Abstract
Background
Spontaneous coronary artery dissection (SCAD) is an uncommon but serious condition presenting as an acute coronary syndrome (ACS) or cardiac arrest. The pathophysiology and outcomes are poorly understood. We investigated the characteristics and outcomes of patients presenting with SCAD.
Methods
In a retrospective study of a large cohort of patients with SCAD, data were collected regarding clinical presentation, patient characteristics, vascular screening, coronary artery involvement and clinical outcomes.
Results
40 patients with SCAD (95% women, mean age 45±10 years) were included. At least 1 traditional cardiovascular risk factor was present in 40% of patients. Migraine was reported in 43% of patients. Events preceding SCAD included parturition (8%), physical stress (13%), emotional stress (10%) and vasoconstrictor substance-use (8%). 65% of patients had a non-ST elevation ACS (NSTEACS) at presentation, 30% had an ST elevation myocardial infarction (STEMI) and 13% had a cardiac arrest. The left anterior descending artery was most frequently involved (68% of patients), and 13% had involvement of multiple coronary territories. Fibromuscular dysplasia (FMD) was identified in 7 (37%) of 19 patients screened. 68% of patients were managed medically, 30% had percutaneous coronary intervention and 5% had coronary artery bypass grafting. Over a median 16-month follow-up period, 8% of patients had at least 1 recurrent SCAD event. There were no deaths.
Conclusions
Patients with SCAD in this study often had multiple coronary territories involved (13%) and extracardiac vascular abnormalities, suggesting a systemic vascular process, which may explain the high incidence of migraine. All patients with SCAD should be screened for FMD and followed closely due to the possibility of recurrence.
Keywords: CORONARY ARTERY DISEASE
Key questions.
What is already known about this subject?
Spontaneous coronary artery dissection (SCAD) tends to occur in younger female patients and has a strong association with extracoronary fibromuscular dysplasia. A recent association has also been reported between SCAD and migraine.
What does this study add?
Our study strengthens the association between spontaneous coronary artery dissection (SCAD) and migraine, which was strictly defined using the International Headache Society criteria. Furthermore, this study describes the experience of SCAD in a real-world setting where there is a low rate and a lack of standardisation with respect to screening for fibromuscular dysplasia, and recurrent SCAD events occur, affecting multiple coronary territories.
How might this impact on clinical practice?
There should be systematic screening of renal and carotid arteries in patients presenting with spontaneous coronary artery dissection, and patients should be followed up closely in the outpatient setting to monitor for recurrent coronary events.
Introduction
Spontaneous coronary artery dissection (SCAD) is an uncommon cause of acute coronary syndrome (ACS) when compared with atherosclerotic plaque rupture, however should no longer be considered rare. In one recent study, 4% of patients presenting with ACS had SCAD on optical coherence tomography (OCT).1 SCAD typically occurs in patients without conventional cardiovascular risk factors, who are predominantly woman (82–98% of SCAD cases) with an average age in their early 40s to early 50s.2–5 SCAD was found to account for 22.5% of women younger than 60 years old with ACS in one recent single-centre study.6 The pathogenesis of SCAD remains incompletely understood. There are two distinct recognised aetiologies of SCAD that may be clinically indistinguishable. A tear in the intimal layer of an artery leading to the creation of a true and false arterial lumen or an intramural haematoma due to disruption of the vasa vasorum with the intima remaining intact.4 7 Myocardial ischaemia may arise with ongoing bleeding into and expansion of the false lumen, or expansion of the intramural haematoma causing external compression of the true arterial lumen.7–10 There is no single unifying disease process leading to SCAD, though there is an association with systemic vascular, inflammatory and connective tissue disorders,3 9 including fibromuscular dysplasia (FMD),4 for which a genetic variant has recently been identified.11
The lack of trial data on SCAD makes its management challenging. Medical management is generally recommended with percutaneous coronary revascularisation reserved for patients who have ongoing ischaemia or those who are haemodynamically unstable.12 Moreover, the outcome of patients presenting with SCAD is not well established. We therefore aimed to investigate, in patients presenting with SCAD, the associated medical issues and the clinical outcomes.
Methods
Study population
This is a retrospective study of patients with SCAD from 31 Australian hospitals, identified using either patient records, including discharge summaries, cardiac catheter reports and cardiologist letters, after diagnosis by the treating cardiologists over a period of 3 years, or identified as a result of a social media survey. The latter self-identified SCAD patients completed two questionnaires, which sought relevant information on the year of SCAD, arteries affected, year(s) of subsequent SCAD, management (medical, percutaneous or surgical intervention), risk factors (traditional and novel risk factors including migraine, carotid dissection and aneurysms) and coronary or vascular outcomes. All patients provided consent for their medical information to be included in the study and provided access to their medical records for validation of the data provided in the surveys. The Human Research and Ethics Committee (HREC) of St Vincent's Hospital approved this study.
