Abstract
Objective
Transcatheter aortic valve implantation (TAVI) is generally more expensive than surgical aortic valve replacement (SAVR) due to the high cost of the device. Our objective was to understand the patient and procedural drivers of cumulative healthcare costs during the index hospitalisation for these procedures.
Design
All patients undergoing TAVI, isolated SAVR or combined SAVR+coronary artery bypass grafting (CABG) at 7 hospitals in Ontario, Canada were identified during the fiscal year 2012–2013. Data were obtained from a prospective registry. Cumulative healthcare costs during the episode of care were determined using microcosting. To identify drivers of healthcare costs, multivariable hierarchical generalised linear models with a logarithmic link and γ distribution were developed for TAVI, SAVR and SAVR+CABG separately.
Results
Our cohort consisted of 1310 patients with aortic stenosis, of whom 585 underwent isolated SAVR, 518 had SAVR+CABG and 207 underwent TAVI. The median costs for the index hospitalisation for isolated SAVR were $21 811 (IQR $18 148–$30 498), while those for SAVR+CABG were $27 256 (IQR $21 741–$39 000), compared with $42 742 (IQR $37 295–$56 196) for TAVI. For SAVR, the major patient-level drivers of costs were age >75 years, renal dysfunction and active endocarditis. For TAVI, chronic lung disease was a major patient-level driver. Procedural drivers of cost for TAVI included a non-transfemoral approach. A prolonged intensive care unit stay was associated with increased costs for all procedures.
Conclusions
We found wide variation in healthcare costs for SAVR compared with TAVI, with different patient-level drivers as well as potentially modifiable procedural factors. These highlight areas of further study to optimise healthcare delivery.
Keywords: Aortic stenosis, health care costs, episode of care, trans-catheter aortic valve implantation, surgical aortic valve replacement
Key questions.
What is already known about this subject?
Transcatheter aortic valve implantation (TAVI) is cost-effective compared with surgical aortic valve replacement (SAVR) for severe aortic stenosis; however, it is on average a more expensive procedure.
What does this study add?
We have identified potentially modifiable factors that drive in-hospital costs for TAVI and SAVR. These include prolonged intensive care unit stay and the use of non-femoral access, for example.
How might this impact on clinical practice?
We have identified potential areas for quality improvement efforts to reduce overall costs, and to improve the efficiency of care delivery for patients with severe aortic stenosis.
Introduction
Valvular heart disease is considered the next cardiovascular epidemic in developed countries, with a prevalence that rises exponentially with the ageing demographic.1 Severe aortic stenosis (AS) is the most common form of valvular disease that requires intervention,2 traditionally with surgical aortic valve replacement (SAVR). Transcatheter aortic valve implantation (TAVI) has rapidly evolved to become the treatment of choice for patients with severe AS, who are either inoperable or at high surgical risk.3 Landmark multicentre randomised controlled trials of TAVI4–10 have shown comparable or superior outcomes compared with SAVR, which has led to the widespread dissemination of this technology.11 Ongoing trials are evaluating the efficacy of TAVI compared with SAVR in low-risk and intermediate-risk AS patient populations.12
As the indications for TAVI expand to lower risk cohorts12 with a corresponding shift in referral patterns from SAVR to TAVI, it is important to understand the relative resource intensity of each procedure. There have been multiple cost-effectiveness analyses that have compared TAVI to SAVR in high-risk cohorts; the majority of these suggest that TAVI is an economic attractive intervention that represents good value.13–16 Investigators have evaluated the impact of complications on the hospitalisation costs associated with TAVI, and have found that complications contribute up to 25% of episode costs.17–20 However, there is a paucity of data on other patient-level and procedural-level drivers of healthcare cost associated with the episode of care for each of these procedures and specifically comparing the drivers for SAVR versus TAVI. Such data are important in order to identify potential modifiable factors, such that the efficiency of healthcare delivery can be improved. In the current economic climate of highly constrained healthcare budgets, it is imperative that the allocation of scarce healthcare resources be optimised.
Accordingly, the objective of our study was to address this gap in knowledge by evaluating healthcare costs associated with the episode of care for patients with severe AS undergoing either SAVR or TAVI in Ontario, Canada, and identifying the drivers of resource usage.
Methods
This retrospective cohort study was conducted in the province of Ontario in Canada. Ontario has a population of ∼13.6 million, all of whom received publicly funded universal medical coverage, provided by a single third party payer, the Ontario Ministry of Health and Long Term Care (MOHLTC).
