Abstract
Objective
Data on early risk of infection in patients receiving their first treatment for type 2 diabetes are limited. We examined rates of community-based antibiotic use and hospital-treated infection in initiators of metformin and other glucose-lowering drugs (GLDs).
Design
Population-based cohort study using medical databases.
Setting
General practice and hospitals in Denmark.
Participants
131 949 patients with type 2 diabetes who initiated pharmacotherapy with a GLD between 2005 and 2012.
Exposure
Initial GLD used for pharmacotherapy.
Main outcome measures
We computed rates and adjusted HRs of community-based antibiotic use and hospital-treated infection associated with choice of initial GLD with reference to metformin initiation, using an intention-to-treat approach.
Results
The rate of community-based antibiotic use was 362 per 1000 patient-years at risk (PYAR) and that for hospital-treated infection was 51 per 1000 PYAR. Compared with metformin, the risk of hospital-treated infection was slightly higher in sulfonylurea initiators (HR 1.12, 95% CI 1.08 to 1.16) and substantially higher in insulin initiators (HR 1.63, 95% CI 1.54 to 1.72) initiators after adjustment for comorbid conditions, comedications and other confounding factors. In contrast, virtually no difference was observed for overall community-based antibiotic use (HR 1.02, 95% CI 1.01 to 1.04, for sulfonylurea initiators; and 1.04, 95% CI 1.01 to 1.07, for insulin initiators).
Conclusions
Rates of community-based antibiotic treatment and hospitalisation for infection were high in patients receiving their first treatment for type 2 diabetes and differed with the choice of initial GLD used for pharmacotherapy.
Keywords: hypoglycaemic agents, type 2 diabetes mellitus, pharmacoepidemiology, infections, antibiotics
Strengths and limitations of this study.
Large nationwide population-based study based on prospectively collected data from hospitals and general practices.
Comprehensive list of infections and antibiotics studied in people receiving their first treatment for type 2 diabetes.
Main limitation was possible residual confounding by differences in diabetes severity.
Introduction
Glucose-lowering drugs (GLDs) are prescribed increasingly in patients with type 2 diabetes,1 with the aim of reducing macrovascular and microvascular complications. Three out of four patients diagnosed with diabetes initiate pharmacotherapy within the following year.2 Although infections are a major clinical problem and an important cause of death in patients with type 2 diabetes,3 4 population-based data are scarce on early infection risk in patients initiating GLD pharmacotherapy.
It has been observed recently that metformin use is associated with reduced risk of infections after surgery5 and reduced risk of septicaemia,6 with improved prognosis following septicaemia and other critical illness,7 and with a beneficial effect on prevention and treatment of respiratory tract infections due to Staphylococcus aureus.8 Limited epidemiological data are available comparing the association of different GLDs with risk of infections.6 9 In a Swedish study based on 51 675 patients with type 2 diabetes treated with GLDs between 2004 and 2007, the HR of hospitalisation for infection with co-occurrence of acidosis was greater for insulin monotherapy users (HR 1.37, 95% CI 1.26 to 1.50) and other oral GLDs users (80% sulfonylurea) (HR 1.16, 95% CI 1.04 to 1.28) compared with metformin users.9 Another study of 43 015 cases with septicaemia and control participants nested in a cohort of newly diagnosed type 2 diabetes patients from Taiwan found that metformin use was associated with reduced risk of developing septicaemia (OR 0.80, 95% CI 0.77 to 0.83) compared with metformin non-users.6 For other infections including those treated by general practitioners, comprehensive data on the risk among users of different GLDs are lacking.
Therefore, we undertook a large cohort study using nationwide Danish population data to investigate rates of community-based antibiotic use and hospital-treated infection associated with initiation of different GLDs in type 2 diabetes patients.
Methods
Data sources
We used the Danish National Patient Registry (DNPR),10 the Danish National Health Service Prescription Database (DNHSPD)11 and the Danish Civil Registration System (CRS)12 to conduct this study. The Danish National Health Service provides Danish residents with universal access to general practice and hospitals and reimburses most of the cost of prescription drugs, including GLDs.11 We used the unique central personal registry (CPR) number to link individual-level data among registries. The CRS began to assign a CPR number to all residents at birth or upon immigration in 1968.12 Since then, the CRS has maintained daily updated records of date of death or emigration, previous and current place of residence, marital status and CPR number for all Danish residents. The DNPR contains nationwide information on all hospitalisations since 1977 and on all outpatient and emergency room visits since 1995.10 It records patients' CPR number, a primary discharge diagnosis and up to 19 secondary discharge diagnoses coded according to the International Classification of Diseases, Eighth Revision (ICD-8) until the end of 1993, and Tenth Revision (ICD-10) thereafter. The DNHSPD collects data from all community pharmacies and hospital-based outpatient pharmacies. It has archived patient-related, drug-related and prescriber-related information on all prescription medications dispensed in Denmark since 2004.11 The drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification system.
Study design and population
We conducted this population-based cohort study in a Danish nationwide cohort of patients with an incident type 2 diabetes diagnosis recorded between 1 January 2005 and 31 December 2012. Incident type 2 diabetes was defined as either the first record in the DNPR of a diabetes-associated inpatient admission (data available from 1977) or outpatient clinic contact (data available from 1995) or the first record of a GLD prescription in the DNHSPD (data available from January 2004), whichever came first.13 To decrease the chance of including patients with type 1 diabetes, we restricted our cohort to patients who were 30 years or older when first diagnosed with diabetes (n=147 396).14 We also excluded patients with a diabetes diagnosis but no recorded GLD prescription during the 2005–2012 study period (n=14 120). Women with a recorded diagnosis of polycystic ovarian disease who were using metformin monotherapy, identified from the DNPR and the DNHSPD, were excluded as well (n=1327). This left a final study cohort of 131 949 patients with incident pharmacotherapy for type 2 diabetes.
We defined exposure as the first record of a redeemed GLD prescription in the DNHSPD (the index date) between 2005 and 2012. We disregarded any change or addition of other GLD afterwards. We established seven mutually exclusive categories of exposure according to the type of first-prescribed GLD: metformin (biguanides); sulfonylurea; insulin; any fixed drug combinations; dipeptidyl peptidase-4 (DPP-4) inhibitors; glucagon-like peptidase-1 (GLP-1) analogue; meglitinides; other (including thiazolidinediones; and α glucosidase inhibitors) (see online supplementary appendix 1 for ATC codes). We followed the study cohort from the index date until death, emigration or end of the study period (31 December 2012), whichever came first.
bmjopen-2016-011523supp_appendix1.pdf (111.1KB, pdf)
Assessment of outcomes
Our outcomes were hospital-treated infections and community-based antibiotic use. Hospital-treated infection was defined as any first inpatient admission or outpatient hospital clinic contact associated with a primary or secondary discharge diagnosis of infection after the index date. We further divided hospital-treated infections into subcategories (see online supplementary appendix 1 for categories and associated ICD codes).
