Abstract
Introduction
Sub-Saharan African (SSA) women with breast cancer (BC) have low survival rates from this potentially treatable disease. An understanding of context-specific societal, health-systems and woman-level barriers to BC early detection, diagnosis and treatment are needed.
Methods
The African Breast Cancer—Disparities in Outcomes (ABC-DO) is a prospective hospital-based study of overall survival, impact on quality of life (QOL) and delays along the journey to diagnosis and treatment of BC in SSA. ABC-DO is currently recruiting in Namibia, Nigeria, South Africa, Uganda and Zambia. Women aged 18 years or older who present at participating secondary and tertiary hospitals with a new clinical or histocytological diagnosis of primary BC are invited to participate. For consented women, tumour characteristics, specimen and treatment data are obtained. Over a 2-year enrolment period, we aim to recruit 2000 women who, in the first instance, will be followed for between 1 and 3 years. A face-to-face baseline interview obtains information on socioeconomic, cultural and demographic factors, QOL, health and BC attitudes/knowledge, and timing of all prediagnostic contacts with caregivers in orthodox health, traditional and spiritual systems. Responses are immediately captured on mobile devices that are fed into a tailored mobile health (mHealth) study management system. This system implements the study protocol, by prompting study researchers to phone women on her mobile phone every 3 months and, failing to reach her, prompts contact with her next-of-kin. At follow-up calls, women provide updated information on QOL, care received and disease impacts on family and working life; date of death is asked of her next-of-kin when relevant.
Ethics and dissemination
The study was approved by ethics committees of all involved institutions. All participants provide written informed consent. The findings from the study will be published in peer-reviewed scientific journals, presented to funders and relevant local organisations and at scientific conferences.
Keywords: EPIDEMIOLOGY, Africa, Survival
Strengths and limitations of this study.
This protocol describes an ongoing prospective cohort study across five countries in sub-Saharan Africa (emphasising a within-African cross-country perspective).
The study uses a mHealth tool for study management and standardisation of data collection in real-time.
The generic set-up of the study's mobile platform means that other funded sites could easily join African Breast Cancer—Disparities in Outcomes (ABC-DO).
The African settings pose many challenges—for example, minimising losses to follow-up, and obtaining valid information on disease progression and mortality.
This is a hospital-based study and, hence, will not capture the experience of women who solely seek care elsewhere (eg, traditional or spiritual healers).
Background
Similar to global patterns, breast cancer is the most common cancer in women in sub-Saharan Africa (SSA),1 with 94 000 new cases of breast cancer and 48 000 deaths due to breast cancer occurring in 2012. This burden is projected to double between 2012 and 2030 due to population ageing and expansion.2 While breast cancer is, on average, a good-prognosis cancer in high-income countries, in SSA its prognosis is considerably lower.3 Excessive deaths due to breast cancer are reflected in a high breast cancer mortality:incidence ratio of 0.50 in SSA in 2012, twice that of Western Europe (0.24).2
Research on survival and outcomes of women having breast cancer in SSA is scarce. Among the studies that have been conducted, the 5-year survival estimates of women having breast cancer reveal that the 5-year survival estimates are near or below 50% in SSA,4–8 contrasting with 73% and 85% among black and white women in the US, respectively.9 However, many of these SSA studies are now either outdated,4–7 were conducted retrospectively,5 10 11 or with no linkable vital status registers and active follow-up; their losses to follow-up were often large adding uncertainty to survival estimates. In addition, prognostic and predictive factors examined were limited to only a few clinical and therapeutic factors.5 8 10–12 Of these factors, improved survival was found for women diagnosed at stages I/II than for those diagnosed at stages III/IV,5 6 8 12 demonstrating the potential to save lives if down staging could be achieved. Nevertheless, the potential for survival gains may be hampered by the relatively young patient profile in SSA, reflecting the young age structure of the underlying population, as younger age is associated with poorer survival.13 Further, while a predominance of more aggressive breast cancer subtypes (eg, triple-negative tumours) in some settings would impede survival prospects,14 15 the totality of evidence across SSA from the largest studies and from a meta-analysis demonstrate a clear predominance of better prognosis oestrogen receptor-positive tumours.16 17
An updated prospective and more comprehensive study of the entire journey of women with breast cancer in SSA is needed, that is, the journey from her first noticing symptoms through to the postdiagnostic treatment period and to subsequent disease cure, recurrence or spread. Influences on certain components of this journey have previously been studied in the SSA setting. Treatment received may depend on its availability, affordability, accessibility, quality and adherence in the presence of other comorbidities, and also its acceptability to patients who use traditional or other healers for healthcare.18 Patients may not complete the recommended treatment courses, due to lack of funds, no bed space in the hospital and particular to some settings, feeling well after initial treatment—the ‘paradox of wellness and non-adherence’.19–21 Further, for the multidisciplinary management of breast cancer, not all SSA health systems have accessible and adequate diagnostic and treatment facilities.22 Radiotherapy facilities in particular are lacking in much of SSA, while centralised oncology facilities are often at large distances from rural health centres and women's homes. Finally, SSA settings have a considerable number of HIV-positive patients with breast cancer23 whose survival and treatment tolerance need further investigation.24
On the basis of the available literature and the research teams' experience in SSA, we hypothesised that:
Poor survival from breast cancer in Africa is largely attributed to, in descending order of magnitude: late stage at diagnosis, lack of access to and compliance with appropriate treatment, tumour aggressiveness and comorbidities.
