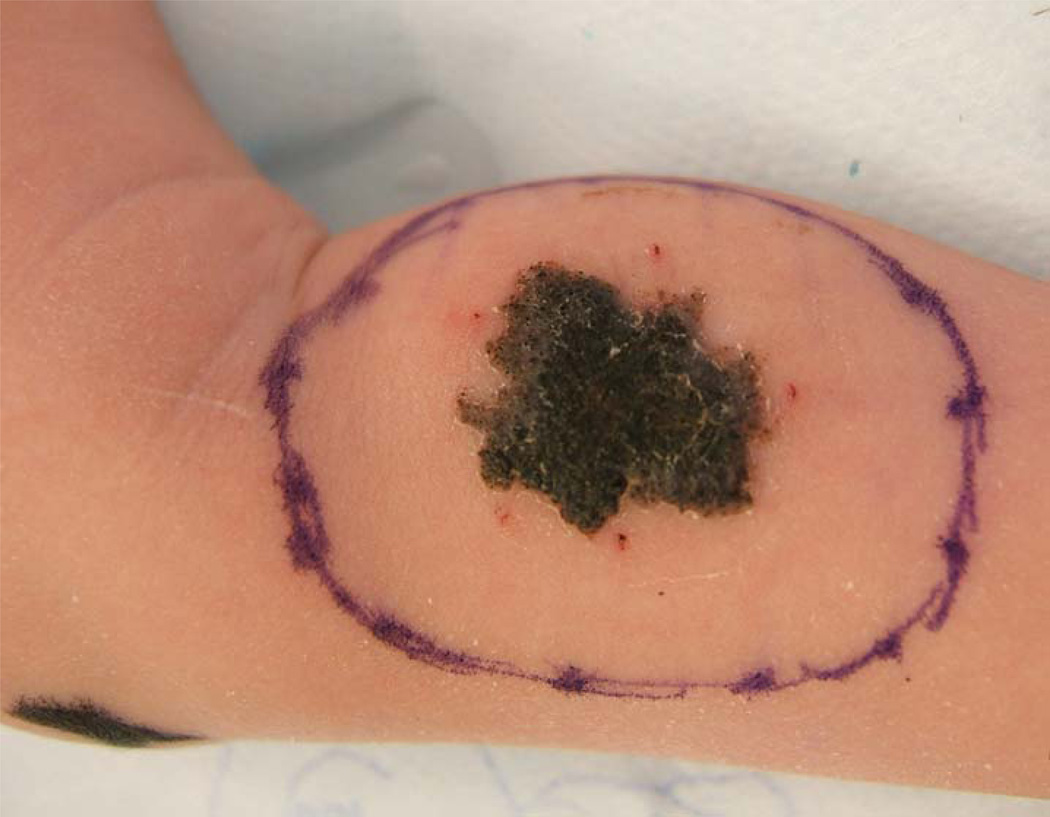
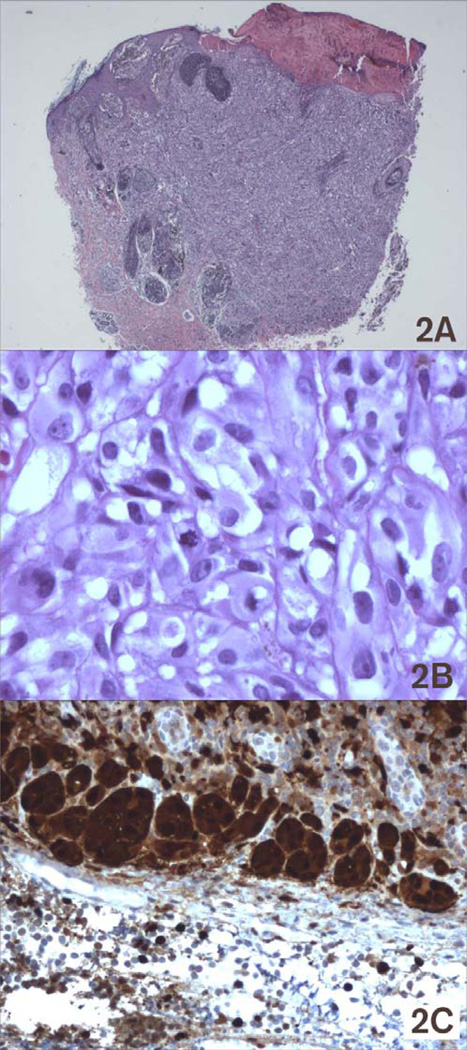
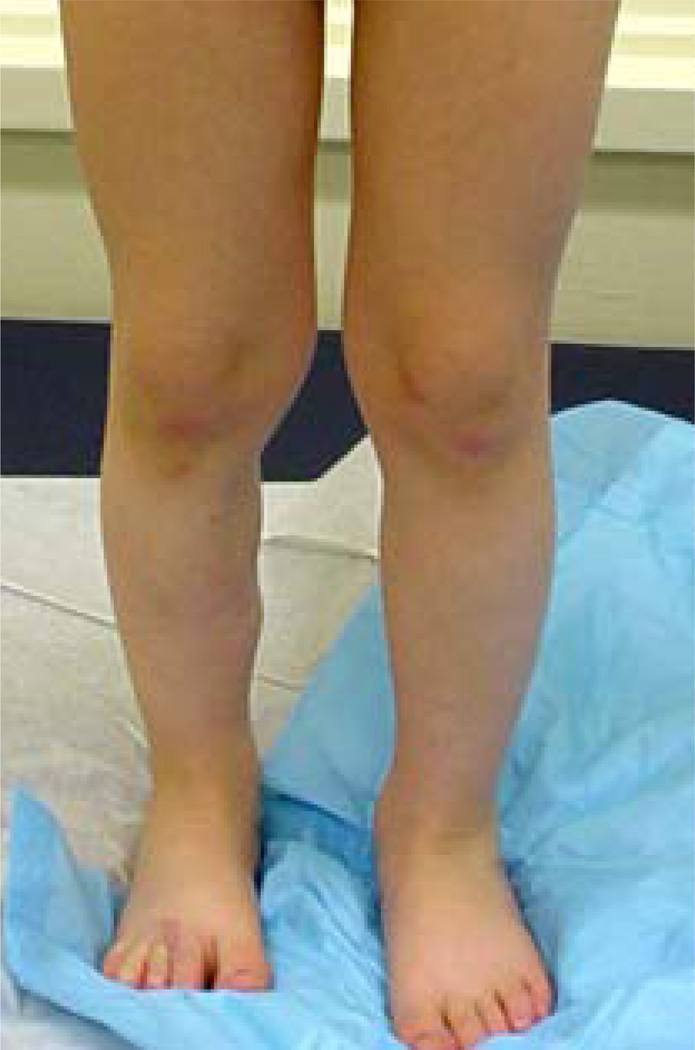
A Caucasian baby girl presented at birth with a dark, irregular, raised skin lesion measuring approximately 2 cm in diameter on the medial aspect of her right leg (Fig 1). There was no history of melanoma in her family and no evidence of melanoma in her mother. At two weeks of age, she underwent an incisional biopsy of the lesion at a community hospital. Histologic review showed a superficial spreading invasive malignant melanoma with Breslow thickness greater than 2.5 mm, with a positive deep margin. There was angiolymphatic invasion but no definite ulceration. She was referred to our institution, where clinical exam revealed an otherwise healthy newborn girl, a black lesion on the right leg with a central scar measuring 1.5 cm in diameter. There were small palpable inguinal nodes in each groin, possibly more prominent on the right but not clinically suspicious. One week later, a wide local excision was performed with a 1 cm margin (Fig 1) and a full thickness skin graft. Lymphoscintigraphy revealed a sentinel node in the right groin, and a sentinel lymph node biopsy was performed. Her final pathology revealed an atypical malignant melanoma arising in a nevus with congenital features with Breslow thickness of 2.58 mm and Clark’s level V (Fig 2A). Margins were widely clear. The lesion was consistent with a primary melanoma, rather than metastatic disease from maternal origin. The sentinel node was positive for metastatic melanoma, with mitotic figures (Fig 2B) and S100 staining (Fig 2C). She underwent staging studies including CT scans of her head, chest, abdomen and pelvis as well as a bone scan. These were negative for metastatic disease. One month later, completion right inguinal lymph node dissection was performed, yielding five lymph nodes without metastatic disease. Her postoperative course was complicated by a wound abscess in the right groin that was drained operatively and healed well. Her final staging, at the age of 9 weeks, was therefore Stage IIIA (T3aN1aM0). No additional treatment was performed. She has been followed to her current age of 32 months without evidence of recurrence by clinical exam, serial chest radiographs, and annual CT scans of the abdomen and pelvis. She has had an excisional biopsy of a suspicious skin lesion from her lower abdomen at age 22 months that was an atypical nevus. She has normal function of her right lower extremity without lymphedema and is healthy and developmentally appropriate (Fig 3).