Community-based antibiotic use was defined as any first record of an antibiotic prescription in the DNHSPD that was redeemed during the study period after the index date. We investigated 10 groups of antibiotics prescribed to treat specific infections according to national Danish guidelines for general practitioners (see online supplementary appendix 1 for ATC codes).15 16
Assessment of covariates
We searched the DNPR for information on 19 major comorbidities included in the Charlson comorbidity index (CCI),17 based on each cohort member's entire hospital contact history during the 10 years prior to his/her index date. We defined three comorbidity levels: low (CCI score of 0), medium (CCI scores of 1 or 2) and high (CCI score ≥3).18 We also collected information on other covariates associated with risk of infection: microvascular and macrovascular diabetes complications not included in the CCI (see online supplementary appendix 1); diabetes duration (if a hospital diagnosis was present before the GLD initiation/index date); presence of alcoholism-related disorders (yes/no); a hospital diagnosis of obesity (yes/no); use of immunosuppressive drugs (yes/no), oral corticosteroids (yes/no) or statins (yes/no); marital status as a marker of social support (married/never married/divorced/widowed); and calendar period of inclusion (2005–2008/2009–2012).
Statistical analysis
We described cohort characteristics at the time the first GLD was redeemed according to GLD categories (table 1). We used an intention-to-treat approach19 and computed incidence rates (IRs) separately for community-based antibiotic use and for hospital-treated infections, by dividing the number of incident outcome events by total exposed patient-time during follow-up (expressed per 1000 patient-years at risk (PYAR)). We then used the Cox regression to compute HRs of community-based antibiotic use and hospital-treated infections (with 95% CIs) associated with the exposure categories described above, using metformin initiation as reference. We computed estimates adjusted for age and sex (Model 1) and estimates fully adjusted for all available confounders (Model 2) (see above). We repeated the analyses for specific infections and antibiotic groups, except for those associated with four or fewer events during complete follow-up.
Table 1.
Baseline characteristics of 131 949 patients with type 2 diabetes, according to initial pharmacotherapy with glucose-lowering drugs (2005–2012)
| Characteristics | Metformin | Sulfonylurea | Insulin | Fixed drug combinations | DPP-4 inhibitors | GLP-1 analogues | Meglitinides | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| n (%)* | 106 424 (81) | 16 703 (13) | 7293 (6) | 553 (<1) | 358 (<1) | 295 (<1) | 231 (<1) | 92 (<1) | 131 949 (100) |
| Sex | |||||||||
| Men | 59 213 (56) | 9879 (59) | 4421 (61) | 355 (64) | 212 (59) | 126 (43) | 128 (55) | 57 (62) | 74 391 (56) |
| Women | 47 211 (44) | 6824 (41) | 3872 (39) | 198 (36) | 146 (41) | 169 (57) | 103 (45) | 35 (38) | 57 558 (44) |
| Age in years | |||||||||
| Median age (IQR) | 62 (52, 70) | 67 (57, 76) | 56 (43, 68) | 62 (52, 70) | 67 (56, 76) | 52 (44, 61) | 62 (53, 72) | 58 (46, 69) | 62 (52, 70) |
| Age-groups (years) | |||||||||
| 30 to <50 | 22 611 (21) | 2026 (12) | 2728 (37) | 124 (22) | 41 (11) | 128 (43) | 49 (21) | 28 (830) | 27 735 (21) |
| 50 to <70 | 58 184 (55) | 7835 (47) | 3050 (42) | 291 (53) | 182 (51) | 143 (48) | 116 (50) | 43 (47) | 69 844 (53) |
| >70 | 25 629 (24) | 6842 (41) | 1515 (21) | 138 (25) | 135 (38) | 24 (8) | 66 (29) | 21 (23) | 34 370 (26) |
| Year of study inclusion | |||||||||
| 2005–2008 | 37 692 (35) | 13 433 (80) | 3702 (51) | 181 (33) | 123 (34) | 5 (2) | 174 (75) | 53 (58) | 55 363 (42) |
| 2009–2012 | 68 732 (65) | 3270 (20) | 3591 (49) | 372 (67) | 235 (66) | 290 (98) | 57 (25) | 39 (42) | 76 586 (58) |
| Marital status | |||||||||
| Married | 64 123 (61) | 9630 (59) | 4062 (58) | 322 (59) | 214 (60) | 196 (66) | 157 (69) | 59 (64) | 78 763 (60) |
| Never married | 13 404 (13) | 1271 (8) | 1211 (17) | 85 (16) | 34 (10) | 55 (19) | 13 (6) | 10 (11) | 16 083 (12) |
| Divorced | 15 457 (15) | 2150 (13) | 1080 (15) | 85 (16) | 46 (13) | 32 (11) | 22 (10) | 17 (18) | 18 889 (14) |
| Widowed | 12 561 (12) | 3269 (20) | 701 (10) | 55 (10) | 60 (17) | 12 (4) | 36 (16) | 6 (7) | 16 700 (13) |
| CCI score | |||||||||
| Low (score of 0) | 75 550 (71) | 10 224 (61) | 3953 (54) | 385 (70) | 202 (56) | 207 (70) | 154 (67) | 54 (59) | 90 729 (69) |
| Medium (scores of 1–2) | 25 957 (24) | 5035 (30) | 2076 (28) | 134 (24) | 110 (31) | 72 (24) | 59 (26) | 28 (30) | 33 471 (25) |
| High (score ≥3) | 4917 (5) | 1444 (9) | 1264 (17) | 34 (6) | 46 (13) | 16 (5) | 18 (8) | 10 (11) | 7749 (6) |
| Diabetes complications | |||||||||
| No complications | 77 981 (73) | 10 968 (66) | 5024 (69) | 417 (75) | 204 (57) | 237 (80) | 168 (73) | 71 (77) | 95 070 (72) |
| Microvascular | 6422 (6) | 1423 (9) | 729 (10) | 33 (6) | 31 (9) | 16 (5) | 22 (10) | 6 (7) | 8682 (7) |
| Macrovascular | 22 021 (21) | 4312 (26) | 1540 (21) | 103 (19) | 123 (34) | 42 (14) | 41 (18) | 15 (16) | 28 197 (21) |
| Alcoholism-related conditions | 2651 (2) | 595 (4) | 742 (10) | 12 (2) | 17 (5) | 4 (2) | 10 (4) | 3 (3) | 4034 (3) |
