Summary
Alcoholic liver disease (ALD) is the most prevalent cause of advanced liver disease in Europe and is the leading cause of death among adults with excessive alcohol consumption. There is a dose-response relationship between the amount of alcohol consumed and the risk of ALD. The relative risk of cirrhosis increases in subjects who consume more than 25 g/day. The burden of alcohol-attributable liver cirrhosis and liver cancer is high and is entirely preventable. Health agencies should develop population-based policies to reduce the prevalence of harmful and/or hazardous alcohol consumption and foster research in this field to provide new diagnostic and therapeutic tools. Disease progression of patients with ALD is heavily influenced by both genetic and environmental factors. Non-invasive methods for the diagnosis of fibrosis have opened new perspectives in the early detection of advanced ALD in asymptomatic patients. Alcoholic hepatitis, the most severe form of ALD, carries a high short-term mortality (around 30–50% at 3 months). Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis but duration of therapy should be adapted to early response. Liver transplantation is the best option for patients with severe liver dysfunction. However, alcohol relapse after transplantation remains a critical issue and drinking habits of transplanted patients need to be routinely screened.
Keywords: Burden of alcoholic liver disease, Natural history, Non-invasive diagnosis, Cirrhosis, Alcoholic hepatitis, Liver transplantation
Burden of alcohol liver disease
Abusive alcohol consumption is a major cause of preventable morbidity and mortality worldwide. Besides organ damage, alcohol consumption is a major cause of accidents and violence [1]. The health consequences of alcohol consumption vary according to the extent and method of usage (excessive or not, acute or chronic), and depend on numerous environmental and individual factors. Demographic characteristics, the amount of alcohol intake, frequency, duration and profile of consumption vary among alcohol drinkers [2]. In men, daily drinking is associated with an increased risk of alcoholic cirrhosis. Interestingly, recent alcohol consumption rather than earlier in life consumption is associated with higher risk of alcoholic cirrhosis [3]. Alcohol drinkers consuming up to 2 drinks/day (men) or 1 drink/day (women) are defined as moderate drinkers and do not disclose increased risk of organ damage compared to abstainers. Daily consumption above those limits can lead to health, personal and social problems. This definition does not capture the pattern of binge drinking that is quite different from that of chronic drinkers. There is wide heterogeneity in the definition of binge drinking, according to the threshold of alcohol intake per episode. The National Institute on Alcohol Abuse and Alcoholism (NIAAA) proposed a consensual definition of binge drinking episodes as the consumption of 5 or more drinks (male) or 4 or more drinks (female) in about 2 h [4]. This definition fails to recognize the amount of alcohol intake per binge episode. Moreover, the possibility of having several binges during the same day is not included. Finally, a binge alcohol drinker can have periods of continuous alcohol use. The phenomenon of binge drinking continues to grow in Western countries, and is particularly striking in beer- and spirit-drinking cultures in the UK and northern Europe [5]. Between 1993 and 2001 in the USA, binge drinking episodes per person per year increased by 17%, with the highest rates occurring among youth aged 18 to 25 years [6]. In the UK, the Health Survey for England (HSE) reported that 57% of young males were binge drinkers [7]. Most European countries exhibit the same trend towards an increase in binge drinking, even in southern countries. As an example, one French 17-year-old population reported having had binge drinking episodes over the previous month, of one, three or ten times in 45.8%, 17.9%, and 2.2% of cases, respectively [8]. Since 1998, American college students aged 18–24 have had a significantly higher increase in alcohol-related deaths than population totals for the same age group [9]. Thus, public health policies should be targeted towards young people, though they may be less receptive to the prevention messages against this pattern of drinking [10]. Up until now, policies have failed to reduce binge drinking in adolescents and adults, which remains an important health objective in Western countries. The question arises as to whether specialists in liver disease should be concerned by the increase in binge drinkers. However, data concerning the impact of binge drinking on the liver are limited because it has been insufficiently investigated [8,11].
There is a significant disparity in the sex distribution of deaths attributable to excessive alcohol consumption. As an example, 11% of deaths in men and 1.8% in women in Europe are attributable to alcohol [2]. Chronic alcohol consumption may lead to cirrhosis and is associated with an increased risk for hepatocellular carcinoma (HCC) [4]. Alcoholic liver disease (ALD) is the most prevalent cause of advanced liver disease in Europe and cirrhosis is the leading cause of death due to alcohol among adults [2]. There is a dose-response relationship between the volume of alcohol consumed with the risk of ALD. Previous epidemiological studies strongly suggested a considerable association between alcohol consumption and cirrhosis and emphasized the correlation between severity and duration of alcohol abuse and the presence of cirrhosis. Above a threshold of daily alcohol consumption, the risk of developing cirrhosis increases exponentially. However, the threshold value of daily alcohol consumption associated with increased risk of cirrhosis has long been controversial. The amount of alcohol intake can be expressed in g/day, number of drinks (each drink contains 14 g of alcohol) or alcohol units (each unit has 8 g of alcohol in the UK or 14 g in USA). A meta-analysis observed that consumption of more than 25 g/day increases the relative risk of cirrhosis [12]. This threshold has been validated in the Dionysos project assessing the prevalence of alcoholic cirrhosis in a cohort derived from a general population of two cities in northern Italy [13]. Six thousand nine hundred and seventeen adult subjects were asked about their alcohol consumption. Twenty-one percent of the population had a daily alcohol consumption of more than 30 g/day. The rate of cirrhosis was significantly higher in patients who consumed ≥30 g/day (2.2%) than among abstainers or those with consumption <30 g/day: 2.2% vs. 0.08%. The risk of cirrhosis increased with the amount of alcohol consumed. Subjects who consumed more than 120 g/day had the highest risk of cirrhosis, with a prevalence of 13.5%.
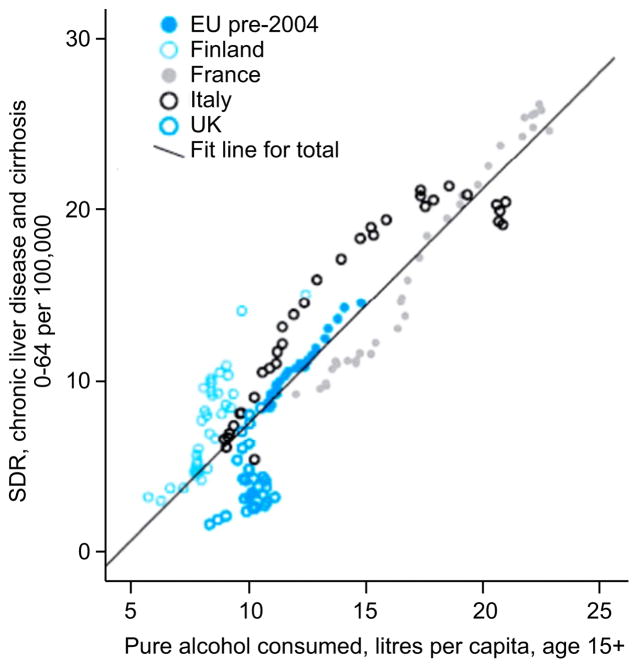
Liver diseases are an important cause of global burden of mortality and morbidity [14,15]. The burden of liver diseases in North America and Europe is mainly attributable to alcohol whereas in Africa and Asia viral hepatitis is the dominant force. In 2010, cirrhosis accounted for more than 493,300 deaths (156,900 female and 336,400 male deaths) and for more than 14.5 million DALYs (disability-adjusted life year) (around 4.1 million DALYs for women and 10.4 million DALYs for men) [14–16]. In addition, around 82,000 deaths (around 14,000 female and 66,000 male deaths) and 2.1 million DALYs were due to alcohol-induced liver cancer [14–16]. The weight of alcohol on liver-related mortality is strongly supported by data showing a relationship between standard liver death rate and overall alcohol consumption in several European countries (Fig. 1) [17].