Data sources
All patients who underwent a SAVR or TAVI procedure from 1 April 2012 to 31 March 2013 were identified through the Cardiac Registry at the Cardiac Care Network (CCN) of Ontario. CCN includes a network of the 19 hospitals that offer invasive cardiac procedures including coronary artery bypass grafting (CABG), TAVI and SAVR.21 22 CCN maintains a registry of all patients who have undergone these procedures, and contains data on patient demographics, comorbidities as well as procedural details including coronary anatomy. Data entry into the CCN Cardiac Registry is a mandatory pre-requisite for provincial funding. The validity of the CCN Cardiac Registry has been evaluated through selected chart audits and core laboratory verification.23 CCN is a prescribed entity under provincial privacy legislation, which permits CCN to collect and use personal patient information without the need for patient consent.
The Ontario Case Costing Initiative (OCCI) is a single source for integrated financial and clinical information that is available via the MOHLTC. Comprehensive primary costing data collection is performed by the accounting/costing centres at hospitals and includes both direct (eg, variable and fixed labour and both general and service-recipient specific supplies) and indirect costs. These are then merged with clinical information, including most responsible diagnosis, case-mix groupers, procedural information and length of hospital stay. During the time period of interest, 7 of the 11 cardiac surgery hospitals (of which 10 performed TAVI and all 11 performed SAVR) in the province were contributing data to OCCI. The cases captured in OCCI represented >50% of TAVI and SAVR volumes in the province.
Patients
We included all patients who underwent TAVI, isolated SAVR and combination SAVR+CABG at the six OCCI participating hospitals from 1 April 2012 to 31 March 2013.
Healthcare costs
Our primary outcome was individual-level cumulative healthcare costs for the index hospitalisation, defined as the acute hospitalisation during which the valve procedure was performed. All costs were reported in 2013 Canadian dollars.
Statistical analysis
Healthcare costs have a number of statistical properties that preclude the use of traditional statistical tools, including a heavily right-skewed distribution.24 We used hierarchical generalised linear models with a logarithmic link and γ distribution to account for these features of costing data.24–26 The models were clustered by hospital. The logarithmic link function restricts predicted costs to positive values, and produces final model coefficients that are straightforward to interpret.27 Specifically, the exponential of the coefficient provides a rate ratio (RR) interpreted as the percentage increase in the mean cost for each unit change in the predictor.
SAS V.9.3 (SAS Institute, Cary, North Carolina, USA) was used for all analyses; p values of <0.05 were considered significant.
Results
Cohort
The cohort consisted of 1310 patients, of whom 585 underwent SAVR, 518 had combination SAVR+CABG, while 207 had TAVI. As seen in table 1, the groups had marked differences in their baseline characteristics, with older and higher risk patients undergoing TAVI. The mean age of patients undergoing isolated SAVR was 67.5 years, with the majority under the age of 75 years. In contrast, the mean age of the patients undergoing TAVI was 81.7 years, and they had a substantially higher comorbidity burden, including prevalence of lung disease, peripheral vascular disease and renal dysfunction, consistent with these being high-risk or inoperable patients.
Table 1.
Baseline characteristics
| Total (n=1310) | Isolated SAVR (n=585) | SAVR with CABG (n=518) | TAVI (n=207) | p Value | |
|---|---|---|---|---|---|
| Patient-level factors | |||||
| Demographics | |||||
| Age | |||||
| 20–74 | 49.4 | 66.5 | 43.8 | 15.0 | <0.0001 |
| 75+ | 50.6 | 33.5 | 56.2 | 85.0 | <0.0001 |
