Abstract
The aminoglycoside antibiotic gentamicin can cause both ototoxicity and nephrotoxicity, the severity of which varies with circadian time of daily treatment. However, it is not yet resolved if such drug-induced adverse effects are independent or dependent phenomena. Two groups of 9 female Sprague-Dawley rats (200-250 g), each housed separately, entrained to a 12h light (06:00 to 18:00h)-12h dark cycle, received a daily subcutaneous injection of 100 mg/kg gentamicin. One group was treated at the beginning of the activity span, 2 HALO (Hours After Lights On), and the other at the beginning of the rest span, 14 HALO. Global toxicity was gauged both by body weight loss relative to the pre-treatment baseline and number of deaths. Ototoxicity, i.e., hearing loss, was assessed by changes in Auditory Brainstem Responses (ABR) for pure tone stimuli of 8, 16, 24, and 32 kHz before and after 2 and 4 weeks of gentamicin treatment. Renal toxicity was evaluated by changes in urinary N-acetyl-β-glucosaminidase (NAG)/creatinine (CR) concentration ratio before and after each week of treatment. In a complementary substudy of separate but comparable 2 and 14 HALO groups of rats, blood samples were obtained before and 30, 60, 120, and 240-mins post-subcutaneous injection of 100 mg/kg gentamicin. Number of animal deaths was greater in the 2 (4 deaths) than 14 HALO (1 death) group, mirroring more severe initial (1st 2 weeks of treatment) body weight losses from baseline, being more than 2-fold greater in animals of the 2 than 14 HALO group. Ototoxicity progressively worsened during treatment; although, the extent of hearing loss varied according to circadian time of treatment across all frequencies (p<0.05), particularly the 24 and 32 kHz ones (both p<0.005), both at the 2 and 4 week assessments. At 32 kHz after 4 weeks of gentamicin dosing, the 2 HALO group showed an average 42 dB hearing loss, while the 14 HALO group exhibited only an average 10 dB loss. ABR response latencies were longer for the 2 than 14 HALO rats. The time course of nephrotoxicity differed from that of ototoxicity. The mean urinary NAG/CR ratio peaked after the 1st week of treatment, averaging 13.64-fold greater than baseline for the 2 HALO-treated animals compared to 7.38-fold greater than baseline for the 14 HALO-treated ones. Ratio values declined thereafter; although even after the 2nd week of dosing, they remained greater in the 2 than 14 HALO group (averaging 8.15-fold greater and 2.23-fold greater than baseline, respectively). Pharmacokinetic analysis of the blood gentamicin values revealed slower clearance, on average by ∼25% (p<0.001), in the rats of the 14 than 2 HALO group (x̄ ± S.E.: 3.22 ± 0.49 and 4.53 ± 0.63 mL/min/kg, respectively). The study findings indicate robust difference of the time course in rats of both treatment groups of gentamicin-induced ototoxicity and nephrotoxicity, supporting the hypothesis these organ toxicities are independent of one another, and further suggest the observed treatment-time differences in gentamicin adverse effects may be more dependent on local cell, tissue, or organ circadian (chrono)pharmacodynamic than (chrono)pharmacokinetic mechanisms.
Keywords: Ototoxicity, Nephrotoxicity, Gentamicin, Circadian Rhythm, Aminoglycosides, Chronopharmacology, Chronotoxicity
Introduction
Aminoglycoside antibiotics have proven to be a very successful treatment for bacterial infection, but they also can cause adverse effects, especially ototoxicity (Dallos & Wang, 1974; Ryan & McGee, 1977) and nephrotoxicity (Kaloyanides & Pastoriza-Munoz, 1980), even in low doses, and these deleterious effects may continue long after discontinuation of drug dosing (Cosgrove et al., 2009). Various methods have been implemented to investigate ototoxicity, including behavioral and electrophysiological ones. The auditory brainstem evoked response (ABR) is the most effective and accurate means of assessing hearing loss in animals, and it does not require animal training or reliance on motor responses that may be impaired (Evans et al., 1983; Yonovitz & Fisch, 1991). Many different methods have been utilized in the past to assess nephrotoxicity, for example, urinary excretion of creatinine, urea, and enzymes indicative of renal tissue injury. Changes in N-acetyl-β-glucosaminidase (NAG), which is ordinarily present in the kidney, have proven to be an effective means of gauging early kidney dysfunction, even before structural kidney damage can be observed by light microscopy (Wellwood et al., 1976).
The literature suggests controversy about the role renal toxicity may play in determining risk for ototoxicity. Arnold (1984) surmised persistent renal impairment increases the risk of ototoxicity, while both Smith et al. (1979) and Lerner et al. (1986) proposed aminoglycoside-induced ototoxicity and nephrotoxicity are independent phenomena that entail different mechanisms. Dulon et al. (1988) simultaneously studied the ototoxic and nephrotoxic effects of long-term gentamicin treatment; guinea pigs were injected with 60 mg/kg gentamicin for 3 weeks with auditory function assessed by click-evoked cortex potentials and nephrotoxicity evaluated by urinary NAG concentration using a fluorometric technique. Renal injury was found to precede noticeable hearing loss, and after cessation of treatment renal damage was reversed while hearing loss progressed. Moreover, the amount of auditory and vestibular dysfunction did not correlate with changes in renal parameters, leading the authors to deduce differences in the magnitude and time course between the expression of hearing loss and renal injury are indicative of separate mechanisms of toxicity.