Clinical characteristics
De-identified data were collected by the administration of the online support group, which included basic demographics, cardiovascular risk factors, associated comorbidities and clinical presentation. The clinical presentation and investigation results were verified from the patient's hospital medical record or letters from the treating cardiologist. The diagnosis of migraine was later confirmed by contacting all patients to ensure that they met the criteria for migraine without aura or migraine with aura from the International Headache Society.13
SCAD diagnosis and coronary territory
A diagnosis of SCAD and the coronary territory or territories affected was made by the treating interventional cardiologist at the time patients presented with an ACS or cardiac arrest, and the cardiologist's records were reviewed by the study authors. SCAD was diagnosed in patients without possible coronary artery trauma who had angiographic features of type 1, 2 or 3 SCAD. Type 1 SCAD was defined as an intimal flap with multiple lumens and contrast staining of the vessel wall. Type 2 SCAD was defined as formation of an intramural haematoma causing a smooth, long stenosis, and type 3 SCAD as an intramural haematoma causing a more focal stenosis.3 SCAD patients in this cohort were not systematically evaluated by intravascular ultrasound (IVUS) or OCT. The decision to perform percutaneous coronary intervention (PCI), or not, was made by the treating interventional cardiologist.
Outcome data
Further SCAD episodes and vascular events were identified through standardised surveys completed by the patient and letters from the treating cardiologist.
Statistics
All statistical analyses were performed using SPSS V.23 (IBM, Armonk, New York, USA). The cohort demographics were characterised using descriptive statistics. Continuous data are presented as mean±SD. Categorical data are presented as numbers (percentages). Fisher's exact test was used to compare categorical data. To better understand the association of migraine and FMD with SCAD, we used a χ2 test to compare the prevalence of these factors within our patient cohort with that in the general population as reported in the journals Neurology and Clinical Journal of the American Society of Nephrology.14 15 Cramer's V was used to test the association between migraine and FMD. The Mann-Whitney U test was used to compare recurrent coronary event status versus age. All statistical tests were two-tailed and a p value of <0.05 was considered statistically significant. A Kaplan-Meier curve was generated to graphically represent the cumulative rate of events on follow-up.
Results
Clinical characteristics
A total of 40 patients with SCAD were included. The average age was 45±10 years and 95% were woman (table 1).
Table 1.
Clinical characteristics
| Age (years±SD) | 45±10 |
| Sex | |
| Female | 38 (95%) |
| Male | 2 (5%) |
| Cardiovascular risk factors | |
| Hypertension | 7 (18%) |
| Smoking | 3 (8%) |
| Dyslipidaemia | 4 (10%) |
| Diabetes | 2 (5%) |
| Family history | 11 (28%) |
| Other conditions | |
| Migraine | 17 (43%) |
| Carotid dissection | 1 (2.5%) |
| Extracoronary aneurysm | 2 (5%) |
| Polycystic kidney disease | 1 (2.5%) |
| Preceding events | |
| Post partum | 3 (7.5%) |
| Preceding physical stress | 5 (12.5%) |
| Preceding emotional stress | 4 (10%) |
| Intravenous methamphetamine use | 1 (2.5%) |
| Vasoconstrictor drug use | 2 (5%) |
| Clinical presentation | |
| STEMI | 12 (30%) |
| NSTEACS | 26 (65%) |
| Cardiac arrest | 5 (12.5%) |
| Coronary vessel involvement | |
| One | 35 (87.5%) |
| Two | 4 (10%) |
| Three | 1 (2.5%) |
| Coronary territory | |
| Left main | 1 (2.5%) |
| LAD system | 27 (67.5%) |
| LAD | 26 |
| Diagonal vessel | 1 |
| Circumflex | 10 (25%) |
| Circumflex | 5 |
| Marginal vessel | 5 |
| RCA | 7 (17.5%) |
| RCA | 3 |
| RCA branches | 4 |
| Ramus intermediate | 1 (2.5%) |
Percentages are expressed as a proportion of the total SCAD population (n=40).
LAD, left anterior descending; NSTEACS, non-ST elevation acute coronary syndrome; RCA, right coronary artery; STEMI, ST elevation myocardial infarction.
Of the 40 patients with SCAD, 24 (60%) had no traditional cardiovascular risk factors. In the 16 patients with one or more risk factors, the most prevalent was a family history of ischaemic heart disease (IHD) with a first- or second-degree relative having had a myocardial infarction or been diagnosed with IHD in 11 (28%) cases. Of the 40 patients with SCAD, 7 (18%) had hypertension, 4 (10%) dyslipidaemia, 3 (8%) were current smokers and 2 (5%) had diabetes. Migraine headache was reported in 17 (43%) of the patients with SCAD (table 1). Two patients had extracoronary aneurysms and one patient each had polycystic kidney disease and a carotid dissection.