| Hospital-diagnosed obesity | 9566 (9) | 602 (4) | 528 (7) | 46 (8) | 28 (8) | 79 (27) | 7 (3) | 17 (18) | 10 873 (8) |
| Hospital outpatient follow-up in first year after study inclusion | 16 463 (15) | 3502 (21) | 1695 (23) | 86 (16) | 62 (17) | 18 (6) | 33 (14) | 11 (12) | 21 870 (17) |
| Therapy change during follow-up | 30 845 (29) | 9977 (60) | 2353 (32) | 259 (47) | 173 (48) | 48 (16) | 135 (58) | 41 (45) | 43 831 (33) |
| Therapy change within 1-year | 16 530 (16) | 3618 (22) | 1752 (24) | 140 (25) | 122 (34) | 31 (11) | 62 (27) | 23 (25) | 22 278 (17) |
| Therapy change within 2 years | 21 877 (21) | 5581 (33) | 1970 (27) | 184 (33) | 147 (41) | 45 (15) | 86 (37) | 33 (36) | 29 923 (23) |
| Number of patients with HbA1c measurement | 27 200 (56) | 4576 (59) | 1649 (61) | 164 (64) | 115 (59) | 35 (43) | 34 (55) | 22 (62) | 33 795 (56) |
| Median % HbA1c (IQR) | 7.1 (6.5, 8.3) | 7.6 (6.9, 9.2) | 10.1 (7.5, 12.1) | 8.3 (7.0, 10.6) | 7.0 (6.5, 7.7) | 6.4 (6.0, 7.3) | 7.1 (6.1, 7.9) | 7.0 (5.9, 7.8) | 7.2 (6.6, 8.7) |
| Other medication use | |||||||||
| Statins | 50 817 (48) | 6230 (37) | 1522 (21) | 230 (42) | 167 (47) | 80 (27) | 63 (27) | 24 (26) | 59 163 (45) |
| Immunosuppressants | 669 (1) | 134 (1) | 85 (1) | 2 (<1) | 3 (1) | 4 (1) | 2 (1) | 5 (5) | 904 (1) |
| Corticosteroids | 3825 (4) | 1163 (7) | 1044 (14) | 20 (4) | 21 (6) | 11 (4) | 15 (6) | 6 (7) | 6105 (5) |
*Parentheses contain percentages unless otherwise specified.
CCI, Charlson comorbidity index; DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; HbA1c, glycated haemoglobin.
Bias and sensitivity analyses
Increased body mass index (BMI) and tobacco smoking may be associated with type 2 diabetes, choice of diabetes therapy and infection risk. As we had data only on hospital-diagnosed obesity and tobacco-related diseases, and no detailed data on smoking or BMI, we computed externally adjusted estimates of unmeasured obesity (BMI ≥30 kg/m2) and smoking, respectively, and compared them with our crude estimates, to assess the proportion of effect possibly explained by obesity or smoking alone, using the array approach as presented by Schneeweiss:20
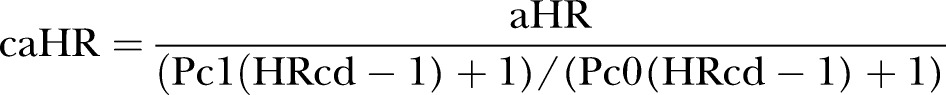 |
1 |
where caHR is the obesity-adjusted HR, aHR the crude rate ratio observed in our study, Pc0 the proportion of patients with obesity in the reference (metformin) group (estimated at 0.49 in the study period based on the study by Ulrichsen et al),21 Pc1 the proportion of patients with obesity in the exposed group (for insulin, 0.19; for sulfonylurea, 0.26)21 and HRcd is the expected rate ratio of infection related to obesity (1.5 for hospital-treated infections and 1.23 for community-based antibiotic use).16 Similarly, we computed externally adjusted estimates for tobacco smoking (Pc0=0.22, Pc1 for insulin=0.26, Pc1 for sulfonylurea=0.30, HRcd for hospital-treated infection=4.1 and HRcd for antibiotic use=1.17).21–23 Additionally, using a rule-out approach,20 we estimated how strongly a single unmeasured binary confounder (eg, BMI, smoking) would need to be associated with the choice of GLD and infection to fully explain our adjusted results. We repeated this sensitivity analysis for the observed lower limit of the 95% CI of the adjusted HR. We describe the details of the methods and the choice of parameter in online supplementary appendix 2. Finally, for a subcohort of our study population (n=33 795), we had additional information on latest glycated haemoglobin (HbA1c) level before GLD initiation (baseline HbA1c). We repeated the analyses for this subcohort including baseline HbA1c categories (reference category: 5.5%–6.5%) as an additional confounder in the fully adjusted model.
bmjopen-2016-011523supp_appendix2.pdf (130.3KB, pdf)
We used SAS software (V.9.1.3; SAS Institute, Cary, North Carolina, USA) for data management. Analyses were carried out using STATA V.12 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, Texas, USA: StataCorp LP). The study was approved by the Danish Data Protection Agency (record numbers 2012-41-0793 and 2013-41-1924).
Results
Cohort characteristics
Of the 131 949 type 2 diabetes patients receiving their first antidiabetic medication, 106 424 (81%) started with metformin, 16 703 (13%) started with sulfonylurea and 7293 (6%) started with insulin. Only 1529 (<1%) individuals used one of the other GLDs as their initial drug (table 1). In our study cohort, 56% (74 391) were men and the median age at inclusion was 62 years (IQR 52–70 years). Compared with type 2 diabetes patients who used metformin as their first drug, sulfonylurea initiators were older (median age 67 years vs 62 years), more likely to be enrolled before 2008 (80% vs 35%), more likely to change therapy within 1 or 2 years (22% and 33% vs 16% and 21%, respectively) and more likely to have comorbidities (39% vs 29%), diabetes-related macrovascular complications (26% vs 21%) or alcoholism-related conditions (4% vs 2%) (table 1). Patients who initiated their therapy with sulfonylurea also had less hospital-diagnosed obesity (4% vs 9%), and were less likely to be using statins at the time of GLD initiation (37% vs 48%).
