Abstract
Neutrophils are essential for innate immunity and inflammation and many neutrophil functions are β2 integrin-dependent. Integrins can extend (E+) and acquire a high-affinity conformation with an ‘open' headpiece (H+). The canonical switchblade model of integrin activation proposes that the E+ conformation precedes H+, and the two are believed to be structurally linked. Here we show, using high-resolution quantitative dynamic footprinting (qDF) microscopy combined with a homogenous conformation-reporter binding assay in a microfluidic device, that a substantial fraction of β2 integrins on human neutrophils acquire an unexpected E−H+ conformation. E−H+ β2 integrins bind intercellular adhesion molecules (ICAMs) in cis, which inhibits leukocyte adhesion in vitro and in vivo. This endogenous anti-inflammatory mechanism inhibits neutrophil aggregation, accumulation and inflammation.
 Integrin β2 attachment regulates inflammation via effects on neutrophil rolling and extravasation through sequential integrin extension then headpiece opening. Here the authors show an alternative open headpiece prior to extension stabilized in cis by ICAM-1 that limits neutrophil adhesion.
Integrin β2 attachment regulates inflammation via effects on neutrophil rolling and extravasation through sequential integrin extension then headpiece opening. Here the authors show an alternative open headpiece prior to extension stabilized in cis by ICAM-1 that limits neutrophil adhesion.
Neutrophils are the most abundant leukocyte population in humans and have essential roles in innate immunity and inflammation. Neutrophils form a primary line of defence against pathogens by recognizing, trapping and eliminating bacteria1, fungae2, viruses3 and helminths4 through mechanisms including phagocytosis5, superoxide production5, granule exocytosis6 and neutrophil extracellular traps1. Congenital deficiencies of neutrophil function, such as leukocyte adhesion deficiency syndrome, can result in fatal infection7. Delay in neutrophil recovery is the main reason for lethal infections in patients receiving allogeneic haematopoietic stem cell transplantation8. Neutrophils are also important in non-infectious or chronic inflammation such as injury-induced sterile inflammation9, and in autoimmune diseases, including rheumatoid arthritis10, multiple sclerosis11 and systemic lupus erythematosus12.
Neutrophils are distributed by the blood circulation, but exert most of their functions outside the vascular system, exiting the circulation at sites of inflammation through the classical adhesion cascade13,14. Neutrophils have evolved specialized systems to allow adhesion under high wall shear stress13,15,16. β2 integrins are vital for neutrophil functions, including neutrophil arrest under flow13, transmigration through the vascular endothelium17, chemotaxis18 and phagocytosis19.
The leukocyte adhesion cascade was first established when Lawrence and Springer showed that neutrophils rolling on P-selectin were induced to arrest under flow by binding to intercellular adhesion molecule-1 ((ICAM-1), a ligand for β2 integrins) when the chemoattractant N-formylmethionine-leucyl-phenylalanine was infused14. Similar results were shown for neutrophil rolling and arrest in vivo20 and in flow chamber studies in which the chemokine interleukin (IL)-8 was co-immobilized with ICAM-1 and P-selectin21. Rolling neutrophils arrest by β2 integrin22 activation through inside-out signalling23, which is thought to be rapid and local24; however, the subcellular distribution of integrin activation is unknown.
Integrins are αβ heterodimers that regulate their adhesiveness through changes in the conformation of their ectodomain25,26. Resting integrins assume a bent conformation with low affinity for ligand. The ‘switchblade' model of integrin activation (Supplementary Fig. 1a)25 suggests a two-step activation process in which integrin extension (E+) is followed by a rearrangement in the ligand-binding site leading to high-affinity (H+). β2 integrin extension is detected by monoclonal antibody (mAb) KIM127, which recognizes a neoepitope27 that is hidden in the bent knee of human β2 (ref. 28). On the other hand, when human β2 integrins acquire high affinity, a neoepitope in the β2 I-like domain29,30,31 is exposed, which is recognized by mAb24 (ref. 32). Thus, KIM127 binding indicates E+ and mAb24 binding indicates H+. KIM127 and mAb24 do not block each other and do not block ligand binding.
Research has shown that P-selectin glycoprotein ligand-1 (PSGL-1) signalling triggers integrin extension33,34. This signalling cascade starts with L-selectin and PSGL-1 (ref. 35), proceeds through various signalling intermediates26,34 and induces the E+ but not the H+ integrin conformation33,34. Another signalling cascade is triggered when a chemokine binds to its cognate G-protein-coupled receptor26. This binding induces dissociation of Gαi2 from Gβγ, and is required for arrest36. A distal signalling cassette involving Rap-1, Rho37, Rap1-GTP-interacting adaptor molecule38,39, talin40,41 and kindlin-3 (refs 7, 41) has been described. G-protein-coupled receptor signalling is thought to trigger H+ or possibly both E+ and H+ (ref. 23).
Quantitative dynamic footprinting (qDF) microscopy, which is based on variable angle total internal reflection fluorescence (TIRF) to provide nanometer resolution in the vertical z axis, was combined with microfluidics15,16 to study neutrophil interaction with defined molecular substrates at the subcellular level. This method provides precise maps of the neutrophil surface and the location of relevant molecules. Here we modify and expand qDF microscopy to three colours and combine it with a homogeneous binding assay33, which introduces soluble, fluorescence-labelled mAb24 and KIM127, to investigate the dynamics of β2 integrin activation during primary human neutrophil rolling and arrest under physiologic conditions. We find an unexpected E−H+ conformation of β2 integrins that interacts with ICAMs in cis. This cis interaction inhibits leukocyte adhesion and aggregation, thus providing an auto-inhibitory mechanism that curbs inflammatory responses.
Results
β2 integrin activation on rolling human neutrophils
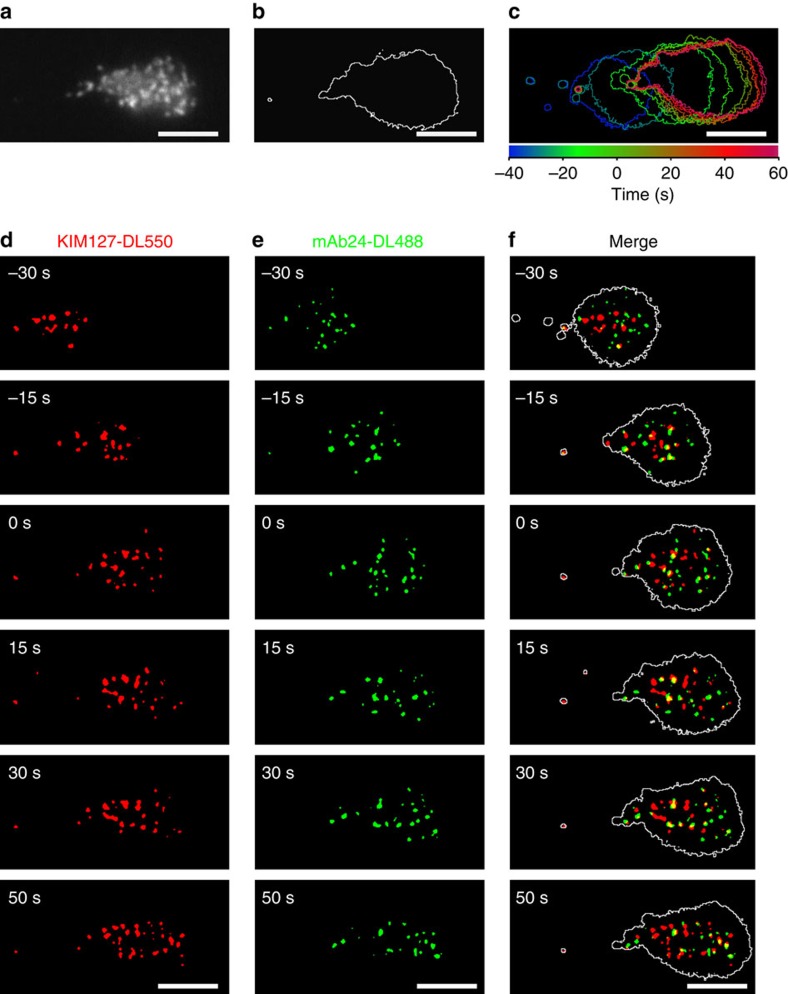
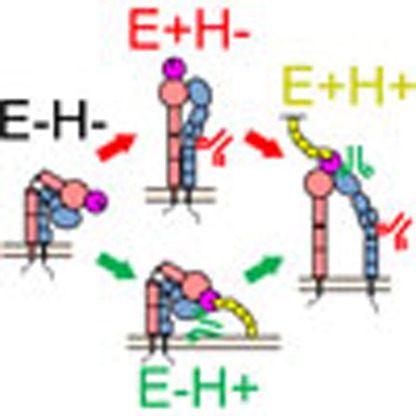
Microfluidic chambers15 were coated with recombinant human P-selectin-Fc (to support rolling), ICAM-1-Fc (a ligand for both αLβ2, lymphocyte function-associated antigen (LFA)-1 and αMβ2, macrophage-1 antigen, Mac-1) and IL-8 with all concentrations titrated so that neutrophils would arrest only when all three molecules were present (Supplementary Fig. 2a). Both LFA-1 and Mac-1 contributed to human neutrophil arrest (Supplementary Fig. 2a). Soluble KIM127 and mAb24 did not shown any significant influence on neutrophil rolling and arrest (Supplementary Fig. 2b–e). Both antibodies bound rapidly (within one video frame) to immobilized activated neutrophils (Supplementary Fig. 3) with no evidence for the loss of binding over time, underscoring the validity of the homogeneous binding assay. Neutrophils isolated from anticoagulated blood and labelled with membrane dye (CellMask DeepRed) were perfused in the presence of DyLight 550 (DL550) conjugated KIM127 and DyLight 488 (DL488) conjugated mAb24 at 6 dyn cm−2 and imaged with a newly developed triple-colour qDF (TqDF) setup. Smart segmentation image processing (Supplementary Fig. 4) was used to remove background and generate binary images of the neutrophil footprint in contact with the substrate (Fig. 1a,b) from raw images (Supplementary Fig. 5a) and reproduce the cluster morphology with better signal-to-noise ratio (Supplementary Fig. 5b). On the P-selectin/ICAM-1/IL-8 substrate, neutrophils rolled and arrested (Fig. 1c). Unlike the nearly homogeneous distribution of total αLβ2 integrins on the cell surface, both KIM127+ and mAb24+ β2 integrins were present in small clusters (Fig. 1d–f, Supplementary Figs 4 and 5 and Supplementary Movie 1) before arrest (time=0 s) and remained in clusters of similar size (Supplementary Fig. 6) after arrest. In the overlaid images (Fig. 1f and Supplementary Movie 1), E+H− (KIM127+mAb24−, red) and E+H+ (KIM127+mAb24+, yellow) β2 integrins were observed during neutrophil rolling and arrest as expected. Unexpectedly, neutrophils also showed clusters of mAb24+KIM127− β2 integrins (E−H+, green). Very few clusters of mAb24+KIM127+ integrins (E+H+, yellow, time before arrest) are observed in rolling neutrophils before arrest. Dye switch experiments excluded nonspecific effects of the fluorochromes used (Supplementary Fig. 7a). These experiments show that neutrophils rolling on ‘complete' substrate (P-selectin/ICAM-1/IL-8) show the complete physiologic transition from rolling to arrest within ∼30 s (Fig. 2a) and express small (∼0.1 μm2) clusters of E+H−, E−H+ and E+H+ β2 integrins.
Figure 1. β2 integrin E+ and H+ conformations on human neutrophil footprint.
(a) A typical image of fluorescently labelled neutrophil membrane. (b) Footprint outline of a neutrophil generated from membrane fluorescence in a. (c) Footprint outlines of a typical cell during rolling on the P-selectin/ICAM-1/IL-8 substrate at a wall shear stress of 6 dyn cm−2 (arrest at time=0 s); time was coded in rainbow colours as shown in colour bar. (d–f) E+ β2 integrins identified by KIM127-DL550 (red in d and f), and H+ by mAb24-DL488 (green in e and f) during neutrophil rolling on P-selectin/ICAM-1/IL-8 substrate. Footprint outlines shown in white in f. Binary images generated by smart segmentation; E+H+, E+H− and E−H+ clusters appear yellow, red and green, respectively, in f; scale bars, 5 μm.