Breast cancer survival probabilities in certain African settings are better than in others, demonstrating realistic improvements that can be achieved to avoid deaths from this disease.
Lack of receptor status testing impacts on survival of women suffering from breast cancer especially for early stage disease.
Inappropriate treatment and lack of treatment adherence, due to poor availability of treatment, its lack of affordability and toxicity (eg, due to coexisting HIV), contribute to poor survival of women suffering from breast cancer.
A woman's socioeconomic position, education and culture influence her health beliefs and health seeking behaviour both prior to and post breast cancer diagnosis.
The African Breast Cancer—Disparities in Outcomes (ABC-DO) study was designed specifically to examine the entire breast cancer journey and test these hypotheses.
Methods and analysis
Study design overview
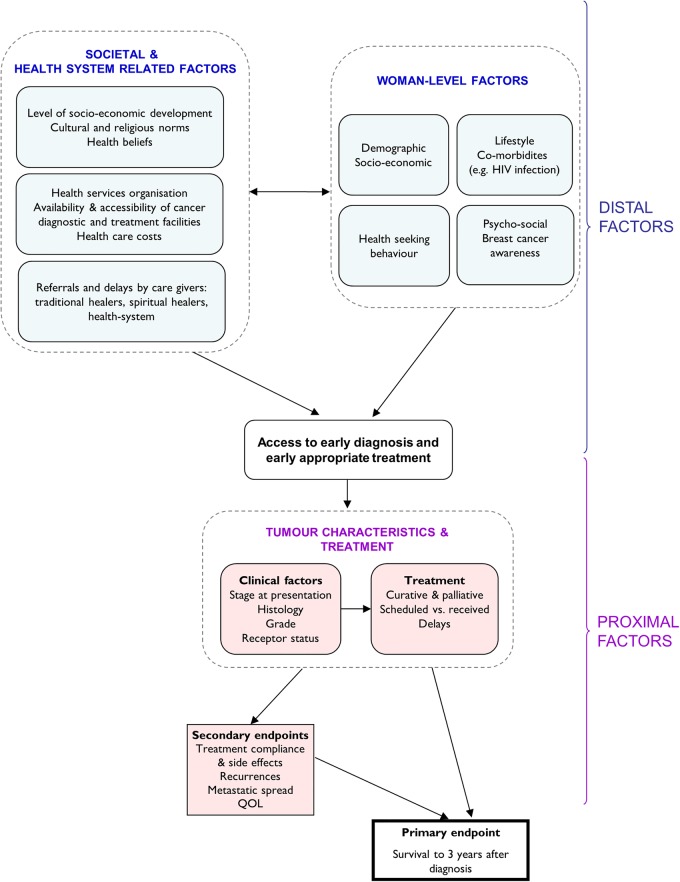
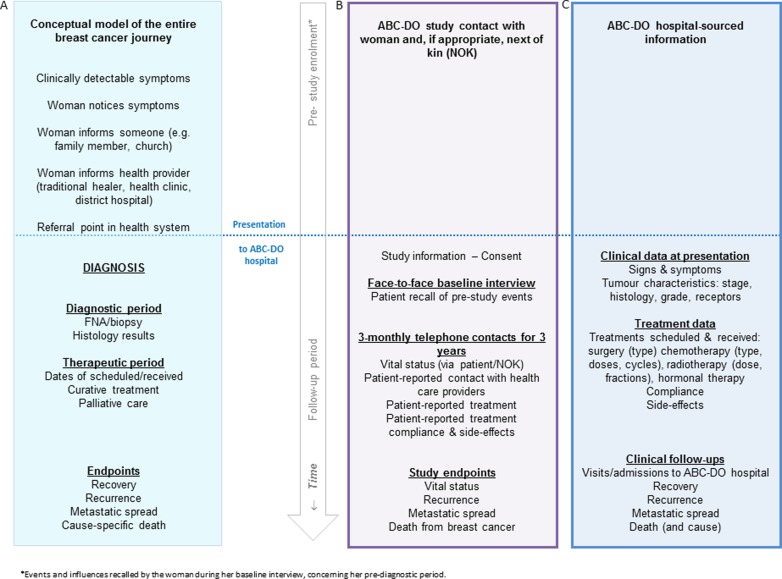
ABC-DO is a hospital-based prospective observational study of outcomes after breast cancer diagnosis in five SSA countries: Namibia and South Africa in Southern Africa, Zambia and Uganda in eastern Africa and Nigeria in western Africa. Recruitment initiation was staggered across the participating hospitals, between September 2014 and early 2016 (table 1). At each site, over a 2-year period of enrolment, all women newly diagnosed with breast cancer are invited to participate in the study. Participation involves a baseline face-to-face interview with a study research assistant (RA) on the woman's presentation at the participating hospital, after which each participant is followed up by mobile phone for, in the first instance, at least one and up to 3 years (ie, with the funding presently in place). Data on factors hypothesised to have a direct impact on survival (proximal factors) such as tumour biology and treatment received are predominantly extracted from hospital records (patient notes, pathology reports, etc), using a standardised study proforma. Factors hypothesised to have an indirect impact on survival (distal factors) such as socioeconomic factors that influence diagnosis delays (figure 1) are obtained directly from the participants during the baseline interview. The full prediagnostic experience from first symptom recognition, every contact with caregivers, through to diagnosis are also obtained at baseline interview (figure 2).