Several investigations demonstrate the extent of beneficial and/or adverse effects of aminoglycoside antibiotics (Beauchamp & Labrecque, 2007; McKinney et al., 2015; Yonovitz & Fisch, 1991) and many other types of medications, including BP-lowering ones (Ayala et al., 2013; Crespo et al., 2013; Hermida et al., 2013a, 2013b, 2013c; Moyá et al., 2013; Reinberg & Smolensky, 1983; Smolensky et al., 2010), differ sometimes markedly according to circadian time of dosing. Such differences in medication effects can be explained by rhythm dependencies of their pharmacokinetics (PK), i.e., chronopharmacokinetics (dosing-time differences in drug absorption, distribution, metabolism, and elimination due to circadian variation in gastrointestinal motility; gastric pH, transport, and emptying; biliary function and hepatic enzyme activity; renal glomerular filtration; and organ blood flow, e.g., of the duodenum, liver, and kidney), and/or chronopharmacodynamics (administration-time differences, independent of drug chronopharmacokinetics, in desired and undesired effects due to circadian patterning, e.g., in blood drug-free fraction, number/conformation of receptors, second messengers, and signaling pathways of drug-targeted cells, tissues, and organs) (Smolensky & Peppas, 2007). The chronopharmacokinetics of many different classes of medications have been evaluated (Lemmer & Bruguerolle, 1994; Reinberg & Smolensky, 1982; Reinberg et al., 1984). Recently, Beauchamp and Labrecque (2007) reviewed the published studies addressing the chronopharmacology, i.e., chronopharmacokinetics and chronopharmacodynamics, of aminoglycoside antibiotics. Results of both animal and patient studies suggest it is possible to significantly attenuate aminoglycoside-induced nephrotoxicity by restricting the administration of high doses to between morning and noon in persons adhering to a normal routine of diurnal activity and nighttime sleep. The goal of our laboratory animal research is to better understand the time course and potential mechanisms of the major adverse effects of aminogylcoside antibiotics -- hearing damage and kidney dysfunction – and to learn how the circadian time of their dosing can be utilized to improve drug safety.
Purpose of the Study
This research proposes to simultaneously examine the relationship between damage to auditory and kidney function induced by gentamicin according to the circadian time of greatest and lowest risk for its toxic effects, respectively, beginning of the rest and activity span, based on the findings of experiments previously conducted by us and others (Beauchamp & Labrecque, 2007; McKinney et al., 2015; Yonovitz & Fisch, 1991). Gentamicin can cause damage to the outer hair cells of the cochlea, resulting in permanent sensorineural hearing loss, and also the nephrons of the renal cortex, resulting in compromised kidney function. In animal models, ABR is the most effective method of assessing auditory function (Evans et al., 1983; Yonovitz & Fisch, 1991), and urine testing for N-acetyl-β-glucosaminidase (NAG) enzyme activity has been substantiated to be a sensitive means of assessing renal injury that correlates well with microscopic renal cortical tissue indices (Wellwood et al., 1976).
Methods
Animals
This study was approved by The University of Maine Institutional Animal Care and Use committee (IACUC). Female Sprague-Dawley rats, obtained from Charles River Laboratories (Boston, Mass.), were individually housed with Purina Rat Chow and tap water available ad libitum and standardized for circadian rhythm study by programming the environment of the vivarium with an alternating 12h light (06:00–18:00h)-12h dark cycle commencing 3 weeks before and continuing throughout the 4-week gentamicin treatment period.
GentamicinTreatment
The female Sprague-Dawley rats were randomly allocated to two groups of 9 animals each for timed treatment once daily either 2 Hours After Lights On (2 HALO), termed the diurnal group, or 2h after lights off (14 HALO), termed the nocturnal group, to assess treatment-time differences in the time course and extent of both gentamicin-induced global measures of toxicity (body weight loss and death) and specific organ toxicities -- ototoxicity and nephrotoxicity. Each rat received a daily subcutaneous injection of gentamicin sulfate (Gentacin) in a dosage of 100 mg/kg body weight (bw) for 4 weeks. Baseline plus bi-monthly ABRs and weekly 24h urine samples were obtained from each animal throughout the 4-week treatment period. This enabled determination of changes in each variable relative to the pretreatment baseline according to the circadian time of dosing, both on an individual animal and treatment-time group basis. In an accompanying substudy (see below), two other groups of female Sprague-Dawley rats of comparable weight and age were investigated for administration-time differences in selected parameters of gentamicin pharmacokinetics (PK).
Electrodes and Surgery
Anesthesia for implantation of the dural electrode and acquisition of the ABR measurements were accomplished as previously described by Wassick & Yonovitz (1985). Rats first received an intramuscular injection of 50 mg/kg bw ketamine hydrochloride (Ketaset) followed by intraperitoneal injection of 21 mg/kg bw of sodium pentobarbital (Nembutal). The chronic dural electrode consisted of a 0.48 cm stainless steel screw soldered to an electrical pin. Following a small sagittal incision, the electrode was implanted 1 mm to the right of the sagittal suture, midway between the lambda and bregma sutures, to a depth where the electrode just touched the dura of the brain. Dental cement was applied to the electrode and surrounding area for securement. The initial baseline ABR was obtained after recovery of the animal, a minimum of 3 days.
Apparatus for ABR Determinations
ABR measurements were obtained at baseline (before treatment) and at 2-week intervals during gentamicin dosing in a sound-attenuated and electrically shielded room. Pure tones were produced with a signal generator and an electronic switch providing an envelope (rise-fall time) of 0.5 msec. Total acoustic stimulus duration was 1 msec with no plateau. The signal was passed through an attenuator to a high frequency electrodynamic transducer (Radio Shack, Model 40-1310). The output transducer was 10 cm distant from the animal's left ear. A 0.625 cm condenser microphone (Bruel & Kjaer, Model 4135), placed at the entrance of the ear canal, was used to calibrate the acoustic stimulus. Signal averaging and stimulus control was provided by a Z-80 microcomputer. All evoked potentials were stored on a disk for later analysis. The active electrode consisted of the durally implanted stainless-steel screw. A subdermal needle electrode (Grass, E2-B) was inserted subcutaneously into the contralateral pinna and served as the reference electrode. A ground lead was clipped to the tail. A biological preamplifier (Grass, Model P511) with a gain of 10k and a bandpass of 100 to 3 kHz amplified the bioelectric signals from the electrodes. An oscilloscope was used to monitor the pre-amplified signal.