| Mean±SD (years) | 72.4±11.3 | 67.5±12.2 | 74.3±8.4 | 81.7±7.2 | |
| Female | 36.3 | 39.5 | 29.2 | 44.9 | <0.0001 |
| Medical comorbidities | |||||
| COPD | 9.4 | 6.3 | 9.7 | 17.4 | <0.0001 |
| CVD | 11.4 | 7.4 | 12.2 | 20.8 | <0.0001 |
| PVD | 12.8 | 5.6 | 16.2 | 24.6 | <0.0001 |
| BMI* | 0.0002 | ||||
| Underweight/normal | 21.1 | 20.2 | 20.1 | 26.1 | |
| Overweight | 71.3 | 70.8 | 75.7 | 61.8 | |
| Missing | 7.6 | 9.1 | 4.2 | 12.1 | |
| Renal function | <0.0001 | ||||
| Creatinine 0–140 μmol/L | 82.8 | 84.1 | 86.5 | 70.0 | |
| Creatinine >140 μmol/L | 10.3 | 7.9 | 10.0 | 17.9 | |
| Missing | 6.9 | 8.0 | 3.5 | 12.1 | |
| Dialysis | 2.0 | 1.2 | 3.1 | 1.4 | 0.0666 |
| Anticoagulant use | 13.4 | 10.8 | 11.2 | 26.6 | <0.0001 |
| Cardiac risk factors | |||||
| Diabetes | 32.1 | 25.6 | 36.1 | 40.1 | <0.0001 |
| Hypertension | 73.5 | 65.5 | 77.0 | 87.4 | <0.0001 |
| Hyperlipidaemia | 62.8 | 49.2 | 73.0 | 75.8 | <0.0001 |
| Smoking | 0.0029 | ||||
| Never/missing/unknown | 50.2 | 54.2 | 44.4 | 53.6 | |
| Former/current smoker | 49.8 | 45.8 | 55.6 | 46.4 | |
| Active endocarditis | 1.5 | 3.2 | 0.2 | 0.0 | <0.0001 |
| Cardiac history | |||||
| History of MI† | 12.7 | 5.0 | 16.4 | 25.6 | <0.0001 |
| Recent MI‡ | 9.2 | 4.3 | 17.0 | 3.4 | <0.0001 |
| Previous PCI | 10.3 | 3.6 | 10.6 | 28.5 | <0.0001 |
| Previous CABG | 7.6 | 2.9 | 3.9 | 30.4 | <0.0001 |
| CHF | 24.7 | 19.5 | 19.9 | 51.7 | <0.0001 |
| LVEF | 0.0058 | ||||
| ≥35% | 86.2 | 85.5 | 86.7 | 87.0 | |
| <34% | 7.9 | 6.3 | 8.5 | 10.6 | |
| Unknown | 6.0 | 8.2 | 4.8 | 2.4 | |
| Procedural factors | |||||
| AV repair | NA | 1.0 | 1.2 | NA | <0.0001 |
| Other procedures§ | NA | 6.7 | 6.6 | NA | 0.0007 |
| Transfemoral approach | NA | NA | NA | 76.8 | <0.0001 |
| Wait location | <0.0001 | ||||
| Outpatient | 77.6 | 81.2 | 67.4 | 93.2 | |
| Inpatient¶ | 22.4 | 18.8 | 32.6 | 6.8 | |
| Inpatient wait length | <0.0001 | ||||
| ≤4 days | 81.5 | 85.0 | 72.4 | 94.2 | |
| ≥5 days | 18.5 | 15 | 27.6 | 5.8 | |
| Short length of stay** | 1.0 | 0.5 | 0.8 | 2.9 | 0.0097 |
| Long ICU length of stay†† | 25.8 | 16.1 | 28.6 | 46.4 | <0.0001 |
Unless otherwise specified, values represent proportions.
*BMI: underweight=<18.5, normal=18.5–24.9, overweight=25–29.9, obese=≥30.
†History of MI: MI >30 days ago.
‡Recent MI: MI within the past 30 days.
§Other procedures: aneurysectomy, myectomy, atrial septal defect closure, ventricular septal defect closure, aortic surgery pericardectomy, arrhythmia surgery, cardiac tumour surgery, other surgery.
¶Wait location—inpatient=if wait location at referral, acceptance or booking is a hospital.
**Short length of Stay: ≤2 days
††Long ICU length of Stay: ≥4 days.
AV, aortic valve; BMI, body mass index; CABG, coronary artery bypass grafting; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; ICU, intensive care unit; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NA, not applicable; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Procedures
Less than 10% of the patients undergoing TAVI were urgent, and instead the major were outpatients who had elective procedures. There was a significantly higher proportion of urgent traditional SAVR cases. In addition, almost 47% of TAVI cases had long intensive care unit (ICU) stays of >4 days, compared with 16.1% and 28.6% of the isolated SAVR and combination SAVR+CABG cases, respectively.
Cost of the index hospitalisation
As expected, in-hospital costs for the episode of care were highly skewed with few outliers with very high costs (figure 1). The mean and median costs for the isolated SAVR hospitalisation were $29 163 and $21 811, respectively, while for combination SAVR+CABG, the mean and median costs were $36 131 and $27 256. The costs for the index TAVI hospitalisation were higher with a mean cost of $49 796 and median cost of $42 742. In addition, the range in TAVI costs was greater with a wider IQR ($37 295–$56 196), compared with either SAVR ($18 148–$30 498) or SAVR+CABG ($21 741–$39 000).
Figure 1.