Table 1.
Characteristics of ABC-DO participating hospital, their catchment populations and expected patient with breast cancer profile
| Country (ordered by date recruitment started) | Namibia | Nigeria | Uganda | South Africa | Zambia |
|---|---|---|---|---|---|
| National estimates of age-standardised breast cancer rates, per 100 000 women* | |||||
| Incidence | 24.4 | 50.4 | 27.5 | 41.5 | 22.4 |
| Mortality | 9.6 | 25.9 | 13.6 | 16.5 | 11.1 |
| National adult HIV prevalence in adults aged 15–49 years, 2011–2015† | 16.0% | 3.2% | 7.3% | 19% | 12.4% |
| ABC-DO recruitment hospital | Windhoek Central Hospital, Windhoek (capital) |
|
Mulago Hospital Complex in Kampala (capital), including:
|
Chris Hani Baragwanath Hospital, Soweto, Gauteng |
|
| Initiation of recruitment | September 2014 | November 2014 | December 2014 | August 2015‡ | April 2016 |
| Type of hospital | National tertiary referral | Federal secondary hospital | National tertiary referral | Tertiary referral | UTH: national tertiary; CDH: cancer-specialised tertiary; KGH: provincial hospital |
| Source population of hospital(s) (both sexes, all ages) | Entire population (2.4 million) of Namibia, residing up to 800 km away | Abia State: 3 million, Imo State: 4 million |
National public referral hospital, serving the entire population (35.6 million) of Uganda, residing up to 500 km away | Population of Soweto (2 million) mostly residing within 50 km | For UTH and CDH: entire population (13.9 million) of Zambia, residing up to 800 km away |
| Majority ethnic groups of patients | Various, including white, Ovambo, Damara. | Igbo | Bantu—Ganda, Nkole | 90% black Xhosa, Sotho, Zulu | Bantu—Bemba, Tonga |
| Expected annual number of newly diagnosed women with breast cancers | 250 | 150 | 250 | 300 | 150 |
| Mean or median age at diagnosis§ | 53 | 43 | 45 | 55 | 49¶ |
| Referral routes | Nationwide referrals from public and private sectors | Nationwide referrals from public and private sectors | Primary hospital referrals and self-referral | Referred from local hospital | Referred from local hospitals and outreach clinics |
| Stage at diagnosis (%)§ I, II, III, IV | 5, 14, 71, 10 | 10, 20, 50, 20 | 5, 15, 40, 40 | 7, 45, 40, 8 | 10¶, 36¶, 54¶ |
| Availability of diagnostic and treatment facilities | |||||
| Mammography–ultrasound | Yes–Yes | Yes–Yes | Yes–Yes | Yes–Yes | Yes–Yes |
| Cytology–histology | Yes–Yes | Yes–Yes | Yes–Yes | Yes–Yes | Yes–Yes |
| Surgery–chemotherapy–radiotherapy | Yes–Yes–Yes | Yes–Yes–Not locallys** | Yes–Yes–Yes | Yes–Yes–Yes | Yes–Yes–Yes |
| Routine receptor status testing (ER, PR, HER2) | Yes | No | No | Yes | No |
| Routine HIV testing at diagnosis | No | No | No | Yes | Yes |
| Treatment costs to the patient | Minimal fee, that can be waived | Patients or relatives | Free chemotherapy and surgery. If drugs run out, patient pays. | US$5, but waived if cannot be paid | Cost shared between patient and government |
*Source of incidence and mortality data.2
†Source of HIV data.30
‡Some retrospective inclusions since January 2015 for women who consented and had already completed a questionnaire, at diagnosis, on barriers to early diagnosis.
¶Sourced from CDH hospital database (stage data corresponds to: early, locally advanced, metastatic).
**Patients are referred to the University of Nigeria Teaching Hospital in Enugu.33
ABC-DO, African Breast Cancer—Disparities in Outcomes; ABSUTH, Abia State University Teaching Hospital; CDH, Cancer Diseases Hospital; ER, oestrogen receptor; HER2, human epidermal growth factor receptor 2; KGH, Kabwe General Hospital; PR, progesterone receptor; UTH, University Teaching Hospital.