ABR Measurement Procedures
ABR data were obtained with the animals anesthetized in an identical manner as described for the surgical procedure. The right ear was blocked by an attenuating ear plug. Auditory responses were obtained at 8, 16, 24, and 32 kHz for intensity levels of 80, 70, 60, 50, 40, 30, and 20 dB. The rapidly acquired ABR recordings by the Z-80 microcomputer (256 data values per 11.95 msec) were stored for future analysis. Graphic records were made of each rat's ABR, and auditory physiological thresholds were determined by visual inspection. The physiological threshold was defined as the lowest intensity level at which an auditory response could be detected visually. Physiological thresholds were obtained from all rats of each group at baseline before and again after 2 and 4 weeks of treatment. In addition to thresholds, each rat's ABR was examined for shifts in the latency of its greatest peak (IV). The utilization of animals with extended high frequency hearing permits brief pure tone stimuli to elicit response in studies of ototoxicity that correlate well with behavioral audiograms (Evans et al., 1983).
Urinary Assay for NAG and Creatinine
Twenty-four-hour urine samples were collected at baseline and at weekly intervals during gentamicin dosing from every animal of the two treatment-time groups by means of Basi (MD-1470) metabolic cages. NAG activity plus creatinine (CR) concentration of every urine sample were determined, respectively, by manual fluorescent assay as described by Powell et al. (1983) and by Sigma Diagnostics Creatinine kits following manufacturer's instructions to derive the NAG/CR ratio as a measure of renal toxicity: the greater the ratio, the greater the extent of renal injury.
Pharmacokinetic Analysis
An automated analyzer enzyme method (Affiliated Laboratories Inc., Bangor, Maine) was used to determine gentamicin concentration of blood samples. Since blood draws could severely compromise the experimental animals, different sets of female rats [2 HALO (n=7) and 14 HALO (n=10)], comparable in age and body weight to those studied for ototoxicity and nephrotoxicity, were used for the PK substudy. Blood (200 μL) samples were obtained by cardiac puncture at baseline and 30, 60, 120, and 240 min following a single subcutaneous injection of 100 mg/kg bw of gentamicin. PK parameters were calculated up to the last observation (240 min post-dose) using non-compartmental analysis (Phoenix WinNonlin 6.4; Certara, Princeton, NJ) to estimate as a function of drug bioavailability apparent clearance (CL/F), apparent volume of distribution (V/F), and terminal elimination half-life (t1/2). Parameter estimates of the 2 and 14 HALO groups were compared by unpaired two-tailed t-testing.
Statistical Analysis
All statistical analyses were accomplished using the Statistical Package for the Social Sciences (SPSS, v14). When the analysis required a large number of multiple comparisons, a Bonferroni correction was made by modifying the confidence interval for the equivalent α level of 0.05.
Results
Global Toxicity Indices
Global toxicity of the timed gentamicin treatment was gauged by number of animal deaths and amount of body weight loss. Four animals of the 2 HALO group died after 13-16 days of treatment, while one animal of the 14 HALO group died on day 11. The initial body weight baseline mean ± standard deviation (S.D.) were comparable for the 2 and 14 HALO treatment groups (p>0.05), being 243 ± 20.8 gm and 245 ± 20.8 gm, respectively. As shown in Figure 1, body weight significantly declined in both groups, especially between treatment days 7 and 15; however, the amount of decline varied with time of drug dosing. The mean decrease in body weight exhibited by the 2 HALO animals on day 15 of drug administration was almost 3-fold greater than that exhibited by the 14 HALO ones. After day 15, the mean body weight of both groups increased and trended in the case of the 2 HALO group toward or to the before-medication baseline level and in the case of the 14 HALO group to or above it. A t-test for body weight differences between the two groups was accomplished for day 1, 8, 15, 22, and 29; only on day 15 was the body weight of the 14 HALO group statistically significant (t=3.55, p<0.005) greater than that of the 2 HALO group. Yet, it is likely that both the differences in body weight and extent of recovery between the treatment-time groups might have been greater than apparent in Figure 1, given that 4 animals of the 2 HALO group vs. only 1 animal of the 14 HALO group, assumedly with severe toxicity and likely highly reduced body weight, were lost to follow-up because of death between treatment days 11-16.
Figure 1.

Change in mean daily body weight of animals from baseline of the 2 and 14 HALO groups throughout the 4 weeks of daily gentamicin treatment (100 mg/kg bw by subcutaneous injection).
Auditory Threshold Changes
Figure 2 presents for one of the studied rats a typical ABR response at 16 kHz for intensity levels of 80, 70, 60, 50, and 40 dB at baseline (left) and again after 2 weeks of daily gentamicin administration (right) at 2 HALO.
Figure 2.

Typical ABRs for a single animal of the 2 HALO treatment group at 16 kHz for the intensity levels of 80, 70, 60, 50, and 40 dB. Pre-drug administration ABRs are shown on the left and ARBs after 2 weeks of daily gentamicin administration are shown on the right. Evident is the depressed ABR response relative to baseline across all intensity levels following 2 weeks of daily gentamicin treatment at 2 HALO.