Fig. 1. Relationship between standard liver death rate and overall alcohol consumption in several European countries.
The relationship between standard liver death rate (SDR) (per 100,000) and overall alcohol consumption (pure alcohol litres per capita, age 15+) in the four countries in the EU (pre-2004) with the largest rises or falls in liver deaths between 1970 and 2008. Data from the World Health Organization, European Health for All database (HFA-DB): http://data.euro.who.int/hfadb/. Reproduced with permission from [17].
Variations in alcohol consumption largely contribute to cirrhosis mortality trends and its variability across countries [18,19]. Also, reduction in alcohol consumption in most countries is followed by a decrease in cirrhosis mortality. In North America, Australia and Southern Europe alcohol consumption decreased in recent years, leading to comparable decline in cirrhosis mortality [18,20–22]. Conversely, the high rates of cirrhosis mortality in Hungary and other countries of central and Eastern Europe are mainly due to their high per capita alcohol consumption [18]. The marked rises in cirrhosis mortality in Ireland and the UK (particularly Scotland) are related to the fact that total recorded alcohol consumption in Britain doubled between 1960 and 2002 [10]. Indeed, one study compared age-standardized mortality rates for cirrhosis in the UK with the rates from 12 Western European countries for the period between 1955 and 2001 [10]. UK standardized mortality rates for cirrhosis increased in men from 3.4 between 1957 and 1961, to 14.1 per 100,000 per year between 1997 and 2001, with the same trend in women, whereas it decreased in other European countries. Cirrhosis mortality rates in Scotland are among the highest in Western Europe, with mortality rates in men and women of 42.2 and 20 per 100,000 per year, respectively [10]. Another study obtained records of all patients in a longitudinal database of 13 million subjects followed in general practice [23]. This study confirmed a 45% increase in the incidence of cirrhosis in the UK during the last decade.
These studies indicate that the burden of alcohol-attributable liver cirrhosis and liver cancer is high and entirely preventable. Health agencies should develop population-based policies to reduce levels of harmful and/or hazardous consumption and provide financial support for research aimed at developing improved therapies for patients with ALD [2,16]. Public health policies should include taxation escalations for alcoholic beverages that should be partially used to cover patient costs and to develop new therapies for alcohol use disorders and ALD. Several approaches have been used, including policies to decrease availability of alcohol by limiting the hours and places of sale and establish minimum age purchase laws [2]. Moreover, several studies have shown that the rising price of alcohol decreases morbidity and mortality [24,25]. The advent of cheap alcohol has had a particularly deleterious effect in the UK and Ireland, which have, in turn, lead to efforts to curb excess consumption by raising the minimum price of alcohol or banning the sale of alcohol below cost (in England and Wales). Recent modeling studies suggest that the former method is more likely to be effective [26]. Other more nuanced approaches may be necessary in societies where alcohol use is linked to production within the family home, especially in Eastern Europe.
Natural history of alcoholic liver disease: from steatosis to cirrhosis
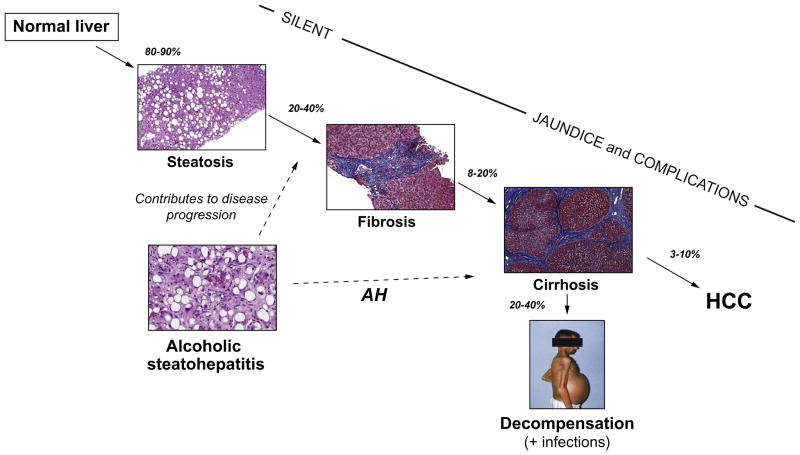
The spectrum of ALD comprises simple steatosis, alcoholic steatohepatitis (ASH), progressive fibrosis, cirrhosis and the development of HCC Fig. 2. Several studies suggested that incidence of HCC is lower in alcoholic cirrhosis than in viral cirrhosis. The risk of HCC persists in cirrhotic patients even among those that remain abstinent. Although up to 90% of heavy drinkers develop steatosis, only a minority of those with steatosis progress to ASH and 10–20% eventually develop cirrhosis [27,28]. Steatosis is usually asymptomatic and rapidly reversible with abstinence [28]. Continued heavy alcohol consumption, however, leads in some patients to liver inflammation characterized by the infiltration of polymorphonuclear cells (PMN), hepatocyte damage often described as ballooning, and Mallory-Denk’s bodies, both of which define ASH. Some patients develop liver fibrosis (20–40%) and cirrhosis (10–20%), which confers a high risk of complications (ascites, variceal bleeding, hepatic encephalopathy, renal failure and bacterial infections) [29,30].
Fig. 2. Natural history of alcoholic liver disease from steatosis to cirrhosis and hepatocellular carcinoma (HCC).
The percentage represents the patients who progress from one stage to the next. Most patients with persistent alcohol abuse develop some degree of hepatic steatosis. If the alcohol abuse persists, liver fibrosis progressively develops, ultimately resulting in cirrhosis. Cirrhosis can lead to severe complications related to portal hypertension (ascites, variceal bleeding and encephalopathy), bacterial infections and also predisposes to HCC. The development of alcoholic steatohepatitis (ASH) predisposes patients to progress to advanced liver fibrosis, and when this develops in patients with severe disease it results in AH.
In the last decades, major advances have been made in our understanding of the factors that influence ALD progression [31–34]. The natural history of ALD cannot be separated the natural history of alcoholism. Alcohol use, remission and relapse comes in many different patterns and needs to be observed over many years [35]. This applies both to those in possession of their native liver, and the post-transplant patient with alcoholism [36]. Disease progression varies according to genetic and environmental factors. The major link of people with a genetic predisposition to develop ALD is supported by the observation of concordance rates for alcoholism and alcohol-induced liver fibrosis in monozygotic twins of 26.3 and 14.6, respectively [37]. Recent studies observed that variations in PNPLA3, which encodes patatin-like phospholipase domain-containing protein 3, strongly influence the development of advanced liver fibrosis among Mexican and Caucasian populations [37–40]. Importantly, PNPLA3 polymorphisms can be considered to be the only confirmed and replicated genetic risk factor for ALD. This genetic factor also influences the development of HCC [41]. Despite the large number of studies that have assessed the role of genetic variation in susceptibility to ALD, a large-scale genome-wide association study of factors associated with ALD remains to be performed given the potential biases of previous studies [42]. Most published reports include a limited number of patients who lack a well-defined phenotype, the investigation of only a few genes and have crucial methodological weaknesses.
Although several translational studies have evaluated the risk of cirrhosis, the natural history of ALD remains only partially explored due to the lack of prospective studies of large cohorts with the goal of assessing progression of liver damage. A translational study comprising over 2000 hospitalized excessive heavy drinkers with modified results in liver laboratory tests investigated by liver biopsy showed that 34% of these subjects presented with alcohol-induced cirrhosis, 46% with fatty liver with/without fibrosis, 9% with acute alcohol-induced hepatitis and 11% with a normal liver [43]. When the progression of fibrosis was expressed in terms of duration of exposure, the progression of fibrosis was estimated by dividing the fibrosis score by the time of exposure. In heavy drinkers, the risk of cirrhosis reached 50% at 61 years of age, and the likelihood of developing cirrhosis in 50% of cases was observed after 35 years, with a shorter time of progression to cirrhosis among women than men [44]. Based on an analysis of all the published data, cirrhosis occurs in approximately 20% of hospitalized patients with excessive alcohol consumption.