Figure 1.
Pathways under investigation for overall survival after breast cancer diagnosis, and for intermediate landmarks on a women's journey with breast cancer. QOL, quality of life.
Figure 2.
(A) Conceptual model of the time-line of the entire journey of a woman with breast cancer. (B) Information sourced from the woman or her next-of-kin in African Breast Cancer—Disparities in Outcomes (ABC-DO). (C) Study information sourced from hospital records.
At three-monthly intervals after the baseline interview, the study RA telephones the woman, or when appropriate, her next-of-kin, to obtain data on vital status, treatment side effects, treatment compliance and health status and possible barriers to healthcare utilisation and treatment adherence. To implement this protocol and to reduce losses to follow-up, mHealth technologies are used throughout.
Study settings
Hospitals participating in ABC-DO to date are settings where: International Agency for Research on Cancer (IARC) had already established,16–25 or were developing, a successful collaboration in breast cancer research; the site has at least 100 newly diagnosed women with breast cancer each year; and the selected sites together span countries and hospitals with contrasting patient profiles, catchment populations and health systems. Notably, ABC-DO countries are at different stages of the breast cancer transition, with breast cancer age-standardised incidence rates per 100 000 women in 2012 ranging from 22–28 in Zambia, Namibia and Uganda, to 42–50 in South Africa and Nigeria.2 The key characteristics of each site are summarised in table 1. All settings are located in the largest metropolitan area of the respective country, with the exception of the Nigerian hospitals, which are based in neighbouring south-eastern states of Abia and Imo. The Namibian and South African settings are similar in terms of routine at-diagnosis triple-receptor assessment, affordable care to most resident patients, and availability of surgery, chemotherapy, radiotherapy and hormonal therapy. Both settings also have a relatively high HIV prevalence among the general population, and thus among younger patients with breast cancer.23 However, these two sites have contrasting catchment populations; Windhoek Central Hospital serves the entire country of Namibia while the Chris Hani Baragwanath Academic Hospital serves a similarly sized local population within a 50 km radius of Soweto. Treatment expenses also vary by setting, with a range of minimal fees, which are often waived, to fees fully paid for by the patient and/or their families. In the Nigerian, Ugandan and Zambian sites, access to standard diagnostic and therapeutic management depends greatly on its availability at the time of treatment, as well as affordability to patients.
Participant inclusion/exclusion
Women eligible for inclusion in ABC-DO are those who have confirmed primary breast cancer, through either a histopathological/cytological or clinical diagnosis, are aged 18 years or over at the time of diagnosis and are being newly seen at the participating hospital. All such patients are invited to participate regardless of their stage at diagnosis, ethnicity/race, country of origin, or place of current or past residence, previous history of no breast cancer or of any treatment considerations (treatment type, intention, affordability, private/public sector). A patient who had preliminary surgery or confirmatory biopsy at another clinic and subsequently referred to the participating ABC-DO hospital for further treatment is eligible. Patients who present with a second primary breast cancer are also eligible. There is the potential that some enrolled women will in fact present with recurrences rather than a second primary cancer—full diagnostic and treatment details of any previous breast problem, including breast cancer, are being obtained from all women so that at a later stage, all women reporting a positive history can be evaluated and excluded where appropriate. A patient who has already started her treatment for breast cancer at a participating hospital is not eligible.
Where tissue/cell sampling is performed, on histo(cyto)pathological confirmation, women diagnosed with either in-situ (International Classification of Diseases, 10th Revision (ICD-10) D05) or invasive breast carcinomas (ICD-10 C50) remain included, whereas if microscopic evaluation indicates a benign lesion (eg, ICD-10 D24), the patient is excluded. If histo(cyto)pathological evaluation is not performed or the microscopic diagnosis, for any reason, remains indeterminable but her physician is treating her for breast cancer, the woman remains in the study. Clinically diagnosed breast cancers are included, because ABC-DO is an observational study, which attempts to capture the reality of the breast cancer situation in each setting; the study itself does not fund, and thus does not require, histo(cyto)pathological confirmation for enrolment, but if tissue is taken as part of the routine diagnostic work-up, consent to use it for research is obtained.
Enrolment and baseline interview
All women presenting with suspected breast cancer diagnosis are invited to participate in ABC-DO; we do not wait until the diagnosis is confirmed to start identification of cases as some women never return for diagnosis and treatment. Through close liaison between clinicians and study RAs, the latter are alerted whenever the clinician identifies a suspected new patient with breast cancer. Before the treatment begins, the RA approaches the woman. After providing study information, the woman can choose to participate at that time, delay her decision or not participate. The number of women who refuse and their reason for refusal are being recorded, as is the number of women who are too ill to participate.