Three (8%) patients had their SCAD events in the postpartum period (9 hours, 9 days and 14 days post partum). Other relevant preceding events or contributing factors included physical stress in five (13%), emotional stress in four (10%), intravenous methamphetamine use in one and vasoconstrictor drug use (ergotamine and sumatriptan) in two patients (table 1).
SCAD most frequently presented as an ACS. In 26 patients (65%), this was most manifested as a NSTEACS. In 12 (30%), the presentation was a STEMI. Five (13%) patients with SCAD had a cardiac arrest. Two of these patients were diagnosed with STEMI, one with NSTEACS and two did not have any ECG changes nor troponin rise recognised prior to their arrest.
Coronary territory involvement
Only a single coronary territory was involved in 35 (88%) cases. A further four (10%) had two coronary territories involved, and one patient had three territories affected by SCAD simultaneously at the time of presentation. The left anterior descending (LAD) coronary artery (figure 1A) was most frequently affected in 27 (68%) patients. The circumflex territory (figure 1B) was affected in 10 (25%), right coronary artery in 7 (18%) and 1 patient each had SCAD of the left main and ramus intermediate arteries (table 1). The unaffected segments of coronary arteries were angiographically normal, aside from an old healing dissection of the LAD in a patient presenting with a right coronary artery territory SCAD.
Figure 1.
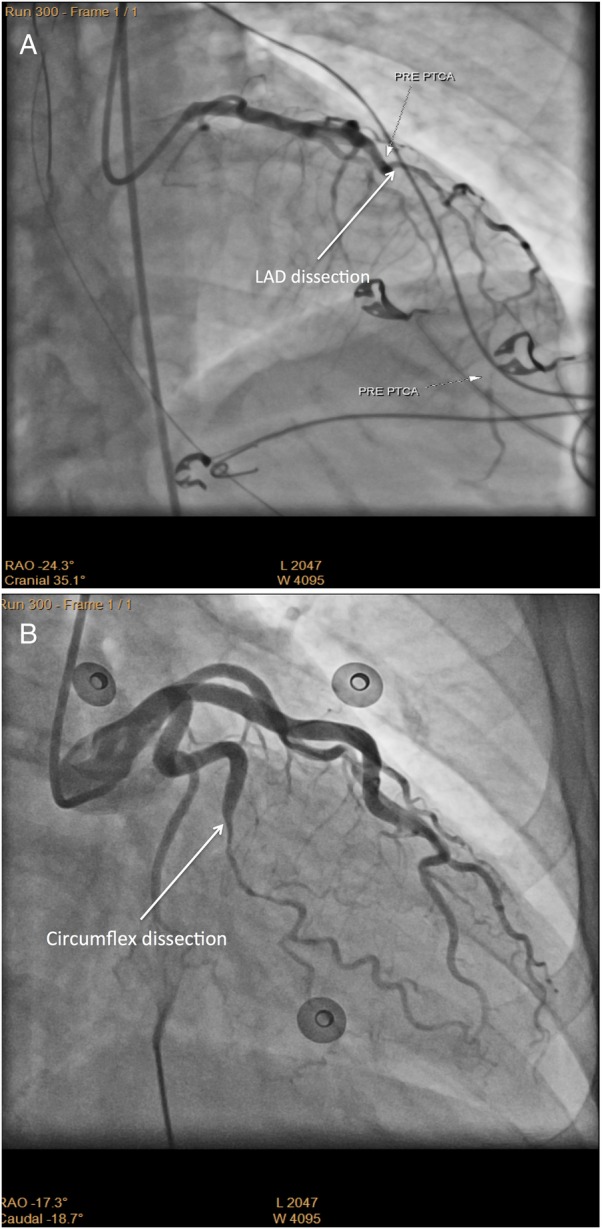
Angiographic findings in spontaneous coronary artery dissection. (A) Coronary angiography of the left anterior descending artery (LAD) demonstrates extensive dissection from proximal to distal involving the proximal and mid-part of a large diagonal branch. (B) Coronary angiography of the obtuse marginal branch of the circumflex artery demonstrates a mid-segment long tubular narrowing consistent with dissection.
Clinical outcomes
Of the 40 patients, 27 (68%) had their initial SCAD managed medically (table 2).
Table 2.