The presence of steatosis typically precedes the development of fibrosis [45] but the influence of fat accumulation on progression of fibrosis is not well known. The long-term survival of patients with isolated steatosis is clearly reduced compared to that of abstinent controls. After a mean follow-up of approximately 10 years, patients with alcoholic steatosis died more frequently than those with non-alcoholic fatty liver disease (74% vs. 25%) and more patients developed cirrhosis (21% vs. 1%) [46]. Twenty-five percent of the observed deaths among heavy drinkers were associated with cirrhosis and 75% were attributed to other diseases related to alcohol.
Patients with underlying severe fibrosis or cirrhosis and heavy alcohol intake can present a form of acute-on-chronic liver failure called alcoholic hepatitis (AH) [29]. This is characterized by an abrupt rise in serum bilirubin levels, jaundice and liver-related complications. Previously, it was considered that AH could occur in patients with mild underlying liver disease. However, more recent studies using tru-cut needles have shown that the majority of patients with severe AH have underlying cirrhosis [47]. Patients with severe forms of AH show high short-term mortality around 30–50% at 3 months. Patients with AH disclosed the fastest progression of fibrosis and consequently an increased risk of liver-related death [45]. Among other factors, obesity is an independent risk factor for progression to cirrhosis. Heavy drinkers who are overweight for at least 10 years have a 2-fold risk of developing cirrhosis [43]. While it is not surprising that obesity predisposes to alcohol-induced fatty liver, it is somehow surprising that it favors the development of AH. This association suggests that insulin resistance probably exacerbates the deleterious effects of alcohol in the liver. In fact, hyperglycemia is an independent predictor of progression of fibrosis in heavy drinkers [48]. Finally, there are some reports suggesting that moderate drinking may have beneficial effect on the livers of obese subjects [49].
There is a clear need to perform prospective large follow-up studies to identify the main genetic and environmental factors that influence disease progression in patients with ALD. There are few clinical trials in patients with early forms of ALD and the natural history of this disease is poorly understood. Disease progression is usually silent until heavy drinkers develop jaundice or liver decompensation that, in many cases, occurs in the context of an episode of AH. The course of ALD frequently alternates between periods of complications and liver failure due to excessive drinking and compensated disease as a result of periods of alcohol abstinence.
Diagnosis of alcoholic liver disease
In its early stages, ALD is a silent disease and can only be detected by laboratory tests or imaging techniques. There are few programs aimed at early detection of ALD at its asymptomatic stages. Some patients with early ALD can show stigmata of alcohol abuse such as bilateral parotid gland hypertrophy, muscle wasting, malnutrition, Dupuytren’s sign, and signs of peripheral neuropathy. In patients with cirrhosis, most physical findings are not specific of the etiology. However, some signs such as gynecomastia and extensive spider angiomas may be more frequently seen in those with alcohol as the main cause of liver disease. The diagnosis of ALD is frequently suspected upon documentation of excessive alcohol consumption (>40–50 g/day) and the presence of clinical and/or biological abnormalities suggestive of liver injury. Laboratory blood tests such as mean corpuscular volume, gamma glutamyl transpeptidase (GGT) and aspartate amino transferase (AST) can indicate early ALD whereas advanced ALD is suspected if there is decreased albumin, increased INR, elevated bilirubin level or low platelet count. There are several laboratory markers that estimate persistent alcohol intake. Among them, carbohydrate deficient transferrin and GGT are the most frequently used markers to detect previous alcohol consumption [50]. In patients with ALD, the AST/ALT ratio typically is greater than 1 [51]. This ratio is typically greater than 2 in AH and can also be found in patients with advanced cirrhosis regardless of the etiology.
Liver biopsy is not clearly indicated in patients with early stages of ALD or when established cirrhosis is revealed by clinical, analytical and imaging data. The liver biopsy can be done percutaneously in most patients but requires a transjugular approach in patients with a low platelet count and/or a prolonged prothrombin time. The precise indications of liver biopsy are not well established in routine practice. However, it is suggested in patients with aggressive forms of ALD such as AH requiring specific therapies (e.g., corticosteroids and/or pentoxifylline) and in patients with other cofactors suspected of contributing to liver disease. In the setting of clinical trials, the assessment of liver histology by performing a liver biopsy is recommended. The typical findings in patients with ALD include steatosis, hepatocellular damage (ballooning and/or Mallory-Denk bodies), an inflammatory infiltrate basically composed of PMN cells that predominates in the lobules, and a variable degree of fibrosis and lobular distortion that may progress to cirrhosis [52].
For the assessment of liver fibrosis in patients with ALD, there are non-invasive methods including serum markers and liver stiffness measurements. Most non-invasive tests have been largely validated in patients with hepatitis C, while few studies have included patients with ALD. Thus, AST to platelet ratio index (APRI), FibroTest®, Fibrometer®, Hepascore®, and Fibrosure® can be useful in patients with ALD [53]. They are useful to distinguish between mild and severe fibrosis, but have limited utility in intermediate degrees of fibrosis. In terms of prognostic value, FibroTest® (AUROC for survival = 0.79 ± 0.04), Fibrometer® (0.80 ± 0.04) and Hepascore® (0.78 ± 0.04) had a prognostic value equivalent to liver biopsy (0.77 ± 0.04) [54]. Transient elastography (FibroScan®) is commonly used to assess fibrosis in patients with chronic liver disease. FibroScan® calculates an estimate, expressed in kPa (kilopascals), for the stiffness from the measurement of wave velocity. The diagnostic threshold with the optimal diagnostic value for the detection of cirrhosis varies between 12.5 and 14.6 kPa in studies including chronic hepatitis C [55]. The diagnostic value of FibroScan® for the detection of cirrhosis has been evaluated in excessive drinkers in two independent studies [56,57]. In these studies, the elasticity was correlated with fibrosis stage and the diagnostic value of FibroScan® confirmed by AUROC close to 0.9. The optimal thresholds for the diagnosis of cirrhosis were respectively 19.5 and 22.6 kPa [56,57]. It is important to note that these thresholds, although close together, are very different from those proposed for the detection of cirrhosis in chronic carriers of the hepatitis B and C virus. Of note, elevated liver stiffness values in patients with ALD and AST serum levels >100 U/L should be interpreted with caution because of the possibility of falsely elevated liver stiffness as a result of superimposed ASH [58]. Moreover, recent alcohol consumption can also elevate liver stiffness, perhaps related to the vasodilatory effects of alcohol [59]. Imaging techniques can also be used to assess the severity of ALD. Ultrasonography, MRI, and CT are useful to detect fatty liver, advanced fibrosis/cirrhosis as well as signs of portal hypertension [60]. Moreover, they are useful for the screening and assessment of complications such as ascites and portal vein thrombosis. Among those methods, ultrasound is the most used due to its low cost. However, its sensitivity and specificity is low especially when steatosis is mild. MRI and MR spectroscopy are reliable tools for assessing the amount of steatosis but their use is limited by high cost [61].
Management of alcoholic hepatitis
Providing more efficacious therapies for patients with AH is one of the most urgent needs in clinical hepatology. The current first line therapy (i.e. prednisolone) was proposed in 1971, but novel targeted therapies are needed [62]. Currently, there are no reliable non-invasive diagnostic tools for AH in patients with decompensated ALD. The classical profile of AH comprises elevated serum AST up to twice the upper limit of the normal range, although rarely above 300 IU/ml, with an AST/ALT ratio typically greater than 2 [29,63]. Biological parameters indicating impaired liver function include hypoalbuminemia, elevated bilirubin, high INR and prothrombin time. The presence of acute kidney injury, often due to superimposed hepatorenal syndrome, is associated with a high risk of death [29,64]. In addition, patients with AH frequently disclose leukocytosis and elevated temperature resulting from a systemic inflammatory response syndrome.