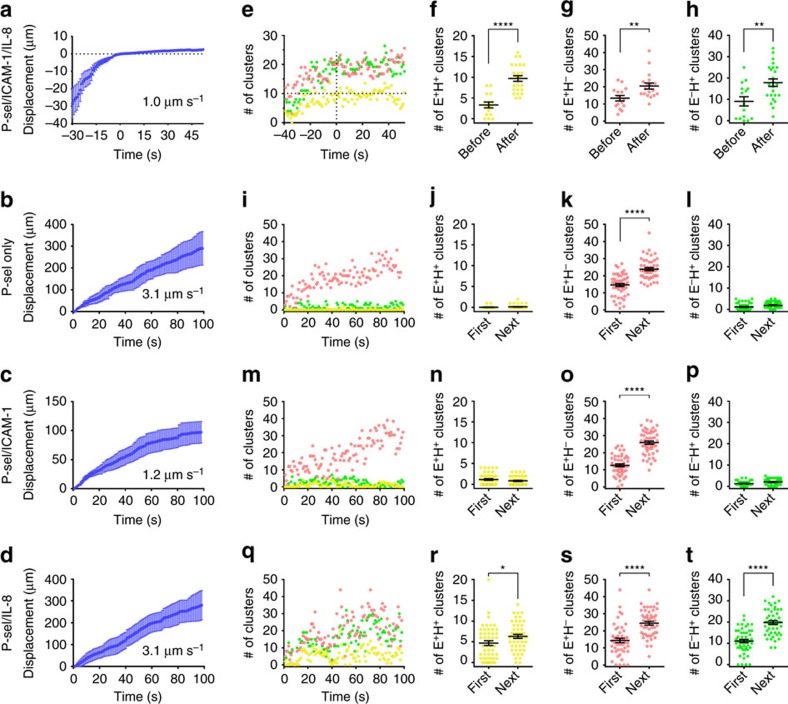
Figure 2. Differential effects of ICAM-1 and IL-8 on integrin activation in human neutrophils.
(a–d) Displacements of typical cells (n=9, mean±s.e.m.) during rolling on P-selectin/ICAM-1/IL-8 (a, arrest at time=0 s), P-selectin only (b), P-selectin/ICAM-1 (c) or P-selectin/IL-8 (d) substrates, respectively. Average rolling velocity (for a: before arrest) determined from linear regression. (e) Dynamics of cluster number per cell (E+H− red, E−H+ green, E+H+ yellow) rolling on P-selectin/ICAM-1/IL-8. (f–h) Number of E+H+ (f), E+H− (g) or E−H+ (h) clusters averaged before (−30 and −15 s, n=16) and after (0, 15 and 30 s, n=24) arrest in eight cells rolling on P-selectin/ICAM-1/IL-8; each time point of each cell represented by one dot, mean±s.e.m. **P<0.01, **** P<0.0001. (i–t) E+H− (red), E−H+ (green) and E+H+ (yellow) clusters for neutrophils rolling on P-selectin only (i), P-selectin/ICAM-1 (m), and P-selectin/IL-8 (q) coated substrates. E+H+ (j,n,r), E+H− (k,o,s) and E−H+ (l,p,t) clusters in the footprint of cells rolling on P-selectin only (j–l), P-selectin/ICAM-1 (n–p) or P-selectin/IL-8 (r–t) in the first 50 s (first) and the next ∼50 s (next) of rolling. Mean values and s.e.ms (error bars) were presented. * P<0.05, **** P<0.0001 in unpaired student t-test.
Different roles of P-selectin and IL-8
To assess which component on the substrate induces integrin activation, we tested neutrophil rolling and adhesion on ‘incomplete' substrates: P-selectin only, P-selectin/ICAM-1 and P-selectin/IL-8 (Fig. 2). On the ‘complete' P-selectin/ICAM-1/IL-8 substrate, neutrophils rolled at a velocity of ∼1.0 μm s−1 (Fig. 2a) before arrest at time=0. As expected34, neutrophils rolled much faster (∼3.1 μm s−1) on P-selectin only (Fig. 2b), whereas the P-selectin/ICAM-1 substrate (Fig. 2c) supported slow rolling (∼1.2 μm s−1), but no arrest. Adding IL-8 to the P-selectin substrate (Fig. 2d) did not reduce rolling velocity (∼3.1 μm s−1) and did not support arrest. Quantitative analysis of the cluster number (Fig. 2e) showed that neutrophils rolling on P-selectin/ICAM-1/IL-8 substrate started with ∼9 E+H−, ∼9 E−H+ and ∼3 E+H+ clusters at −30 s. As the cells continued rolling, the number of E+H+ clusters increased and reached 9±1 when the cells arrested (time=0 s, Fig. 2e and Supplementary Fig. 6a). The step change from pre-arrest to arrest was highly significant (Fig. 2f). The number of E+H− clusters (Fig. 2g) and E−H+ clusters (Fig. 2h) also significantly increased upon arrest. The total area of E+H−, E−H+ and E+H+ clusters increased in proportion to the cluster number (Supplementary Fig. 6b) and the size of each cluster did not change significantly (Supplementary Fig. 6c).
When neutrophils were rolling on P-selectin only (Fig. 2i–l), E+H− clusters were induced (red, Fig. 2i,k), as expected33,34, but no E+H+ clusters (yellow, Fig. 2i,j) or E−H+ clusters (green, Fig. 2i,l) were observed. Induction of E+H− clusters but not E−H+ or E+H+ clusters was highly significant when comparing the first 50 s and the next ∼50 s of rolling (Fig. 2j–l). Rolling neutrophils on P-selectin/ICAM-1 substrate (no chemokine, Fig. 2m–p) produced a similar increase in E+H− integrin (red, Fig. 2m,o) as on P-selectin. As expected, the cells rolled more slowly because the E+H− integrin was able to bind to ICAM-1 with intermediate affinity. Neither E+H+ integrin (yellow, Fig. 2m,n) nor E−H+ integrin (green, Fig. 2m,p) were observed. This changed drastically when chemokine was available on the P-selectin/IL-8 substrate (no ICAM-1, Fig. 2q–t). Strikingly, E+H+ clusters (yellow, Fig. 2r) and E−H+ clusters (green, Fig. 2t) were induced along with the expected E+H− clusters (red, Fig. 2s). Taken together, these data suggest that P-selectin binding is sufficient to induce integrin extension and chemokine is necessary to induce headpiece-opening.
E+H+ clusters derived from both E+H− and E−H+ clusters
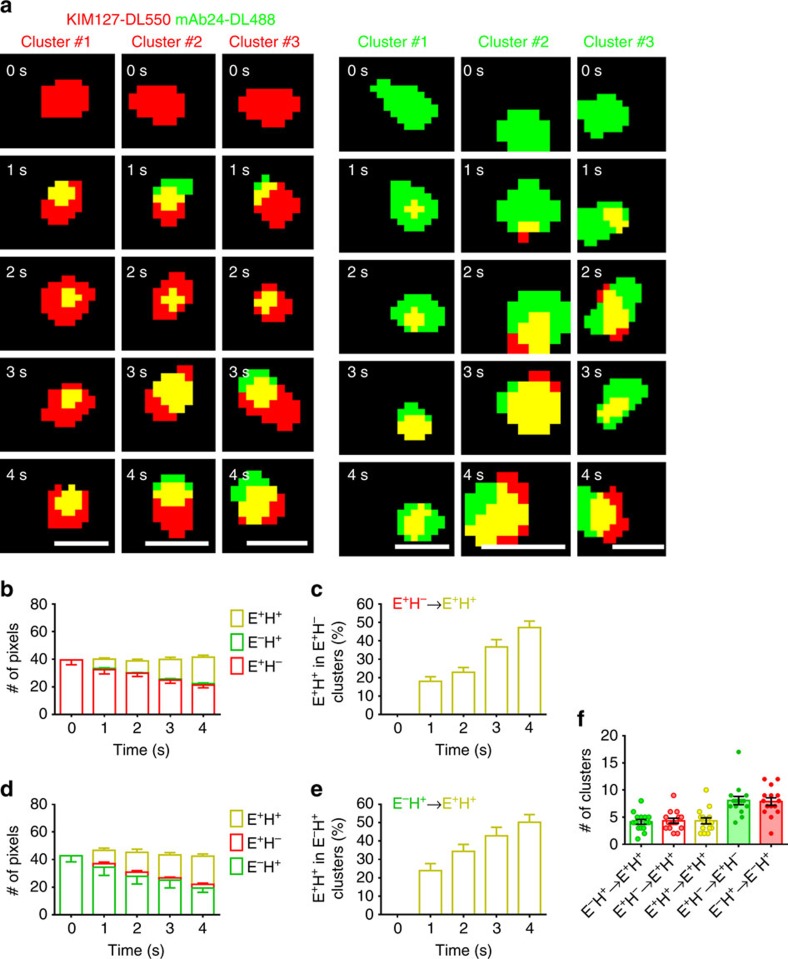
The strong dependence of arrest on the appearance of ∼9 E+H+ clusters (Fig. 2e and Supplementary Fig. 6a) suggests that E+H+ integrins are the functional entity for binding ICAM-1 in trans. When focusing on individual clusters labelled with KIM127-DL550 or mAb24-DL488, we observed that both E+H− integrins (red) and E−H+ integrins (green) transitioned to E+H+ (yellow, Fig. 3a). Dye switch experiments excluded nonspecific effects of the fluorochromes used (Supplementary Fig. 7b). About one-third of E+H− clusters became E+H+ within 4 s (Fig. 3b,c, n=15). E−H+ clusters also became E+H+ at a similar rate (Fig. 3d,e, n=15). When tracking the history of the clusters on arrested cells, many E−H+ and E+H− clusters remained E−H+ or E+H−, respectively, but some clusters (∼5 per neutrophil) converted from E+H− or E−H+ to E+H+ (Fig. 3f). This was caused by an increase in the number of double positive (red and green) pixels over time and independent of small random fluctuations in background intensity (Supplementary Fig. 8). These findings suggest a new alternative pathway (Supplementary Fig. 1b) in which integrin undergoes a conformational change from E−H− to E−H+ first and then to E+H+, clearly different from the canonical pathway suggested by the switchblade model. These two pathways both contributed to fully activated (E+H+) integrins on neutrophils during transition from rolling to arrest on P-selectin/ICAM-1/IL-8 substrate.
Figure 3. Two pathways of conformational transitions during β2 integrin activation.
(a) Three examples of KIM127-DL550 (red, E+H−) or mAb24-DL488 (green, E−H+) clusters transitioning to E+H+ (yellow) over 4 s in the footprint of primary human neutrophils rolling on P-selectin, ICAM-1 and IL-8; scale bars, 0.5 μm. (b–e) Mean values of pixel numbers per cluster in colours as indicated (b,d) and percentage of E+H+ pixels (c,e) of 15 clusters starting as E+H− (b,c) or 15 clusters starting as E−H+ (d,e); s.e.ms were shown as error bars. Data collected from static cells (pre-arrest and arrested). (f) Transition history of the clusters on arrested cells (n=15, one dot per cell). Mean values and s.e.ms (error bars) were presented.