Clinical outcomes
| Management of initial SCAD | |
| Percutaneous coronary intervention | 12 (30%) |
| Medical management | 27 (67.5%) |
| Coronary artery bypass grafting | 2 (5%) |
| Fibromuscular dysplasia | |
| Screened | |
| Positive | 7 (17.5%) |
| Negative | 12 (30%) |
| Not screened | 17 (43%) |
| Uncertain | 4 (10%) |
| Event recurrence | |
| None | 34 (85%) |
| Further SCAD events | 3 (7.5%) |
| Other vascular events | 3 (7.5%) |
| Stent thrombus | 1 (2.5%) |
| Coronary artery aneurysm | 2 (5%) |
| Death | 0 |
Percentages are presented as a proportion of the total SCAD population (n=40).
SCAD, spontaneous coronary artery dissection.
There was no standardised protocol for medical management. The proportion of patients managed medically was not significantly different between the patients presenting with NSTEACS and those presenting with STEMI (p=NS). Medical, interventional and surgical management were not mutually exclusive and patients could have more than one management type. In the 27 medically managed patients, 78% were prescribed a β-blocker, 89% aspirin, 74% an additional antiplatelet agent, 59% an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, 41% a statin and 7% a calcium channel blocker. A PCI was performed in 12 (30%) patients presenting with SCAD. Of these patients, four had cardiac arrest, five had STEMI (one had cardiac arrest and STEMI) and five had NSTEACS (one had cardiac arrest and NSTEACS). Of note, seven patients with STEMI (one with STEMI and cardiac arrest) did not receive a percutaneous intervention. One patient presenting with simultaneous SCAD in three coronary territories received a coronary artery stent for a right coronary artery SCAD and a trial of medical management for the LAD and circumflex SCADs. This patient subsequently required rescue coronary artery bypass grafting following a ventricular fibrillation cardiac arrest. Another patient required immediate two-vessel coronary artery bypass grafting when presenting with LAD and right coronary artery SCADs. Thus, urgent coronary artery bypass grafting was performed in two (5%) patients.
FMD screening was undertaken in 19 (48%) patients presenting with SCAD. The imaging modality used for screening was variable. Duplex ultrasonography was used to scan the carotid arteries in 14 (35%) and the renal arteries in 13 (33%) patients (figure 2A, B). Invasive angiography of the renal arteries was performed in two (5%) patients and CT angiography or magnetic resonance angiography in four (10%) patients. Of the 19 patients who were screened, 7 (37%) patients had a positive screening result for FMD (table 2). The prevalence of FMD in the cohort (screened and unscreened) was 18%. There was no association between migraine and FMD (p=NS), although given that only 19 patients were screened for FMD, the generalisability of this finding is limited.
Figure 2.

Screening for fibromuscular dysplasia (FMD). (A) Duplex ultrasonography of the right renal artery in a patient who presented with a dissection of the obtuse marginal branch of the circumflex coronary artery demonstrates severe stenosis of the mid-section consistent with FMD. (B) Duplex ultrasonography of the distal segment of the left internal carotid artery in the same patient demonstrates mild FMD.
Follow-up events
The follow-up period ranged from 6 months to over 4 years, with a median time of 16 months (IQR 8–28.5 months). In this cohort of patients, three (8%) had at least one recurrent SCAD event each presenting as a myocardial infarction. All episodes of recurrent SCAD occurred in medically managed patients. One patient who presented with a posterior descending artery SCAD had a recurrent SCAD in the marginal branch of the circumflex coronary artery 2 months later (figure 3A, B). Interestingly, at initial presentation, this patient also had evidence of a healing SCAD in her LAD coronary artery, which likely occurred as a silent event several years prior to her presentation. Another patient had multiple recurrent SCAD events in all three coronary artery territories 2 days after, then 4 and 6 years after the initial event in the LAD coronary artery. Another patient had a recurrent SCAD in the right coronary artery territory 5 years after an initial SCAD in the LAD coronary artery. Multiple coronary territories were affected by SCAD in seven (18%) patients, either at presentation or follow-up.
Figure 3.