Insulin initiators were younger (median age 56 vs 62 years); more likely to have been included in the study before 2008 (51% vs 35%); more likely to have comorbidities (45% vs 29%), microvascular complications (10% vs 6%) and alcoholism-related conditions (10% vs 2%); less likely to be using statins (21% vs 48%); and more likely to have changed their therapy within 1 or 2 years (24% and 27% vs 16% and 21%, respectively) than metformin initiators (table 1).
Rates of community-based antibiotic use and hospital-treated infections
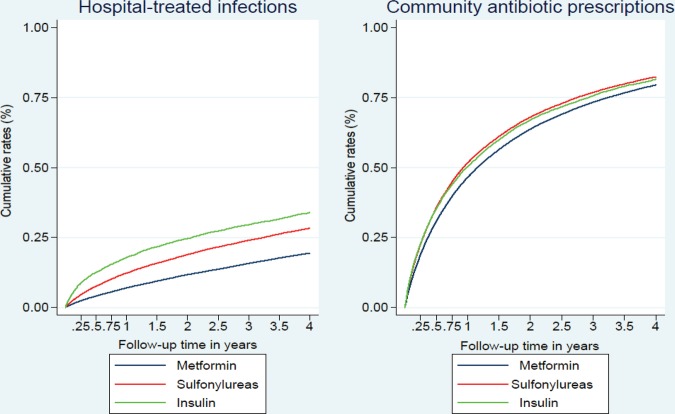
During 218 032 PYAR, we identified 78 847 events (60% of all patients), yielding an IR of 361.8 per 1000 PYAR (95% CI 359.2 to 364.3). The IRs of community-based antibiotic use were higher in patients who initiated their treatment with insulin compared with those who initiated with sulfonylurea or metformin (see online supplementary table S1). We identified 20 308 (15%) initial-onset hospital-treated infection events during 395 171 PYAR, yielding an overall IR of 51.4 per 1000 PYAR (95% CI 50.7 to 52.1). IRs of hospital-treated infections were highest in patients who initiated their treatment with insulin, followed by patients who initiated with sulfonylurea and metformin (see online supplementary table S1). Cumulative rates of community-based antibiotic prescriptions and hospital-treated infections within the first 4 years in patients who initiated their treatment with metformin, sulfonylurea or insulin are illustrated in figure 1. The figure shows that infection rates increased most sharply shortly after GLD treatment initiation. The unadjusted curves for the three treatment modalities diverged early during follow-up, with insulin initiators experiencing more infections than sulfonylurea initiators throughout follow-up, and sulfonylurea initiators experiencing more infections than metformin initiators (log-rank test for equality of survival function between the three exposure groups, p<0.00001 for both outcomes) (figure 1).
Figure 1.
Kaplan-Meier curves showing cumulative rates of community-based antibiotic prescriptions and hospital-treated infections as percentages within the first 4 years following treatment initiation with metformin, sulfonylurea or insulin.
bmjopen-2016-011523supp_tables.pdf (239.9KB, pdf)
Community-based antibiotic use
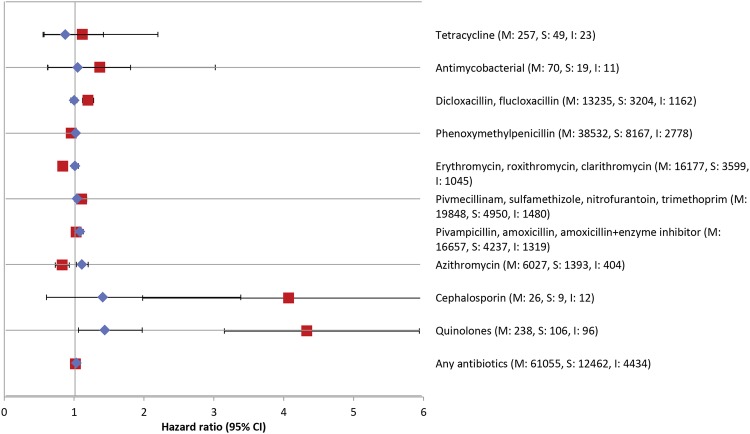
Compared with patients who initiated their treatment with metformin, the crude risk of subsequent community-based antibiotic prescriptions was increased in patients who initiated treatment with sulfonylurea (crude HR 1.06, 95% CI 1.04 to 1.08). The HR remained stable even after adjusting for age and sex (HR 1.05, 95% CI 1.03 to 1.07), but reduced to 1.02 (95% CI 1.01 to 1.04) in the fully adjusted model (table 2). For specific antibiotic groups, patients who initiated antidiabetic treatment with sulfonylurea were at increased risk of treatment for infection with azithromycin (adjusted HR 1.10, 95% CI 1.03 to 1.17), quinolones (adjusted HR 1.43, 95% CI 1.12 to 1.84), antibiotics used to treat urinary tract infection (UTI) (adjusted HR 1.07, 95% CI 1.03 to 1.11) and other broad-spectrum antibiotics (adjusted HR 1.07, 95% CI 1.03 to 1.11) (figure 2 and see online supplementary table S2).
Table 2.