If women consent to participate in the study, at a minimum they are asked to complete the baseline interview, provide access to their medical records and be followed up through telephonic contact thereafter. The baseline interview obtains information on breast cancer risk factors, socioeconomic information, health attitudes, knowledge and beliefs. Women are asked about the dates they first noticed their symptoms, and all further dates when they informed another person or made contact with any service, system or professional for help/treatment/relief, including public and private health facilities, traditional healers, faith healers and religious institutions. The woman's own knowledge of breast cancer symptoms and causes are ascertained. At enrolment women are given a study contact card with the RA's telephone number.
Clinical, pathological and treatment information
Standard proformas are used to record all clinical, pathological and treatment information, or, to extract these from medical notes, as per the local site's preference. During the diagnostic period, this information includes clinical presentation of the breast mass, its size and extension, and where available, histo(cyto)pathological diagnosis and the status of receptors (oestrogen, progesterone and HER2 via immunohistochemistry (IHC) or fluorescent in-situ hybridisation (FISH)). If any of this information is not available, we also record the reason why (eg, no capacity to perform test, patient cannot pay for test, results indeterminable). The study enrolment date is the date of baseline interview. Thereafter, there may be significant delays during the diagnostic period, thus so as to not exclude this important time window where survival gains could be made, the study RA obtains each of the dates at first presentation, date of biopsy/cytology and date of histocytological confirmation. Patient management information is also ascertained, including on surgical procedures, chemotherapy, hormonal therapy and palliative care. For each treatment, the intended course is documented as well as the actual received treatment, and where these deviate, the reasons why. Updated disease status, including recurrence and metastases, will be recorded if and when the woman returns to the hospital.
Biospecimen
At enrolment, women are also asked to provide consent for their tumour specimen to be used for research purposes (if such a specimen exists), including for genomic studies and, if not already performed as part of the diagnostic work-up, for IHC. The type and timing of the tumour specimen collection in relation to chemotherapy initiation is individually recorded for each specimen. Formalin-fixed paraffin embedded tumour blocks will be sent to IARC for histopathological review and complementary studies. At the end of the study, morphological types, receptors' status and molecular subtypes will be independently evaluated at IARC and compared across sites. In some sites, women are also asked to provide a saliva sample for DNA extraction (Oragene DNA research kits). The convenient room-temperature storage of such samples makes them a viable solution for standardised DNA collection.
Patient and next-of-kin contact: follow-up methodology
Most SSA countries have no national identification systems or linkable electronic records of vital status thus ABC-DO requires a proactive approach to follow-up. Every 3 months, women are contacted to repeatedly update contact details for their next-of-kin. This telephone call is also used to obtain information on health, side-effects during treatments (neuropathy, hair loss), indications of potential recurrence (pain in chest, breathlessness, pain in armpit), care she is receiving and any changes to her family and work life. If the woman cannot be contacted repeatedly, the mHealth system prompts the RA to telephone her next-of-kin; if they inform the RA that the woman has passed away, information on the date of death and cause of death are obtained. In the instance where a death is reported by the woman's next-of-kin, while vital status and date of death may be accurate, cause of death information will be limited especially for patients who have multiple chronic morbidities.
Maintaining contact with patients by mobile phone is a novel methodological component, and, over the short-term has been successful in Africa,26 but the present study will evaluate the performance of this method over longer periods of time. Contact rates may be compromised by frequent changes of SIM cards or problems with mobile phone network connectivity.
ABC-DO mHealth application
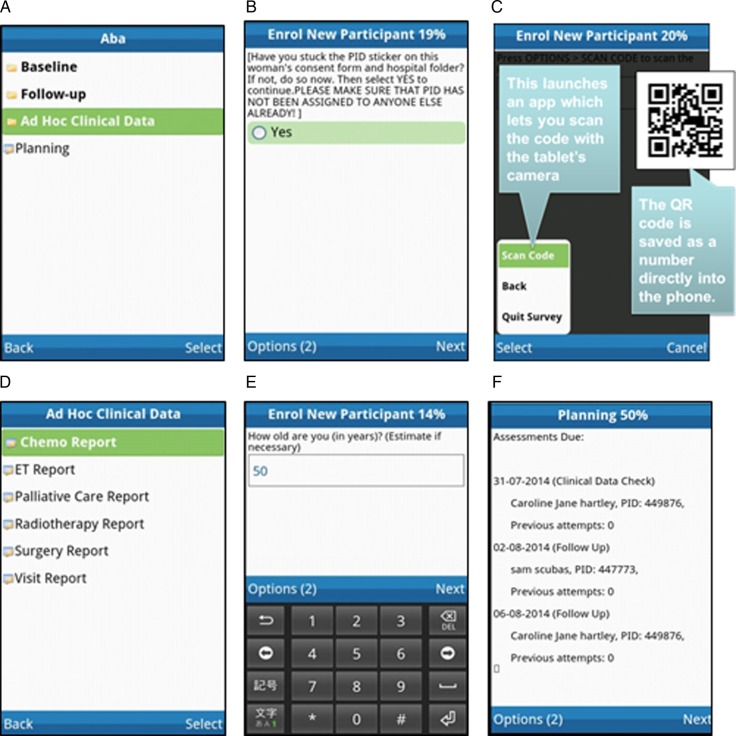
To facilitate data collection and assist in the complex study management of timely contact with woman and/or their next-of kin, a specialised mHealth study tool is being employed. This application was specifically designed for the purpose of the ABC-DO study by Mobenzi Technologies (Pty), a South African mHealth solutions company specialised in m-application (app) development and support for health research. This m-technology has been successfully implemented in several studies.27 28 Screen shots of the app are shown in figure 3. Some of its features are:
The app's mobile interface is simple to use. Each RA has a mobile phone (or tablet with call capability) for data capture at the face-to-face baseline interview. At follow-up interviews, the RA's phone is used with a headset to call the patient's mobile or fixed line, with the RA immediately entering the woman's responses given over the telephone onto the app. All questionnaires/forms for each stage of data capture are contained on the app and administered through the app. Regardless of the complexity of the questionnaire, RAs simply respond to each question one-by-one. Automatic validation checks are incorporated, for example, to check patient eligibility, and most questions are in the format of predefined single or multiple options. There are few free-text fields and standardised data are captured in easily analysable formats.