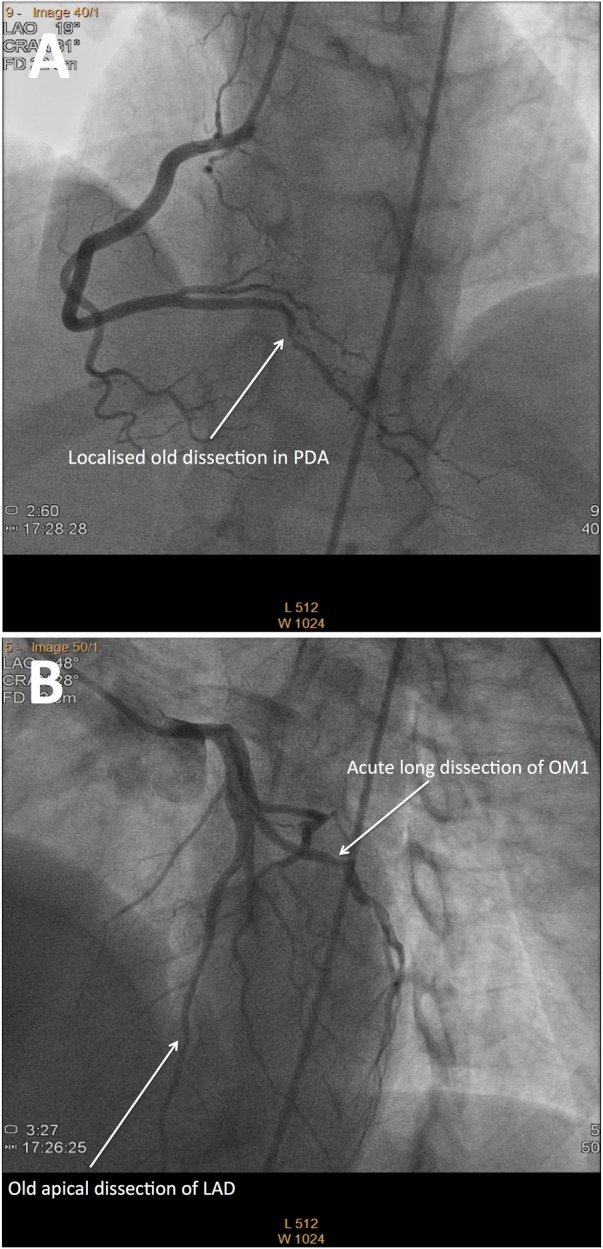
Multiple recurrent spontaneous coronary artery dissections of varying chronicity in one patient. (A) Coronary angiography of the posterior descending artery (PDA) demonstrates diffuse irregularity with a focal ulceration or dissection in its first centimetre from original presentation 2 months prior. (B) Coronary angiography of the left anterior descending artery demonstrates an old healing dissection of the terminal segment and a new dissection of a single large obtuse marginal (OM) branch of the circumflex.
A further three (8%) patients had another coronary vascular event over the follow-up period. The other vascular events included a bare metal stent thrombosis at 5 months post the initial SCAD event, which required emergency coronary artery bypass grafting. Two (5%) patients developed coronary artery aneurysms detected on routine follow-up angiography. In one patient, a right coronary artery aneurysm developed 2 years after the SCAD event and required PCI. A total of four (10%) patients had recurrent myocardial infarction. Recurrent coronary events are graphically represented in the Kaplan-Meier curve (figure 4).
Figure 4.
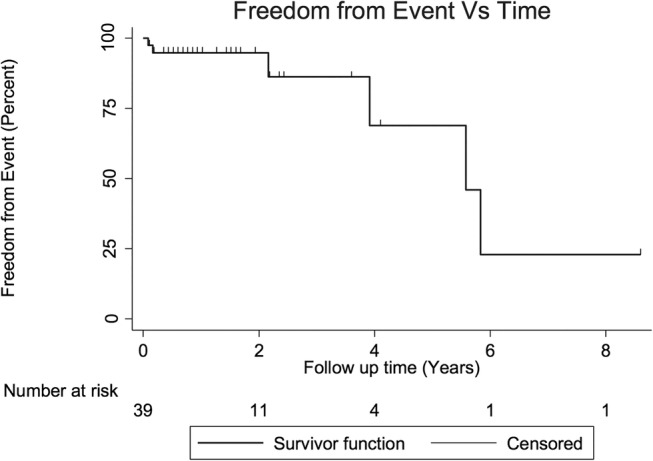
Kaplan-Meier curve of freedom from event. Each step in the curve indicates that a recurrent spontaneous coronary artery dissection (SCAD) or a non-SCAD coronary event has occurred in the cohort. The censored data points represent patients who have been lost to follow-up but had not experienced an additional event when last seen.
The mean age of the six patients with recurrent coronary vascular events was 40 years, compared with the mean age of the patients without recurrent coronary vascular events, which was 46 years (p=NS). All patients with recurrent events had evidence of LAD involvement at presentation compared with 62% of patients without recurrent events; however, this was not significant (p=NS). Half of the patients with recurrent coronary vascular events had two territories involved at initial presentation.
Discussion
We have demonstrated that patients with SCAD are predominantly middle-aged women, with a relatively low incidence of traditional risk factors for IHD and an 8% recurrence rate of SCAD. Of the SCAD cases, 13% had multiple coronary territories involved at presentation and patients with SCAD had a high proportion of renal and carotid features in keeping with FMD. We believe these findings suggest that at least in some instances, and probably not uncommonly, SCAD may be part of a systemic vascular disease process.