Figure 1.
Figure 2.
Figure 3.
Congenital melanoma is a rare entity, with only a few cases reported in the literature. We are unaware of prior reports of congenital melanoma metastatic to the inguinal lymph node basin treated with subsequent inguinal lymph node dissection in the neonatal period. Because of the rarity of the disease entity, there was little published guidance for the treatment of this child once her sentinel lymph node was reported positive.
The first reported case of congenital melanoma involved a newborn with a scalp lesion which grew rapidly and metastasized to regional lymph nodes, resulting in the baby’s death at 4 months.1 Since then, we could find only sixteen other reports of congenital melanoma reported in the literature.2–3 The most recent report described a case of melanoma in situ in a giant congenital nevus in a neonate with Down’s Syndrome. This was treated with resection and SLNB.4 The incidence of congenital melanoma at this time is unknown. However, the SEER database, which captures data on cancer in areas representing approximately 14% of the U.S. population, recorded only 5 cases of melanoma diagnosed in the first year of life over a period of 12 years between 1992 and 2004.5 Mortality rates are likewise unknown. Over a broader expanse of time, a study of pediatric deaths from mortality based upon death certificates showed only 12 deaths in children under the age of one from melanoma over 37 years between 1968 and 2004 in the U.S6. Presumably, the incidence and mortality from congenital melanoma is even smaller, though under-reporting may result from failure to recognize small or slow-growing lesions at birth or misdiagnosis of biopsy specimens as Spitz nevi.
Evaluation of a neonate for melanoma can be very difficult. From 1 to 3% of neonates may have congenital pigmented nevi. The size of the lesion is important in determining the risk of malignancy. The lifetime risk of melanoma in patients with giant melanocytic nevi, defined as measuring larger than 10 cm in diameter, is reported to range from 5% to 40%,7–8 with short-term prospective studies indicating risks closer to 5%9. Transformation occurs during the first decade 60% of the time, frequently before age 3 years. As such, current recommendations are to excise fully before the age of 10 years if feasible. However, all concerning lesions should be biopsied as early as possible. Our patient’s lesion was approximately 2 cm in size and had malignant transformation. Generally, medium-sized congenital nevi have slightly lower rates of transformation which usually do not occur until adulthood.10 Even with a high level of suspicion and early evaluation, the diagnosis may still not be easily made. Even skilled dermatopathologists may have difficulty distinguishing between melanoma and melanocytic nevi or Spitz nevi, with concordance rates ranging from 54% to 66%.11 Importantly, when this diagnosis is made, metastatic disease from the mother should be considered. We could find only three cases of malignant melanoma metastatic from the mother to the fetus, though other cancers have also metastasized in this fashion.12–13 In our case, the mother was examined thoroughly to ensure that this did not represent placental transmittal of metastatic melanoma.
Prognosis for congenital melanoma must be extrapolated to some degree from reports on childhood melanoma. Earlier beliefs that childhood melanoma was more benign probably resulted from overdiagnosis of Spitz nevi as melanoma. Recent reports suggest that, like adult melanoma, the prognosis of childhood melanoma is related to tumor thickness and clinical stage at the time of diagnosis.11 There have been too few cases of congenital melanoma to make any valid predictions regarding survival.