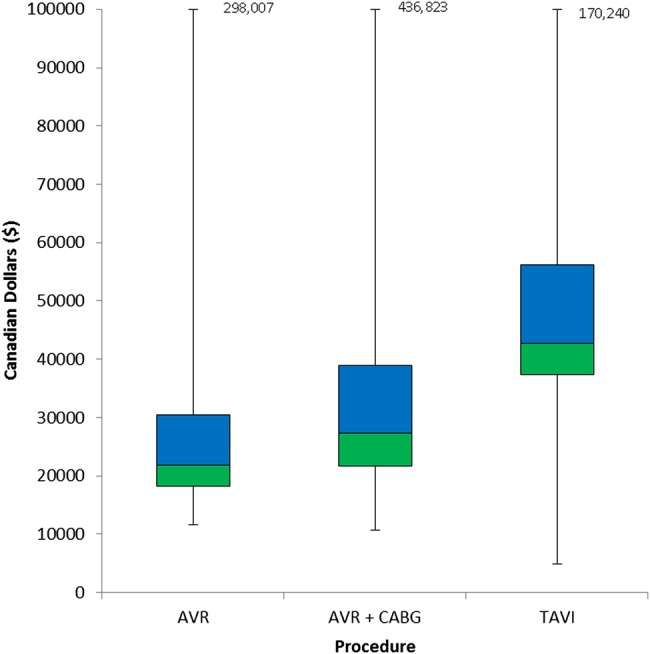
Box plots of costs for the index hospitalisation. The upper and lower margins of the boxes represent the first and third quartiles of cost. The middle line represents the median. For all procedures, the upper limit has been truncated and represented by the figure in the text box.
Drivers of costs
Several patient-level factors were associated with an increased cost for the index hospitalisation (table 2). Age over 75 years was a significant predictor for increased costs for isolated SAVR and TAVI. For SAVR, renal dysfunction and in particular being on dialysis was an important driver of higher costs associated with a 35% increase in mean costs, as was active endocarditis (31% increase in costs). For TAVI, lung disease was the comorbidity with the greatest impact on costs with an RR of 1.26 (95% CI 1.12 to 1.40; p<0.001), while active heart failure was a strong driver for combination CABG+SAVR (RR 1.28; 95% CI 1.16 to 1.41; p<0.001).
Table 2.
Factors associated with total cost in SAVR, SAVR with CABG and TAVI
| Isolated SAVR |
SAVR with CABG |
TAVI |
||||
|---|---|---|---|---|---|---|
| RR (95% CI) | p Value | RR (95% CI) | p Value | RR (95% CI) | p Value | |
| Patient-level factors | ||||||
| Demographics | ||||||
| Age | ||||||
| 20–74 | Referent | |||||
| 75+ | 1.12 (1.05 to 1.19) | 0.0007 | 1.04 (0.97 to 1.12) | 0.3015 | 1.13 (1.01 to 1.26) | 0.0295 |
| Male | Referent | |||||
| Female | 1.01 (0.95 to 1.07) | 0.7772 | 1.04 (0.96 to 1.13) | 0.3139 | 1.06 (0.97 to 1.16) | 0.2298 |
| Medical comorbidities | ||||||
| COPD | 1.13 (1.00 to 1.28) | 0.0515 | 1.10 (0.97 to 1.24) | 0.1332 | 1.26 (1.12 to 1.40) | 0.0001 |
| CVD | 1.21 (1.09 to 1.35) | 0.0006 | 1.01 (0.90 to 1.13) | 0.8652 | 1.04 (0.94 to 1.15) | 0.4138 |
| PVD | 1.04 (0.91 to 1.18) | 0.5960 | 1.05 (0.96 to 1.16) | 0.2876 | 0.97 (0.88 to 1.07) | 0.5304 |
| BMI* | ||||||
| Underweight/normal | Referent | |||||
| Overweight | 1.00 (0.93 to 1.07) | 0.9645 | 1.07 (0.97 to 1.17) | 0.1604 | 0.93 (0.85 to 1.02) | 0.1291 |
| Missing | 0.99 (0.87 to 1.13) | 0.9130 | 1.06 (0.88 to 1.28) | 0.5572 | 0.95 (0.79 to 1.14) | 0.5678 |
| Renal function | ||||||
| Creatinine 0–140 μmol/L | Referent | |||||
| Creatinine >140 μmol/L | 1.13 (1.01 to 1.26) | 0.0371 | 1.08 (0.94 to 1.23) | 0.2616 | 1.00 (0.90 to 1.12) | 0.9813 |
| Missing | 0.99 (0.88 to 1.11) | 0.8393 | 0.92 (0.76 to 1.12) | 0.4126 | 1.05 (0.91 to 1.21) | 0.5361 |
| Dialysis | 1.35 (1.02 to 1.78) | 0.0347 | 0.90 (0.72 to 1.13) | 0.3565 | 1.19 (0.86 to 1.66) | 0.2855 |
| Anticoagulant use | 1.01 (0.92 to 1.11) | 0.8365 | 1.00 (0.90 to 1.12) | 0.9422 | 1.01 (0.92 to 1.10) | 0.8295 |
| Cardiac risk factors | ||||||
| Diabetes | 1.09 (1.02 to 1.17) | 0.0157 | 0.98 (0.91 to 1.06) | 0.6466 | 1.01 (0.93 to 1.10) | 0.7275 |