The average age of patients in our study was 45 years at the time of their first SCAD event and 98% were woman, which is similar to previously reported cohorts.2 4 Traditional cardiovascular risk factors were relatively uncommon in the patients in our study with 60% of patients having no risk factors. By comparison, the population 5-year risk of cardiovascular disease for Australian women in the age group of 45–54 years without hypertension, diabetes, smoking or hypercholesterolaemia is <5%.16 This finding is in keeping with the risk profile of non-atherosclerotic SCAD patients previously described by Saw et al.3 Therefore, SCAD should be considered as a differential diagnosis in younger, female patients who present with an ACS, particularly in the postpartum period.
Multiple coronary territory involvement, the frequent finding of extracardiac vascular disease and association with migraine suggest that SCAD is part of a systemic vascular disease process and not merely a localised weakness in the wall of a single coronary artery. Over the duration of the study, 18% of patients with SCAD had more than one coronary territory affected, either at presentation or during the follow-up period. The major associated vascular condition is FMD. Polycystic kidney disease has a reported association with SCAD and one patient in our cohort had polycystic kidney disease.3 SCAD also occurs not infrequently in the peri-partum period, and in association with hormonal fluxes.17 In the current cohort, 8% of patients had postpartum SCAD. In 23% of patients in our study, a physical or emotional stress preceded their SCAD event. This is lower than previous reports, where up to half of patients with SCAD have an antecedent physical or emotional stress.3 Over 40% of the current cohort suffered from migraine headaches,13 a higher proportion than the population prevalence of 12% (p<0.001).14 In a previous study by Saw et al,3 a migraine history was reported at a similar incidence; 37.5% of patients presenting with SCAD. This association is not surprising, given that migraine has a reported association with cerebrovascular FMD.18 This association may be due to the shared genetic basis of migraine and FMD through the phosphatase and actin regulator 1 (PHACTR1) gene.11 Structural endothelial vulnerabilities and impaired vasomotor tone are possible mechanisms for migraine development in this patient group via the functions of the phosphatase and actin regulator 1 protein.19 To date, there is no association of SCAD with this genetic variant. In two patients, SCAD was preceded by the use of vasoconstrictor agents that had been prescribed to treat migraine.
Our cohort prevalence of FMD of 18% represents a significantly higher proportion than previously reported in the population, at 3% (p<0.001).15 In the current study, the screening rate for FMD was low, with less than half of the patients undergoing FMD screening. When FMD screening was performed, it was often incomplete with only one extracoronary vascular bed being investigated. This is despite strong evidence of an association between FMD and SCAD. In systematically screened SCAD populations (renal, carotid and iliac vascular beds), the incidence of FMD is 50–86%.3 4 20 Despite the high proportion of SCAD patients with FMD, myocardial infarction remains an uncommon event in FMD patients, affecting only 3% of patients in the US FMD Registry.21 Screening systematically for FMD in patients presenting with SCAD remains important for prognostication. FMD in the renal arteries may contribute to the development of hypertension, while in the carotid arteries, it may predispose to stroke. None of the patients in the current study reported a history of stroke and none had a stroke in the follow-up period.
The distribution of SCAD across coronary territories in this study is similar to that described in the literature, with the greatest proportion occurring in the LAD coronary artery and its branches.4 12 Angiographic support for a diagnosis of SCAD includes the distribution in the artery affecting the mid to distal portion (compared with proximal for atherosclerosis), absence of atherosclerotic disease or calcification and tortuosity of the coronary vessels.22 In the situation where there is uncertainty regarding a diagnosis of SCAD on coronary angiography and a high clinical index of suspicion, it is suggested that the patients be further investigated by IVUS or OCT to better assess the arterial wall and establish a diagnosis of SCAD.7 8 23 24 These techniques should be used with caution; however, since, as with percutaneous intervention in this patient group, this might result in extension of the coronary artery dissection.