Table 1 lists the means ± S.D. of the physiologic threshold changes in dB, i.e., difference between the thresholds per test frequency determined at baseline versus weeks 2 and 4 of gentamicin administration for the diurnal and nocturnal treatment groups, and Figure 3 graphically depicts the same data to visualize the differential progressive loss of hearing according to circadian time of dosing. The differences from baseline increased progressively during the 4-week study, with obvious large discrepancy in mean ABR threshold per test frequency between the 2 and 14 HALO treatment groups. Hearing loss per auditory frequency was more profound in animals of the 2 HALO group, particularly at the higher frequencies of 24 and 32 kHz. ANOVA substantiated significant difference in the amount of change in the physiologic thresholds from baseline according to the HALO time of gentamicin administration after both 2 and 4 weeks of treatment for the high frequencies of 24 and 32 kHz (both p<0.005) and also middle frequencies of 8 and 16 kHz (both p<0.05).
Table 1. Mean ± S.D. decibel change in physiological ABR threshold from baseline per auditory frequency after 2 and 4 weeks of gentamicin treatment of different but comparable groups of female rats at either 2 or 14 HALO.
| Test Frequency (Hz) | ||||
|---|---|---|---|---|
|
| ||||
| ABR Testing | 8 k | 16 k | 24 k | 32 k |
| 2 HALO | ||||
| 2 weeks | 15.0 ± 13.1 | 25.0 ± 20.0 | 27.5 ± 15.8 | 31.3 ± 15.5 |
| 4 weeks | 24.0 ± 17.4 | 34.0 ± 17.0 | 40.0 ± 14.1 | 42.0 ± 9.9 |
|
| ||||
| 14 HALO | ||||
| 2 weeks | 3.8 ± 7.4 | 2.5 ± 4.6 | 8.8 ± 13.6 | 7.5 ± 14.9 |
| 4 weeks | 10.0 ± 17.7 | 7.5 ± 15.8 | 12.5 ± 12.8 | 10.0 ± 20.0 |
Figure 3.
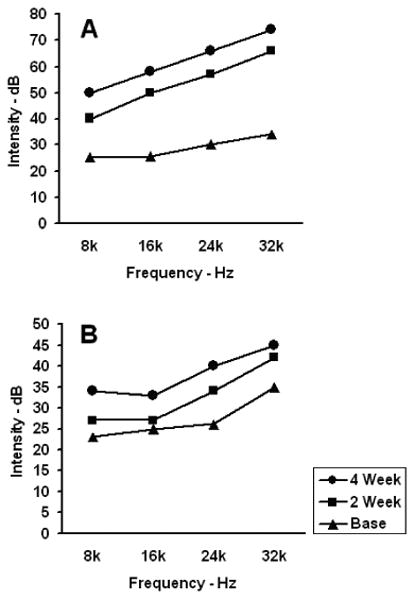
Mean ABR threshold per test frequency, i.e., 8, 16, 24, and 32 kHz, is shown for the 2 and 14 HALO gentamicin treatment groups: A represents the 2 HALO group and B the 14 HALO group.
Auditory Latency Changes
Auditory latency determinations were performed at 2 and 4 weeks of gentamicin administration at all frequencies (8, 16, 24, and 32 kHz) and intensity levels for which an ABR occurred. Figure 4 shows the mean values (typically the S.D.s were small, between 0.065 and 0.490 msec) of all the measured latencies at baseline and at the 2 and 4-week assessments for animals of the 2 and 14 HALO groups. The 4-week test latencies are not presented because of insufficient response across all intensity levels due to the extensive progressive hearing losses, particularly in animals of the 2 HALO group. Generally, as the intensity in dB was decreased, the latency in msec was increased. Rats of both the diurnal and nocturnal treatment groups exhibited latency changes by the second week of gentamicin administration. Definitive differences in the magnitude of such changes according to the circadian time of dosing was observed, with the rats of the 2 HALO group showing greatest latency change per test frequency.
Figure 4.

Mean auditory latencies of gentamicin-treated animals are shown for each frequency (8, 16, 24, and 32 kHz) and across different designated intensity levels shown (primarily for 40, 50, 60, 70, and 80 dB) at baseline (circles) and after 2 weeks of drug administration: 2 HALO (squares) and 14 HALO (triangles).
Renal Toxicity: NAG/CR Ratio
NAG fluorescence and spectrophotometric CR were determined from 24h urine samples collected from each rat at baseline and after each week of treatment to derive changes in the NAG/CR ratio per animal and thereafter collectively for the diurnal and nocturnal groups as a measure of renal toxicity. Mean change (± S.E.) in NAG/CR ratio per week of drug administration for the 2 and 14 HALO groups relative to the respective initial baseline level is presented in Figure 5. Although the NAG/CR ratio varied greatly between rats, the overall general trend is for a sharp initial rise in both the 2 and 14 HALO animals by the end of the first week of treatment and marked decline thereafter. Difference in the mean magnitude of change between the two treatment-time groups is clearly evident after the first week of gentamicin dosing, the mean NAG/CR ratio for the 2 HALO group being almost twice that of the 14 HALO group, i.e., 13.64 vs. 7.38. Extensive treatment-time difference is also obvious after the second week of dosing when the mean NAG/CR ratio is somewhat reduced, i.e., when both groups exhibited recovery of the NAG/CR ratio. By the second week of gentamicin administration, the 14 HALO animals recovered toward the baseline ratio value more rapidly than the 2 HALO ones. Differences in the rate of recovery between the diurnal and nocturnal groups might have been even greater, since 4 animals of the diurnal compared to only 1 animal of the nocturnal group were lost to follow-up due to death before or around the second week of dosing, presumably because of severe drug-induced renal toxicity.
Figure 5.

Bar graph illustration of the mean (± S.E.) change in NAG/CR ratio from the before-treatment baseline and after each week of timed (2 or 14 HALO) gentamicin dosing. Mean NAG/CR ratio is substantially elevated relative to baseline at both week 1 and 2 of treatment, particularly of the 2 HALO compared to the 14 HALO group, but much less so at weeks 3 and 4 of treatment.