| Hypertension | 1.00 (0.94 to 1.07) | 0.9826 | 1.04 (0.95 to 1.13) | 0.4144 | 1.03 (0.92 to 1.17) | 0.5974 |
| Hyperlipidaemia | 1.00 (0.94 to 1.07) | 0.9042 | 1.05 (0.96 to 1.14) | 0.2696 | 0.96 (0.87 to 1.06) | 0.3841 |
| Smoking | ||||||
| Never/missing/unknown | Referent | |||||
| Former/current smoker | 0.95 (0.89 to 1.01) | 0.0733 | 0.97 (0.90 to 1.04) | 0.3670 | 1.00 (0.92 to 1.09) | 0.9593 |
| Active endocarditis | 1.31 (1.09 to 1.56) | 0.0033 | 1.09 (0.49 to 2.40) | 0.8332 | NA | |
| Cardiac history | ||||||
| History of MI† | 1.08 (0.93 to 1.24) | 0.3303 | 1.06 (0.95 to 1.17) | 0.2962 | 1.02 (0.93 to 1.13) | 0.6109 |
| Recent MI‡ | 0.81 (0.69 to 0.94) | 0.0054 | 1.16 (1.03 to 1.30) | 0.0126 | 1.24 (0.99 to 1.56) | 0.0633 |
| Previous PCI | 0.93 (0.79 to 1.10) | 0.4010 | 1.09 (0.97 to 1.23) | 0.1634 | 1.00 (0.91 to 1.09) | 0.9574 |
| Previous CABG | 1.03 (0.87 to 1.22) | 0.7336 | 0.95 (0.79 to 1.14) | 0.5784 | 1.09 (0.99 to 1.20) | 0.0938 |
| CHF | 1.05 (0.97 to 1.14) | 0.1971 | 1.28 (1.16 to 1.41) | <0.0001 | 0.97 (0.88 to 1.06) | 0.4490 |
| LVEF | ||||||
| ≥35% | Referent | |||||
| <34% | 1.03 (0.90 to 1.16) | 0.6973 | 0.95 (0.83 to 1.08) | 0.4247 | 1.07 (0.93 to 1.23) | 0.3282 |
| Unknown | 0.89 (0.79 to 0.99) | 0.0374 | 1.04 (0.88 to 1.23) | 0.6591 | 0.98 (0.76 to 1.26) | 0.8787 |
| Procedural factors | ||||||
| AV repair | 1.26 (0.94 to 1.69) | 0.1179 | 0.90 (0.65 to 1.24) | 0.5074 | NA | |
| Other procedures§ | 1.14 (1.02 to 1.28) | 0.0260 | 1.09 (0.96 to 1.25) | 0.239 | NA | |
| Transfemoral approach | ||||||
| Yes | Referent | |||||
| No | NA | NA | 1.31 (1.18 to 1.45) | <0.0001 | ||
| Wait location | ||||||
| Outpatient | Referent | |||||
| Inpatient¶ | 1.41 (1.20 to 1.66) | <0.0001 | 1.02 (0.87 to 1.21) | 0.7914 | 0.87 (0.60 to 1.27) | 0.4768 |
| IP wait length | ||||||
| IP ≤4 days | Referent | |||||
| IP ≥5 days | 0.94 (0.79 to 1.12) | 0.4842 | 1.02 (0.87 to 1.21) | 0.7768 | 1.44 (0.95 to 2.17) | 0.0814 |
| Short length of stay** | ||||||
| Yes | Referent | |||||
| No | 1.28 (0.86 to 1.92) | 0.2276 | 1.29 (0.87 to 1.92) | 0.2068 | 1.42 (1.14 to 1.78) | 0.0023 |
| Long ICU length of stay†† | ||||||
| No | Referent | |||||
| Yes | 2.16 (1.99 to 2.35) | <0.0001 | 2.05 (1.89 to 2.21) | <0.0001 | 1.30 (1.20 to 1.41) | <0.0001 |
Bold values are statistically significant.
*BMI: underweight=<18.5, normal=18.5–24.9, overweight=25–29.9, obese=≥30.
†History of MI: MI >30 days ago.
‡Recent MI: MI within the past 30 days.
§Other procedures: aneurysectomy, myectomy, atrial septal defect closure, ventricular septal defect closure, aortic surgery pericardectomy, arrhythmia surgery, cardiac tumour surgery, other surgery.
¶Wait location—inpatient=if wait location at referral, acceptance or booking is a hospital or EMS Direct.
**Short length of stay: ≤2 days.
††Long ICU length of Stay: ≥4 days.