The majority of SCAD patients in the study were managed medically; albeit that this management was highly variable among the patients. Most patients received aspirin, a second antiplatelet agent and a β-blocker. There was no apparent difference in the medical management of the patients who had recurrent SCAD episodes and the broader cohort, and unfortunately no data were available on patient compliance. Optimal medical management of SCAD has not been described but often includes short-term inpatient cardiac monitoring and administration of an antiplatelet agent, a β-blocker and, if dyslipidaemia is diagnosed, a statin.7 10 24 25 These treatments are largely empirical and have not been studied in prospective clinical trials of SCAD patients.25 A conservative treatment approach should be the default management option in patients with SCAD as there are high rates of complications from PCI and similar 5-year outcomes when compared with conservative treatment.25 26 In unstable patients or those with ongoing ischaemia, revascularisation of the coronary artery in the form of PCI or surgery may be required. The complication rate from PCI in SCAD is high due to vessel fragility and technical difficulties.2 24 A retrospective study of 189 patients presenting with their first SCAD event, of whom 87 underwent initial PCI, reported a PCI failure rate of 53%.26
At follow-up, 8% of our patients had at least one new coronary dissection. An additional 8% of patients had another coronary vascular event at follow-up. Patients with SCAD therefore require close outpatient surveillance. Early recurrence is commonly due to extension of dissection in the affected coronary artery, whereas recurrence more than 2 months after the initial SCAD tends to be in a remote coronary vessel.27 SCAD recurrence was reported at 6% in a recent small retrospective study.28 Another retrospective study of 75 patients reported a recurrence rate of 24% over a 15-year period.29 SCAD recurrence was 13% over a variable follow-up period in a mixed retrospective (median follow-up 6.9 years) and prospective (median follow-up 0.8 years) cohort.3 A prospective study of 115 patients with SCAD found a recurrence rate of 28% over a median follow-up of 21 months.5
Limitations of this study include the retrospective study design with variable follow-up period and the lack of a control group. There was a low rate of screening for FMD and a non-standardised screening approach. The majority of patients screened had ultrasound screening for FMD, which we acknowledge is highly operator-dependent, requiring adequate skill in detecting FMD in the distal carotid/renal vessels, hence resulting in the low overall sensitivity. Central adjudication of the diagnostic angiograms was not able to be performed as participants spanned a large area of Australia. In the light of these limitations, a prospective controlled study is needed to evaluate the association of SCAD with FMD, other systemic diseases and appropriate treatment strategies. Although there were no deaths in our study, by virtue of the fact that many cases were accrued as a result of self-reporting to a support group, patients where SCAD caused death would not have been captured unless a relative reported this to the support group. Hence, the frequency of SCAD-induced death remains unclear, but is likely not insignificant.
Conclusion
In a retrospective study of 40 patients with SCAD, the vast majority were women, with a low rate of risk factors for IHD. There was a strong association of SCAD with FMD, although only 48% of the cohort was screened. An interesting association is that with migraine headache, which may be pathophysiologically linked. We recommend that all patients with SCAD are screened for FMD and close follow-up is required given the risk of recurrent events. Further studies are needed to assess the pathogenesis, association with migraines and appropriate management strategies.
Footnotes
Contributors: LM-C performed the literature review, collated the data and wrote the original research article titled Outcomes of Patients with Spontaneous Coronary Artery Dissection. PMK collected data from study participants through the use of questionnaires. SE performed all statistical analyses. DWMM, head of Interventional Cardiology at St Vincent’s Hospital, provided cases to the study and corrections to the manuscript, in particular reviewing the coronary angiographic images. RMG, Executive Director Victor Chang Cardiac Research Institute, and CJH, Cardiologist St Vincent’s Hospital, were senior authors on the study. They jointly determined the direction and focus of the study and provided regular revisions to the manuscript.
Competing interests: None declared.
Ethics approval: The Human Research and Ethics Committee (HREC) of St Vincent's Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Nishiguchi T, Tanaka A, Ozaki Y et al. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2016;5:263–70. 10.1177/2048872613504310 [DOI] [PubMed] [Google Scholar]
- 2.Tweet MS, Hayes SN, Pitta SR et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012;126:579–88. 10.1161/CIRCULATIONAHA.112.105718 [DOI] [PubMed] [Google Scholar]
- 3.Saw J, Aymong E, Sedlak T et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014;7:645–55. 10.1161/CIRCINTERVENTIONS.114.001760 [DOI] [PubMed] [Google Scholar]