Three treatment dilemmas related to her neonatal status were addressed in her management. The first related to the margin of resection. The standard recommendation for an adult with a lesion of this depth is a 2 cm margin. Given the small circumference of this patient’s lower leg, a 2 cm margin would have been more than hemi-circumferential. A 1 cm margin was chosen to minimize morbidity and deformity, and to scale proportionately to the patient’s body size. The second was whether to perform inguinal node dissection after identification of metastatic melanoma in the sentinel node. Inguinal lymph node dissection in the developing and growing leg of a two month old baby carried unknown risk. However, without other treatment options, an aggressive surgical approach was chosen. Postoperatively, she did not develop any long-term morbidity from the procedure and continued to develop appropriately. The third question was whether to treat with adjuvant high-dose interferon, as the only approved adjuvant treatment for stage III melanoma. Our institutional experience with interferon in neonates has been that there can be particularly severe morbidity. In consultation with our pediatric medical oncologists, we felt that the toxicity would likely outweigh the potential benefit. There were no available clinical trials for neonatal melanoma at that time.
Our patient had successful surgical management of metastatic congenital melanoma with wide local excision and inguinal lymph node dissection. There has been no detectable long-term morbidity of inguinal dissection. This experience supports application of standard surgical management for melanoma, even in a neonate.
References
- 1.Coe H. Malignant pigmented mole in an infant. Northwest Med. 1925;24:181–182. [Google Scholar]
- 2.Lyall D. Malignant melanoma in infancy. JAMA. 1967;202:1153. [Google Scholar]
- 3.Richardson SK, Tannous ZS, Mihm MC. Congenital and infantile melanoma: Review of the literature and report of an uncommon variant, pigment-synthesizing melanoma. J Am Acad Dermtatol. 2002;47:77–90. doi: 10.1067/mjd.2002.120602. [DOI] [PubMed] [Google Scholar]
- 4.Almaraz RL, Alvarex CV, Luis JR, Martinez ED. Neoplasias neonatales:experiencia de un centro. An Pediatric (Barc) 2006;65(6):529–535. doi: 10.1157/13095844. [DOI] [PubMed] [Google Scholar]
- 5.Linaberry AM, Ross JA. Trends in Childhood Cancer Incidence in the U.S. (1992–2004) Cancer. 2008;112:416–432. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 6.Lewis KG. Trends in Pediatric Melanoma Mortality in the United States, 1968 through 2004. Dermatologic Surgery. 2008;34:152–159. doi: 10.1111/j.1524-4725.2007.34032.x. [DOI] [PubMed] [Google Scholar]
- 7.Sober AJ, Burstein JM. Precursors to skin cancer. Cancer. 1995;75:645–650. doi: 10.1002/1097-0142(19950115)75:2+<645::aid-cncr2820751405>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Rhodes AR, Wood WC, Sober AJ, Mihm MC. Nonepidermal origin of malignant melanoma associated with a giant congenital nevocellular nevus. Plastics and Reconstructive Surgery. 1981;67:782–790. doi: 10.1097/00006534-198106000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Marghoob AA, Schoenbach SP, Kopf AW, Orlow SJ, Nossa R, Bart RS. Large congenital melanocytic nevi and the risk for the development of malignant melanoma. A prospective study. Archives of Dermatology. 1996;132:170–175. [PubMed] [Google Scholar]
- 10.De Sa BC, Rezze GG, Scramim AP, Landman G, Neves RI. Cutaneous melanoma in childhood and adolescence: retrospective study of 32 patients. Melanoma Research. 2004;14(6):487–492. doi: 10.1097/00008390-200412000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Wechsler J, Bastuji-Garin S, Spatz A, et al. Reliability of the histopathological diagnosis of malignant melanoma in childhood. Archives of Dermatology. 2002;138:625–628. doi: 10.1001/archderm.138.5.625. [DOI] [PubMed] [Google Scholar]
- 12.Weber FP, Schwartz E, Hellenschmeid R. Spontaneous inoculation of melanotic sarcoma from mother to fetus. British Medical Journal. 1930;1:529–537. doi: 10.1136/bmj.1.3611.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brodsky I, Baren M, Kahn SB, Lewis G, Jr, Tellem M. Metastatic malignant melanoma from mother to fetus. Cancer. 1965;18:1048–1054. doi: 10.1002/1097-0142(196508)18:8<1048::aid-cncr2820180817>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]