Pharmacokinetic (PK) Analysis
Estimates of the gentamicin PK were derived in a preliminary small-scale substudy entailing a limited blood sampling protocol utilizing a different set of female Sprague-Dawley rats of comparable age and body weight as those assessed for adverse effects. Blood samplings were obtained by cardiac puncture before and 30, 60, 120 and 240 min after a single subcutaneous 100 mg/kg bw injection of the medication at 2 HALO in one group of rats and at 14 HALO in another group. The mean concentration-time gentamicin data for the rats of the respective treatment-time groups are shown in Figure 6. Although blood sampling was too infrequent to enable determination of peak drug concentration (Cmax) and time to peak concentration (Tmax), the data of Figure 6 show the drug concentration of the two drug-administration groups both at the 30 and 60-min post-treatment intervals is comparable, suggesting absence of a dosing-time difference upon these parameters. At the 120 and 240-min post-dosing samplings, blood gentamicin concentration is greater in the animals of the 14 than 2 HALO treatment group, indicative of administration-time differences in drug elimination rate. Mean ± S.E. of the analyzed gentamicin PK parameters are shown in Table 2. Gentamicin, which is eliminated from the body via renal mechanisms, was cleared in a statistically significantly slower rate (p<0.005) when injected subcutaneously at 14 than 2 HALO. Even though the mean terminal half-life was longer and mean volume of distribution greater in animals of the 14 than 2 HALO group, differences were not statistically significant.
Figure 6.

Mean (± S.E.) concentration-time profile of gentamicin (acute single subcutaneous 100 mg/kg bw injection) for animals of the 2 (squares) and 14 HALO (triangles) treatment groups.
Table 2. Pharmacokinetic analysis (x̄ ± S.E.) of gentamicin (acute single subcutaneous 100 mg/kg bw injection) for animals of the 2 and 14 HALO groups.
| 2 HALO | 14 HALO | |
|---|---|---|
|
|
||
| CL/F (mL/min/kg) | 4.53 ± 0.63 | 3.22 ± 0.49* |
| V/F (mL/kg) | 384 ± 127 | 488 ± 230 |
| t1/2 (min) | 61.7 ± 29.3 | 107 ± 52 |
p<0.005
Discussion
Using the Sprague-Dawley laboratory rodent model, our investigation explored differences according to circadian time of treatment in the occurrence and intensity of the major adverse effects of the aminoglycoside antibiotic gentamicin. The medication was administered chronically, i.e., daily during a 4-week period, either at the beginning of the rodent's diurnal rest span (2 HALO) or beginning of its activity span (14 HALO), and the global indices of body weight maintenance and animal viability plus the specific indices of ototoxicity and nephrology relative to drug PK were examined. Overall global toxicity -- changes in body weight from the baseline level and animal deaths that occurred during the first 2 weeks of treatment -- was greater in rats of the diurnal than nocturnal group. Four animal deaths took place in the 2 HALO group versus only 1 animal death in the 14 HALO group, and this mirrored the differential extent of mean weight loss from baseline in rats of the corresponding treatment-time groups. Toyoda et al. (1977) reported administration of the aminoglycoside antibiotic kanamycin in a dose of 400 mg/kg bw compromised the nutritional maintenance of their animals, and they and Ryan & McGee (1977) reported animal deaths with high doses of kanamycin. In the present study, the gentamicin dosage of 100 mg/kg bw caused immediate and rapid decline in body weight, which in the surviving animals recovered to approximately baseline levels over the remaining 2 weeks of the 4-week course of treatment. The deaths and weight loss of the animals most likely resulted, at least in part, from renal toxicity and failure. In this regard, Ryan & McGee (1977) surmised the deaths of half of the chinchillas in their study resulted from kanamycin-induced renal failure. Dulon et al. (1988) found renal function recovers after cessation of aminoglycoside administration, and Kosek et al. (1974) reported a possible compensatory strategy (reduced production of myeloid bodies) by the kidneys to circumvent renal failure. Perhaps, this explains why in our study after approximately 2 weeks of daily gentamicin dosing the health of the animals, as gauged by body weight as a nonspecific global index of overall drug-induced toxicity and animal well-being, recovered. It is of relevance that the NAG/CR ratio was almost 2-fold greater, i.e., nephrotoxicity was almost 2-fold more severe, in the 2 than 14 HALO rats after the first week of gentamicin dosing. This finding is consistent with the hypothesis that the differential extent of gentamicin-induced renal toxicity according to its administration time plays a significant role in the differential amount of animal mortality according to its administration time.
The results of the ABR assessments substantiate findings of previous circadian rhythm studies entailing this and other aminoglycoside antibiotics (Beauchamp & Labrecque, 2007; McKinney et al., 2015; Yonovitz & Fisch, 1991), i.e., that there is indeed a difference in induced ototoxicity with respect to drug-administration time. The ABR assessments revealed substantially greater hearing losses across all the test frequencies in rats of the 2 compared to the 14 HALO group, with threshold shifts in the former group in the range of 30 to 50 dB. The results of this study entailing gentamicin are in close agreement with those of Yonovitz & Fisch (1991) entailing kanamycin. The shift in hearing threshold was most severe in the high 24 and 32 kHz frequencies, as found in previous studies (Dallos & Wang, 1974; Ryan & McGee, 1977), and although the amplitudes of the ABR waves varied between animals, the relative morphology and peaks of the component waves and latencies were consistent within the same animal under normal conditions (Schwent et al., 1980). Analysis of the auditory latencies in the present study entailing gentamicin and those of Wassick & Yonovitz (1985) entailing methyl mercury showed remarkable pre- and post-treatment differences. The animals of the low-dose group of the latter study incurred a high frequency loss for the 80 dB signal and reduction in all peaks of every measured wave component. In the high-dose group, there was a similar response, but not for all peaks and all test times during the course of study; that is, the absolute latency tended to be shortened for the majority of frequencies at the different test times during methyl mercury exposure.