In routine practice as in many published clinical trials, the diagnosis of AH relies on clinical and biological criteria. However, such strategy carries a 10–50% risk of misclassifying patients with or without ASH [65–67]. In the setting of clinical trials, the diagnosis of AH through liver biopsy has been recommended by EASL guidelines [2], and liver biopsy may be considered in patients with aggressive forms of ALD requiring specific interventions or when the diagnosis remains in doubt. A transjugular route is often preferred due to frequent coexisting ascites and/or coagulopathy. Recently, a histological scoring system has been proposed for predicting short-term survival in patients with AH. The resulting Alcoholic Hepatitis Histological Score comprises four parameters that are independently associated with patients’ survival: fibrosis stage, PMN infiltration, type of bilirubinostasis and the presence of megamitochondria (Fig. 3). By combining these parameters in a semi-quantitative manner, this histological score is able to stratify patients into low, intermediate, or high risk for death within 90 days [47].
Fig. 3. The Alcoholic Hepatitis Histological Score (AHHS) allows prognostic stratification of patients with biopsy-proven AH.
AHHS categories are as follows: mild, 0–3; intermediate, 4–5; severe, 6–9. The Kaplan-Maier graph shows 90-day survival in each the three categories. Reproduced with permission from [47].
Viral hepatitis and bacterial infections should be ruled out, particularly spontaneous bacterial peritonitis. Importantly, the clinical manifestations of spontaneous bacterial peritonitis and the episode of AH are often similar (abdominal pain, fever, leukocytosis). Doppler ultrasound is required to exclude biliary or vascular disorders and HCC. Severe AH may progress to multisystem organ failure and as mentioned above the advent of acute kidney injury is associated with a bad prognosis [68]. In this setting, the use of AKIN criteria for acute kidney injury is more sensitive than the traditional criteria of renal failure (i.e. creatinine >1.5 mg/dl) for predicting multiorgan dysfunction and death [69]. AH patients are also at risk of acute kidney injury due to the use of nephrotoxic radiocontrast agents, aminoglycosides and non-steroidal anti-inflammatories.
Significant mortality and morbidity in Europe and North America are attributable to AH [70,71], with around 57,000 hospital admissions for AH in the US in 2007, which accounted to 0.71% of all admissions [70,71]. However, the diagnosis of AH is frequently overlooked, especially in patients admitted for gastrointestinal bleeding or sepsis. In-hospital mortality fluctuates from 6.8% to 15% [70,71]. Patients at significant risk of early death can be identified by prognostic models. The available prognostic models include the Maddrey’s discriminant function (DF), the Model for End-Stage Liver Disease (MELD), the Glasgow AH (GAH) score, and the ABIC score [72–74]. The most widely used is the DF, which is calculated as 4.6 × (prothrombin time patient − prothrombin time control) + serum bilirubin [75]. A DF value ≥32 is indicative of evaluated short-term mortality around 20–35% at 1 month. The MELD discloses accuracy similar to DF [73] and a MELD of 21 has 75% sensitivity and specificity in predicting 90-day mortality. The GAH score incorporates age, serum bilirubin, blood urea nitrogen, prothrombin time, and peripheral white blood cell count, and has been shown to accurately predict 28-day mortality [74]. The ABIC uses age, bilirubin, INR, and creatinine to estimate a 90-day risk of mortality, and can categorize patients into low (0%), intermediate (30%), and high (75%) risk of death [72]. The MELD has been evaluated in US cohorts, the GAH in populations from the UK, and the ABIC in Spain.
Early identification of patients with a substantial improvement in hepatic function is of interest in the management of severe AH [76]. After 7 days of medical therapy with prednisolone, physicians may identify responders using the Lille model [76]. The Lille model is highly predictive of death at 6 months and a score above 0.45 predicted 75% of deaths [76,77]. Clinical practice guidelines therefore recommend stopping corticosteroids after one week in those with an unfavorable Lille score, as the risks of continued therapy likely outweigh the benefits [2,76,78].
The therapeutic management of AH patients includes general and specific measures. The achievement of alcohol abstinence is the cornerstone of treatment of AH patients and requires active management of alcohol use disorders. Symptoms of alcohol withdrawal should be monitored, prevented and treated. Intensive nutritional intervention is required to correct protein-calorie malnutrition [79] through high calorie meals and supplements, or through enteral feedings if necessary and thiamine should be administered when considering the increased risk of Wernicke’s encephalopathy in alcoholic patients [80]. Patients with severe AH should be admitted for initial management when considering the increased risk of early deaths, and often need admission to an intensive care unit. Infections are exceedingly common in severe AH [78] and should be routinely screened with at least chest x-ray, urinoanalysis, and cultures of blood, urine and ascites [78]. Empiric antibiotics may be administered if there is a high suspicion of infection. Prevention of acute kidney injury, another frequent complication indicating increased risk of early mortality [64], should be performed using volume expansion with crystalloid/albumin. Interestingly, patients with systemic inflammatory response are at high risk of developing acute kidney injury [64].
The European and American guidelines proposed prednisolone or pentoxifylline [81] as first line therapeutic options for patients with severe AH [82,83]. However, two recent studies called into question the use of pentoxifylline. A head-to-head randomized study showed that the use of pentoxifylline does not have beneficial effects on survival compared to prednisolone [84]. In a large randomized controlled trial (STOPAH) that includes more than 1000 patients that was presented during the 2014 AASLD The Liver Meeting®, pentoxifylline was no better than placebo in terms of short-term mortality [85]. However, this study excluded patients with more severe forms of AH (acute kidney injury, severe sepsis, severe encephalopathy, etc.). Therefore, it is unclear if pentoxifylline is also ineffective in patients with very severe forms.
Corticosteroids have been used in the treatment of AH for more than 40 years [75,86–90]. The most studied formulation is prednisolone 40 mg daily for one month, with or without a taper. Although the clinical trials suffer from heterogeneity and high risk of bias, a meta-analysis from individual data observed that corticosteroids improved survival in patients with a high DF [91]. This study confirmed the need for adapting corticosteroid therapy to response to treatment. A subgroup analysis was performed according to the percentile distribution of the Lille score. This approach identified three patterns of responses; complete, partial and null, with significant differences in survival benefit: 91% vs. 79% vs. 53%, respectively. Survival impact of corticosteroids was significant in complete and partial responders, whereas it appeared negligible in null responders [91]. This new classification raises questions concerning management of severe AH. There are two issues that should be raised. First, the survival benefit from corticosteroid therapy is transient, as suggested by the recent STOPAH trial [85]. And second, the use of prednisolone increases the risk of pulmonary infections, especially invasive aspergillosis [92]. Therefore, it is important to develop new therapies that offer a sustained response and do not favor potentially lethal infections.
It seems clear that corticosteroids may be sufficient in complete responders and that novel pharmacological therapies are required for intermediate responders. Several attempts have been made to combine steroids with other drugs. The combination of both pentoxifylline and prednisolone offers no benefit [93] and there are no available rescue medical therapies for non-responders [29,77]. A recent randomized trial showed that the combination of N-acetylcysteine with prednisolone reduced 1-month mortality (8% vs. 24%) and incidence of hepatorenal syndrome and infection [94]. The favorable safety profile of N-acetylcysteine makes it an appealing option, although confirmatory studies are needed. In non-responders to corticosteroids, a liver transplantation was traditionally contraindicated, since patients could not complete a 6-month period of abstinence. When following a strict 6-month rule for transplant eligibility, most patients die before a transplant can be indicated [95]. This fact prompted several experts in the field to challenge this policy and to perform a prospective study of early liver transplantation in highly selected patients with good social support and positive prognosis from a psychological point of view. Performing a salvage liver transplant in selected patients with AH non-responding to medical therapy dramatically improve survival [96]. These results support future evaluation of early liver transplantation in a carefully-selected subgroup of patients with severe AH non-responding to medical therapy [2]. This controversial indication is increasingly being accepted by liver centers yet confirmatory studies are needed.