Three-dimensional localization of integrin activation
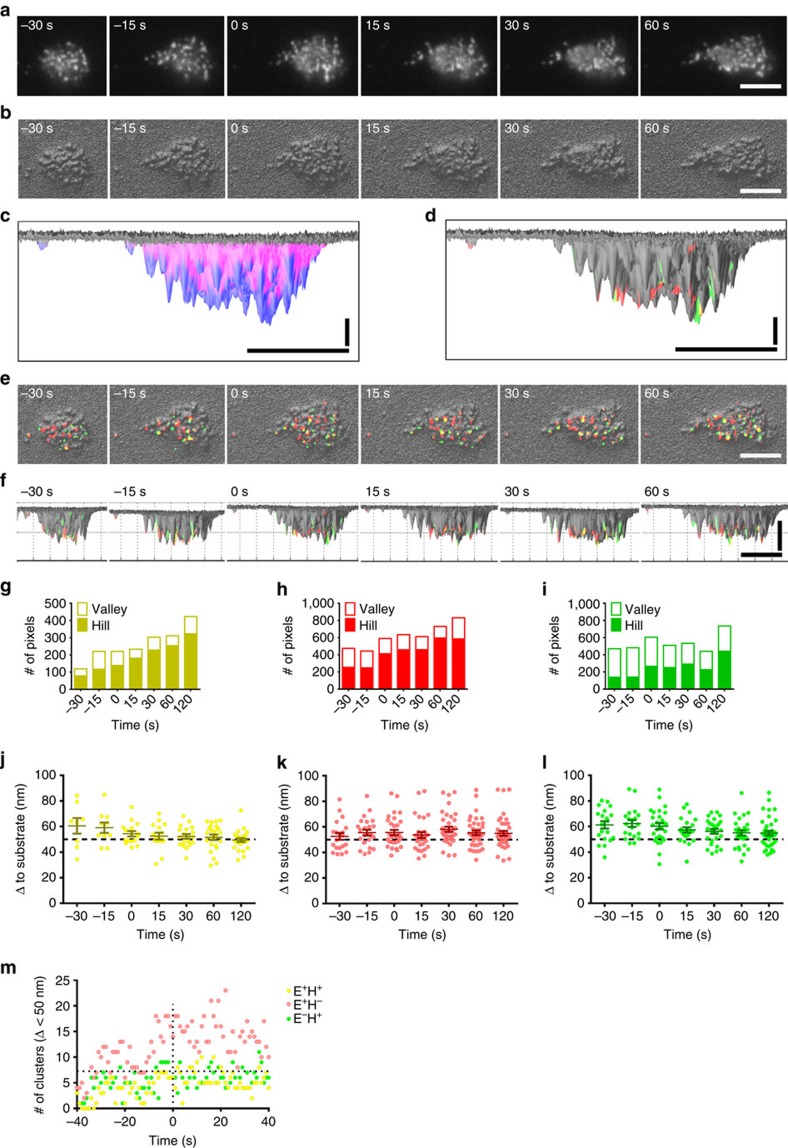
E+H+ integrins can bind ligand in trans with high affinity. The E+H+ conformation is a necessary, but not sufficient condition for binding, since the ligand-binding I domain of αL or αM is only about 23 nm42 above the plasma membrane when extended. The extended β2 integrin-ICAM-1-assembly is about 42 nm long43. Neutrophils have microvilli that are ∼200 nm high44, and β2 integrins are known to be located both on microvilli (hills) and in the ‘valleys' between microvilli45. For E+H+ β2 integrins to reach ligand in trans, they effectively need to be near the top of the microvilli. To test what fraction of integrin clusters met these criteria, we converted15 the raw membrane data (Fig. 4a) into 3D footprints (Fig. 4b). Automated smart segmentation showed 27±1% hills and 73±1% valleys (Fig. 4c and Supplementary Fig. 9). Next, we superimposed E+H+ (yellow), E+H− (red) and E−H+ (green) integrin clusters (Fig. 4d,e) on the 3D topography. Rotation by 90 degrees (Fig. 4d,f and Supplementary Movie 2) allowed us to map all clusters within ∼100 nm from the surface. Interestingly, significantly more (P<0.05) of the E+H+ (Fig. 4g, 70±4%) and E+H− (Fig. 4h, 68±4%) clusters were on hills and thus close to the substrate than E−H+ clusters (Fig. 4i). The fraction of E+H+ and E+H− integrin on hills significantly increased with time of rolling and continued to increase after arrest (time=0 s).
Figure 4. 3D distributions of β2 integrin activation clusters in primary human neutrophils.
(a) Neutrophil membrane (CellMask DeepRed) before and after arrest (0 s) of one representative neutrophil rolling on P-selectin, ICAM-1 and IL-8. (b) Membrane signal converted to hills (microvilli) and valleys (space between microvilli). (c,d) Hills (blue) and valleys (magenta) segmented (c) or E+H− (red), E−H+ (green) or E+H+ (yellow) clusters (d) were identified at time of arrest (0 s). (e,f) Top-view (e) and side-view (f) of the 3D topography overlaid with E+H− (red), E−H+ (green) or E+H+ (yellow) clusters; binary images. Horizontal scale bars, 5 μm, vertical scale bar, 20 nm for d, scale bar, 50 nm for f. (g–i) Most E+H+ (g, 70±4%) and E+H− (h, 68±4%) cluster pixels were on hills. Most E−H+ cluster pixels (i, 71±0%) were in valleys before arrest (P<0.01 and P<0.05 compared with E+H− and E+H− cluster pixels in unpaired student t-test, respectively) and more E−H+ cluster pixels (52±6%) localized to the hills after arrest (P<0.05 and P<0.01 compared with E+H− and E+H− cluster pixels in unpaired student t-test, respectively). The E+H+ (g), E+H− (h) and E−H+ (i) cluster pixels on the hills increased with time (the slopes were significantly non-zero, F-test, P<0.01). (j–l) Distance (Δ) of E+H+ (yellow, j), E+H− (red, k) or E−H+ (green, l) integrin clusters to the substrate. The dashed line at 50 nm separates the integrin clusters within reach (≤50 nm) from those beyond reach (>50 nm). Each cluster represented by one dot, mean values and s.e.ms (error bars) were presented. (m) Number of clusters within 50 nm to the substrate per cell (E+H− red, E−H+ green, E+H+ yellow) during rolling on the substrate of P-selectin/ICAM-1/IL-8 (arrest at 0 s).
Integrin can bind ICAM-1 on the substrate only when the integrin is within ∼50 nm from the substrate (Supplementary Fig. 10). Analyzing the number of E+H−, E+H+ and E−H+ clusters within 50 nm of the substrate shows that during rolling, about three E+H+ clusters are ‘within reach', and the number of E+H+ clusters close to the substrate (Fig. 4j, yellow) continues to increase until arrest. The number of E+H− clusters (Fig. 4k, red) within 50 nm of the substrate also increases during rolling. Some E−H+ clusters (Fig. 4l, green) are also within 50 nm, but this is irrelevant to ligand binding, because the bent conformation is not expected to bind ligand in trans even if the headpiece is ‘open' (Supplementary Fig. 10). The dynamics of integrin conformations within 50 nm to the substrate over time of rolling and arrest is shown in Fig. 4m, which shows that arrest is triggered by ∼7 E+H+ clusters that are close enough to the substrate to bind ICAM-1 in trans.
To directly measure the number of fully activated E+H+ β2 integrins that are needed for arrest, we measured the sites of KIM127 and mAb24 on the neutrophil surface by calibration beads with known antibody-binding sites using flow cytometry. Calculating from the fluorescence intensity integral of E+H+ clusters at the time of arrest (t=0), we find that 258±41 E+H+ integrin molecules are present in the neutrophil footprint at the time of arrest.
E−H+ β2 integrins bind ICAM-1 on neutrophils in cis
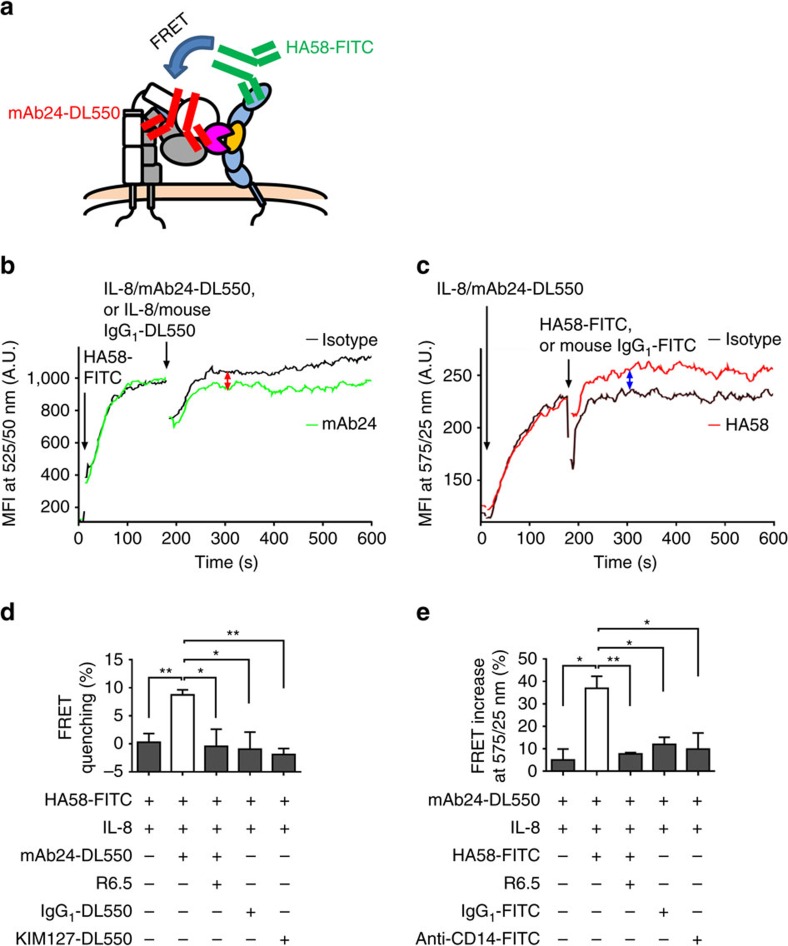
The discovery of E−H+ β2 integrins on human neutrophils is the first report of E−H+ integrins on any physiological relevant cell. We reasoned that such bent high-affinity integrins may have a specific function. Since E−H+ integrin is not expected to bind ligand in trans, we considered whether E−H+ integrin may bind ligand in cis, that is, ICAM-1 expressed on the neutrophil. Human LFA-1 and Mac-1 bind domain 1 (ref. 46) and domain 3 (ref. 47) of human ICAM-1, respectively. To directly test whether E−H+ LFA-1 and Mac-1 could bind ICAM-1 in cis (on the neutrophil), we conducted Förster resonance energy transfer (FRET) experiments that report proximity of molecules within 1–10 nm (Fig. 5). When FRET occurs, emission at the shorter wavelength donor fluorochrome (for example, fluorescein isothiocyanate, FITC) is reduced (quenching at 525/50 nm), because some energy is transferred to the higher wavelength acceptor fluorochrome (for example, DL550). Conversely, FRET increases the emission of the higher wavelength fluorochrome (for example, DL550, measured at 575/25 nm). We reasoned that FRET should occur between mAb24 (binding β2 H+) and ICAM-1 domain 1 detected by mAb HA58 (Fig. 5a). Since mAb HA58 is function-blocking48 (disables ICAM-1 domain 1 binding to LFA-1), this assay directly tests the interaction of Mac-1 with domain 3 of ICAM-1. We indeed observed a significant decrease in donor fluorescence (Fig. 5b) and significant increase in acceptor fluorescence (Fig. 5c). This was specific, because FRET quenching did not occur when the acceptor mAb24-DL550 was absent or replaced by an isotype control antibody or KIM127-DL550, or when Mac-1 binding to ICAM-1 was blocked by mAb R6.5 (Fig. 5d)49. Similarly, the gain of acceptor fluorescence was blocked by adding R6.5, or when an irrelevant donor was used (anti-CD14-FITC, or isotype control, Fig. 5e) instead of HA58-FITC. Based on the FRET efficiency as measured, the average distance between the β2 I-like domain (mAb24-binding site) and ICAM-1 domain 1 (HA58-binding site) is calculated to be ∼9.9 nm, which is consistent with the known molecular dimensions.
Figure 5. E−H+ Mac-1 binds ICAM-1 in cis.
