E., Zhang L., Yeroushalmi A., Huang D. & Hamilton D. R. Effect of mitomycin-C on the variance in refractive outcomes after photorefractive keratectomy. J Cataract Refract Surg 40, 1980–1984, doi: 10.1016/j.jcrs.2014.02.048 (2014). [DOI] [PubMed] [Google Scholar]
- Hanna K. D. et al. Corneal wound healing in monkeys after repeated excimer laser photorefractive keratectomy. Arch Ophthalmol 110, 1286–1291 (1992). [DOI] [PubMed] [Google Scholar]
- Lohmann C. P. et al. Corneal haze after excimer laser refractive surgery: objective measurements and functional implications. Eur J Ophthalmol 1, 173–180 (1991). [DOI] [PubMed] [Google Scholar]