We used the NAG/CR ratio as an index of nephrotoxicity. In the past, both NAG and CR have been used as separate measures of kidney status and function. According to Horak et al. (1981), NAG excretion exhibits little circadian fluctuation, but urine formation does. However, these and other temporal variations in urine flow can be reliably compensated for by relating urine NAG activity to urine CR concentration. In an earlier study, Wellwood et al. (1976) reported NAG activity to be as much as 30-fold greater in gentamicin-dosed than control animals after 5 days of treatment. The urinary NAG/CR ratio data of the present study reveal gentamicin dosing induces considerable nephrotoxicity essentially within the same time frame, after 7 days of drug administration, but with the ratio being almost 2-fold greater in the animals treated at 2 vs. 14 HALO and with somewhat more rapid recovery to baseline level by the 14 than 2 HALO rats at the second week of treatment. The ratio further declined after 2 weeks of treatment, but it was still several-fold greater for rats of the 2 than 14 HALO group. After 3 weeks of treatment the NAG/CR ratio, expressed relative to baseline values, of both the diurnal and nocturnal treatment groups further declined, although it still remained elevated in some individual rats by 2 to 5-fold over baseline. Overall, the NAG/CR ratio in terms of peak values and time course of their reduction over the 4-week treatment span indicates gentamicin induces greater nephrotoxicity when injected daily at 2 than 14 HALO. The findings are the same for ototoxicity. Animals administered gentamicin during the beginning of their rest span at 2 HALO than during the beginning of their activity span at 14 HALO experienced significantly greater, i.e., more severe, hearing deficits.
Analysis of the data from our PK substudy demonstrated the clearance rate (x̄ ± S.E.) of gentamicin in animals of the 2 and 14 HALO groups differs significantly: 4.53 ± 0.63 and 3.22 ± 0.49 mL/min/kg, respectively. The slower clearance of drug in the animals of the 14 HALO group resulted in a non-statistically significant longer gentamicin terminal half-life (1.73-fold longer than animals of the 2 HALO group). Although it may be argued the faster drug clearance by animals of the 2 HALO vs. 14 HALO group perhaps translated into higher renal tissue gentamicin exposure per unit time and thus greater risk for renal dysfunction, it does not explain the differential ototoxicity observed between the two treatment-time groups. Dulon et al. (1988) proposed different mechanisms are responsible for the damage to hair cells of the cochlea and nephrons of the kidney. The results of this study do not suggest otherwise. Indeed, major nephrotoxicity, as evidenced by change in the NAG/CR ratio from baseline, developed rapidly, being evident by the seventh day of treatment, and thereafter it slowly regressed. In contrast, ototoxicity, as evidenced by change in the ABR threshold and latency responses from baseline, was displayed by the seventh day of gentamicin dosing and continued to progress throughout the 4-week treatment protocol.
Unique to our study is exploration of the role that circadian rhythms plays in gentamicin toxicity. The untoward effects of ototoxicity and nephrotoxicity examined herein with respect to the different circadian times of drug administration indicate the relationship between deficits in hearing and deficits in renal function is similar in direction. Furthermore, findings of the present study indicate that both of the drug-induced indices of global toxicity -- body weight loss and mortality -- as well as both of the specific organ toxicities -- nephrotoxicity and ototoxicity -- do not seem to be predicted by treatment-time differences in gentamicin PK parameters, particularly its rate of clearance, and perhaps Cmax, Tmax, and t1/2; thus, the data of our pilot substudy of gentamicin PK suggest the hypothesis that chronopharmacodynamic phenomena at the local cell, tissue, and organ level may be more responsible than chronopharmacokinetic phenomena for the observed administration-time-dependent differences in the extent of the recorded adverse effects. More complete PK data. i.e., more frequent and dense blood samplings obtained soon after gentamicin administration, are necessary to fully interpret the relationship between treatment-time differences in gentamicin PK parameters plus global and specific organ toxicity. The collective results of this study, nonetheless, substantiate the hypothesis that circadian rhythms can exert definitive influence on the adverse effects of aminoglycoside antibiotics. This implicates the need to research how best to schedule gentamicin and other aminoglycoside antibiotics to minimize the risk of their adverse effects, particularly in patients who require long-term or recurrent therapy for management of bacterial infection.
Summary
The aminoglycoside antibiotic gentamicin was administered to different groups of 9 rats each at 2 different circadian times (2 and 14 HALO). ABR tests and urine assays, to assess hearing sensitivity and presence of NAG, respectively, were used to simultaneously investigate the relationship between ototoxicity and nephrotoxicity relative to the circadian time of drug administration. The methods of the ABR and NAG analysis provide sensitive measures that allowed, within the 4-week dosing period, demonstrable effects upon both systems. At baseline and at regular intervals during treatment, ABRs for the frequencies of 8, 16, 24, and 32 kHz were obtained (2-week intervals) and 24h urine voidings were collected (1-week intervals) separately from each animal. Changes both in hearing sensitivity and NAG/CR ratio were examined as a function of the circadian time of drug dosing. The results of this investigation involving gentamicin, as do others involving this and other aminoglycoside antibiotics (Beauchamp & Labrecque, 2007; McKinney et al., 2015; Yonovitz & Fisch, 1991), indicate their circadian timing plays a very significant role in the risk and severity of drug-induced adverse effects.
From the data obtained in this study the following conclusions can be made.