(a) Schematic of assessing the cis interaction between E−H+ Mac-1 and neutrophil ICAM-1 by FRET between ICAM-1 domain 1 (HA58-FITC, donor) and H+ integrin (mAb24-DL550, acceptor). (b,c) Donor fluorescence decrease (green in b) and acceptor fluorescence increase (red in c) shows FRET of HA58-FITC with mAb24-DL550, but not with isotype controls (IgG1-DL550 as acceptor, black in b; and IgG1-FITC as donor, black in c). (d,e) Donor fluorescence decrease (d) and acceptor fluorescence increase (e) of HA58-FITC-mAb24-DL550 pairs and controls measured at 2–3 min after adding IL-8 and acceptor or donor, respectively. Blocking of E−H+ Mac-1-ICAM-1 interactions (mAb R6.5) eliminated the donor fluorescence decrease and acceptor fluorescence increase. n=3, mean values and s.e.ms (error bars) were presented. * P<0.05, ** P<0.01 in unpaired student t-test. A.U., arbitrary unit.
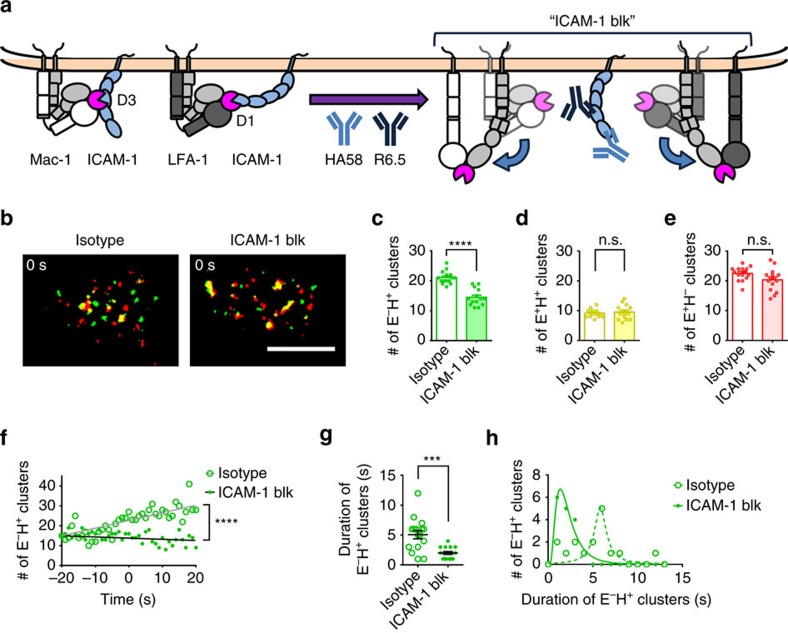
Having shown that E−H+ neutrophil β2 integrins directly bind ICAM-1 in cis, we reasoned that this binding may stabilize E−H+ clusters. Thus, E−H+ clusters should be decreased when ICAM-1 binding to LFA-1 (using mAb HA58) and Mac-1 (using mAb R6.5) were blocked (Fig. 6a). Indeed, blocking ICAM-1 binding in cis (ICAM-1 blk) reduced the number of E−H+ clusters (Fig. 6b,c) at the time of neutrophil arrest (0 s). We found no significant difference in E+H+ (Fig. 6d) or E+H− (Fig. 6e) clusters when ICAM-1 was blocked on the neutrophils, but ICAM-1 blk neutrophils reach the number of E+H+ clusters needed for arrest faster. Under control conditions, the number of E−H+ clusters increased with time, and this did not happen when ICAM-1 was blocked (Fig. 6f). If indeed β2 integrin interaction with ICAM-1 in cis stabilized the E−H+ conformation, then the duration of E−H+ clusters (time before having E+H+ on the cluster) should be reduced. Indeed, the average duration of E−H+ clusters was reduced from >5 s to <2 s (Fig. 6g,h).
Figure 6. Blocking E−H+ integrin binding to neutrophil ICAM-1 promotes its transition to E+H+.
(a) Schematic showing the hypothesis that the cis interactions of E−H+ integrin (both LFA-1 and Mac-1) and neutrophil ICAM-1 may stabilize the E−H+ integrin. (b) Integrin clusters (E+H− red, E−H+ green, E+H+ yellow) on arresting neutrophils rolling on P-selectin/ICAM-1/IL-8 with or without neutrophil ICAM-1 blocking by mAbs HA58 and R6.5; scale bar, 5 μm. (c–e) ICAM-1 blocking decreased the number of E−H+ clusters at arrest (c, n=15 cells). The number of E+H+ (d, n=15 cells) and E+H− clusters (e, n=15 cells) at arrest with or without ICAM-1 blocking. (f) Dynamics of E−H+ clusters with or without ICAM-1 blockade on cells rolling on P-selectin/ICAM-1/IL-8. (g,h) ICAM-1 blocking decreased the duration of E−H+ clusters before transitioning to E+H+ clusters (g, n=16 clusters). Duration histograms (h, bin=1 s). Mean values and s.e.ms (error bars) were presented in (c–e) and (g). Log Gaussian (ICAM-1 blk) and Lorentzian (isotype) fits were used in h. n.s. P>0.05, ** P<0.01, *** P<0.001, **** P<0.0001 in unpaired student t-test. n.s., not significant.
E−H+ β2 integrins inhibit neutrophil adhesion
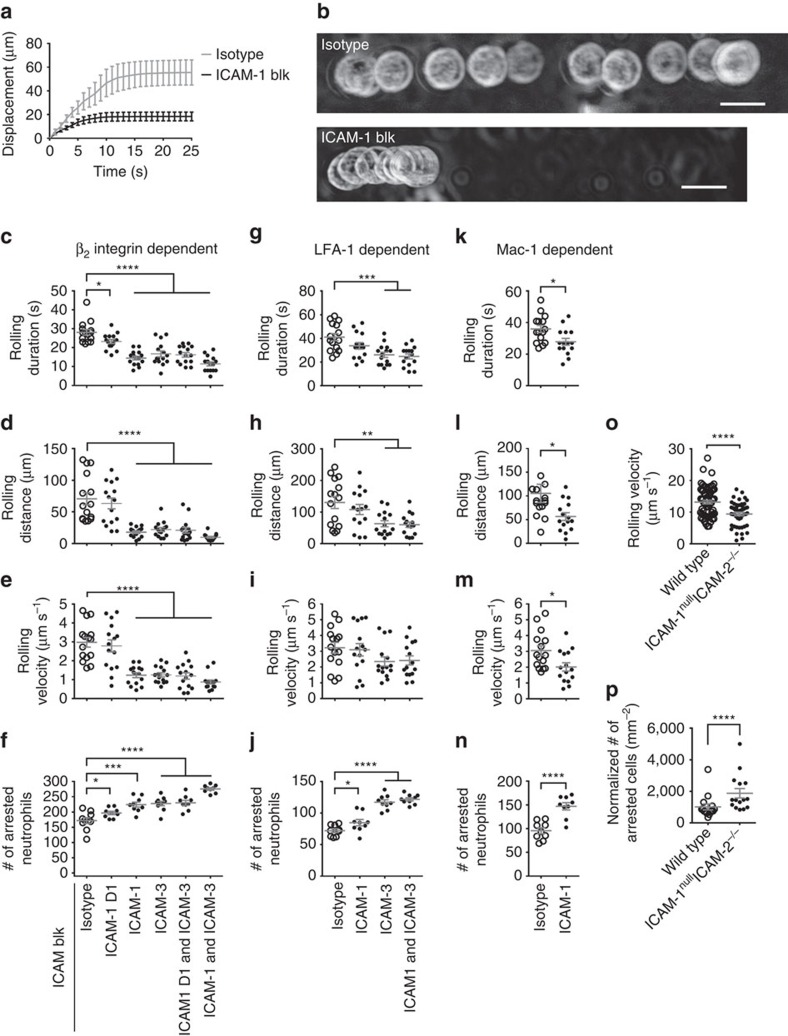
Since β2 integrin interaction with ICAM-1 in cis stabilized the E−H+ conformation, we hypothesized that this may represent an auto-inhibitory pathway, because E−H+ integrins are not available for ligand binding in trans and thus are not expected to support cell adhesion under flow. Indeed, when ICAM-1 was blocked, human neutrophils arrested much quicker and rolled for a shorter distance than untreated human neutrophils on P-selectin/ICAM-1/IL-8 substrate (Fig. 7a,b). Human neutrophils express ICAM-1, a ligand for LFA-1 and Mac-1, and ICAM-3, a ligand for LFA-1, but not ICAM-2 (Supplementary Fig. 11). Therefore, we tested the rolling duration and distance (until arrest, Fig. 7c,d, respectively), the rolling velocity (Fig. 7e) and the resulting number of arrested neutrophils (Fig. 7f) with or without ICAM-1 and/or ICAM-3 blockade. Blocking ICAM-1 domain 1 (the LFA-1-binding site) was sufficient to make neutrophils adhere significantly faster (shorter rolling duration), resulting in more arrested neutrophils. Blocking both LFA-1 and Mac-1-binding sites on ICAM-1 further accelerated neutrophil accumulation. The effect of blocking ICAM-1 was similar to the effect of blocking ICAM-3, and blocking both ICAM-1 and ICAM-3 further reduced rolling duration, distance and velocity and further increased the number of arrested neutrophils.
Figure 7. Blocking E−H+ integrin binding to neutrophil ICAMs promotes leukocyte adhesion.
(a) Displacements of human neutrophils (n=5) with or without blockade of neutrophil ICAM-1 during rolling on P-selectin/ICAM-1/IL-8. (b) Maximum intensity projection of a typical bright-field-imaged human neutrophil with (13 frames) or without (30 frames) blockade of neutrophil ICAM-1 rolling on P-selectin/ICAM-1/IL-8. Flow direction is from left to right. Scale bar, 10 μm. (c–f) Rolling duration until arrest (c, n=15 cells per group), rolling distance until arrest (d, n=15 cells per group), rolling velocity before arrest (e, n=15 cells per group) and the number of arrested human neutrophils (f, n=9 observations per group) with or without blockade of neutrophil ICAM-1 D1 (domain 1, binds LFA-1, mAb HA58), or ICAM-1 (binds both LFA-1 and Mac-1, mAbs HA58 and R6.5), and/or ICAM-3 (binds LFA-1, mAb CBR-IC3/1). (g–j) Upon blockade of Mac-1 by mAb ICRF44, LFA-1-dependent cis binding was analysed with or without blockade of LFA-1 cis binding to neutrophil ICAM-1 (mAb HA58), and/or ICAM-3 (mAb CBR-IC3/1). n=15, 15, 15 and 9 cells per group in g,h,i and j, respectively. (k–n) Upon blockade of LFA-1 (mAb TS1/22), Mac-1-dependent cis binding was analysed with or without blockade of Mac-1 cis binding to neutrophil ICAM-1 (mAb R6.5). n=15, 15, 15 cells, and nine observations per group in k,l,m and n. (o) Leukocyte rolling velocity (n=48 for ICAM-1nullICAM-2−/− and 65 for wild typeDsRed cells) in mouse cremaster muscle venules of ICAM-1nullICAM-2−/−/wild typeDsRed mixed chimeric mice before intravenous injection of CXCL1. (p) The number of adherent leukocytes (n=15 observations) in mouse cremaster muscle venules of the mixed chimeric mice 1 min after intravenous injection of 600 ng CXCL1. Mean values and s.e.ms (error bars) were presented in a,c–p. *P<0.05, **P<0.01, *** P<0.001, **** P<0.0001 in unpaired student t-test for a,c–o and paired student t-test for p.
To specifically look at the contributions of LFA-1 and Mac-1, we blocked Mac-1 by mAb ICRF44 (Fig. 7g–j) or LFA-1 by mAb TS1/22 (Fig. 7k–n). Since LFA-1 binds both ICAM-1 and ICAM-3, we tested the contribution of both ligands and found that blocking ICAM-3 alone had a stronger effect on the number of arrested neutrophils than blocking ICAM-1 alone. This is consistent with the higher expression of ICAM-3 than ICAM-1 on human neutrophils (Supplementary Fig. 11). We conclude that ICAM-3 is the dominant LFA-1 ligand in cis. Since Mac-1 binds only to ICAM-1, we tested its contribution by blocking the Mac-1 binding to ICAM-1 using mAb R6.5 (ref. 49). This intervention significantly decreased rolling duration, distance and velocity and significantly increased Mac-1-dependent neutrophil arrest. These data show that Mac-1 interaction with ICAM-1 in cis significantly contributes to limiting neutrophil accumulation.