HRs of infection associated with initial use of glucose-lowering drugs in patients with type 2 diabetes, according to drug category
| Metformin | Sulfonylurea | Insulin | Fixed drug combinations | DPP-4 inhibitors | GLP-1 analogues | Meglitinides | Other | |
|---|---|---|---|---|---|---|---|---|
| Community-based antibiotic use | ||||||||
| Number of events | 61 055 | 12 462 | 4434 | 317 | 213 | 146 | 183 | 64 |
| Crude HR (95% CI) | 1.00 (referent) | 1.06 (1.04 to 1.08) | 1.13 (1.09 to 1.16) | 1.03 (0.92 to 1.15) | 1.16 (1.01 to 1.32) | 1.31 (1.12 to 1.55) | 1.07 (0.92 to 1.24) | 1.17 (0.92 to 1.50) |
| Model 1* HR (95% CI) | 1.00 (referent) | 1.05 (1.03 to 1.07) | 1.18 (1.15 to 1.22) | 1.06 (0.95 to 1.18) | 1.16 (1.01 to 1.32) | 1.29 (1.09 to 1.51) | 1.06 (0.92 to 1.23) | 1.17 (0.92 to 1.50) |
| Model 2† HR (95% CI) | 1.00 (referent) | 1.02 (1.00 to 1.04) | 1.04 (1.01 to 1.07) | 1.04 (0.93 to 1.16) | 1.11 (0.97 to 1.27) | 1.20 (1.02 to 1.41) | 1.01 (0.87 to 1.17) | 1.07 (0.84 to 1.36) |
| Hospital-treated infections | ||||||||
| Number of events | 13 949 | 4350 | 1785 | 74 | 53 | 18 | 61 | 18 |
| Crude HR (95% CI) | 1.00 (referent) | 1.41 (1.36 to 1.46) | 1.96 (1.87 to 2.06) | 1.06 (0.85 to 1.34) | 1.28 (0.98 to 1.68) | 0.85 (0.54 to 1.36) | 1.40 (1.09 to 1.79) | 1.29 (0.81 to 2.05) |
| Model 1* HR (95% CI) | 1.00 (referent) | 1.20 (1.16 to 1.24) | 2.28 (2.17 to 2.39) | 1.05 (0.84 to 1.33) | 1.14 (0.87 to 1.49) | 1.05 (0.66 to 1.66) | 1.34 (1.04 to 1.72) | 1.28 (0.81 to 2.03) |
| Model 2† HR (95% CI) | 1.00 (referent) | 1.12 (1.08 to 1.16) | 1.63 (1.54 to 1.72) | 1.03 (0.82 to 1.30) | 1.05 (0.80 to 1.38) | 0.93 (0.58 to 1.47) | 1.27 (0.98 to 1.64) | 1.04 (0.66 to 1.65) |
*Model 1 adjusted for age and sex.
†Model 2 adjusted for age, sex, comorbidity (CCI score), hospital-diagnosed obesity, alcoholism-related conditions, marital status, microvascular and macrovascular diabetes complications not included in the CCI, diabetes duration, concurrent use of statins/corticosteroids/immunosuppressive drugs and calendar period of study inclusion.
DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1.
Figure 2.
Adjusted HRs of specific antibiotic therapies associated with pharmacotherapy initiation with sulfonylureas versus metformin (shown as blue diamonds) and insulin versus metformin (shown as red squares), in patients with type 2 diabetes. M, S and I denote total number or hospital-treated infections in metformin, sulfonylurea and insulin initiators, respectively.
Similarly, the risk of community-based antibiotic use in patients who initiated their treatment with insulin decreased from 1.13 (95% CI 1.09 to 1.16) to 1.04 (95% CI 1.01 to 1.07) in the fully adjusted model (table 2). For specific antibiotic groups, insulin initiators had increased risks of subsequent treatment of infections with quinolones (adjusted HR 4.36, 95% CI 3.36 to 5.65), cephalosporins (adjusted HR 4.65, 95% CI 2.16 to 10.01), dicloxacillin/flucloxacillin (adjusted HR 1.16, 95% CI 1.09 to 1.24) and with antibiotics used to treat UTI (adjusted HR 1.17, 95% CI 1.11 to 1.24) (figure 2 and see online supplementary table S2).
In sensitivity analyses for sulfonylurea versus metformin, external adjustment for unmeasured obesity (lower with sulfonylurea) changed the crude HR from 1.06 to 1.11 and for smoking (higher with sulfonylurea) changed the crude HR from 1.06 to 1.05, respectively. For insulin versus metformin, external adjustment for unmeasured obesity changed the crude HR from 1.13 to 1.20 and for smoking changed the crude HR from 1.13 to 1.12, respectively. The rule-out sensitivity analysis suggested that had we been able to account for obesity, we would likely have observed an association of antibiotic use with sulfonylurea or insulin compared with metformin that was stronger than we observed, as obesity is more prevalent among metformin users than the other treatment (see online supplementary appendix 2 for details). In contrast, had we been able to account for more smoking in sulfonylurea or insulin compared with metformin users, this might have nullified our weakly increased antibiotic HRs. For example, if smoking were 1.3-fold more prevalent among sulfonylurea than metformin users, the relatively likelihood of being prescribed antibiotics would have to be about 50% greater in those who smoke for the HR to be ≤1, which is plausible from findings in the literature21 (see online supplementary figure S1.4 in appendix 2).
We performed further analyses on the subcohort of patients with baseline HbA1c information. Compared with those who initiated their treatment with metformin, patients first treated with sulfonylurea and insulin had adjusted HRs of community-based antibiotic use of 1.05 (95% CI 1.01 to 1.10) and 1.03 (95% CI 0.96 to 1.11), respectively (vs 1.02, 95% CI 1.00 to 1.04, and 1.04, 95% CI 1.01 to 1.07 in the full cohort). After additional adjustment for baseline HbA1c, the HRs did not change for sulfonylurea initiators (adjusted HR 1.05, 95% CI 1.01 to 1.10), but increased slightly for insulin initiators (adjusted HR 1.08, 95% CI 1.00 to 1.17) (see online supplementary table S3).
The HRs were not increased for the rest of the rarer GLD categories (table 2). For GLDs other than sulfonylurea and insulin, the HRs and number of infections (if ≤4) treated with specific antibiotic groups are provided in online supplementary table S2.
Hospital-treated infections
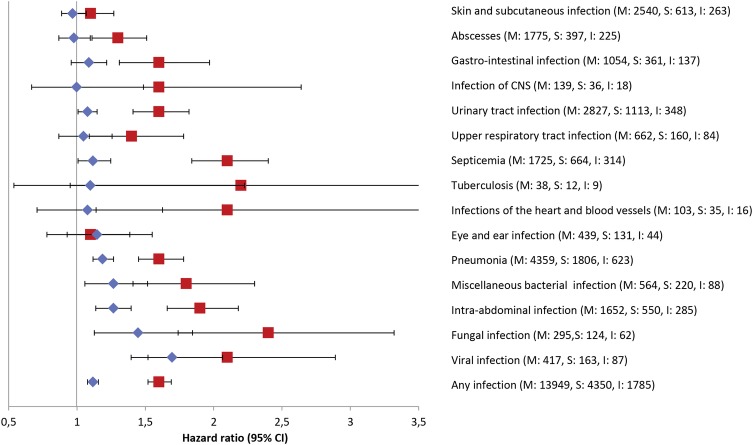
Compared with patients who initiated treatment with metformin, the risk of hospital-treated infections was higher in patients who initiated treatment with sulfonylurea (HR 1.41, 95% CI 1.36 to 1.46). The HR was reduced to 1.20 (95% CI 1.16 to 1.24) in Model 1 and further reduced to 1.12 (95% CI 1.08 to 1.16) in fully adjusted Model 2 (table 2). Patients who initiated their treatment with sulfonylurea had increased risk of hospitalisation for viral infections (adjusted HR 1.70, 95% CI 1.40 to 2.07), fungal infections (adjusted HR 1.45, 95% CI 1.15 to 1.83), intra-abdominal infections (adjusted HR 1.27, 95% CI 1.14 to 1.41), bacterial infections (adjusted HR 1.27, 95% CI 1.07 to 1.50), UTI (adjusted HR 1.08, 95% CI 1.01 to 1.17), pneumonia (adjusted HR 1.19, 95% CI 1.12 to 1.26) and septicaemia (adjusted HR 1.12, 95% CI 1.02 to 1.24) compared with those who initiated treatment with metformin (figure 3 and see online supplementary table S4).