The ABC-DO app is installed on the RAs' mobiles and automatically communicates with the Mobenzi web platform. Network connectivity is not required at the time of data capture, but is needed to automatically upload captured data, after which no data remain on the mobile phone.
The web platform provides automatic coordination and remote monitoring of the fieldwork. Each RA's mobile phone downloads a ‘to do’ list daily with relevant information on which patients are due follow-up. Investigators are able to remotely track the progress of the fieldwork in real-time and keep in touch with the RAs on the ground, creating a closer motivated team.
Data are encrypted and securely stored with access control codes defining levels of access (eg, local investigators have access to the data from their centre; while the study coordinator has access to all the data).
Figure 3.
(A) African Breast Cancer—Disparities in Outcomes (ABC-DO) mobile phone application: appearance of outer folder. (B) A reminder to the research assistant to assign a new participating woman a new ID number. (C) Scanning of two-dimensional ID barcodes avoids data entry errors. (D) Reports for treatment information. (E) Example of data entry screen for a numeric response (age). (F) A constantly updated list of follow-up calls that are due (the names shown here do not correspond to the real names of any of the study participants).
The above app is being used with one exception, the South African site. Their breast clinic already had a successfully operational database which contains information for the clinical management of patients, and treatment, as well as questions on barriers to diagnosis. Fields for the follow-up have been added so as to implement the ABC-DO design.
Data analysis plan
Overall survival analysis will be carried out using time since first presentation at the hospital as the relevant timescale. Absolute survival probabilities overall, and stratified by baseline characteristics, will be examined using Kaplan-Meier curves for sites and subsets of women of interest for example, by stage and for invasive breast carcinoma only. Relative survival estimates29 will be used to compare overall survival between sites as background mortality rates differ greatly between countries, especially in HIV-endemic populations.
To investigate determinants of survival within sites, Cox proportional hazard models will be used to estimate relative risks of survival time since baseline interview associated with explanatory variables including baseline factors (eg, age, ethnicity, tumour characteristics) as well as time-changing explanatory variables (eg, having received chemotherapy, number of chemotherapy rounds). Effects will be compared across sites before pooling the data across sites and estimating effects in a single Cox model stratified by site to account for between-site differences in the baseline hazards of death. In doing this, power will be gained to examine less-common exposures (eg, the effect of non-adherence to treatment, or survival in less common stages). To investigate the indirect determinants of survival (eg, socioeconomic factors, distance to hospital), crude HRs will be compared with those adjusted for relevant clinical variables to identify confounding and/or the pathways through which these indirect factors operate. We will also investigate the duration of the prediagnostic journey and its determinants using linear regression models and logistic regression models for a diagnostic journey of over 6 months, and for late-stage diagnosis.
Sample size
The study aims to evaluate proximal and distal determinants of overall survival after breast cancer diagnosis, and to do so separately in each participating country. In each country, over 2 years of recruitment, smaller and larger centres are expected to have, respectively, ∼300 and 500 women recruited, with an average 2-year follow-up time. As diagnostic stage is a major prognostic factor, assuming 2-year survival probabilities of 0.85 for stage I/II and 0.5 for stage III/IV, the number of deaths at a site with 50% stage I/II disease is 100 and 160 for the smaller and larger sites, respectively. With 100 deaths, for an exposure prevalence of 30% and 50%, the study will have 80% power to detect HRs of 1.7 and 2.6; and in larger sites with 160 deaths, we have the power to detect HRs of at least 1.5 and 2.1, respectively.
Potential for expansion
The generic set-up of the ABC-DO mHealth study platform via an easily translatable and transportable m-platform means that other funded sites, especially in unrepresented francophone or lusophone Africa, are welcome to join this initiative.