To directly address the in vivo relevance of our findings, we performed in vivo mouse experiments. ICAM-1 and 2 are both expressed on mouse neutrophils16,50. We performed in vivo cremaster imaging on mice reconstituted with mixed ICAM-1/2-double-knockout51,52 and DsRed-wild type (1:1) bone marrow. ICAM-1/2-double-knockout leukocytes (non-fluorescent) showed significantly decreased rolling velocity in cremaster venules (Fig. 7o). After injecting the chemokine CXCL1, the number of arrested ICAM-1/2-double-knockout leukocytes was twice that of wild-type neutrophils (Fig. 7p). These data show that the binding of E−H+ β2 integrin to ICAM-1 and 2 in cis strongly inhibits leukocyte adhesion in vivo.
Based on the finding that integrin activation blockade by interaction of E−H+ β2 integrins with ICAMs in cis is relevant in vitro and in vivo, we asked whether it would also limit neutrophil aggregation. To test this, we performed an aggregation assay (Supplementary Fig. 12), where we stained human neutrophils with two different dyes (carboxyfluorescein succinimidyl ester (CFSE) and cell tracker orange (CMRA)) and tested the aggregation between the two populations. When ICAMs were blocked on the CMRA population, thus effectively blocking the cis interaction and liberating β2 integrins, the percentage of heteroaggregates increased about threefold. When we further blocked β2 integrins on the other (CFSE) population, which released the cis-binding ICAMs, CFSE-CMRA aggregates increased by a further factor of two. Therefore, without the inhibition of integrin extension by binding ICAMs in cis, neutrophil aggregation would be expected to be sixfold higher than it actually is. These results directly demonstrate that the cis interaction between E−H+ β2 integrin and ICAMs provides a relevant mechanism that inhibits neutrophil aggregation in suspension.
Taken together, our data support a new model (Supplementary Fig. 1b) where resting E−H− LFA-1 and Mac-1 are stimulated by chemokine to assume the E−H+ conformation that binds mAb24, but not KIM127. This conformation is stabilized by interaction with ICAM-1 on the neutrophil in cis. When extension occurs, this converts E−H+ to E+H+ integrin, which is now able to bind ICAM-1 in trans (on the substrate, endothelium or other leukocytes) and thus promote arrest.
Discussion
Here we elucidate the molecular mechanism of β2 integrin-dependent neutrophil arrest. As expected, rolling neutrophils express some β2 integrins in the E+H− conformation. Unexpectedly, the E+H− integrins are organized in clusters with an average size of ∼25 pixels (∼0.1 μm2). Unlike bulk β2 integrins, most of these E+H− clusters are on the tips of microvilli and thus able to reach ICAM-1 on the flat substrate. In vivo, neutrophil hills and valleys may interdigitate with endothelial hills and valleys, but this cannot be imaged with sufficient resolution by intravital microscopy. Very few clusters of high-affinity (E+H+) integrin are observed on rolling neutrophils. When immobilized chemokine is added, both E−H+ and E+H+ clusters are induced. When the number of E+H+ clusters reaches ∼9 (∼7 within 50 nm from substrate), the cell stops rolling and arrests. We estimate that 258 fully activated E+H+ β2 integrin molecules are sufficient to arrest a human neutrophil at 6 dyn cm−2.
According to the switchblade model23,25 of integrin activation, E+ precedes H+. Thus, the appearance of E−H+ clusters was completely unexpected. The E−H+ conformation was suggested by an electron microscopic image of αVβ3 integrin53, a crystal structure of αXβ2 stabilized by introduced disulfide bridges54, and three Mac-1 mutants55. However, these first two constructs lacked transmembrane and cytoplasmic domains and the third introduced physiologically irrelevant mutations. Thus, the physiological significance remained unclear. Here we show that the E−H+ integrin conformation exists on primary human cells. Our findings suggest that integrin H+ and E+ are regulated individually, which disagrees with the canonical switchblade model25, where integrin H+ requires integrin extension (E+). We show that PSGL-1 signalling is sufficient to induce E+, whereas chemokine signalling necessary for H+.
Beyond these results that are relevant to our understanding of basic integrin biology, we discovered that E−H+ integrins bind ICAMs in cis, which effectively inhibits cell adhesion as evidenced by prolonged rolling distance, prolonged rolling time and reduced number of adherent neutrophils. This is a new, powerful and unexpected endogenous anti-inflammatory mechanism that impacts inflammation and immunity. This new mechanism not only limits neutrophil adhesion under flow, but also curbs neutrophil aggregation, which is associated with acute lung injury56, sepsis57 and coronary artery disease58. E−H+ LFA-1 binds to both ICAM-1 and ICAM-3 in cis; E−H+ Mac-1 binds to ICAM-1 in cis. Thus, both major β2 integrins on neutrophils are inhibited by binding ICAMs in cis. The FRET assay provides direct evidence of close proximity between H+E− β2 integrins and ICAMs. The functional data (adhesion and aggregation assays) are consistent with our interpretation. Direct evidence to demonstrate the existence of the cis interaction of the β2 integrins with ICAMs might ultimately be provided by co-crystallization.
It is known that Mac-1 on neutrophils is increased upon chemokine stimulation by degranulation33. However, the degranulation does not occur until ∼5 min after chemokine. In our investigation, we considered the conformational changes of β2 integrins only less than 1 min after exposure to IL-8. Thus, Mac-1 degranulation does not influence our findings. Whether freshly recruited Mac-1 can interact with ICAMs in cis is unknown.
An alternative possibility that would explain the observation of KIM127− mAb24+ clusters is steric exclusion. If extended integrin molecules were clustered so tightly that KIM127 cannot find its binding site, extended high-affinity integrin would be falsely classified as bent. If this were the case, clustered extended high-affinity integrin would be available to bind ligand in trans and not available to bind ligand in cis. Therefore, the steric antibody exclusion hypothesis is not supported by our functional data (Figs 6 and 7 and Supplementary Fig. 12), which clearly show that liberating β2 integrins from interactions in cis increases binding to ligand in trans and consequently neutrophil adhesion and aggregation.
Our finding also suggests a possible new class of drugs that target β2 integrins. Currently, six integrin-targeting drugs are on the market, five of which are antibodies or small molecules that inhibit the ligand-binding site59. The present data suggest that a new class of allosteric inhibitors that stabilize E−H+ may be useful as anti-inflammatory drugs. Conversely, molecules that release E−H+ to E+H+ could be used to fight infections where more neutrophil recruitment might be needed.
Beyond neutrophils, β2 integrins are widely expressed and functional in other leukocyte subsets. LFA-1 on lymphocytes is involved in trafficking to lymph nodes60 and in the formation of the immunological synapse61. The newly discovered E−H+ conformation thus may also be involved in the regulation of adaptive immunity. The recruitment of patrolling monocytes62 is also β2 integrin-dependent. Finally, other integrins may also have E−H+ conformations, which may be involved in other immunological processes, like β1 integrin-dependent60 or β7 integrin-dependent63 leukocyte recruitment.
In conclusion, we show that H+E− β2 integrins exist on rolling neutrophils, where they bind ICAM-1 in cis, thus limiting neutrophil adhesion by preventing ICAM-1 binding in trans. These data support a revised model of β2 integrin activation separating headpiece opening from extension (Supplementary Fig. 1). Beside the significance in β2 integrin activation during neutrophil adhesion in particular, our finding provides insights into integrin function in general.
Methods
Reagents
Recombinant human P-selectin-Fc, ICAM-1-Fc and IL-8 were purchased from R&D Systems. Murine CXCL1 was purchased from Pepro Tech. Casein blocking buffer was purchased from Thermo Fisher Scientific. The conformation specific antibody mAb24 to human β2-I-like-domain, which reports the headpiece-opening29,30,31,32, was purchased from Abcam. The KIM127 mAb to human β2-IEGF-domain, which reports the ectodomain extension27,28, was purified at the Lymphocyte Culture Center at the University of Virginia from hybridoma supernatant (American Type Culture Collection). Purified CD11a blocking mAb TS1/22 was purchased from Thermo Fisher Scientific. Purified CD11b blocking mAb ICRF44, purified CD11a non-blocking mAb TS2/4, purified and FITC-conjugated ICAM-1 domain 1 mAb HA58, purified ICAM-3 blocking mAb CBR-IC3/1, purified ICAM-2 blocking mAb CBR-IC2/2, FITC-conjugated anti-mouse IgG1 mAb, and purified isotype control mAbs were purchased from Biolegend. The CD18 blocking mAb IB4, and human Fc receptor (FcR) blocking reagents were purchased from Miltenyi Biotec. Purified ICAM-1 domain 2 mAb R6.5 was purchased from eBioscience. FITC-conjugated CD14 mAb was purchased from Invitrogen. DL488- or DL550-conjugated isotype control mAbs were purchased from Novus Biologicals. FITC-conjugated isotype control mAbs was purchased from BD Bioscience. mAb24, KIM127 or TS2/4 were directly labelled by DL488 or DL550 using DyLight antibody labelling kits from Thermo Fisher Scientific. CellMask DeepRed was purchased from Molecular Probes. CellTrace CFSE and CellTracker Orange CMRA were purchased from Thermo Fisher Scientific. Quantum Simply Cellular anti-Mouse (calibration beads) was purchased from Bangs Laboratories. Polymorphprep was purchased from Accurate Chemical. Roswell Park Memorial Institute (RPMI) medium 1,640 without phenol red and phosphate-buffered saline (PBS) without Ca2+ and Mg2+ were purchased from Gibco. Human serum albumin (HSA) was purchased from Gemini Bio Products. Paraformaldehyde (PFA) was purchased from Thermo Fisher Scientific.
Mice
C57BL/6J wild-type mice (000664; JAX) and DsRed mice (006051; JAX; wild-type C57BL/6J background) were obtained from the Jackson Laboratory. Mice were fed a standard rodent chow diet and were housed in microisolator cages in a pathogen-free facility. Mice were euthanized by CO2 inhalation. All experiments followed guidelines of the La Jolla Institute for Allergy and Immunology Animal Care and Use Committee, and approval for use of rodents was obtained from the La Jolla Institute for Allergy and Immunology according to criteria outlined in the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health.
ICAM-1null and ICAM-2−/− mice were described before51,52. They were backcrossed to the C57BL/6 background for at least eight generations64. ICAM-1 and ICAM-2 double-deficient (ICAM-1null/ ICAM-2−/−) C57BL/6 mice were generated by crossing ICAM-1null and ICAM-2−/− C57BL/6 mice in the specific pathogen-free animal facility, University of Bern, Switzerland. Animal procedures were performed in accordance with the Swiss legislation on the protection of animals and approved by the veterinary office of the Kanton of Bern.
Bone marrow transplantation for mixed chimera
Recipient mice (C57BL/6J wild-type male, 8-weeks old, Jackson Labs) were irradiated in two doses of 550 rads each (for a total of 1,100 rads) 4 h apart. Bone marrow cells from both femurs and tibias of donor mice (ICAM-1nullICAM-2−/− male and DsRed wild-type male, 11 weeks old) were collected under sterile conditions. Bones were centrifuged for the collection of marrow, unfractionated bone marrow cells were washed, resuspended in PBS, mixed at a ratio of 1:1 (2.5 million cells each) in 200 μl, confirmed by flow cytometry and injected retro-orbitally into the lethally irradiated mice. Recipient mice were housed in a barrier facility under pathogen-free conditions before and after bone marrow transplantation. After bone marrow transplantation, mice were provided autoclaved acidified water with antibiotics (trimethoprim-sulfamethoxazole) and were fed autoclaved food. Mice were used for experiments 6 weeks after bone marrow reconstitution.