Liver transplantation in alcoholic cirrhotic patients
ALD is a major indication for liver transplantation worldwide. Patients needing a transplant due to ALD often present at the time of transplantation multisystemic effects of long-term ethanol abuse [97]. These comorbidities include malnutrition, vitamin deficiencies, non-immune hemolytic anemia, peripheral and central neural system abnormalities, nephropathy, muscle wasting due to alcoholic myopathy, and others. Therefore, the integral care of the transplanted patient with ALD ideally requires a multidisciplinary approach.
Most liver programs require a 6-month period of abstinence prior to evaluation of alcoholic patients [82,98,99]. The rationale of this strategy is to allow disease regression in patients with recent alcohol abstinence and to ensure proper alcohol counseling in order to prevent relapse after the liver transplantation. As for other causes of decompensated liver disease, patients are listed if the MELD score is ≥15. In patients with ALD, the survival benefit among transplant recipients arises only from this threshold [100]. Alcohol abstinence is a critical issue and drinking habits of transplanted patients need to be routinely screened by physicians with tools of proven reliability. However, there is no consensus concerning the definition of alcohol relapse [101,102]. For some experts, any drinking is considered a relapse, whereas others have defined excessive drinking as relapse because only this drinking pattern is associated with alcohol-induced liver injury. The definition of a relapse as any alcohol intake after liver transplantation contrasts with the literature on addiction medicine in which relapse is considered only in the presence of heavy drinking recurrence. This lack of consensus in definition of alcohol relapse explains why the rate of relapse after liver transplantation varies among studies ranging from 10% to 50% [103,104]. A meta-analysis showed no differences in the proportion of transplant recipients with ALD that drank after a liver transplant compared with those with non-ALD: 4% vs. 5% at 6 months and 17% vs. 16% at 12 months [105]. However, transplant patients with ALD were more likely to drink excessively [105]. In terms of liver injury, occasional or moderately heavy drinking does not impact graft function or patient survival [106,107] whereas the deleterious effect of excessive drinking is established at long-term. Indeed, recipients who resume abusive drinking have shorter long-term survival than abstinent recipients or patients with minor relapse [108] and recurrence of ALD is the main cause of death.
Mortality and morbidity after liver transplantation in ALD patients is similar to patients with other etiologies [109]. A recent study demonstrated that survival outcome of living donor liver transplantation in ALD patients is comparable with that of deceased donor liver transplantation. Interestingly, less than 2% of patients died of alcohol abuse, suggesting that alcohol relapse is not a major problem in patients receiving a living donor liver transplantation [110]. The cause of death after transplantation for ALD differs compared to non-ALD recipients. In particular, cardiovascular causes and de novo malignancies are significantly over-represented in the patients transplanted for ALD [111]. There is not a clear association between new-onset cancers and alcohol relapse, suggesting that other environmental factors such as cigarette smoking and obesity can certainly play a role.
There are few data demonstrating the effectiveness of preventive measures in the development of comorbidities in these patients. Psychosocial efforts during the pre- and post-liver transplantation periods should be focused not only on alcohol relapse but also on preventing and treating modifiable risk factors such as obesity and cigarette smoking. A recent proof of concept study showed that smoking withdrawal after liver transplantation had a protective effect against the development of neoplasia in ALD transplant patients [112]. Further studies assessing the impact of specific measures and programs for smoking cessation should be performed in this patient population.
There is a current trend to tailor the immunosuppression in patients with ALD by minimizing the exposure to calcineurin inhibitors and promoting the use of mTOR inhibitors (rapamycin and everolimus). The rationale is that calcineurin inhibitors are known to favor cardiovascular events and mTOR inhibitors may promote decreased tumor growth and angiogenesis. Although some recent retrospective studies support this strategy in patients with ALD [113], well-designed studies should assess the optimal immunosuppressive regimens in these patients.
Acknowledgments
Financial support
This work was supported by NIAAA grants 1U01AA021908 and 1U01AA020821.
Abbreviations
- AH
alcoholic hepatitis
- ALD
Alcoholic liver disease
- ASH
alcoholic steatohepatitis
- DF
discriminant function
- HCC
hepatocellular carcinoma
- LPS
lipopolysaccharide
- NIAAA
National Institute on Alcohol Abuse and Alcoholism
Footnotes
Conflict of interest
Ramon Bataller has provided consulting services to Sandhill Scientific. Philippe Mathurin has nothing to disclose.
References
- 1.Rehm J, Room R, Monteiro M, et al. Alcohol as a risk factor for global burden of disease. Eur Addict Res. 2003;9:157–164. doi: 10.1159/000072222. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57:399–420. doi: 10.1016/j.jhep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Askgaard G, Gronbaek M, Kjaer MS, et al. Alcohol drinking pattern and risk of alcoholic liver cirrhosis: a prospective cohort study. J Hepatol. 2015 doi: 10.1016/j.jhep.2014.12.005. http://dx.doi.org/10.1016/j.jhep.2014.12.005, in press. [DOI] [PubMed]
- 4.Zakhari S, Li T. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46:2032–2039. doi: 10.1002/hep.22010. [DOI] [PubMed] [Google Scholar]
- 5.Pincock S. Binge drinking on rise in UK and elsewhere. Lancet. 2003;362:1126–1127. doi: 10.1016/s0140-6736(03)14502-3. [DOI] [PubMed] [Google Scholar]
- 6.Naimi TS, Brewer R, Mokdad A, Denny C, Serdula MK, Marks JS. Binge drinking among US adult. JAMA. 2003;289:70–75. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- 7.McAlaney J, McMahon J. Establishing rates of binge drinking in the UK: anomalies in the data. Alcohol Alcohol. 2006;41:355–357. doi: 10.1093/alcalc/agl025. [DOI] [PubMed] [Google Scholar]
- 8.Mathurin P, Delterne P. Effect of binge drinking on the liver: an alarming public health issue? Gut. 2009;58:613–617. doi: 10.1136/gut.2007.145573. [DOI] [PubMed] [Google Scholar]
- 9.Hingson R, Heeren T, Winter M, Wechsler H. Magnitude of alcohol-related mortality and morbidity among U.S. college students ages 18–24: changes from 1998 to 2001. Annu Rev Public Health. 2005;26:259–279. doi: 10.1146/annurev.publhealth.26.021304.144652. [DOI] [PubMed] [Google Scholar]
- 10.Leon DA, McCambridge J. Liver cirrhosis mortality rates in Britain from 1950 to 2002: an analysis of routine data. Lancet. 2006;367:52–56. doi: 10.1016/S0140-6736(06)67924-5. [DOI] [PubMed] [Google Scholar]
- 11.Miller JW, Naimi T, Brewer RD, Jones SE. Binge drinking and associated health risk behaviors among high school students. Pediatrics. 2007;119:76–85. doi: 10.1542/peds.2006-1517. [DOI] [PubMed] [Google Scholar]