More animal deaths occurred in the 2 (4 animals) than 14 HALO (1 animal) group. Weight loss from baseline was greatest in rats of the 2 than 14 HALO group after 2 weeks of treatment, although the mean body weights of the surviving animals of both groups recovered to approximately baseline levels by the third and fourth weeks of drug administration.
Hearing losses, which progressively became more severe during the 4-week treatment protocol, were greater in rats of the 2 than 14 HALO group. The average hearing losses for the 2 HALO animals after 4 weeks of gentamicin dosing were 24, 34, 40, and 42 dB for the 8, 16, 24, and 32 kHz frequencies, respectively. In comparison, the average hearing losses in the 14 HALO rats were 10, 7.5, 12.5, and 10 dB for the 8, 16, 24, and 32 kHz frequencies, respectively.
Peak values of the NAG/CR ratio relative to the before-treatment baseline that occurred after the first week of gentamicin dosing, were greater in the 2 than 14 HALO rats. The average ratio after 1 week of treatment was 13.64-fold greater than baseline in animals of the 2 HALO group, and it was as great as 26-fold higher in 1 animal. In comparison, the average ratio after 1 week of treatment was only 7.38-fold greater than baseline in animals of the 14 HALO group, with 1 rat showing as much as 14.28-fold increase. After the first week of dosing, the NAG/CR began to decrease. The decline in NAG/CR ratio commencing at the second week of drug administration occurred more rapidly in the 14 than 2 HALO group; after the second week of treatment the ratio decreased in the 14 HALO group to 2.23-fold the baseline level, while in the 2 HALO animals it was still extensively elevated to 8.15-fold baseline level.
Blood gentamicin concentration was comparable between treatment groups at the 30 and 60-min post-subcutaneous, drug-injection time intervals, but it was higher for the 14 than 2 HALO group at the 120 and 240-min time intervals. The estimated clearance rate (x̄ ± S.E.) of gentamicin in the animals of the 2 and 14 HALO groups differed significantly: 4.53 ± 0.63 and 3.22 ± 0.49 mL/min/kg, respectively. The average half-life of gentamicin in the animals of the 14 HALO group was correspondingly 1.73-fold longer than it was in those of the 2 HALO group.
Results of this research study confirm speculation that separate mechanisms govern the renal and auditory toxicity induced by gentamicin and most likely other aminoglycoside antibiotics. They also substantiate the circadian timing of gentamicin administration plays a role in its PK and, most importantly, toxicity/animal tolerance. Thus, the findings suggest circadian rhythm-dependent effects be taken into consideration by investigators when studying the adverse toxic effects and mechanisms of gentamicin and other aminoglycoside antibiotic medications. Moreover, clinicians should be mindful that not only the dose but the schedule relative to circadian time of gentamicin and other aminoglycoside medications are important determinants of their adverse effects. The chronotherapy of aminoglycoside and other antibiotic therapies have yet to be rigorously explored. The results of this and other aminoglycoside circadian rhythm studies (Beauchamp & Labrecque, 2007; McKinney et al., 2015; Yonovitz & Fisch, 1991) suggest late evening and before-bedtime dosing of humans should be reduced or avoided whenever feasible to minimize undesired drug-induced outcomes.
References
- Arnold W. Inner ear and renal diseases. Ann Otol Rhinol Laryngol (Suppl) 1984;112:119–24. doi: 10.1177/00034894840930s420. [DOI] [PubMed] [Google Scholar]
- Ayala DE, Ucieda R, Hermida RC. Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiol Int. 2013;30:260–79. doi: 10.3109/07420528.2012.717455. [DOI] [PubMed] [Google Scholar]
- Beauchamp D, Labrecque G. Chronobiology and chronotoxicology of antibiotics and aminoglycosides. Adv Drug Del Rev. 2007;59:896–903. doi: 10.1016/j.addr.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Cosgrove SE, Vigliani GA, Campion M, Fowler VG, Jr, Abrutyn E, Corey GR, Levine DP, Rupp ME, Chambers HF, Karchmer AW, Boucher HW. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin Infect Dis. 2009;48:713–21. doi: 10.1086/597031. [DOI] [PubMed] [Google Scholar]
- Crespo JJ, Piñeiro L, Otero A, Castiñeira C, Ríos MT, Regueiro A, Mojón A, Lorenzo S, Ayala DE, Hermida RC on behalf of the Hygia Project Investigators. Administration-time-dependent effects of hypertension treatment on ambulatory blood pressure in patients with chronic kidney disease. Chronobiol Int. 2013;30:159–75. doi: 10.3109/07420528.2012.701459. [DOI] [PubMed] [Google Scholar]
- Dallos P, Wang C. Bioelectric correlates of Kanamycin Intoxication. Audiology. 1974;13:277–89. doi: 10.3109/00206097409071685. [DOI] [PubMed] [Google Scholar]
- Dulon D, Aurousseau C, Erre J-P, Aran J-M. Relationship between the nephrotoxicity and ototoxicity induced by gentamicin in the guinea pig. Acta Otolaryngol. 1988;106:219–25. doi: 10.3109/00016488809106429. [DOI] [PubMed] [Google Scholar]
- Evans B, Yonovitz A, Fox DA. Ototoxic effects of triethyltin: Electrophysiological and histological correlates. Toxicol. 1983;3:169. [Google Scholar]