Neutrophil isolation
Heparinized whole blood was obtained from healthy human donors after informed consent, as approved by the Institutional Review Board of the La Jolla Institute of Allergy & Immunology in accordance with the Declaration of Helsinki. Informed consent was obtained from all donors. Neutrophils were isolated by using Polymorphprep (a mixture of sodium metrizoate and Dextran 500) density gradient. Briefly, human blood was applied onto Polymorphprep, centrifuged at 500g for 35 min at 20–25 °C, resulting in neutrophils concentrated in a layer between peripheral blood mononuclear cells and erythrocytes. After washing with PBS without Ca2+ and Mg2+ twice, the neutrophils (>95% purity by flow cytometry, no visible activation by microscopy) were re-suspended in RPMI-1640 without phenol red plus 2% HSA and were used within 4 h. Neutrophils were incubated with FcR blocking reagents for 10 min at room temperature before all the experiments.
Microfluidic device
The assembly of the microfluidic devices used in this study and the coating of coverslips with recombinant human P-selectin-Fc, ICAM-1-Fc and IL-8 has been described previously15,16,33. Briefly, coverslips were coated with P-selectin-Fc (2 μg ml−1), ICAM-1-Fc (10 μg ml−1), and IL-8 (10 μg ml−1) for 2 h and then blocked for 1 h with casein (1%) at room temperature. In some experiments (Fig. 2), coverslips were coated with P-selectin-Fc only, P-selectin-Fc plus ICAM-1-Fc, or P-selectin-Fc plus IL-8. After coating, coverslips were sealed to polydimethylsiloxane chips by magnetic clamps to create flow chamber channels ∼29 μm high and ∼300 μm across. By modulating the pressure between the inlet well and the outlet reservoir, 6 dyn cm−2 wall shear stress was applied in all experiments.
Microfluidic perfusion assay
To study the arrest of neutrophils, isolated human primary neutrophils (5 × 106 cells ml−1) were perfused in the microfluidic device over a substrate of recombinant human P-selectin-Fc with or without recombinant human ICAM-1-Fc and/or IL-8 under shear stress of 6 dyn cm−2. In some experiments (Supplementary Fig. 2a), neutrophils were incubated with anti-CD11a (TS1/22, blocking, 10 μg ml−1) mAb, anti-CD11b (ICRF44, blocking, 10 μg ml−1) mAb, anti-CD18 (IB4, blocking, 10 μg ml−1 mAb for 20 min at room temperature before being perfused into the microfluidic devices, as described previously33. In some experiments (Supplementary Fig. 2b–e), neutrophils were incubated with isotype mAb (10 μg ml−1), KIM127 and isotype (5 μg ml−1 each), mAb24 and isotype (5 μg ml−1 each) or KIM127 and mAb24 (5 μg ml−1 each) for 3 min at room temperature before being perfused into the microfluidic devices. In ICAM-1/ICAM-3 blocking experiments (Fig. 7c–n), neutrophils were incubated with ICAM-1 domain 1 blocking mAb HA58 (10 μg ml−1), or/and domain 3 blocking mAb R6.5 (10 μg ml−1), and/or ICAM-3 blocking mAb CBR-IC3/1 (10 μg ml−1), or/and isotype control mAbs for 20 min at room temperature, with two washes before being perfused into the microfluidic devices. IB4, TS1/22 and ICRF44 mAbs were used in some data sets to assess the contribution of β2 integrins, LFA-1 and Mac-1 in the cis interactions. The microfluidic devices were perfused with neutrophils for 10 min and washed with RPMI-1640 without phenol red plus 2% HSA for 5 min. Then, the arrested neutrophils were counted in nine fields-of-view per group. In some experiments, time-lapse images (one frame per second) were taken during the profusion. Then the rolling duration and rolling distance were acquired from the images by analysing 15 cells starting rolling to arrest.
Homogeneous binding qDF imaging
The homogeneous binding assay (that is, the continuous real-time measurement without separation of soluble antibody33) and qDF imaging15,16 were combined here. Briefly, the conformation reporting antibody mAb24 or KIM127 were conjugated with DL488 or DL550, respectively, using the DyLight antibody labelling kits according to the manufacturer's instructions. In some experiments (Supplementary Fig. 7), the fluorochrmes of mAb24 and KIM127 were switched to test for possible nonspecific effects of the fluorochromes. In neutrophil ICAM-1 blocking experiments (Fig. 6), neutrophils were incubated with both ICAM-1 domain 1 mAb HA58 (10 μg ml−1) and domain 2 mAb R6.5 (10 μg ml−1), which will block both LFA-1 and Mac-1 binding49, or isotype control mAbs for 20 min at room temperature, with two washes before performing the homogeneous binding assay.
During the homogeneous binding assay, neutrophils (2.5 × 106 cells ml−1) were incubated with fluorochrome-conjugated reporting mAbs (5 μg ml−1 each) for 3 min at room temperature and immediately perfused through the microfluidic device at a wall shear stress of 6 dyn cm−2 without separation of the soluble mAbs. The plasma membrane of neutrophils was labelled with CellMask DeepRed according to the manufacturer's instructions before the incubation with mAbs. When neutrophils were observed rolling on the substrate, acquisition was started using TqDF microscopy to acquire the dynamics of integrin activation on neutrophil footprint during rolling (∼30 s), arrest and ∼30–100 s following arrest.
To assess the binding kinetics and stability of binding of the mAbs, neutrophils were perfused through the microfluidic device at a wall shear stress of 6 dyn cm−2 for 5 min to allow them to roll and arrest on the substrate. Then 1% PFA was perfused through the device to fix the neutrophils for 5 min. After washing the device by PBS perfusion for 5 min, 5 μg ml−1 of fluorochrome-conjugated reporting mAbs KIM127 or mAb 24 were perfused through the device. Footprints of the fixed cells were recorded by TqDF microscopy.
TqDF microscopy
The qDF set up and the theory of qDF have been described previously in detail15. Here, we expended qDF to three channels (TqDF). The set up consisted of an IX71 inverted TIRF research microscope (Olympus America) with a 100 × NA 1.45 plan-apochromatic oil immersion TIRF microscopy objective and 10 mW blue (λ=488 nm), 10 mW yellow-green (λ=561 nm), and 5 mW red (λ=641 nm) diode-pumped solid-state lasers (CVI Melles Griot) as TIRF excitation light sources. Images were captured at a rate of 0.2–1 frames per second using a QV2 (Photometrics) QuadView video coupler and a 16-bit digital charge coupled device camera (Hamamatsu C10600-10B ORCA-R2). The laser shutters and camera were controlled with the SlideBook5.5 software (Intelligent Imaging Innovations). The absorption and emission peaks of the fluorochromes used in this study were, respectively, 493 and 518 nm for DL488, 562 and 576 nm for DL550, 649 and 666 nm for CellMask DeepRed. There was no bleed-through between channels. A TIRF incidence angle of θ=70° was used for all three lasers in all TqDF experiments.
Intravital microscopy
For in vivo imaging of leukocyte rolling and arrest, intravital microscopy of postcapillary venules of the cremaster muscle was performed on five mixed chimeric mice as described before36,41. Anesthesia was induced with an intraperitoneal injection of ketamine hydrochloride (125 mg kg−1) and xylazine (12.5 mg kg−1), and was maintained with inhalation of isoflurane/oxygen gas mixture. The left carotid artery was cannulated with a polyethylene-10 tube (BD) and the left cremaster muscle was exteriorized and prepared for imaging through a × 20 water immersion objective with simultaneous bright-field and epifluorescent imaging, where the wild-type (DsRed) leukocytes appeared bright and the ICAM-1nullICAM-2−/− leukocytes appeared dim. On each cremaster muscle, three different postcapillary venular sites with rolling leukocytes were recorded. After one minute of recording rolling, 600 ng murine CXCL1 was injected through the carotid cannula. The numbers of arrested DsRed wild-type and ICAM-1nullICAM-2−/− leukocytes were counted within 2 min after injections. The arrest counts were normalized by the number of rolling cells before murine-CXCL1 injection and the square of imaged venules.
Δ map and footprint binary images
The distance (Δ) from any region in the neutrophil footprint within ∼200 nm to the total internal reflective interface can be calculated from fluorescence intensity of membrane dye using the equation described previously15. Membrane fluorescence images (Supplementary Fig. 4a) were converted to Δ maps (Supplementary Fig. 4b) that encode Δ as pixel intensity, using the ‘Math' function in FIJI-ImageJ2. The neutrophil footprint binary images were generated from Δ maps by setting a threshold of 95 (the distance to the interface ⩽95 nm, Supplementary Fig. 4c), which excluded the background not associated with the footprint. The footprint outline images (Fig. 1b,c,f, Supplementary Fig. 4d,g and Supplementary Movie 1) were generated from footprint binary images using the ‘Outline' function in FIJI-ImageJ2.
Displacements of the neutrophils and definition of the arrest
The time-lapse footprint binary images were used to compute the cell velocities and displacements (Fig. 2a–d) using ‘TrackMate' in FIJI-ImageJ2. Cell arrest was defined as the time when the velocity dropped below 0.1 μm s−1.
Binary images of integrin clusters
Binary images of integrin clusters (Fig. 1d–f, Fig. 3a, Fig. 4d–f, Fig. 6b, Supplementary Figs 4f,g and 7 and Supplementary Movies 1 and 2) were generated from raw images (Supplementary Figs 4e,5) by using ‘Smart Segmentation' in ImagePro Premier 9.1 (Media Cybernetics), which is widely used for identifying objects65. Smart Segmentation is a pixel classification algorithm66, which uses reference objects to define classes based on pixel intensities. Subsequently, each pixel in the image is analysed and compared with the values of the reference objects and the pixel is assigned to the class of the closest reference object. Different from simple thresholding, Smart Segmentation can identify clusters by comparing each cluster with its local background. Final binary images for integrin clusters (Supplementary Fig. 4g) were prepared by subtracting background noise not associated with neutrophil footprints using ‘image calculator' in FIJI-ImageJ2.
Dual colour binary images of integrin clusters were split into binary images for yellow (E+H+), red (E+H−), and green (E−H+) clusters, respectively. Raw images were masked with the binary clusters and mean fluorescence intensity was quantified using the ‘analyze particles' function in FIJI-ImageJ2. The mean fluorescence intensities (MFI) were normalized by background intensities and highest fluorescence intensities in the recording. Quantification of the KIM127 and mAb24 MFI of yellow (E+H+, Supplementary Fig. 4h), red (E+H−, Supplementary Fig. 4i), or green (E−H+, Supplementary Fig. 4j) clusters demonstrated the accuracy of the cluster binary images generated by ‘Smart Segmentation'. KIM127 and mAb24 MFI for each cluster were plotted against each other to identify the three populations of clusters (Supplementary Fig. 4k). To measure the cluster number (Fig. 2e–t, Fig. 4m, Fig. 6c–f, Supplementary Fig. 6a), total area (Supplementary Fig. 6b) and average size (Supplementary Fig. 6c), the cluster binary images were analysed by ‘analyze particles' in FIJI-ImageJ2.
Colour transition history of the clusters
The cluster binary images were analysed manually to reveal the colour transition history (representing integrin conformation changes) of the clusters. We analysed the E+H+ clusters after cell arrest. 15 clusters, which transitioned from E+H− clusters (Fig. 3b,c), and 15 clusters, which transitioned from E−H+ clusters (Fig. 3d,e), were analysed by acquiring the pixel colours over 4 s. KIM127 and mAb24 fluorescence intensity of individual pixels in these clusters were analysed within these 4 s (Supplementary Fig. 8). In some analyses, 15 arrested cells were selected to reveal their colour transition history of the clusters (Fig. 3f). The colours when the clusters were first observed were defined as their initial colour. In some analyses, the durations of 16 E−H+ clusters each on ICAM-1 blocked or isotype mAb-treated neutrophils were calculated (Fig. 6g,h). The durations were the time from the appearing of the green clusters to appearing of yellow pixels in the clusters.
