Abstract
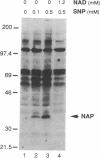
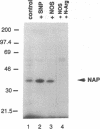
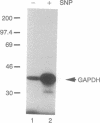
Nitric oxide generation in brain cytosolic fractions markedly enhances ADP-ribosylation of a single 37-kDa protein. By utilizing a biotinylated NAD and avidin affinity chromatography, we purified this protein. Partial amino acid sequencing establishes its identity as glyceraldehyde-3-phosphate dehydrogenase (GAPDH). This is further confirmed by detection of GAPDH enzymatic activity in the purified 37-kDa protein. GAPDH is ADP-ribosylated in the absence of brain extract. This auto-ADP-ribosylation is enhanced by nitric oxide generation. ADP-ribosylation appears to involve the cysteine where NAD interacts with GAPDH so that ADP-ribosylation likely inhibits enzymatic activity. Such inhibition may play a role in nitric oxide-mediated neurotoxicity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Just I., Rosenthal W. Different types of ADP-ribose protein bonds formed by botulinum C2 toxin, botulinum ADP-ribosyltransferase C3 and pertussis toxin. Biochem Biophys Res Commun. 1988 Oct 14;156(1):361–367. doi: 10.1016/s0006-291x(88)80849-0. [DOI] [PubMed] [Google Scholar]
- Bode J., Blumenstein M., Raftery M. A. 19F nuclear magnetic resonance studies of structure and function relationships in trifluoroacetonylated rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 1975 Mar 25;14(6):1153–1160. doi: 10.1021/bi00677a009. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide, a novel neuronal messenger. Neuron. 1992 Jan;8(1):3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- Brüne B., Lapetina E. G. Activation of a cytosolic ADP-ribosyltransferase by nitric oxide-generating agents. J Biol Chem. 1989 May 25;264(15):8455–8458. [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., London E. D., Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R. S., Terwilliger R. Z., Nestler E. J. Endogenous ADP-ribosylation in brain: initial characterization of substrate proteins. J Neurochem. 1991 Dec;57(6):2124–2132. doi: 10.1111/j.1471-4159.1991.tb06431.x. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- Kots AYa, Skurat A. V., Sergienko E. A., Bulargina T. V., Severin E. S. Nitroprusside stimulates the cysteine-specific mono(ADP-ribosylation) of glyceraldehyde-3-phosphate dehydrogenase from human erythrocytes. FEBS Lett. 1992 Mar 23;300(1):9–12. doi: 10.1016/0014-5793(92)80153-8. [DOI] [PubMed] [Google Scholar]
- Lamas S., Marsden P. A., Li G. K., Tempst P., Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein C. J., Glatt C. S., Bredt D. S., Snyder S. H. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6711–6715. doi: 10.1073/pnas.89.15.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig H. S., von Brauchitsch K. L., Chan J., Greenberg D. A. Cyclic GMP modulators and excitotoxic injury in cerebral cortical cultures. Brain Res. 1992 Apr 17;577(2):343–346. doi: 10.1016/0006-8993(92)90295-k. [DOI] [PubMed] [Google Scholar]
- Lyons C. R., Orloff G. J., Cunningham J. M. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem. 1992 Mar 25;267(9):6370–6374. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moss J., Vaughan M. ADP-ribosylation of guanyl nucleotide-binding regulatory proteins by bacterial toxins. Adv Enzymol Relat Areas Mol Biol. 1988;61:303–379. doi: 10.1002/9780470123072.ch6. [DOI] [PubMed] [Google Scholar]
- Nakane M., Ichikawa M., Deguchi T. Light and electron microscopic demonstration of guanylate cyclase in rat brain. Brain Res. 1983 Aug 22;273(1):9–15. doi: 10.1016/0006-8993(83)91088-0. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Reisler E., Tauber-Finkelstein M., Shaltiel S. Evidence for an interprotomerial active site in D-glyceraldehyde-3-phosphate dehydrogenase. FEBS Lett. 1975 Jul 1;54(3):315–318. doi: 10.1016/0014-5793(75)80929-x. [DOI] [PubMed] [Google Scholar]
- Ryzlak M. T., Pietruszko R. Heterogeneity of glyceraldehyde-3-phosphate dehydrogenase from human brain. Biochim Biophys Acta. 1988 Jun 13;954(3):309–324. doi: 10.1016/0167-4838(88)90086-6. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Yoshihara K., Kamiya T. Enzymic and nonenzymic mono ADP-ribosylation of proteins in skeletal muscle. Biochem Biophys Res Commun. 1989 Sep 15;163(2):1063–1070. doi: 10.1016/0006-291x(89)92329-2. [DOI] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]