AV, aortic valve; BMI, body mass index; CABG, coronary artery bypass grafting; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; ICU, intensive care unit; IP, inpatient; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NA, not applicable; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; RR, rate ratio; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Procedural factors of importance included the wait-time location and urgency (table 2). For TAVI, a non-transfemoral access was associated with an almost 30% increase in total costs. Patients undergoing TAVI who were discharged within 2 days of their procedure had a substantially lower cost compared with those with longer admissions (RR 1.42; 95% CI 1.14 to 1.78; p=0.0023). For all procedures, a long intensive care stay of >3 days was a strong driver of increased costs.
Discussion
In this multicentre cohort study, we found wide variation in the cumulative healthcare costs associated with the index hospitalisation between SAVR, SAVR+CABG and TAVI and identified a number of potentially modifiable procedural drivers of healthcare costs. These represent areas for further study to determine if quality improvement initiatives targeting these areas will improve the efficiency of care delivery and translate to lower overall costs.
Although we found that the median costs for SAVR were substantially less than those for TAVI, it is important to recognise that the patients undergoing each of the procedures were vastly different, as is consistent with the high-risk/inoperable indication for TAVI. Multiple cost-effectiveness analyses have evaluated TAVI and SAVR for either inoperable or high-risk patients.13–15 28–32 These have generally found that TAVI is cost-effective. That said, even in these populations, previous literature has generally found TAVI to have higher overall costs.13 This issue, coupled with a limited funding envelope and restricted capacity, reinforces the need to understand the major cost drivers. The transcatheter heart valve (THV) prosthesis itself has a substantial acquisition cost that is 5–6 times that of a surgical prosthesis. The bulk of the previous work on TAVI17 18 20 33 and SAVR34 related costs has focused on the importance of complications and the incremental costs associated with them.
Our study contributes a number of novel insights to this body of literature. First, we found that the procedures had substantially different drivers of overall cost. There were a number of important patient-related factors that drove SAVR cost, including age, renal disease, lung disease, diabetes, recent myocardial infarction and endocarditis. In contrast, for TAVI, only older age and lung disease were statistically significant patient-level drivers. We hypothesise that the greater relative importance of patient factors for SAVR-related costs is because patients undergoing SAVR more likely represent the full spectrum of operative risk from low, intermediate and high risk. In contrast, those for TAVI are almost exclusively either at high or prohibitive risk. The fact that there is a greater spectrum of patients would translate to a potentially greater impact of patient selection on SAVR cost. Lung disease is increasingly recognised as a poor prognostic factor for patients undergoing TAVI—our finding that it is one of the few patient factors that influence TAVI-related costs is consistent with these previous findings.
Second, we found that procedural factors were key cost drivers in SAVR and TAVI. This is particularly important as these are modifiable. Both in TAVI and SAVR, the majority of the costs associated with the index episode of care are not prosthesis related. This is especially relevant to TAVI, given the high cost of THV currently. Although the acquisition cost of the THV prosthesis will decrease as technology improves and more transcatheter systems enter the marketplace, this is likely to be a protracted process. In contrast, the non-prosthesis-associated costs, in particular the potentially modifiable procedural drivers, are ones that may be more readily addressed in the short term. Indeed, there are a number of ongoing initiatives that are already targeting some of these drivers. Specifically, in TAVI, there is an emphasis on a ‘minimalist’ approach that uses local anaesthesia/conscious sedation rather than general anaesthesia.35 36 The goal is that a less invasive procedure, coupled with explicit clinical care pathways that aim to mobilise patients early, result in shorter ICU stays and earlier discharge, both of which were major drivers of lower costs. Our finding that non-transfemoral approaches were associated with higher resource use reinforces this point.
Our study must be interpreted in the context of several limitations that merit discussion. First, our healthcare cost estimates were from fiscal year 2012–2013. For TAVI, where there have been rapid technological and procedural refinements, these costs may not be representative of current costs. However, we would expect that many of the patient and procedural drivers highlighted are likely to continue to be important. Second, we focused solely on the costs associated with the index hospitalisation and these represent only a fraction of the overall healthcare costs associated with each of these procedures. Both SAVR and TAVI require an extensive preprocedural workup including evaluation by a multidisciplinary Heart Team and the need for multiple diagnostic modalities, including coronary angiography and CT imaging often. For TAVI, excluded costs would also include any preprocedural revascularisation by angioplasty, which would typically occur at a separate sitting. Finally, ours was an observational data set, and thus there was most likely residual confounding that we could not account for despite the use of advanced statistical techniques. As such, our findings should be considered hypothesis generating.
In conclusion, we identified a number of patient and procedural factors that impact on healthcare costs for the index hospitalisation; some of these are potentially modifiable and may represent areas for quality improvement to optimise healthcare delivery.