- 4.Saw J, Ricci D, Starovoytov A et al. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013;6:44–52. 10.1016/j.jcin.2012.08.017 [DOI] [PubMed] [Google Scholar]
- 5.Prasad M, Tweet MS, Hayes SN et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol 2015;115:1672–7. 10.1016/j.amjcard.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 6.Rashid HN, Wong DT, Wijesekera H et al. Incidence and characterisation of spontaneous coronary artery dissection as a cause of acute coronary syndrome—a single-centre Australian experience. Int J Cardiol 2016;202:336–8. 10.1016/j.ijcard.2015.09.072 [DOI] [PubMed] [Google Scholar]
- 7.Alfonso F, Bastante T. Spontaneous coronary artery dissection: novel diagnostic insights from large series of patients. Circ Cardiovasc Interv 2014;7:638–41. 10.1161/CIRCINTERVENTIONS.114.001984 [DOI] [PubMed] [Google Scholar]
- 8.Vrints CJM. Spontaneous coronary artery dissection. Heart 2010;96:801–8. 10.1136/hrt.2008.162073 [DOI] [PubMed] [Google Scholar]
- 9.Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013;29:1027–33. 10.1016/j.cjca.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 10.Giacoppo D, Capodanno D, Dangas G et al. Spontaneous coronary artery dissection. Int J Cardiol 2014;175:8–20. 10.1016/j.ijcard.2014.04.178 [DOI] [PubMed] [Google Scholar]
- 11.Kiando SR, Tucker N, Katz A et al. Genetic study identifies common variation in phactr1 to associate with fibromuscular dysplasia (Best of Basic Science Abstract). Circulation 2015;132:A15370. [Google Scholar]
- 12.Alfonso F, Paulo M, Lennie V et al. Spontaneous coronary artery dissection: long-term follow-up of a large series of patients prospectively managed with a ‘conservative’ therapeutic strategy. JACC Cardiovasc Interv 2012;5:1062–70. 10.1016/j.jcin.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 13.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders 3rd edition (beta version). Cephalalgia 2013;33:629–808. 10.1177/0333102413485658 [DOI] [PubMed] [Google Scholar]
- 14.Lipton RB, Bigal ME, Diamond M et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68:343–9. 10.1212/01.wnl.0000252808.97649.21 [DOI] [PubMed] [Google Scholar]
- 15.Lorenz EC, Vrtiska TJ, Lieske JC et al. Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clin J Am Soc Nephrol 2010;5:431–8. 10.2215/CJN.07641009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Vascular Disease Prevention Alliance. Absolute cardiovascular disease risk management. Quick reference guide for health professionals, 2012. [Google Scholar]
- 17.Eng L, Starovoytov A, Heydari M et al. Spontaneous coronary artery dissection in women and association with hormonal stressors. J Am Coll Cardiol 2015;1:B2 10.1016/j.jacc.2015.08.904 [DOI] [Google Scholar]
- 18.Olin JW, Gornik HL, Bacharach JM et al. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation 2014;129:1048–78. 10.1161/01.cir.0000442577.96802.8c [DOI] [PubMed] [Google Scholar]
- 19.Winsvold BS, Nelson CP, Malik R et al. Genetic analysis for a shared biological basis between migraine and coronary artery disease. Neurol Genet 2015;1:e10 10.1212/NXG.0000000000000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eleid MF, Guddeti RR, Tweet MS et al. Coronary artery tortuosity in spontaneous coronary artery dissection angiographic characteristics and clinical implications. Circ Cardiovasc Interv 2014;7:656–62. 10.1161/CIRCINTERVENTIONS.114.001676 [DOI] [PubMed] [Google Scholar]
- 21.Olin JW, Froehlich J, Gu X et al. The United States Registry for Fibromuscular Dysplasia: results in the first 447 patients. Circulation 2012;125:3182–90. 10.1161/CIRCULATIONAHA.112.091223 [DOI] [PubMed] [Google Scholar]
- 22.Saw J, Bezerra H, Gornik HL et al. Angiographic and intracoronary manifestations of coronary fibromuscular dysplasia. Circulation 2016;133:1548–59. 10.1161/CIRCULATIONAHA.115.020282 [DOI] [PubMed] [Google Scholar]
- 23.Saw J, Poulter R, Fung A. Intracoronary imaging of coronary fibromuscular dysplasia with OCT and IVUS. Catheter Cardiovasc Interv 2013;82:E879–83. 10.1002/ccd.24640 [DOI] [PubMed] [Google Scholar]
- 24.Alfonso F, Bastante T, Rivero F et al. Spontaneous coronary artery dissection—from diagnosis to management. Circulation 2014;78:2099–110. 10.1253/circj.CJ-14-0773 [DOI] [PubMed] [Google Scholar]
- 25.Tweet MS, Gulati R, Hayes SN. What clinicians should know about spontaneous coronary artery dissection. Mayo Clin Proc 2015;90:1125–30. 10.1016/j.mayocp.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 26.Tweet MS, Eleid MF, Best PJ et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014;7:777–86. 10.1161/CIRCINTERVENTIONS.114.001659 [DOI] [PubMed] [Google Scholar]
- 27.Main A, Starovoytov A, Aymong A et al. Recurrent spontaneous coronary artery dissection. J Am Coll Cardiol 2016;67:565 10.1016/S0735-1097(16)30566-6 [DOI] [Google Scholar]
- 28.Garcia Bermudez M, Tizon Marcos H, Miranda Guardiola F et al. Incidence, management and prognosis of spontaneous coronary dissection in our center from 2003 to 2015. Eur Heart J Acute Cardiovasc Care 2015;4:230–1. 10.1177/2048872614547689 [DOI] [PubMed] [Google Scholar]
- 29.Shivapour DM, Erwin P, Gornik HL et al. Spontaneous coronary artery dissection: characterizing presentation, management, and outcomes at a large referral cardiovascular center. Circulation 2015;132(Suppl 3):A15492–A15492. [Google Scholar]


