Abstract
Background and purpose
Preoperative 5-fluorouracil-based chemoradiotherapy is a standard treatment for locally advanced lower rectal cancer (LALRC). We performed a phase I study to develop a new regimen combining irinotecan and S-1.
Materials and methods
Patients with LALRC (T3-4, N0-2) were studied. The radiation dose was 45 Gy in 25 fractions. S-1 (80 mg/m2/day) was administered on days 1–5, 8–12, 22–26, and 29–33. Irinotecan was administered on days 1, 8, 22, and 29. The dose of irinotecan was initially 60 mg/m2 (level 1). Surgery was performed 6–10 weeks after the chemoradiotherapy.
Results
Twenty patients were enrolled, of whom 18 patients were analyzed. Dose-limiting toxicity (DLT) did not occur in the first 3 patients treated with irinotecan at 80 mg/m2 (level 2), but developed in 3 of the 6 patients who received irinotecan at 90 mg/m2 (level 3). Then DLT occurred in 3 other patients at level 2. At level 2 or 3, DLT comprised neutropenia, thrombocytopenia, and diarrhea. Level 2 was designated as the maximum tolerated dose, and level 1 as a recommended dose (RD). The pathological complete response rate was 28%, and the down-staging rate was 56%.
Conclusions
Our results suggested that the RD of irinotecan when combined with preoperative S-1 and pelvic radiation was 60 mg/m2.
Keywords: Neoadjuvant chemoradiotherapy, S-1, Irinotecan, Rectal cancer
Many studies have evaluated the effectiveness of preoperative chemo/radiotherapy in patients with locally advanced rectal cancer because it potentially offers advantages such as tumor shrinkage, lower toxicity than postoperative radiotherapy, and a higher sphincter preserving rate [1]. In a prospective randomized trial from the German Rectal Cancer Study Group, fluorouracil-based preoperative chemoradiotherapy showed improved local control rate and reduced treatment-related toxicities in compared with postoperative chemoradiotherapy for clinical stage II/III rectal cancer, although OS were similar results in both groups [2]. Thus, the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology recommend 5-fluorouracil (5-FU)-based preoperative chemoradiotherapy as a standard treatment option for patients with rectal cancer who have T3, N0 disease, any T, N1-2 disease, or T4 disease [3].
Recently, several randomized controlled trials have been conducted to evaluate the effect of adding oxaliplatin to 5-FU-based regimens for preoperative chemoradiotherapy. However, oxaliplatin has been reported to increase toxicity. Some studies reported that oxaliplatin did not improve tumor response [4], [5], whereas others reported that oxaliplatin improved tumor response [6] and disease-free survival [7]. The development of new regimens for preoperative chemoradiotherapy is thus needed to further enhance treatment response.
S-1 is an oral fluoropyrimidine anticancer agent combining tegafur, a prodrug of 5-FU, with gimeracil and oteracil potassium in a molar ratio of 1:0.4:1. Gimeracil is dihydropyrimidine dehydrogenase (DPD) inhibitor that acts to maintain high levels of 5-FU in plasma and has been suggested to have radiosensitizing activity [8], [9]. Oteracil potassium decreases gastrointestinal toxicity caused by 5-FU.
As for combined chemotherapy with S-1 plus irinotecan (IRIS), a randomized controlled trial (FIRIS study) showed that IRIS is non-inferior to a combination of fluorouracil, folinic acid, and irinotecan (FOLFIRI) in terms of progression-free survival time as second-line therapy for unresectable colorectal cancer [10]. IRIS is expected to become a useful regimen for the management of unresectable colorectal cancer because of several potential benefits, including a shorter infusion time and fewer hospital visits.
In a phase II trial performed by Sato et al., chemoradiotherapy with S-1 plus irinotecan had a histopathological complete response (pCR) rate of 34.7% in patients with locally advanced rectal cancer [11]. However, the clinical target volume for the primary tumor used typically included the perirectal lymph nodes. The target volumes used for radiotherapy in that study were much smaller than those generally used in North American and European practice, in which the internal iliac nodes and often the external iliac nodes were electively irradiated.
To further evaluate the safety and effectiveness of preoperative chemoradiotherapy with S-1 plus irinotecan, we extended the irradiated field to the standard range and performed a multicenter phase I study in patients with locally advanced lower rectal cancer (SAMRAI-1). Our primary purpose was to determine the RD of irinotecan. We also studied whether extension of the radiation field leads to increased toxicity (particularly gastrointestinal toxicity).