- 12.Corrao G, Bagnardi V, Zambon A, Torchio P. Meta-analysis of alcohol intake in relation to risk of liver cirrhosis. Alcohol Alcohol. 1998;33:381–392. doi: 10.1093/oxfordjournals.alcalc.a008408. [DOI] [PubMed] [Google Scholar]
- 13.Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, et al. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut. 1997;41:845–850. doi: 10.1136/gut.41.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 16.Rehm J, Samokhvalov A, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013:160–168. doi: 10.1016/j.jhep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Jewell J, Sheron N. Trends in European liver death rates: implications for alcohol policy. Clin Med. 2010;10:259–263. doi: 10.7861/clinmedicine.10-3-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosetti C, Levi F, Lucchini F, Zatonski WA, Negri E, La Vecchia C. Worldwide mortality from cirrhosis: an update to 2002. J Hepatol. 2007;46:827–839. doi: 10.1016/j.jhep.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 19.Leon DA, Shkolnikov V, McKee M. Alcohol and Russian mortality: a continuing crisis. Addiction. 2009;104:1630–1636. doi: 10.1111/j.1360-0443.2009.02655.x. [DOI] [PubMed] [Google Scholar]
- 20.Corrao G, Ferrari P, Zambon A, Torchio P, Arico S, Decarli A. Trends of liver cirrhosis mortality in Europe, 1970–1989: age-period-cohort analysis and changing alcohol consumption. Int J Epidemiol. 1997;26:100–109. doi: 10.1093/ije/26.1.100. [DOI] [PubMed] [Google Scholar]
- 21.La Vecchia C. Alcohol in the Mediterranean diet: benefits and risks. Int J Vitam Nutr Res. 2001;71:210–213. doi: 10.1024/0300-9831.71.3.210. [DOI] [PubMed] [Google Scholar]
- 22.Ramstedt M. Per capita alcohol consumption and liver cirrhosis mortality in 14 European countries. Addiction. 2001;96:S19–S33. doi: 10.1080/09652140020021152. [DOI] [PubMed] [Google Scholar]
- 23.Adam R, McMaster P, O’Grady JG, et al. Evolution of liver transplantation in Europe: report of the European Liver Transplant Registry. Liver Transpl. 2003;9:1231–1243. doi: 10.1016/j.lts.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Wagenaar AC, Tobler AL, Komro KA. Effects of alcohol tax and price policies on morbidity and mortality: a systematic review. Am J Public Health. 2010;100:2270–2278. doi: 10.2105/AJPH.2009.186007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagenaar AC, Salois MJ, Komro KA. Effects of beverage alcohol price and tax levels on drinking: a meta-analysis of 1003 estimates from 112 studies. Addiction. 2009;104:179–190. doi: 10.1111/j.1360-0443.2008.02438.x. [DOI] [PubMed] [Google Scholar]
- 26.Brennan A, Meng Y, Holmes J, et al. Potential benefits of minimum unit pricing for alcohol versus a ban on below cost selling in England 2014: modelling study. BMJ. 2014;349:g5452. doi: 10.1136/bmj.g5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teli MRDC, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet. 1995;346:987–990. doi: 10.1016/s0140-6736(95)91685-7. [DOI] [PubMed] [Google Scholar]
- 28.Lefkowitch JH. Morphology of alcoholic liver disease. Clin Liver Dis. 2005;9:37–53. doi: 10.1016/j.cld.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Lucey M, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 30.Adachi M, Brenner D. Clinical syndromes of alcoholic liver disease. Dig Dis Sci. 2005;23:255–263. doi: 10.1159/000090173. [DOI] [PubMed] [Google Scholar]
- 31.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsukamoto H, Cheng S, Blanner WS. Effects of dietary polyunsaturated fat on ethanol-induced Ito cell activation. Am J Physiol. 1996;270:G581–G586. doi: 10.1152/ajpgi.1996.270.4.G581. [DOI] [PubMed] [Google Scholar]
- 33.Tsukamoto H, Horne W, Kamimura S, Niemelä O, Parkkila S, Ylä-Herttuala S, et al. Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest. 1995;96:620–630. doi: 10.1172/JCI118077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsukamoto H, Takei Y, McClain CJ, Joshi-Barve S, Hill D, Schmidt J, et al. How is the liver primed or sensitized for alcoholic liver disease? Alcohol Clin Exp Res. 2001;25:171S–181S. doi: 10.1097/00000374-200105051-00029. [DOI] [PubMed] [Google Scholar]
- 35.Vaillant GE. A 60-year follow-up of alcoholic men. Addiction. 2003;98:1043–1051. doi: 10.1046/j.1360-0443.2003.00422.x. [DOI] [PubMed] [Google Scholar]
- 36.DiMartini A, Dew MA, Day N, et al. Trajectories of alcohol consumption following liver transplantation. Am J Transplant. 2010;10:2305–2312. doi: 10.1111/j.1600-6143.2010.03232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hrubec Z, Omenn G. Evidence of genetic predisposition to alcoholic cirrhosis and psychosis: twin concordances for alcoholism and its biological end points by zygosity among male veterans. Alcohol Clin Exp Res. 1981;5:207–215. doi: 10.1111/j.1530-0277.1981.tb04890.x. [DOI] [PubMed] [Google Scholar]
- 38.Stickel F, Buch S, Lau K, Meyer zu Schwabedissen H, Berg T, Ridinger M, et al. Genetic variation in the PNPLA3 gene is associated with alcoholic liver injury in caucasians. Hepatology. 2010;53:86–95. doi: 10.1002/hep.24017. [DOI] [PubMed] [Google Scholar]
- 39.Trépo E, Gustot T, Degré D, Lemmers A, Verset L, Demetter P, et al. Common polymorphism in the PNPLA3/adiponutrin gene confers higher risk of cirrhosis and liver damage in alcoholic liver disease. J Hepatol. 2011;55:906–912. doi: 10.1016/j.jhep.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 40.Tian C, Stokowski R, Kershenobich D, Ballinger DG, Hinds DA. Common polymorphism in the PNPLA3/adiponutrin gene confers higher risk of cirrhosis and liver damage in alcoholic liver disease. Nat Genet. 2010;42:21–23. doi: 10.1038/ng.488. [DOI] [PubMed] [Google Scholar]
- 41.Liu YL, Patman GL, Leathart JB, et al. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61:75–81. doi: 10.1016/j.jhep.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 42.Bataller R, North K. Brenner DA Genetic polymorphisms and the progression of liver fibrosis: a critical appraisal. Hepatology. 2003;37:493–503. doi: 10.1053/jhep.2003.50127. [DOI] [PubMed] [Google Scholar]
- 43.Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108–111. doi: 10.1002/hep.510250120. [DOI] [PubMed] [Google Scholar]
- 44.Poynard T, Mathurin P, Lai CL, Guyader D, Poupon R, Tainturier MH, et al. A comparison of fibrosis progression in chronic liver diseases. J Hepatol. 2003;38:257–265. doi: 10.1016/s0168-8278(02)00413-0. [DOI] [PubMed] [Google Scholar]
- 45.Mathurin P, Beuzin F, Louvet A, Carrie-Ganne N, Balian A, Trinchet JC, et al. Fibrosis progression occurs in a subgroup of heavy drinkers with typical histological features. Aliment Pharmacol Ther. 2007;25:1047–1054. doi: 10.1111/j.1365-2036.2007.03302.x. [DOI] [PubMed] [Google Scholar]
- 46.Dam-Larsen S, Franzmann M, Andersen IB, Christoffersen P, Jensen LB, Sørensen TI, et al. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53:750–755. doi: 10.1136/gut.2003.019984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Altamirano J, Miquel R, Katoonizadeh A, Abraldes JG, Duarte-Rojo A, Louvet A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014;146:1231–1239. doi: 10.1053/j.gastro.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, et al. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635–638. doi: 10.1053/jhep.2002.31782. [DOI] [PubMed] [Google Scholar]