Acknowledgments
The authors acknowledge that the clinical registry data used in this publication are from CCN and its participating hospitals. CCN serves as an advisory body to the MOHLTC and is dedicated to improving the quality, efficiency, access and equity of adult cardiovascular services in Ontario, Canada. CCN is funded by the MOHLTC.
Footnotes
Contributors: HCW contributed to the conception, acquisition and analysis of the work, and drafted the manuscript. LL contributed to the acquisition and analysis of the work, while all other authors contributed to the interpretation of data. All authors revised the manuscript critically for important intellectual content and have provided final approval of the version to be published. They also agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. HCW had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Funding: HCW is supported by a Distinguished Clinical Scientist Award from the Heart and Stroke Foundation of Canada.
Disclaimer: The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources.
Competing interests: HCW received research funding from Edwards Lifesciences.
Ethics approval: Cardiac Care Network of Ontario.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.d'Arcy JL, Prendergast BD, Chambers JB et al. Valvular heart disease: the next cardiac epidemic. Heart 2011;97:91–3. 10.1136/hrt.2010.205096 [DOI] [PubMed] [Google Scholar]
- 2.Iung B, Baron G, Butchart EG et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231–43. 10.1016/S0195-668X(03)00201-X [DOI] [PubMed] [Google Scholar]
- 3.Nishimura RA, Otto CM, Bonow RO et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2014;148:e1–e132. 10.1016/j.jtcvs.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 4.Kodali SK, Williams MR, Smith CR et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686–95. 10.1056/NEJMoa1200384 [DOI] [PubMed] [Google Scholar]
- 5.Leon MB, Smith CR, Mack M et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–607. 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 6.Makkar RR, Fontana GP, Jilaihawi H et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012;366:1696–704. 10.1056/NEJMoa1202277 [DOI] [PubMed] [Google Scholar]
- 7.Smith CR, Leon MB, Mack MJ et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–98. 10.1056/NEJMoa1103510 [DOI] [PubMed] [Google Scholar]
- 8.Adams DH, Popma JJ, Reardon MJ et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790–8. 10.1056/NEJMoa1400590 [DOI] [PubMed] [Google Scholar]
- 9.Popma JJ, Adams DH, Reardon MJ et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014;63:1972–81. 10.1016/j.jacc.2014.02.556 [DOI] [PubMed] [Google Scholar]
- 10.Reardon MJ, Adams DH, Kleiman NS et al. 2-Year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol 2015;66:113–21. 10.1016/j.jacc.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 11.Généreux P, Head SJ, Wood DA et al. Transcatheter aortic valve implantation 10-year anniversary: review of current evidence and clinical implications. Eur Heart J 2012;33:2388–98. 10.1093/eurheartj/ehs220 [DOI] [PubMed] [Google Scholar]
- 12.Leon MB, Smith CR, Mack MJ et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609–20. 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 13.Gadey G, Reynolds MR. Cost-effectiveness considerations in transcatheter management of valvular heart disease. Can J Cardiol 2014;30:1058–63. 10.1016/j.cjca.2014.03.005 [DOI] [PubMed] [Google Scholar]
- 14.Hancock-Howard RL, Feindel CM, Rodes-Cabau J et al. Cost effectiveness of transcatheter aortic valve replacement compared to medical management in inoperable patients with severe aortic stenosis: Canadian analysis based on the PARTNER Trial Cohort B findings. J Med Econ 2013;16:566–74. 10.3111/13696998.2013.770747 [DOI] [PubMed] [Google Scholar]
- 15.Iannaccone A, Marwick TH. Cost effectiveness of transcatheter aortic valve replacement compared with medical management or surgery for patients with aortic stenosis. Appl Health Econ Health Policy 2015;13:29–45. 10.1007/s40258-014-0141-6 [DOI] [PubMed] [Google Scholar]
- 16.Simons CT, Cipriano LE, Shah RU et al. Transcatheter aortic valve replacement in nonsurgical candidates with severe, symptomatic aortic stenosis: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes 2013;6:419–28. 10.1161/CIRCOUTCOMES.113.000280 [DOI] [PubMed] [Google Scholar]