Methods and materials
Eligibility criteria
Eligible patients had to satisfy all of the following criteria: (1) a histologically confirmed diagnosis of rectal cancer (adenocarcinoma); (2) resectable clinical stage T3 or T4, N0-2 disease with the primary tumor located either above or below the peritoneal reflection, the inferior tumor margin located distally to the peritoneal reflection, and no enlarged nodules measuring ⩾10 mm in diameter suggesting extramesorectal metastasis on computed tomography (slice width, ⩽5 mm) (i.e., no distinct metastasis to lateral lymph nodes), as confirmed by imaging studies performed within 4 weeks before enrollment; (3) no hepatic, peritoneal, or distant metastasis; (4) an age of 20–80 years at enrollment; (5) no previous treatment; and (6) no severe compromise of main organ functions, with a white cell count of 4000/μL or more and less than 12,000/μL, a platelet count of 100 × 103/μL or more, a hemoglobin level of 9.0 g/dL or more, a total bilirubin level of 1.5 mg/dL or less, aspartate aminotransferase and alanine aminotransferase levels of less than twice the institutional upper limit of normal, a serum creatinine level of less than the institutional upper limit of normal, and an creatinine clearance rate of 50 mL/min or more. Patients also had to have ECOG performance status of 0 or 1 and to be able to orally receive drugs. Written informed consent was obtained from all patients before enrollment.
Exclusion criteria
Patients were excluded from the study if they had any of the following conditions: a history of serious drug hypersensitivity; active double cancer or multiple colorectal cancers; a genotype of UGT1A1*6/*6, UGT1A1*28/*28, or were heterozygous for both (UGT1A1*6/*28); active infection (fever of 38.0 °C or higher); serious complications (e.g., intestinal paralysis or intestinal obstruction); a history of interstitial pneumonia; diarrhea (watery stool); or positive test results for HBs antigen. In patients with a genotype of UGT1A1*6/*6 or UGT1A1*28/*28 or who were heterozygous for both UGT1A1*6/*28, treatment with irinotecan has been reported to possibly cause serious adverse events, especially neutropenia [12]. Therefore, patients with these genotypes were excluded from the present study.
Treatment
Radiotherapy
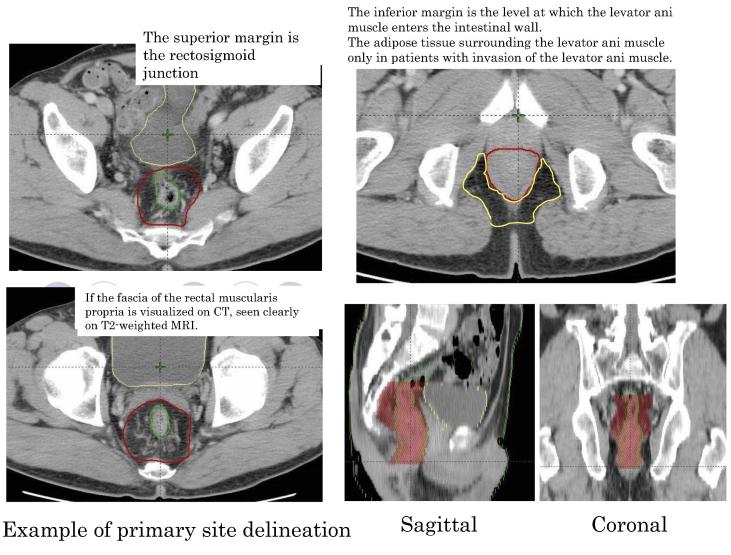
Radiotherapy was delivered with 10 MV X-rays in 1.8 Gy daily standard fractionation for a total dose of 45 Gy. Fig. 1 shows the target volume.
-
(1)
Gross tumor volume
Fig. 1.
An example of primary site delineation.
The gross tumor volume included the primary tumor, enlarged lymph nodes, and suspected sites of tumor invasion of adjacent organs. The primary lesion was evaluated on barium enema examination and MRI. To maintain consistency with the results of a study by Sato et al. [11], an enlarged lymph node with a diameter of 1 cm or more was defined as nodal metastasis in accordance with RECIST 1.0.