Figure 3.
Adjusted HRs of specific hospital-treated infections associated with pharmacotherapy initiation with sulfonylureas versus metformin (shown as blue diamonds) and insulin versus metformin (shown as red squares), in patients with type 2 diabetes. M, S and I denote total number or hospital-treated infections in metformin, sulfonylurea and insulin initiators, respectively.
The risk of hospital-treated infections was twice as high in patients initiating treatment with insulin compared with metformin initiators (HR 1.96, 95% CI 1.87 to 2.07), and the association strengthened after adjusting for age and sex (HR 2.28, 95% CI 2.17 to 2.39). After inclusion of other confounders, the HR decreased to 1.63 (95% CI 1.54 to 1.72) in the full model (table 2). Type 2 diabetes patients who initiated treatment with insulin had a greater risk of hospitalisation for nearly all examined infections in particular fungal infections (adjusted HR 2.45, 95% CI 1.82 to 3.29), tuberculosis (adjusted HR 2.39, 95% CI 1.11 to 5.14), infections of the heart and blood vessels (adjusted HR 2.13, 95% CI 1.21 to 3.75) and septicaemia (adjusted HR 2.10, 95% CI 1.84 to 2.40), compared with patients who initiated treatment with metformin (figure 3 and see online supplementary table S4).
In sensitivity analyses for sulfonylurea versus metformin, external adjustment for unmeasured obesity changed the crude HR from 1.41 to 1.55, while external adjustment for smoking decreased the HR to 1.23, respectively. For insulin versus metformin, external adjustment for unmeasured obesity (lower with insulin) increased the crude HR from 1.96 to 2.23 and decreased to 1.83 after external adjustment for smoking (more with insulin). The rule-out approach of sensitivity analyses illustrated that for hospital-treated infections, neither obesity nor smoking could completely explain the observed association in our study (see online supplementary appendix 2 for details). For example, if obesity were 1.6-fold more frequent among sulfonylurea users than metformin users, the relative likelihood of hospital-treated infections would have to be increased by a factor of three or more to explain our findings fully, if no increased risk actually existed, which is unlikely based on available literature21 (see online supplementary figure S2.2 in appendix 2).
In the subcohort with available baseline HbA1c information, pre-treatment HbA1c decreased from insulin to sulfonylurea to metformin initiators; however, baseline HbA1c per se was not a strong predictor of infection risk (data not shown). When using treatment initiation with metformin as the comparator, adjusted HRs of hospital-treated infection associated with sulfonylurea and insulin initiation in the subcohort were 1.17 (95% CI 1.08 to 1.26) and 1.88 (95% CI 1.69 to 2.1), respectively (vs 1.12 and 1.63 in the full cohort). Additional adjustment for baseline HbA1c did not change the adjusted HR for sulfonylurea initiators (adjusted HR 1.17, 95% CI 1.08 to 1.26), and increased it slightly for insulin initiators (adjusted HR 1.96, 95% CI 1.73 to 2.22) (see online supplementary table S3).
Few episodes of infection occurred in patients taking medication in the remaining small GLD categories, and we did not detect a clear difference compared with metformin (table 2). For GLDs other than sulfonylurea and insulin, the HRs and number of hospital contacts (if ≤4) for specific infections are provided in online supplementary table S4.
Discussion
In this study of patients with type 2 diabetes treated pharmacologically for the first time, we found high rates of community-based antibiotic treatment and hospitalisations for infection during follow-up. We also found that patients who initiated pharmacotherapy with insulin, and to less extent those who initiated sulfonylurea, were at increased risk of hospital-treated infection compared with those who initiated pharmacotherapy with metformin. In contrast, there was little difference in rates of community-based antibiotic use between initiators of different GLDs.
Our results corroborate findings from the Swedish study that reported an increased risk of hospitalisation for infection among patients who initiated their pharmacotherapy with insulin alone (HR 1.37, 95% CI 1.26 to 1.50) or with other oral GLDs (other than metformin) (HR 1.16, 95% CI 1.04 to 1.28), compared with metformin.9 Furthermore, our results are in line with the observed reduced odds of septicaemia in metformin users versus metformin never users (OR 0.80, 95% CI 0.77 to 0.83) and increased odds in sulfonylurea users versus sulfonylurea never users (OR 1.06, 95% CI 1.03 to 1.10) in the nationwide cohort of GLD-treated type 2 diabetes patients from Taiwan.6 Few comparative studies have examined newer second-line GLDs.24 25 Although statistically imprecise, our results are in line with those from a double-blind randomised study of 807 type 2 diabetes patients, in which 3% of patients treated with metformin and 6% of patients treated with DPP-4 inhibitors experienced an upper respiratory tract infection (URTI) event during a follow-up period of 52 weeks (p >0.05).25 Our results support a recent systematic review and meta-analysis of 19 randomised controlled trials that found no difference in risk of UTI (RR 0.86, 95% CI 0.51 to 1.45) between patients receiving DPP-4 inhibitors and those receiving metformin.24
Hyperglycaemia may be a risk factor for infections in patients with type 2 diabetes.26–30 Therefore, GLDs in theory might influence risk of infections via their different glucose-lowering mechanisms and effectiveness. Hyperglycaemia seems to weaken innate immunity via its negative influence on polymorphonuclear neutrophil function and intracellular bactericidal and opsonic activity.31 Insulin is more effective in reducing blood glucose than sulfonylureas and metformin;32 and insulin has been suggested to enhance innate and cell-mediated immunity33 and promote macrophage function.31 34 This contrasts with our observation that insulin initiators had the highest risk of infections. Other non-glycaemic effects of GLDs on the immune system might be at play.31 33 35–37 It has been suggested that the 5′ AMP-activated protein kinase activation property of metformin facilitates neutrophil-dependent bacterial uptake and killing associated with inhibition of neutrophil activation and chemotaxis.36 37 This mechanism might contribute to the lower risk of infections in patients taking metformin versus insulin or sulfonylureas.9 Apart from the inhibitory effect of sulfonylureas on inflammasome assembly, evidence is sparse on their association with immune regulation.35 Thus, while the mechanisms underlying the association of different GLDs with infection remain unclear,38 our results support metformin as the preferred first-line drug in treatment algorithms from the point of view of infections.