Creation of 3D reconstructions/footprint topography
Raw CellMask DeepRed qDF images were used to create 3D reconstructions (3D topography, Fig. 4a–f, Supplementary Fig. 9 and Supplementary Movie 2) by custom scripts in Matlab (MathWorks) as described previously15. Hills (microvilli) and valleys (the space between microvilli) were identified from CellMask DeepRed images by using ‘Smart Segmentation' in ImagePro. Hills and valleys were psuedocoloured blue and magenta, respectively, to generate hill-valley maps (Fig. 4c, Supplementary Fig. 9) by custom scripts in Matlab.
3D localization of the clusters
To reveal the 3D localization of the clusters, the cluster binary images were superimposed on the 3D topography (Fig. 4d–f and Supplementary Movie 2) by custom scripts in Matlab. We masked the hill-valley maps and the Δ maps with the yellow (E+H+), red (E+H−), or green (E−H+) cluster areas, respectively, using ‘image calculator' in FIJI-ImageJ2. The hill or valley location of each cluster was analysed by using ‘measure' in FIJI-ImageJ2. Similarly, the Δ of every cluster Fig. 4j–l) was analysed by the ‘analyze particles' function in FIJI-ImageJ2.
Integrin sites measurements
To measure the integrin expression/epitope sites, we used QuantumTM Simply Cellular anti-Rat IgG beads coated with anti-rat IgG antibodies at five known antibody binding capacities. In all, 106 ml−1 human neutrophils were incubated with FcR blocking reagents for 10 min at room temperature, and then incubated with 5 μg ml−1 KIM127, mAb24, which are both mouse anti-human IgG1 isotype, and isotype control, respectively, for 5 min at room temperature. After fixation by 1% PFA on ice for 10 min, the cells were washed twice and incubated with FITC-conjugated rat anti-mouse IgG1 mAb on ice for 30 min. The calibration beads were incubated with the same FITC-conjugated rat anti-mouse IgG1 mAb according to the manufacturer's instruction. After measuring the fluorescence intensity of calibration beads, a linear correlation between the fluorescence intensity and antibody-binding sites was established.
KIM127 and mAb24 epitope sites on the footprint of rolling cells were determined by dividing the number of antibody molecules bound per cell by the cell surface and multiplying by the footprint area. Cell surface area was estimated based on the average diameter of 64 neutrophils captured by microscopy. The fluorescence intensity integral of both KIM127 and mAb24 on the footprint were calculated and converted to KIM127 and mAb24 epitope sites.
FRET assay using flow cytometry
Flow cytometry with compensation (BD FACSDiva) for bleed-through based FRET were used for detecting molecular interaction with high sensitivity35,67. To test whether E−H+ integrin can interact with endogenous ICAM-1 in cis, FRET between H+ (mAb24-550 as acceptor) and ICAM-1 (domain 1 mAb HA58-FITC as donor) was measured. This assay tests the cis interaction of neutrophil ICAM-1 and E−H+ Mac-1, which binds ICAM-1 domain 3. Isolated neutrophils (106 cells ml−1) were incubated with FcR blocking reagents (1:100) for 10 min at room temperature, followed by incubating with 5 μg ml−1 purified isotype control or mAb R6.5, which blocks Mac-1-ICAM-1-binding49, for 20 min at room temperature. Live cells were tested by time-resolved flow cytometry. The 488 nm laser excited the FRET donor HA58-FITC (525/50 nm), which excited the FRET acceptor mAb24-DL550 (575/25 nm).
To quantify the quenching of FRET donor fluorescence, HA58-FITC (2 μg ml−1) was added at 10 s after starting recording, with 3 min recording to reach saturation, followed by adding IL-8 (1 μg ml−1) inducing the mAb24 epitope (mAb24-DL550, 5 μg ml−1, Fig. 5b,d). mAb24-DL550 was replaced by vehicle, non-binding isotype control mAb (IgG1-DL550, 5 μg ml−1) or KIM127-DL550 (5 μg ml−1), respectively, as negative controls. ICAM-1 blocked neutrophils served as control to test whether the blockade of Mac-1-ICAM-1 in cis interaction will eliminate the quenching of FRET donor HA58-FITC.
To quantify the increase in fluorescence of the FRET acceptor, IL-8 and mAb24-DL550 (1.5 μg ml−1) were added at 10 s after starting recording, with 3 min recording to reach saturation, followed by adding HA58-FITC (2 μg ml−1, Fig. 5c,e). HA58-FITC was replaced by vehicle, isotype control mAb (IgG1-FITC, 2 μg ml−1) or anti-CD14-FITC (2 μg ml−1), respectively, as negative controls. ICAM-1 blocked neutrophils served as control to test whether the blockade of Mac-1-ICAM-1 in cis interaction will eliminate the fluorescence increase of FRET acceptor mAb24-DL550.
Distance calculation of the FRET pairs
From the data obtained by the flow cytometry-based FRET assay, we obtain the value of FRET efficiency (E) as
 |
in which F′D and FD is the fluorescence intensity of the FRET donor (FITC) in the presence and in the absence of the FRET acceptor (DL-550), respectively.
E depends on the donor-to-acceptor separation distance r with an inverse sixth power law due to the dipole–dipole coupling mechanism:
 |
in which R0 is the Förster distance of this pair of donor and acceptor, defined as the distance at which the energy transfer efficiency is 50%. R0 depends on the overlap integral of the donor emission spectrum with the acceptor absorption spectrum and their mutual molecular orientation as expressed by the following equation:
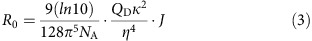 |
in which NA is Avogadro's number; QD is the fluorescence quantum yield of the donor in the absence of acceptor, which is 0.92 of FITC in physiological solutions68; κ2 is the dipole orientation factor (2/3 when both dyes are freely rotating and isotropically oriented); η (refractive Index) of medium is ∼1.338 (ref. 69) and J is the spectral overlap integral of the donor-acceptor pair. The calculation of J was done by using a|e-ultraviolet-Vis-infrared Spectral Software 2.2 (FluorTools, www.fluortools.com). Spectra were obtained from Thermo Fisher Scientific.
Aggregation assay using flow cytometry
To assess whether the cis binding of β2 integrins to ICAMs affects neutrophil aggregation, we performed a flow cytometry based aggregation assay70. We separated isolated neutrophils into two populations labelled with CFSE and CMRA, respectively. Aggregation of a CFSE-labelled neutrophil with a CMRA-labelled neutrophil appears as a double-positive event in flow cytometry. ICAM-1 (10 μg ml−1 HA58 and 10 μg ml−1 R6.5), ICAM-2 (10 μg ml−1 CBR-IC2/2) and ICAM-3 (10 μg ml−1 CBR-IC3/1) were all blocked (room temperature, 30 min) to eliminate the cis binding on CMRA population. In some experiments, β2 integrins on the CFSE population were blocked by 10 μg ml−1 IB4 at room temperature for 30 min.
Statistics
Statistical analysis was performed with Prism 6 (GraphPad). Data (Fig. 2a–d,f–h,j–l,n–p,r–t, Fig. 3b–f, Fig. 4j–l, Fig. 5d,e, Fig. 6c–e,g, Fig. 7a,c–p, Supplementary Figs 2,3,4h–j and 6) are presented as mean±s.e.m. Single data points are presented in some graphs (Fig. 2e–t, Fig. 3f, Fig. 4j–m, Fig. 6c–h, Fig. 7c–p, Supplementary Fig. 2,4h–k and 6). The means for the data sets were compared using paired (Fig. 7p) or unpaired student t-tests with equal variances (n<30, Fig. 2f–h,j–l,n–p, r–t, Fig. 3f, Fig. 4j–l, Fig. 5d,e, Fig. 6c–e,g, Fig. 7c–n, Supplementary Fig. 2) or Chi-square test (n⩾30, Fig. 7o). Log-Gaussian, Gaussian and Lorentizian fits were applied, and the best fit for the data sets were shown in Fig. 6h. Linear regression fits were applied for the data sets in Fig. 2a–d, Fig. 4g–i, Fig. 6f. The slopes of the linear regression for the data sets in Fig. 4g–i were tested against zero and the slopes of the linear regression for the data sets in Fig. 6f were tested against each other using an F-test. P values <0.05 were considered significant. Post hoc analysis (SPSS 22.0 software, IBM) was performed to ensure that the sample size we chose had adequate power.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Additional information
How to cite this article: Fan, Z. et al. Neutrophil recruitment limited by high-affinity bent β2 integrin-binding ligand in cis. Nat. Commun. 7:12658 doi: 10.1038/ncomms12658 (2016).
Supplementary Material
Supplementary Figures 1-12.
Dynamics of β2 integrin extension (KIM127) and headpiece-opening (mAb24) during arrest of primary human neutrophils rolling on P-selectin, ICAM-1 and IL-8: Isolated primary human neutrophils were perfused through the microfluidic chamber (wall shear stress 6 dyn/cm2) coated with P-selectin, ICAM-1 and IL-8. A typical cell rolled for 30 s, then arrested at time = 0 s. Footprints of the cell (see Extended Data Fig. 3a-d) shown in grey outlines. The extended conformation of β2 integrins were identified by DL550 conjugated KIM127 (red), and the open headpiece conformation of β2 integrins were identified by DL488 conjugated mAb24 (green). Binary images; scale bar 5 μm. Clusters classified as E+H- (red), E-H+ (green), and E+>H+ (yellow).
3D distributions of different β2 integrin activation clusters in primary human neutrophils arresting on P-selectin, ICAM-1 and IL-8. Binary images of E+H- (red), E-H+ (green) or E+H+ (yellow) clusters overlaid with 3D topography of neutrophil footprint, derived from membrane fluorescence signal as in Fig. 4a and presented as hills (microvilli) and valleys (space between microvilli). Rotations of 3D topography with clusters at the time of arrest (time = 0) about the flow axis (x).
Acknowledgments
This research was supported by funding from the National Institutes of Health, USA (NIH, HL078784) and WSA postdoctoral fellowship from the American Heart Association, USA (AHA, 16POST31160014). We acknowledge Dr Michael Abadier from the Division of Inflammation Biology, La Jolla Institute for Allergy and Immunology for finding the ICAM-1/2 double knockout mice. We acknowledge Jackie Miller from the Division of Inflammation Biology, La Jolla Institute for Allergy and Immunology for preparing materials for bone marrow transplantation and mouse husbandry. We thank Dr Terry Sasser from Thermo Fisher Scientific for providing the excitation spectrum of DL550.
Footnotes
Author contributions Experiments were designed by Z.F. and K.L. Most of the experiments were performed by Z.F. In vivo cremaster imaging was performed by A.M. Data analysis was performed by Z.F. Image processing was performed by Z.F., S.M. and Z.M. FRET distance calculation was performed by Z.F. and S.M. Some of the custom scripts on MatLab were coded by S.M. The microfluidic device was designed by E.G. and A.G. The bones of ICAM-1/2 double-deficient mice were provided by B.E. and U.D. The manuscript was written by Z.F., K.L. and M.G. The project was supervised by K.L. All authors discussed the results and commented on the manuscript.