Ethics and dissemination
ABC-DO does not involve invasive procedures or the alteration of standard clinical care. Patient information sheets are available in local languages, and explain in lay language the aims of the study, what the study involves and what will be asked from the participants. To avoid women agreeing to participate under false impressions of benefits to their treatment, the information sheets emphasise that a woman's participation: (1) will be entirely voluntary and that the woman will be entitled to withdraw at any time; (2) will not alter the standard clinical care that she will be offered and (3) will not cover diagnostic or treatment costs. Informed written consent is obtained from literate women. Local/institutional ethics guidelines are followed for obtaining proper consent from illiterate women (eg, patient's fingerprint as an alternative to a signature).
Findings from the study will be disseminated through presentations at both international and African-focused health conferences (eg, African Organisation for Research and Training in Cancer), and submitted for publication to international peer-reviewed journals.
Acknowledgments
The authors thank the women who participated in this study. They also thank the dedicated team of study research assistants, notably Johanna Pontac, Allen Naamala, Teopista Nakazibwe, Agnes Kaggwa, Anne Nteziryayo, Chris Sule Oyamienlen, Kingsley Iwuoha, Esther Ezeigbo, Evelyn Bakengesa, Mirriam Mudolo and Mildred Lusaka.
Footnotes
Contributors: VM had the original idea for this study. VM and IdSS designed the core study. FM wrote the first version of the manuscript. All the authors contributed to the development of the current study design, critically revised the manuscript and approved the final submitted version.
Funding: ABC-DO is supported by the Susan G Komen for the Cure Foundation (IIR 13264158 to IARC and as part of ‘Implementing breast cancer care efficiency in Zambia through specialised health provider training and m-health evaluation of patient outcomes’ for the Zambian site) and by IARC.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The study was approved by ethics committees of all involved institutions: IARC (IEC 13-19, IEC15-18), LSHTM (6459), Federal Medical Centre Owerri, Abia State University Teaching Hospital, University of Zambia Biomedical Research Ethics Committee (004-08-15), University of Witwatersrand (SA), Uganda National Council for Science and Technology (HS 1588) and the Ministry of Health and Social Services of Namibia (17/3/3).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Parkin DM, Bray F, Ferlay J et al. Cancer in Africa 2012. Cancer Epidemiol Biomarkers Prev 2014;23:953–66. 10.1158/1055-9965.EPI-14-0281 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 3.Kantelhardt EJ, Cubasch H, Hanson C. Taking on breast cancer in East Africa: global challenges in breast cancer. Curr Opin Obstet Gynecol 2015;27:108–14. 10.1097/GCO.0000000000000139 [DOI] [PubMed] [Google Scholar]
- 4.Sankaranarayanan R, Swaminathan R. Cancer survival in Africa, Asia, the Caribbean and Central America. 2011. Sankaranarayanan, R and Swaminathan, R (Eds). IARC Scientific Publications No. 162. Lyon: International Agency for Research on Cancer IARC Sci Publ 2011;162:1–5. [PubMed] [Google Scholar]
- 5.Popoola AO, Ogunleye OO, Ibrahmi NA et al. Five year survival of patients with breast cancer at the Lagos State University Teaching Hospital, Nigeria. J Med Med Sci Res 2012;1:24–31. [Google Scholar]
- 6.Galukande M, Wabinga H, Mirembe F. Breast cancer survival experiences at a tertiary hospital in sub-Saharan Africa: a cohort study. World J Surg Oncol 2015;13:220 10.1186/s12957-015-0632-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker AR, Walker BF, Tshabalala EN et al. Low survival of South African urban black women with breast cancer. Br J Cancer 1984;49:241–4. 10.1038/bjc.1984.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anyanwu SN. Survival following treatment of primary breast cancer in eastern Nigeria. East Afr Med J 2000;77:539–43. [PubMed] [Google Scholar]
- 9.Coleman MP, Quaresma M, Berrino F et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol 2008;9:730–56. 10.1016/S1470-2045(08)70179-7 [DOI] [PubMed] [Google Scholar]
- 10.Kene TS, Odigie VI, Yusufu LM et al. Pattern of presentation and survival of breast cancer in a teaching hospital in north Western Nigeria. Oman Med J 2010;25:104–7. 10.5001/omj.2010.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gakwaya A, Kigula-Mugambe JB, Kavuma A et al. Cancer of the breast: 5-year survival in a tertiary hospital in Uganda. Br J Cancer 2008;99:63–7. 10.1038/sj.bjc.6604435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kantelhardt E, Zerche P, Mathewos A et al. Breast cancer survival in Ethiopia: a cohort study of 1,070 women. Int J Cancer 2013;135:702–9 10.1002/ijc.28691. [DOI] [PubMed] [Google Scholar]