The main strengths of our study are its population-based design, the large nationwide cohort of patients with type 2 diabetes and virtually no loss to follow-up (<1%). The use of high-quality medical databases to identify infections treated in the community and in the hospital setting ensured inclusion of nearly all diagnosed infections.
Nonetheless, observational studies of the comparative effects of diabetes drugs have several major methodological challenges.39 Therefore, our results for different therapies should be interpreted with caution, bearing in mind the limitations of this routine registry-based study. A main limitation was lack of accurate data on clinical severity of diabetes, which might have led to residual confounding by indication.40 Nevertheless, increased clinical severity of type 2 diabetes (including complications such as early signs of renal disease, or indicators of less insulin production), other contraindications to metformin and/or anticipated worse glucose derangement may have led physicians to initiate treatment with sulfonylurea and particularly insulin instead of metformin. This may be supported by our observation that sulfonylurea and insulin initiators had more subsequent therapy shifts than metformin initiators, possibly related to glycaemic control problems. However, our regional subcohort analysis suggested that differences in pre-treatment HbA1c (highest with insulin initiation) did not explain observed drug differences. It is also possible that a pre-existing predisposition to infections may have led physicians to choose insulin versus other drugs as initial pharmacotherapy. Furthermore, unmeasured confounding due to combination of other factors such as those related to unhealthy lifestyle and less social support might have influenced the risk of infections. Our sensitivity analyses suggested that the observed weak associations between non-metformin GLDs and increased antibiotic use may have been explained by differences in smoking, although on the other hand, differences in BMI and baseline HbA1c may have led to an underestimation of the associations.
Our results for infections treated in the hospital suggest either increased severity of infections associated with specific GLDs or a lower threshold for hospitalising a patient with a given infection, for example, due to anticipated problems with glycaemic control or more comorbidity/frailty among patients in these treatment groups (surveillance bias). However, since we observed consistent results for hospitalisations for severe infections, such as septicaemia, for which all patients are likely to receive inpatient care, it is unlikely that our results can be explained by increased surveillance alone. As well, the initial GLD therapy choice may be altered, which may lead to increasing exposure misclassification with longer follow-up periods. However, we observed that less than one-quarter of our patients changed therapy within the first year of starting treatment with an antidiabetic drug. Changes were most likely for patients treated with insulin and sulfonylurea and thus unlikely to explain their increased infection risk compared with metformin users.
In conclusion, the present study provides evidence that rates of infection are high in type 2 diabetes patients during early treatment, and that pharmacotherapy initiation with metformin may be associated with reduced risk of hospital-treated infections, compared with other GLDs.
Footnotes
Twitter: Follow Anil Mor at @dr_anilmor
Contributors: AM, IP, HTS and RWT designed the study. IP advised on the design and implementation of the data analysis. AM did the data analysis. AM, IP and RWT wrote the report. HTS, IP and RWT contributed to interpretation of results. All authors revised the manuscript for intellectual content and approved the final version for submission. AM is the guarantor of this work and, as such, had full access to all the study data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This work was supported by the Danish Centre for Strategic Research in Type 2 Diabetes (DD2) and the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck Foundation and the Novo Nordisk Foundation (HTS).
Competing interests: The DD2 is supported by the Danish Agency for Science (grant numbers 09-067009 and 09-075724), the Danish Health and Medicines Authority, the Danish Diabetes Association and an unrestricted donation from Novo Nordisk A/S. Project partners are listed on the website http://www.DD2.nu.
Ethics approval: The Danish Data Protection Agency approved the study (record numbers 2012-41-0793 and 2013-41-1924). As this registry-based study did not include human biological material, approval by the Danish Scientific Ethical Committee was not needed, according to Danish legislation.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Sharma M, Nazareth I, Petersen I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: a retrospective cohort study. BMJ Open 2016;6:e010210 10.1136/bmjopen-2015-010210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mor A, Berencsi K, Svensson E et al. Prescribing practices and clinical predictors of glucose-lowering therapy within the first year in people with newly diagnosed Type 2 diabetes. Diabet Med 2015;32:1546–54. 10.1111/dme.12819 [DOI] [PubMed] [Google Scholar]
- 3.Seshasai SR, Kaptoge S, Thompson A et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–41. 10.1056/NEJMoa1008862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomsen R, Mor A. Diabetes and risk of community-acquired respiratory tract infection, urinary tract infections, and bacteraemia: a review. Open Infect Dis J 2012;6:27–39. 10.2174/1874279301206010027 [DOI] [Google Scholar]
- 5.Duncan AI, Koch CG, Xu M et al. Recent metformin ingestion does not increase in-hospital morbidity or mortality after cardiac surgery. Anesth Analg 2007;104:42–50. 10.1213/01.ane.0000242532.42656.e7 [DOI] [PubMed] [Google Scholar]
- 6.Shih CJ, Wu YL, Chao PW et al. Association between use of oral anti-diabetic drugs and the risk of sepsis: a nested case–control study. Sci Rep 2015;5:15260 10.1038/srep15260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christiansen CF, Johansen MB, Christensen S et al. Preadmission metformin use and mortality among intensive care patients with diabetes: a cohort study. Crit Care 2013;17:R192 10.1186/cc12886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garnett JP, Baker EH, Naik S et al. Metformin reduces airway glucose permeability and hyperglycaemia-induced Staphylococcus aureus load independently of effects on blood glucose. Thorax 2013;68:835–45. 10.1136/thoraxjnl-2012-203178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekström N, Schiöler L, Svensson AM et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open 2012;2:e001076 10.1136/bmjopen-2012-001076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt M, Schmidt SA, Sandegaard JL et al. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johannesdottir SA, Horváth-Puhó E, Ehrenstein V et al. Existing data sources for clinical epidemiology: the Danish national database of reimbursed prescriptions. Clin Epidemiol 2012;4:303–13. 10.2147/CLEP.S37587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol 2014;29:541–9. 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 13.Thomsen RW, Sørensen HT. Using registries to identify type 2 diabetes patients. Clin Epidemiol 2014;7:1–3. 10.2147/CLEP.S75572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carstensen B, Kristensen JK, Ottosen P et al. The Danish national diabetes register: trends in incidence, prevalence and mortality. Diabetologia 2008;51:2187–96. 10.1007/s00125-008-1156-z [DOI] [PubMed] [Google Scholar]
- 15.Gahrn-Hansen B, Gerstoft J, Helweg-Larsen J et al. Vejledning i brug af antibiotika 2015. http://pro.medicin.dk/Specielleemner/Emner/318019 (accessed 21 Jul 2015).