- 49.Dunn W, Sanyal AJ, Brunt EM, et al. Modest alcohol consumption is associated with decreased prevalence of steatohepatitis in patients with non-alcoholic fatty liver disease (NAFLD) J Hepatol. 2012;57:384–391. doi: 10.1016/j.jhep.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hock B, Schwarz M, Domke I, et al. Validity of carbohydrate-deficient transferrin (%CDT), gamma-glutamyltransferase (gamma-GT) and mean corpuscular erythrocyte volume (MCV) as biomarkers for chronic alcohol abuse: a study in patients with alcohol dependence and liver disorders of non-alcoholic and alcoholic origin. Addiction. 2005;100:1477–1486. doi: 10.1111/j.1360-0443.2005.01216.x. [DOI] [PubMed] [Google Scholar]
- 51.Alatalo P, Koivisto H, Puukka K, et al. Biomarkers of liver status in heavy drinkers, moderate drinkers and abstainers. Alcohol Alcohol. 2009;44:199–203. doi: 10.1093/alcalc/agn099. [DOI] [PubMed] [Google Scholar]
- 52.MacSween RN, Burt AD. Histologic spectrum of alcoholic liver disease. Semin Liver Dis. 1986;6:221–232. doi: 10.1055/s-2008-1040605. [DOI] [PubMed] [Google Scholar]
- 53.Chrostek L, Panasiuk A. Liver fibrosis markers in alcoholic liver disease. World J Gastroenterol. 2014;20:8018–8023. doi: 10.3748/wjg.v20.i25.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naveau S, Gaudé G, Asnacios A, Agostini H, Abella A, Barri-Ova N, et al. Diagnostic and prognostic values of noninvasive biomarkers of fibrosis in patients with alcoholic liver disease. Hepatology. 2009;49:97–105. doi: 10.1002/hep.22576. [DOI] [PubMed] [Google Scholar]
- 55.Castera L, Pinzani M. Biopsy and non-invasive methods for the diagnosis of liver fibrosis: does it take two to tango? Gut. 2010;59:861–866. doi: 10.1136/gut.2010.214650. [DOI] [PubMed] [Google Scholar]
- 56.Nahon P, Kettaneh A, Tengher-Barna J, Ziol M, de Lédinghen V, Douvin C, et al. Assessment of liver fibrosis using transient elastography in patients with alcoholic liver disease. J Hepatol. 2008;49:1062–1068. doi: 10.1016/j.jhep.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen-Khac E, Saint-Leger P, Tramier B, Coevoet H, Capron D, Dupas JL. Noninvasive diagnosis of large esophageal varices by Fibroscan: strong influence of the cirrhosis etiology. Alcohol Clin Exp Res. 2010;34:1146–1153. doi: 10.1111/j.1530-0277.2010.01191.x. [DOI] [PubMed] [Google Scholar]
- 58.Mueller S, Millonig G, Sarovska L, et al. Increased liver stiffness in alcoholic liver disease: differentiating fibrosis from steatohepatitis. World J Gastroenterol. 2010;16:966–972. doi: 10.3748/wjg.v16.i8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gelsi E, Dainese R, Truchi R, et al. Effect of detoxification on liver stiffness assessed by Fibroscan(R) in alcoholic patients. Alcohol Clin Exp Res. 2011;35:566–570. doi: 10.1111/j.1530-0277.2010.01374.x. [DOI] [PubMed] [Google Scholar]
- 60.Penny SM. Alcoholic liver disease. Radiol Technol. 2013;84:577–592. quiz 593–595. [PubMed] [Google Scholar]
- 61.d’Assignies G, Fontes G, Kauffmann C, et al. Early detection of liver steatosis by magnetic resonance imaging in rats infused with glucose and intralipid solutions and correlation to insulin levels. Metabolism. 2013;62:1850–1857. doi: 10.1016/j.metabol.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Porter HP, Simon FR, Pope CE, 2nd, et al. Corticosteroid therapy in severe alcoholic hepatitis. A double-blind drug trial. N Engl J Med. 1971;284:1350–1355. doi: 10.1056/NEJM197106172842404. [DOI] [PubMed] [Google Scholar]
- 63.Cohen JA, Kaplan M. The SGOT/SGPT ratio – An indicator of alcoholic liver disease. Dig Dis Sci. 1979;24:835–838. doi: 10.1007/BF01324898. [DOI] [PubMed] [Google Scholar]
- 64.Altamirano J, Fagundes C, Dominguez M, Garcia E, Michelena J, Cardenas A, et al. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol. 2012;10:e3. doi: 10.1016/j.cgh.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 65.Kryger P, Schlichting P, Dietrichson O, Juhl E. The accuracy of the clinical diagnosis in acute hepatitis and alcoholic liver disease. Clinical versus morphological diagnosis. Scand J Gastroenterol. 1983;18:691–696. doi: 10.3109/00365528309181659. [DOI] [PubMed] [Google Scholar]
- 66.Mookerjee RP, Lackner C, Stauber R, Stadlbauer V, Deheragoda M, Aigelsreiter A, et al. The role of liver biopsy in the diagnosis and prognosis of patients with acute deterioration of alcoholic cirrhosis. J Hepatol. 2011;55:1103–1111. doi: 10.1016/j.jhep.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 67.Hardy T, Wells C, Kendrick S, Hudson M, Day CP, Burt AD, et al. White cell count and platelet count associate with histological alcoholic hepatitis in jaundiced harmful drinkers. BMC Gastroenterol. 2013;2013:55–59. doi: 10.1186/1471-230X-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mathurin P, Lucey M. Management of alcoholic hepatitis. J Hepatol. 2012;56:S39–S45. doi: 10.1016/S0168-8278(12)60005-1. [DOI] [PubMed] [Google Scholar]
- 69.Altamirano J, Fagundes C, Dominguez M, et al. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol. 2012;10:e3. doi: 10.1016/j.cgh.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 70.Sandahl TD, Jepsen P, Thomsen KL, Vilstrup H. Incidence and mortality of alcoholic hepatitis in Denmark 1999–2008: a nationwide population based cohort study. J Hepatol. 2011;54:760–764. doi: 10.1016/j.jhep.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 71.Liangpunsakul S. Clinical characteristics and mortality of hospitalized alcoholic hepatitis patients in the United States. J Clin Gastroenterol. 2011;45:714–719. doi: 10.1097/MCG.0b013e3181fdef1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dominguez M, Rincon D, Abraldes JG, Miquel R, Colmenero J, Bellot P, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747–2756. doi: 10.1111/j.1572-0241.2008.02104.x. [DOI] [PubMed] [Google Scholar]
- 73.Dunn W, Jamil L, Brown LS, Wiesner RH, Kim WR, Menon KVN, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353–358. doi: 10.1002/hep.20503. [DOI] [PubMed] [Google Scholar]
- 74.Forrest EH, Evans C, Stewart S, Phillips M, Oo YH, McAvoy NC, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54:1174–1179. doi: 10.1136/gut.2004.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carithers RL, Jr, Herlong H, Diehl AM, Shaw EW, Combes B, Fallon HJ, et al. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann Intern Med. 1989;110:685–690. doi: 10.7326/0003-4819-110-9-685. [DOI] [PubMed] [Google Scholar]
- 76.Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45:1348–1354. doi: 10.1002/hep.21607. [DOI] [PubMed] [Google Scholar]
- 77.Louvet A, Diaz E, Dharancy S, Coevoet H, Texier F, Thévenot T, et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol. 2008;48:465–470. doi: 10.1016/j.jhep.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 78.Louvet A, Wartel F, Castel H, Dharancy S, Hollebecque A, Canva-Delcambre V, et al. Prospective screening of infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology. 2009;137:541–548. doi: 10.1053/j.gastro.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 79.Singal AK, Charlton MR. Nutrition in alcoholic liver disease. Clin Liver Dis. 2012;16:805–826. doi: 10.1016/j.cld.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 80.Butterworth RF. Pathophysiology of alcoholic brain damage: synergistic effects of ethanol, thiamine deficiency and alcoholic liver disease. Metab Brain Dis. 1995;10:1–8. doi: 10.1007/BF01991777. [DOI] [PubMed] [Google Scholar]