- 13.Keegan TH, Press DJ, Tao L et al. Impact of breast cancer subtypes on 3-year survival among adolescent and young adult women. Breast Cancer Res 2013;15:R95 10.1186/bcr3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huo D, Ikpatt F, Khramtsov A et al. Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. J Clin Oncol 2009;27:4515–21. 10.1200/JCO.2008.19.6873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Der EM, Gyasi RK, Tettey Y et al. Triple-negative breast cancer in Ghanaian women: the Korle Bu Teaching Hospital experience. Breast J 2015;21:627–33. 10.1111/tbj.12527 [DOI] [PubMed] [Google Scholar]
- 16.Dickens C, Joffe M, Jacobson J et al. Stage at breast cancer diagnosis and distance from diagnostic hospital in a peri-urban setting: a South African public hospital case series of over 1000 women. Int J Cancer 2014;135:2173–82 10.1002/ijc.28861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eng A, McCormack V, dos-Santos-Silva I. Receptor-defined subtypes of breast cancer in indigenous populations in Africa: a systematic review and meta-analysis. PLoS Med 2014;11:e1001720 10.1371/journal.pmed.1001720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kemfang Ngowa JD, Yomi J, Kasia JM et al. Breast cancer profile in a group of patients followed up at the Radiation Therapy Unit of the Yaounde General Hospital, Cameroon. Obstet Gynecol Int 2011;2011:143506 10.1155/2011/143506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egwuonwu OA, Anyanwu SN, Nwofor AM. Default from neoadjuvant chemotherapy in premenopausal female breast cancer patients: what is to blame? Niger J Clin Pract 2012;15:265–9. 10.4103/1119-3077.100618 [DOI] [PubMed] [Google Scholar]
- 20.Anyanwu SN, Egwuonwu OA, Ihekwoaba EC. Acceptance and adherence to treatment among breast cancer patients in Eastern Nigeria. Breast 2011;20(Suppl 2):S51–3. 10.1016/j.breast.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 21.Adisa AO, Lawal OO, Adesunkanmi AR. Paradox of wellness and nonadherence among Nigerian women on breast cancer chemotherapy. J Cancer Res Ther 2008;4:107–10. 10.4103/0973-1482.42640 [DOI] [PubMed] [Google Scholar]
- 22.Edge J, Buccimazza I, Cubasch H et al. The challenges of managing breast cancer in the developing world—a perspective from sub-Saharan Africa. S Afr Med J 2014;104:377–9. 10.7196/SAMJ.8249 [DOI] [PubMed] [Google Scholar]
- 23.Cubasch H, Joffe M, Hanisch R et al. Breast cancer characteristics and HIV among 1,092 women in Soweto, South Africa. Breast Cancer Res Treat 2013;140:177–86. 10.1007/s10549-013-2606-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coghill AE, Newcomb PA, Madeleine MM et al. Contribution of HIV infection to mortality among cancer patients in Uganda. AIDS 2013;27:2933–42. 10.1097/01.aids.0000433236.55937.cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickens C, Duarte R, Zietsman A et al. Racial comparison of receptor-defined breast cancer in Southern African women: subtype prevalence and age-incidence analysis of nationwide cancer registry data. Cancer Epidemiol Biomarkers Prev 2014;23:2311–21. 10.1158/1055-9965.EPI-14-0603 [DOI] [PubMed] [Google Scholar]
- 26.Talbot E, Munseri P, Teixeira P et al. Test characteristics of urinary lipoarabinomannan and predictors of mortality among hospitalized HIV-infected tuberculosis suspects in Tanzania. PLoS ONE 2012;7:e32876 10.1371/journal.pone.0032876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naik R, Tabana H, Doherty T et al. Client characteristics and acceptability of a home-based HIV counselling and testing intervention in rural South Africa. BMC Public Health 2012;12:824 10.1186/1471-2458-12-824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnabas RV, van Rooyan H, Tumwesigye E et al. Initiation of antiretroviral therapy and viral suppression after home HIV testing and counselling in KwaZulu-Natal, South Africa, and Mbarara district, Uganda: a prospective, observational intervention study. Lancet HIV 2014;1:e68–76. 10.1016/S2352-3018(14)70024-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickman PW, Sloggett A, Hills M et al. Regression models for relative survival. Stat Med 2004;23:51–64. 10.1002/sim.1597 [DOI] [PubMed] [Google Scholar]
- 30.World Bank. Prevalence of HIV, total (% of population ages 15–49). Secondary Prevalence of HIV, total (% of population ages 15–49) 2016. http://data.worldbank.org/indicator/SH.DYN.AIDS.ZS.
- 31.Anele AA, Okoro IO, Oparaocha DC et al. Pattern of breast diseases in Owerri, Imo State, Nigeria. Port Harcourt Med J 2009;4:1. [Google Scholar]
- 32.McCormack VA, Joffe M, van den Berg E et al. Breast cancer receptor status and stage at diagnosis in over 1,200 consecutive public hospital patients in Soweto, South Africa: a case series. Breast Cancer Res 2013;15:R84 10.1186/bcr3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nwankwo KC, Dawotola DA, Sharma V. Radiotherapy in Nigeria: current status and future challenges. West Afr J Radiol 2013;20:84–8. 10.4103/1115-1474.121099 [DOI] [Google Scholar]