-
(2)
Clinical target volume
The clinical target volume was derived by adding a 1 cm margin to the gross tumor volume and included the mesorectal lymph nodes (pararectal lymph nodes), internal iliac lymph nodes, and the obturator lymph nodes.
-
(3)
Planning target volume
The planning target volume was derived by adding a margin of 1 cm to the clinical target volume. The superior margin of the typical irradiation field was defined as the level between the fifth lumbar and first sacral vertebrae. The inferior margin was 3 to 4 cm below the inferior edge of the primary lesion, as defined by a line to the inferior margin of the ischial tuberosity, in principle. Irradiation of the skin of the anal region was avoided as much as possible. The lateral margins were 1 cm lateral to the cavity of the lesser pelvis. The anterior margin was the posterior margin of the pubic symphysis, and the posterior margin was the center of the sacral bone on the lateral view. Completely different subgroups might be intermingled among patients who have enlarged lateral lymph nodes 1 cm or more in diameter. In the present study, we therefore studied patients with no lateral lymph node metastasis and did not perform prophylactic lymph-node dissection, which is commonly done in Japan. Because radiotherapy was given preoperatively, the same radiation dose was delivered to the planned target volume (PTV) in all patients.
The dose was prescribed to the beam isocenter or near to this point with tissue inhomogeneity correction for dose calculation. No tissue inhomogeneity correction was performed if there was too much air in the rectum.
To decrease the intestinal volume included in the treated field, the prone position was recommended for irradiation. To minimize exposure of the small intestine to radiation, the use of a belly board device was recommended, although fixation of body position was not required.
Chemotherapy
The dose of S-1 was determined according to body surface area (BSA) as follows: BSA <1.25 m2, 80 mg/day; BSA ⩾1.25 m2 to <1.5 m2, 100 mg/day; and BSA ⩾1.5 m2, 120 mg/day. S-1 was given orally twice daily after breakfast and dinner for 5 days followed by a 2-day rest, on days 1–5, 8–12, 22–26, and 29–33. Irinotecan was given as a continuous intravenous infusion on days 1, 8, 22, and 29. The first 3 patients received irinotecan in a dose of 60 mg/m2 (level 1). The dose was increased in a stepwise fashion 80 mg/m2 (level 2), 90 mg/m2 (level 3), and 100 mg/m2 (level 4) until the maximum tolerated dose (MTD) was reached. If level 1 met the conditions for the MTD, the dose was reduced to 40 mg/m2 (level 0).
Operation
Surgery was performed at least 6 weeks after the completion of radiotherapy. Patients underwent abdominoperineal resection (APR) or lower anterior resection (LAR). A temporary ileostomy was created in patients who underwent LAR. Total mesorectal excision was performed. Lateral lymph-node dissection was not performed unless distinct metastasis to the lateral lymph nodes was confirmed on imaging studies after completion of the protocol treatment (immediately before surgery) or intraoperatively.
Dose-limiting toxicity (DLT)
Adverse events were evaluated according to the Common Terminology Criteria for Adverse Events, version 3.0. DLT was defined as any of the following events or conditions occurring within 28 days after the completion of chemoradiotherapy: grade 4 hematologic toxicity; grade 3 neutropenia associated with a fever of ⩾38 °C; grade 3 or higher thrombocytopenia, grade 3 or higher nonhematologic toxicity (excluding anorexia nausea, and vomiting); the dose of S-1 was decreased or S-1 was given for 13 days or less because of adverse events; treatment with irinotecan was skipped on days 8, 22, and 29 or the dose of irinotecan was reduced because of adverse events; chemotherapy scheduled for day 22 had to be postponed for 1 week or longer because of adverse events; or radiotherapy had to be discontinued for 1 week or longer because of adverse events.
Dose-escalation scheme
The MTD was defined as the dose level that produced DLT in at least 3 of 6 patients. If DLT occurred in 1 or 2 of the first 3 patients, 3 additional patients were assigned to receive the same dose level. If none of the first 3 patients receiving a given dose level had DLT or if 1 or 2 of 6 patients had DLT, the dose was increased to the next level. Dose escalation was not allowed in the same patient. The dose level immediately below the MTD was considered the recommended dose (RD) for Phase II studies.
Assessment
The primary endpoint of the study was to estimate the MTD and RD of irinotecan. Secondary endpoints were safety (incidences of adverse events and complications), the curative resection (R0) rate, down-staging rate, and pathological complete response (pCR) rate.