- 17.Arnold SV, Lei Y, Reynolds MR et al. Costs of periprocedural complications in patients treated with transcatheter aortic valve replacement: results from the Placement of Aortic Transcatheter Valve trial. Circ Cardiovasc Interv 2014;7:829–36. 10.1161/CIRCINTERVENTIONS.114.001395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutmann A, Kaier K, Sorg S et al. Analysis of the additional costs of clinical complications in patients undergoing transcatheter aortic valve replacement in the German Health Care System. Int J Cardiol 2015;179:231–7. 10.1016/j.ijcard.2014.11.095 [DOI] [PubMed] [Google Scholar]
- 19.Reinöhl J, Gutmann A, Kollum M et al. Transfemoral aortic valve implantation: bleeding events, related costs and outcomes. J Thromb Thrombolysis 2013;35:469–75. 10.1007/s11239-012-0829-0 [DOI] [PubMed] [Google Scholar]
- 20.Van Gestel R, De Graeve D, Vrints C et al. Hospitalization costs of TAVI in one Belgian university hospital. Acta Cardiol 2013;68:263–70. 10.2143/AC.68.3.2983420 [DOI] [PubMed] [Google Scholar]
- 21.Bagust A, Grayson AD, Palmer ND et al. Cost effectiveness of drug eluting coronary artery stenting in a UK setting: cost-utility study. Heart 2006;92:68–74. 10.1136/hrt.2004.053850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annual Report 2014–2015. Secondary annual report 2014–2015 2015. http://www.ccn.on.ca/ccn_public/uploadfiles/files/CCN_Annual_Report_2014_15.pdf
- 23.Gurevich Y, McFarlane A, Morris K et al. Estimating the number of coronary artery bypass graft and percutaneous coronary intervention procedures in Canada: a comparison of cardiac registry and Canadian Institute for Health Information data sources. Can J Cardiol 2010;26:249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mihaylova B, Briggs A, O'Hagan A et al. Review of statistical methods for analysing healthcare resources and costs. Health Econ 2011;20:897–916. 10.1002/hec.1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC, Ghali WA, Tu JV. A comparison of several regression models for analysing cost of CABG surgery. Stat Med 2003;22:2799–815. 10.1002/sim.1442 [DOI] [PubMed] [Google Scholar]
- 26.Bennell MC, Qiu F, Micieli A et al. Identifying predictors of cumulative healthcare costs in incident atrial fibrillation: a population-based study. J Am Heart Assoc 2015;4:e001684 10.1161/JAHA.114.001684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blough DK, Ramsey SD. Using generalized linear models to assess medical care costs. Health Serv Outcomes Res Methodol 2000;1:185–202. 10.1023/A:1012597123667 [DOI] [Google Scholar]
- 28.Fairbairn TA, Meads DM, Hulme C et al. The cost-effectiveness of transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with severe aortic stenosis at high operative risk. Heart 2013;99:914–20. 10.1136/heartjnl-2013-303722 [DOI] [PubMed] [Google Scholar]
- 29.Cao C, Indraratna P, Ang SC et al. Cost-effectiveness of transcatheter aortic valve implantation versus surgery for high-risk patients with aortic stenosis. J Am Coll Cardiol 2013;61:1747–8. 10.1016/j.jacc.2012.11.066 [DOI] [PubMed] [Google Scholar]
- 30.Orlando R, Pennant M, Rooney S et al. Cost-effectiveness of transcatheter aortic valve implantation (TAVI) for aortic stenosis in patients who are high risk or contraindicated for surgery: a model-based economic evaluation. Health Technol Assess 2013;17:1–86. 10.3310/hta17330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doble B, Blackhouse G, Goeree R et al. Cost-effectiveness of the Edwards SAPIEN transcatheter heart valve compared with standard management and surgical aortic valve replacement in patients with severe symptomatic aortic stenosis: a Canadian perspective. J Thorac Cardiovasc Surg 2013;146:52–60.e3. 10.1016/j.jtcvs.2012.06.018 [DOI] [PubMed] [Google Scholar]
- 32.Reardon MJ, Reynolds MR. Cost-effectiveness of TAVR in the non-surgical population. J Med Econ 2013;16:575–9. 10.3111/13696998.2013.772753 [DOI] [PubMed] [Google Scholar]
- 33.Chevreul K, Brunn M, Cadier B et al. Cost of transcatheter aortic valve implantation and factors associated with higher hospital stay cost in patients of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Arch Cardiovasc Dis 2013;106:209–19. 10.1016/j.acvd.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 34.Abstracts: Pulse: a report on the UHC Annual Conference 2013, October 17–18, Atlanta, GA. Am J Med Qual 2014;29(2 Suppl):3S–28S. 10.1177/1062860613519567 [DOI] [PubMed] [Google Scholar]
- 35.Wood DA. Could a “simplified” transcatheter aortic valve replacement procedure eliminate post-operative delirium? JACC Cardiovasc Interv 2016;9:169–70. 10.1016/j.jcin.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 36.Lauck SB, Wood DA, Achtem L et al. Risk stratification and clinical pathways to optimize length of stay after transcatheter aortic valve replacement. Can J Cardiol 2014;30:1583–7. 10.1016/j.cjca.2014.07.012 [DOI] [PubMed] [Google Scholar]