References
- Yipp B. G. et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 18, 1386–1393 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. R. et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat. Immunol. 15, 143–151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen M. B. et al. An innate antiviral pathway acting before interferons at epithelial surfaces. Nat. Immunol. 17, 150–158 (2016). [DOI] [PubMed] [Google Scholar]
- Chen F. et al. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat. Immunol. 15, 938–946 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autenrieth S. E. et al. Depletion of dendritic cells enhances innate anti-bacterial host defense through modulation of phagocyte homeostasis. PLoS Pathog. 8, e1002552 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlic J. et al. Regulation of tyrosine kinase activation and granule release through β-arrestin by CXCR1. Nat. Immunol. 1, 227–233 (2000). [DOI] [PubMed] [Google Scholar]
- Moser M. et al. Kindlin-3 is required for β2 integrin-mediated leukocyte adhesion to endothelial cells. Nat. Med. 15, 300–305 (2009). [DOI] [PubMed] [Google Scholar]
- Zimmerli W., Zarth A., Gratwohl A. & Speck B. Neutrophil function and pyogenic infections in bone marrow transplant recipients. Blood 77, 393–399 (1991). [PubMed] [Google Scholar]
- Lammermann T. et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498, 371–375 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps M. et al. Blockade of PI3Kγ suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat. Med. 11, 936–943 (2005). [DOI] [PubMed] [Google Scholar]
- Gunawan M. et al. The methyltransferase Ezh2 controls cell adhesion and migration through direct methylation of the extranuclear regulatory protein talin. Nat. Immunol. 16, 505–516 (2015). [DOI] [PubMed] [Google Scholar]
- Pisitkun P. et al. Interleukin-17 cytokines are critical in development of fatal lupus glomerulonephritis. Immunity 37, 1104–1115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K., Laudanna C., Cybulsky M. I. & Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 (2007). [DOI] [PubMed] [Google Scholar]
- Lawrence M. B. & Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell 65, 859–873 (1991). [DOI] [PubMed] [Google Scholar]
- Sundd P. et al. Quantitative dynamic footprinting microscopy reveals mechanisms of neutrophil rolling. Nat. Methods 7, 821–824 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundd P. et al. ‘Slings' enable neutrophil rolling at high shear. Nature 488, 399–403 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann G., Weber K. S., Zernecke A., Schroder A. & Weber C. JAM-1 is a ligand of the β2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nat. Immunol. 3, 151–158 (2002). [DOI] [PubMed] [Google Scholar]
- Filippi M. D. et al. Localization of Rac2 via the C terminus and aspartic acid 150 specifies superoxide generation, actin polarity and chemotaxis in neutrophils. Nat. Immunol. 5, 744–751 (2004). [DOI] [PubMed] [Google Scholar]
- Schnitzler N., Haase G., Podbielski A., Lutticken R. & Schweizer K. G. A co-stimulatory signal through ICAM-β2 integrin-binding potentiates neutrophil phagocytosis. Nat. Med. 5, 231–235 (1999). [DOI] [PubMed] [Google Scholar]
- von Andrian U. H. et al. Two step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte β2 integrins in vivo. Proc. Natl Acad. Sci. USA 88, 7538–7542 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiVietro J. A. et al. Immobilized IL-8 triggers progressive activation of neutrophils rolling in vitro on P-selectin and intercellular adhesion molecule-1. J. Immunol. 167, 4017–4025 (2001). [DOI] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C. & Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J. Clin. Invest. 83, 2008–2017 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon R. & Feigelson S. W. Chemokine-triggered leukocyte arrest: force-regulated bi-directional integrin activation in quantal adhesive contacts. Curr. Opin. Cell Biol. 24, 670–676 (2012). [DOI] [PubMed] [Google Scholar]
- Laudanna C. & Alon R. Right on the spot. Chemokine triggering of integrin-mediated arrest of rolling leukocytes. Thromb. Haemost. 95, 5–11 (2006). [PubMed] [Google Scholar]
- Luo B. H., Carman C. V. & Springer T. A. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25, 619–647 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z. & Ley K. Leukocyte arrest: biomechanics and molecular mechanisms of β2 integrin activation. Biorheology 52, 353–377 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. K. et al. Antibody against the Leu-CAM b-chain (CD18) promotes both LFA-1-dependent and CR3-dependent adhesion events. J. Immunol. 148, 1080–1085 (1992). [PubMed] [Google Scholar]
- Lu C., Ferzly M., Takagi J. & Springer T. A. Epitope mapping of antibodies to the C-terminal region of the integrin β2 subunit reveals regions that become exposed upon receptor activation. J. Immunol. 166, 5629–5637 (2001). [DOI] [PubMed] [Google Scholar]
- Kamata T. et al. The role of the CPNKEKEC sequence in the β2 subunit I domain in regulation of integrin αLβ2 (LFA-1). J Immunol. 168, 2296–2301 (2002). [DOI] [PubMed] [Google Scholar]
- Lu C., Shimaoka M., Zang Q., Takagi J. & Springer T. A. Locking in alternate conformations of the integrin αLβ2 I domain with disulfide bonds reveals functional relationships among integrin domains. Proc. Natl Acad. Sci .USA 98, 2393–2398 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Shimaoka M., Chen J. & Springer T. A. Activation of integrin β-subunit I-like domains by one-turn C-terminal α-helix deletions. Proc. Natl Acad. Sci .USA 101, 2333–2338 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield I. & Hogg N. Regulated expression of Mg2+ binding epitope on leukocyte integrin α subunits. EMBO J. 8, 3759–3765 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano Y., Spelten O., Zhang H., Ley K. & Zarbock A. Rolling on E- or P-selectin induces the extended but not high-affinity conformation of LFA-1 in neutrophils. Blood 116, 617–624 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A., Lowell C. A. & Ley K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced αLβ2 integrin mediated rolling on intercellular adhesion molecule-1. Immunity 26, 773–783 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtmann A. et al. The PSGL-1-L-selectin signaling complex regulates neutrophil adhesion under flow. J. Exp. Med. 210, 2171–2180 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A., Deem T. L., Burcin T. L. & Ley K. Gαi2 is required for chemokine-induced neutrophil arrest. Blood 110, 3773–3779 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montresor A. et al. JAK tyrosine kinases promote hierarchical activation of Rho and Rap modules of integrin activation. J. Cell Biol. 203, 1003–1019 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarrigue F. et al. A RIAM/lamellipodin-talin-integrin complex forms the tip of sticky fingers that guide cell migration. Nat. Commun. 6, 8492 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. et al. Conformational activation of talin by RIAM triggers integrin-mediated cell adhesion. Nat. Commun. 5, 5880 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro S. et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science 302, 103–106 (2003). [DOI] [PubMed] [Google Scholar]
- Lefort C. T. et al. Distinct roles for talin-1 and kindlin-3 in LFA-1 extension and affinity regulation. Blood 119, 4275–4282 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. D. & Humphries M. J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 3, a004994 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L. & Shaw A. S. Costimulation: building an immunological synapse. Science 283, 649–650 (1999). [DOI] [PubMed] [Google Scholar]
- Bruehl R. E., Springer T. A. & Bainton D. F. Quantitation of L-selectin distribution on human leukocyte microvilli by immunogold labeling and electron microscopy. J. Histochem. Cytochem. 44, 835–844 (1996). [DOI] [PubMed] [Google Scholar]
- Borregaard N. et al. Changes in subcellular localization and surface expression of L-selectin, alkaline phosphatase, and Mac-1 in human neutrophils during stimulation with inflammatory mediators. J. Leukoc. Biol. 56, 80–87 (1994). [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Erickson H. P. & Springer T. A. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell 61, 243–254 (1990). [DOI] [PubMed] [Google Scholar]
- Diamond M. S., Garcia-Aguilar J., Bickford J. K., Corbi A. L. & Springer T. A. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J. Cell Biol. 120, 1031–1043 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard A. L., Igakura T., Tanaka Y., Taylor G. P. & Bangham C. R. Engagement of specific T-cell surface molecules regulates cytoskeletal polarization in HTLV-1-infected lymphocytes. Blood 106, 988–995 (2005). [DOI] [PubMed] [Google Scholar]
- Diamond M. S. et al. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J. Cell Biol. 111, 3129–3139 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfin A. et al. ICAM-1-expressing neutrophils exhibit enhanced effector functions in murine models of endotoxemia. Blood 127, 898–907 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard D. C. et al. Intercellular adhesion molecule-1 expression is required on multiple cell types for the development of experimental autoimmune encephalomyelitis. J. Immunol. 178, 851–857 (2007). [DOI] [PubMed] [Google Scholar]
- Gerwin N. et al. Prolonged eosinophil accumulation in allergic lung interstitium of ICAM-2 deficient mice results in extended hyperresponsiveness. Immunity 10, 9–19 (1999). [DOI] [PubMed] [Google Scholar]
- Adair B. D. et al. Three-dimensional EM structure of the ectodomain of integrin αVβ3 in a complex with fibronectin. J. Cell Biol. 168, 1109–1118 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M., Yuki K. & Springer T. A. An internal ligand-bound, metastable state of a leukocyte integrin, αXβ2. J. Cell Biol. 203, 629–642 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V. et al. The β-tail domain (βTD) regulates physiologic ligand binding to integrin CD11b/CD18. Blood 109, 3513–3520 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A., Singbartl K. & Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J. Clin. Invest. 116, 3211–3219 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschenbaum L. A., Aziz M., Astiz M. E., Saha D. C. & Rackow E. C. Influence of rheologic changes and platelet-neutrophil interactions on cell filtration in sepsis. Am. J. Respir. Crit. Care Med. 161, 1602–1607 (2000). [DOI] [PubMed] [Google Scholar]
- Nijm J., Wikby A., Tompa A., Olsson A. G. & Jonasson L. Circulating levels of proinflammatory cytokines and neutrophil-platelet aggregates in patients with coronary artery disease. Am. J. Cardiol. 95, 452–456 (2005). [DOI] [PubMed] [Google Scholar]
- Ley K., Rivera-Nieves J., Sandborn W. J. & Shattil S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat. Rev. Drug Discov. 15, 173–183 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood L. G. et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat. Immunol. 9, 42–53 (2008). [DOI] [PubMed] [Google Scholar]
- Katagiri K., Maeda A., Shimonaka M. & Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat. Immunol. 4, 741–748 (2003). [DOI] [PubMed] [Google Scholar]
- Auffray C. et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670 (2007). [DOI] [PubMed] [Google Scholar]
- Peer D., Park E. J., Morishita Y., Carman C. V. & Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science 319, 627–630 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorina R., Lyck R., Vestweber D. & Engelhardt B. β2 integrin-mediated crawling on endothelial ICAM-1 and ICAM-2 is a prerequisite for transcellular neutrophil diapedesis across the inflamed blood-brain barrier. J. Immunol. 192, 324–337 (2014). [DOI] [PubMed] [Google Scholar]
- Shaw T. N. et al. Perivascular arrest of CD8+ T Cells is a signature of experimental cerebral malaria. PLoS Pathog. 11, e1005210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. D., Jiang X. H., Sun Y. & Wang J. Color image segmentation: advances and prospects. Pattern Recogn. 34, 2259–2281 (2001). [Google Scholar]
- Chigaev A. et al. FRET detection of lymphocyte function-associated antigen-1 conformational extension. Mol. Biol. Cell 26, 43–54 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming G. R., Knight A. W. E., Morris J. M., Morrison R. J. S. & Robinson G. W. Picosecond fluorescence studies of xanthene dyes. J. Am. Chem. Soc. 99, 4306–4311 (1977). [Google Scholar]
- Chan C. J., Whyte G., Boyde L., Salbreux G. & Guck J. Impact of heating on passive and active biomechanics of suspended cells. Interface Focus 4, 20130069 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelamegham S., Taylor A. D., Shankaran H., Smith C. W. & Simon S. I. Shear and time-dependent changes in Mac-1, LFA-1, and ICAM-3 binding regulate neutrophil homotypic adhesion. J. Immunol. 164, 3798–3805 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-12.
Dynamics of β2 integrin extension (KIM127) and headpiece-opening (mAb24) during arrest of primary human neutrophils rolling on P-selectin, ICAM-1 and IL-8: Isolated primary human neutrophils were perfused through the microfluidic chamber (wall shear stress 6 dyn/cm2) coated with P-selectin, ICAM-1 and IL-8. A typical cell rolled for 30 s, then arrested at time = 0 s. Footprints of the cell (see Extended Data Fig. 3a-d) shown in grey outlines. The extended conformation of β2 integrins were identified by DL550 conjugated KIM127 (red), and the open headpiece conformation of β2 integrins were identified by DL488 conjugated mAb24 (green). Binary images; scale bar 5 μm. Clusters classified as E+H- (red), E-H+ (green), and E+>H+ (yellow).
3D distributions of different β2 integrin activation clusters in primary human neutrophils arresting on P-selectin, ICAM-1 and IL-8. Binary images of E+H- (red), E-H+ (green) or E+H+ (yellow) clusters overlaid with 3D topography of neutrophil footprint, derived from membrane fluorescence signal as in Fig. 4a and presented as hills (microvilli) and valleys (space between microvilli). Rotations of 3D topography with clusters at the time of arrest (time = 0) about the flow axis (x).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.