- 81.Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–1648. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 82.EASL. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 83.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105:14–32. doi: 10.1038/ajg.2009.593. [DOI] [PubMed] [Google Scholar]
- 84.Park SH, Kim DJ, Kim YS, Yim HJ, Tak WY, Lee HJ, et al. Pentoxifylline vs corticosteroid to treat severe alcoholic hepatitis: a randomised, non-inferiority, open trial. J Hepatol. 2014;61:792–798. doi: 10.1016/j.jhep.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 85.Thursz M, Richardson P, Allison ME, Austin A, Bowers M, Day CP, et al. Steroids or pentoxifylline for alcoholic hepatitis: results of the STOPAH trial. Hepatology. 2014;60:LB1. [Google Scholar]
- 86.Imperiale TF, McCullough A. Do corticosteroids reduce mortality from alcoholic hepatitis? Ann Intern Med. 1990;113:299–307. doi: 10.7326/0003-4819-113-4-299. [DOI] [PubMed] [Google Scholar]
- 87.Maddrey WC, Boitnott J, Bedine MS, Weber FL, Mezey E, White RI. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199. [PubMed] [Google Scholar]
- 88.Mathurin P, Mendenhall C, Carithers RL, Jr, Ramond MJ, Maddrey WC, Garstide P, et al. Corticosteroids improve short term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials. J Hepatol. 2002;36:480–487. doi: 10.1016/s0168-8278(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 89.Mendenhall CL, Anderson S, Garcia-Pont P, Goldberg S, Kiernan T, Seef LB, et al. Short-term and long-term survival in patients with alcoholic hepatitis treated with oxandrolone and prednisolone. N Engl J Med. 1984;311:1464–1470. doi: 10.1056/NEJM198412063112302. [DOI] [PubMed] [Google Scholar]
- 90.Ramond MJ, Poynard T, Rueff B, Mathurin P, Theodore C, Chaput JC, et al. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med. 1992;326:507–512. doi: 10.1056/NEJM199202203260802. [DOI] [PubMed] [Google Scholar]
- 91.Mathurin P, O’Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60:255–260. doi: 10.1136/gut.2010.224097. [DOI] [PubMed] [Google Scholar]
- 92.Gustot T, Maillart E, Bocci M, et al. Invasive aspergillosis in patients with severe alcoholic hepatitis. J Hepatol. 2014;60:267–274. doi: 10.1016/j.jhep.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 93.Mathurin P, Louvet A, Duhamel A, Nahon P, Carbonell N, Boursier J, et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. JAMA. 2013;310:1033–1041. doi: 10.1001/jama.2013.276300. [DOI] [PubMed] [Google Scholar]
- 94.Nguyen-Khac E, Thevenot T, Piquet MA, Benferhat S, Goria O, Chatelain D, et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781–1789. doi: 10.1056/NEJMoa1101214. [DOI] [PubMed] [Google Scholar]
- 95.Donckier V, Lucidi V, Gustot T, Moreno C. Ethical considerations regarding early liver transplantation in patients with severe alcoholic hepatitis not responding to medical therapy. J Hepatol. 2014;60:866–871. doi: 10.1016/j.jhep.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 96.Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790–1800. doi: 10.1056/NEJMoa1105703. [DOI] [PubMed] [Google Scholar]
- 97.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beresford TP, Everson GT. Liver transplantation for alcoholic liver disease: bias, beliefs, 6-month rule, and relapse—but where are the data? Liver Transpl. 2000;6:777–778. doi: 10.1053/jlts.2000.19027. [DOI] [PubMed] [Google Scholar]
- 99.Consensus conference: indications for liver transplantation, January 19 and 20 Lyon-Palais Des Congrès: text of recommendations (long version) Liver Transpl. 2005;2006:998–1011. doi: 10.1002/lt.20765. [DOI] [PubMed] [Google Scholar]
- 100.Merion RM, Schaubel DE, Dykstra DL, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 101.Lucey MR. Liver transplantation for alcoholic liver disease: past, present, and future. Liver Transpl. 2007;13:190–192. doi: 10.1002/lt.21014. [DOI] [PubMed] [Google Scholar]
- 102.Lucey MR. How will patients be selected for transplantation in the future? Liver Transpl. 2004;10:S90–S92. doi: 10.1002/lt.20256. [DOI] [PubMed] [Google Scholar]
- 103.Burra P, Lucey MR. Liver transplantation in alcoholic patients. Transpl Int. 2005;1:491–498. doi: 10.1111/j.1432-2277.2005.00079.x. [DOI] [PubMed] [Google Scholar]
- 104.Burra P, Mioni D, Cillo U, Fagiuoli S, Senzolo M, Naccarato R, et al. Long-term medical and psycho-social evaluation of patients undergoing orthotopic liver transplantation for alcoholic liver disease. Transpl Int. 2000;13:S174–S178. doi: 10.1007/s001470050320. [DOI] [PubMed] [Google Scholar]
- 105.Bravata DM, Olkin I, Barnato AE, Keeffe EB, Owens DK. Employment and alcohol use after liver transplantation for alcoholic and nonalcoholic liver disease: a systematic review. Liver Transpl. 2001;7:191–203. doi: 10.1053/jlts.2001.22326. [DOI] [PubMed] [Google Scholar]
- 106.DiMartini A, Day N, Dew MA, Javed L, Fitzgerald MG, Jain A, et al. Alcohol consumption patterns and predictors of use following liver transplantation for alcoholic liver disease. Liver Transpl. 2006;12:813–820. doi: 10.1002/lt.20688. [DOI] [PubMed] [Google Scholar]
- 107.Pageaux G, Bismuth M, Perney P, Costes V, Jaber S, Possoz P, et al. Alcohol relapse after liver transplantation for alcoholic liver disease: does it matter? J Hepatol. 2003;38:629–634. doi: 10.1016/s0168-8278(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 108.Pfitzmann R, Schwenzer J, Rayes N, Seehofer D, Neuhaus R, Nussler NC. Long-term survival and predictors of relapse after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl. 2007;13:197–205. doi: 10.1002/lt.20934. [DOI] [PubMed] [Google Scholar]
- 109.Burra P, Senzolo M, Adam R, et al. Liver transplantation for alcoholic liver disease in Europe: a study from the ELTR (European Liver Transplant Registry) Am J Transplant. 2010;10:138–148. doi: 10.1111/j.1600-6143.2009.02869.x. [DOI] [PubMed] [Google Scholar]
- 110.Ahn CS, Hwang S, Kim KH, et al. Long-term outcome of living donor liver transplantation for patients with alcoholic liver disease. Transplant Proc. 2014;46:761–766. doi: 10.1016/j.transproceed.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 111.Gaglio PJ, Jr, Gaglio PJ., Sr Complications in patients with alcohol-associated liver disease who undergo liver transplantation. Clin Liver Dis. 2012;16:865–875. doi: 10.1016/j.cld.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 112.Herrero JI, Pardo F, D’Avola D, et al. Risk factors of lung, head and neck, esophageal, and kidney and urinary tract carcinomas after liver transplantation: the effect of smoking withdrawal. Liver Transpl. 2011;17:402–408. doi: 10.1002/lt.22247. [DOI] [PubMed] [Google Scholar]
- 113.Jimenez-Romero C, Manrique A, Marques E, et al. Switching to sirolimus monotherapy for de novo tumors after liver transplantation. A preliminary experience. Hepatogastroenterology. 2011;58:115–121. [PubMed] [Google Scholar]