- Hermida C, Ayala DE, Fernández JR, Artemio M, Smolensky MH, Fabbian F, Portaluppi F. Administration-time-differences in effects of hypertension medications on ambulatory blood pressure regulation. Chronobiol Int. 2013a;30:280–314. doi: 10.3109/07420528.2012.709448. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Crespo JJ, Mojón A, Chayán L, Fontao MJ, Fernández JR. Influence of age and hypertension treatment-time on ambulatory blood pressure in hypertensive patients. Chronobiol Int. 2013b;30:176–91. doi: 10.3109/07420528.2012.701131. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ríos MT, Crespo JJ, Moya A, Domínguez-Sardiña M, Otero A, Sánchez JJ, Mojón A, Fernández JR, Ayala DE on behalf of the Hygia Project Investigators. Treatment-time regimen of hypertension medications significantly affects ambulatory blood pressure and clinical characteristics of patients with resistant hypertension. Chronobiol Int. 2013c;30:192–206. doi: 10.3109/07420528.2012.701460. [DOI] [PubMed] [Google Scholar]
- Horak E, Hopfer SM, Sunderman FW. Spectrophotometric assay for urinary N-acetyl-β-D- glucosaminidase activity. Clin Chem. 1981;27:1180–5. [PubMed] [Google Scholar]
- Kaloyanides GJ, Pastoriza-Munoz E. Aminoglycoside nephrotoxicity. Kidney Int. 1980;18:571–82. doi: 10.1038/ki.1980.175. [DOI] [PubMed] [Google Scholar]
- Kosek JC, Mazze RI, Cousins MJ. Nephrotoxicity of gentamicin. Lab Invest. 1974;30:48–57. [PubMed] [Google Scholar]
- Lemmer B, Bruguerolle B. Chronopharmacokinetics. Are they clinically relevant? Clin Pharmacokinet. 1994;26:419–27. doi: 10.2165/00003088-199426060-00001. [DOI] [PubMed] [Google Scholar]
- Lerner SA, Schmitt BA, Seligsohn R, Mats GJ. Comparative study of ototoxicity and nephrotoxicity in patients randomly assigned to treatment with amikacin or gentamicin. Am J Med. 1986;80(Suppl. 6B):98–104. doi: 10.1016/0002-9343(86)90486-9. [DOI] [PubMed] [Google Scholar]
- McKinney W, Yonovitz A, Smolensky MH. Circadian rhythm in gentamicin toxicity in rats. Laryngoscope. 2015;125:e252–6. doi: 10.1002/lary.25116. [DOI] [PubMed] [Google Scholar]
- Moyá A, Crespo JJ, Ayala DE, Ríos MT, Pousa L, Callejas PA, Salgado JL, Mojón A, Fernández JR, Hermida RC on behalf of the Hygia Project Investigators. Effects of time-of-day of hypertension treatment on ambulatory blood pressure and clinical characteristics of patients with type 2 diabetes. Chronobiol Int. 2013;30:116–31. doi: 10.3109/07420528.2012.702587. [DOI] [PubMed] [Google Scholar]
- Powell SC, Scaro J, Wilson E, Shihabi ZK. Assay of urinary N-acetyl-β-glucosaminidase in a centrifugal analyzer. Clin Chem. 1983;29:1717–19. [PubMed] [Google Scholar]
- Reinberg A, Smolensky MH. Circadian changes of drug disposition in man. Clin Pharmocokin. 1982;7:401–20. doi: 10.2165/00003088-198207050-00002. [DOI] [PubMed] [Google Scholar]
- Reinberg A, Smolensky MH. Biological rhythms and medicine. Springer‐Verlag; NY: 1983. pp. 211–263. [Google Scholar]
- Reinberg A, Levi F, Smolensky MH. Clinical Chronopharmacokinetics. J Pharmacol (Paris) 1984;15(Supp l):95–124. [PubMed] [Google Scholar]
- Ryan A, McGee TJ. Development of hearing loss in kanamycin treated chinchillas. Ann Otology Rhinol Laryngol. 1977;86:176–82. doi: 10.1177/000348947708600207. [DOI] [PubMed] [Google Scholar]
- Schwent VL, Williston JS, Jewett DL. The effects of ototoxicity on the auditory brainstem response and the scalp-recorded cochlear microphonic in guinea pigs. Laryngoscope. 1980;90:1350–9. [PubMed] [Google Scholar]
- Smith CA, Lipsky JJ, Lietman PS. Relationship between aminoglycoside-induced nephrotoxicity and auditory toxicity. Antimicrob Agents Chemother. 1979;15:780–82. doi: 10.1128/aac.15.6.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolensky MH, Peppas NA. Chronobiology, drug delivery, and chronotherapeutics. Adv Drug Del Res. 2007;59:828–51. doi: 10.1016/j.addr.2007.07.001. (2007) [DOI] [PubMed] [Google Scholar]
- Smolensky MH, Hermida R, Ayala DE, Portaluppi F. Administration-time-dependent effects of antihypertension medications: Basis for the chronotherapy of hypertension. Blood Press Monitor. 2010;15:173–80. doi: 10.1097/MBP.0b013e32833c7308. [DOI] [PubMed] [Google Scholar]
- Toyoda Y, Saito H, Matsuoka H, Takenaka H, Oshima W, Mizukoso O. Quantitative analysis of kanamycin ototoxicosis. Acta Otolaryngol. 1977;84:202–12. doi: 10.3109/00016487709123958. [DOI] [PubMed] [Google Scholar]
- Wassick KH, Yonovitz A. Methyl mercury ototoxicity in mice determined by auditory brainstem evoked responses. Acta Otolaryngol. 1985;99:35–45. doi: 10.3109/00016488509119143. [DOI] [PubMed] [Google Scholar]
- Wellwood JM, Lovell D, Thompson AE, Tighe JR. Renal damage caused by gentamicin: a study of the effects on renal morphology and urinary enzyme excretion. J Pathol. 1976;118:171–82. doi: 10.1002/path.1711180307. [DOI] [PubMed] [Google Scholar]
- Yonovitz A, Fisch J. Circadian rhythm dependent kanamycin-induced hearing loss in rodents assessed by auditory brainstem responses. Acta Otolaryngol. 1991;111:1006–12. doi: 10.3109/00016489109100749. [DOI] [PubMed] [Google Scholar]