- 16.Kaspersen KA, Pedersen OB, Petersen MS et al. Obesity and risk of infection: results from the danish blood donor study. Epidemiology 2015;26:580–9. 10.1097/EDE.0000000000000301 [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 18.Thomsen RW, Riis A, Nørgaard M et al. Rising incidence and persistently high mortality of hospitalized pneumonia: a 10-year population-based study in Denmark. J Intern Med 2006;259:410–17. 10.1111/j.1365-2796.2006.01629.x [DOI] [PubMed] [Google Scholar]
- 19.Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. Int J Epidemiol 1992;21:837–41. 10.1093/ije/21.5.837 [DOI] [PubMed] [Google Scholar]
- 20.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf 2006;15:291–303. 10.1002/pds.1200 [DOI] [PubMed] [Google Scholar]
- 21.Ulrichsen SP, Mor A, Svensson E et al. Lifestyle factors associated with type 2 diabetes and use of different glucose-lowering drugs: cross-sectional study. PLoS ONE 2014;9:e111849 10.1371/journal.pone.0111849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuorti JP, Butler JC, Farley MM et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med 2000;342:681–9. 10.1056/NEJM200003093421002 [DOI] [PubMed] [Google Scholar]
- 23.Blix HS, Hjellvik V, Litleskare I et al. Cigarette smoking and risk of subsequent use of antibacterials: a follow-up of 365,117 men and women. J Antimicrob Chemother 2011;66:2159–67. 10.1093/jac/dkr273 [DOI] [PubMed] [Google Scholar]
- 24.Karagiannis T, Paschos P, Paletas K et al. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ 2012;344:e1369 10.1136/bmj.e1369 [DOI] [PubMed] [Google Scholar]
- 25.Umpierrez G, Tofé Povedano S, Pé et al. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care 2014;37:2168–76. 10.2337/dc13-2759 [DOI] [PubMed] [Google Scholar]
- 26.Hamilton EJ, Martin N, Makepeace A et al. Incidence and predictors of hospitalization for bacterial infection in community-based patients with type 2 diabetes: the fremantle diabetes study. PLoS ONE 2013;8:e60502 10.1371/journal.pone.0060502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis TM, Weerarathne T, Foong Y et al. Community-acquired infections in type 2 diabetic patients and their nondiabetic partners. The Fremantle Diabetes Study. J Diabetes Complications 2005;19:259–63. 10.1016/j.jdiacomp.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 28.Benfield T, Jensen JS, Nordestgaard BG. Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia 2007;50:549–54. 10.1007/s00125-006-0570-3 [DOI] [PubMed] [Google Scholar]
- 29.Thomsen RW, Riis AH, Kjeldsen S et al. Impact of diabetes and poor glycaemic control on risk of bacteraemia with haemolytic streptococci groups A, B, and G. J Infect 2011;63:8–16. 10.1016/j.jinf.2011.05.013 [DOI] [PubMed] [Google Scholar]
- 30.McKane CK, Marmarelis M, Mendu ML et al. Diabetes mellitus and community-acquired bloodstream infections in the critically ill. J Crit Care 2014;29:70–6. 10.1016/j.jcrc.2013.08.019 [DOI] [PubMed] [Google Scholar]
- 31.Vanhorebeek I, Langouche L, Van den Berghe G. Glycemic and nonglycemic effects of insulin: how do they contribute to a better outcome of critical illness? Curr Opin Crit Care 2005;11:304–11. 10.1097/01.ccx.0000170506.61281.94 [DOI] [PubMed] [Google Scholar]
- 32.Thomsen RW, Baggesen LM, Svensson E et al. Early glycaemic control among patients with type 2 diabetes and initial glucose-lowering treatment: a 13-year population-based cohort study. Diabetes Obes Metab 2015;17:771–80. 10.1111/dom.12484 [DOI] [PubMed] [Google Scholar]
- 33.Sun Q, Li J, Gao F. New insights into insulin: the anti-inflammatory effect and its clinical relevance. World J Diabetes 2014;5:89–96. 10.4239/wjd.v5.i2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyun E, Ramachandran R, Hollenberg MD et al. Mechanisms behind the anti-inflammatory actions of insulin. Crit Rev Immunol 2011;31:307–40. 10.1615/CritRevImmunol.v31.i4.30 [DOI] [PubMed] [Google Scholar]
- 35.Koh GC, Maude RR, Schreiber MF et al. Glyburide is anti-inflammatory and associated with reduced mortality in melioidosis. Clin Infect Dis 2011;52:717–25. 10.1093/cid/ciq192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park DW, Jiang S, Tadie JM et al. Activation of AMPK enhances neutrophil chemotaxis and bacterial killing. Mol Med 2013;19:387–98. 10.2119/molmed.2013.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearce EL, Walsh MC, Cejas PJ et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 2009;460:103–7. 10.1038/nature08097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koh GC, Peacock SJ, van der Poll T et al. The impact of diabetes on the pathogenesis of sepsis. Eur J Clin Microbiol Infect Dis 2012;31:379–88. 10.1007/s10096-011-1337-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patorno E, Patrick AR, Garry EM et al. Observational studies of the association between glucose-lowering medications and cardiovascular outcomes: addressing methodological limitations. Diabetologia 2014;57:2237–50. 10.1007/s00125-014-3364-z [DOI] [PubMed] [Google Scholar]
- 40.Signorello LB, McLaughlin JK, Lipworth L et al. Confounding by indication in epidemiologic studies of commonly used analgesics. Am J Ther 2002;9:199–205. 10.1097/00045391-200205000-00005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-011523supp_appendix1.pdf (111.1KB, pdf)
bmjopen-2016-011523supp_appendix2.pdf (130.3KB, pdf)
bmjopen-2016-011523supp_tables.pdf (239.9KB, pdf)