The antitumor effects of preoperative chemoradiotherapy were evaluated according to the tumor-node-metastasis classification [13], and the down-staging rate was calculated. Baseline evaluations were performed on imaging studies before the start of chemoradiotherapy. Tumor response was evaluated immediately before surgery at least 4 weeks after the completion of chemoradiotherapy. The histopathological response was also evaluated. In patients with measurable tumors, histological response was graded according to the “General Rules for Clinical and Pathological Studies on Cancer of the Colon, Rectum and Anus (7th Edition), and the results were included in evaluation of the effectiveness of preoperative chemoradiotherapy [14]. These evaluations were performed in each participating hospital. Adverse events were evaluated according to the Common Terminology Criteria for Adverse Events, version 3.0.
Overall survival (OS) was calculated from the time of enrollment to death from any cause or the last follow-up, and relapse-free survival (RFS) was defined as the time from enrollment to recurrence or death. Overall survival and RFS were estimated by using Kaplan–Meier method.
The present study was conducted in accordance with the ethical principles of the Declaration of Helsinki and the Japanese “Ethical Guidelines for Clinical Study” to maximally ensure the human rights, welfare, and safety of the subjects. Written informed consent was obtained from all patients. The protocol was approved by the relevant institutional review boards or ethics committees at each center after a careful review of the ethical and scientific aspects of the study.
Results
From February 2009 through December 2011, a total of 20 patients were enrolled at 7 hospitals. Two patients violated the criteria for dosage and treatment with the study drugs. The other 18 patients were included in data analysis. The clinical characteristics of the patients are shown in Table 1. The subjects ranged in age from 34 to 77 years and comprised 15 men and 3 women. The performance status was 0 in 17 patients and 1 in 1.
Table 1.
Patient characteristics.
| Variable | Total (N = 18) | Level 1 (N = 6) | Level 2 (N = 6) | Level 3 (N = 6) |
|---|---|---|---|---|
| Age | ||||
| Median | 58 | 54 | 60.5 | 57.5 |
| Range | [34–77] | [42–66] | [47–77] | [34–71] |
| Sex | ||||
| Male | 15 | 6 | 4 | 5 |
| Female | 3 | 0 | 2 | 1 |
| ECOG PS | ||||
| 0 | 17 | 5 | 6 | 6 |
| 1 | 1 | 1 | 0 | 0 |
| Tumor site | ||||
| Rab | 5 | 1 | 4 | 0 |
| Rba | 4 | 2 | 0 | 2 |
| Rb | 9 | 3 | 2 | 4 |
| Stage | ||||
| II A | 7 | 4 | 0 | 3 |
| III B | 10 | 2 | 5 | 3 |
| IIIC | 1 | 0 | 1 | 0 |
Abbreviations: Ra denotes the rectum above the peritoneal reflection; Rb, the rectum below the peritoneal reflection; Rab, the rectum above and below peritoneal reflection; and Rba, the rectum below and above peritoneal reflection.
Table 2 summarizes the DLT according to dose level. At level 1 (irinotecan 60 mg/m2), the first patient and third patient had DLT, and 3 additional patients were studied. In total, DLT developed in 2 of the 6 patients, and the dose level was increased to level 2 (irinotecan 80 mg/m2). None of the first 3 patients who received level 2 (irinotecan 60 mg/m2) had DLT, and the dose was increased to level 3 (irinotecan 90 mg/m2). At level 3, DLT developed in the second patient, and 3 patients were additionally studied. In total, 3 of 6 the patients who received level 3 had DLT.
Table 2.
Dose-limiting toxicity.
| Patient No. | Level | No. of patients with DLT | Type of DLT⁎ | Adverse events |
|---|---|---|---|---|
| 1-1 | 1 | 2/6 | (3) | Diarrhea |
| 1-3 | (4–6) | Diarrhea, Leukopenia, Neutropenia | ||
| 2-4 | 2 | 3/6 | (1,2,4,5) | Neutropenia, Leukopenia, Thrombocytopenia |
| 2-5 | (3,7) | Radiation-associated pneumonitis | ||
| 2-6 | (4,5) | Leukopenia, Neutropenia | ||
| 3-2 | 3 | 3/6 | 5) | Neutropenia |
| 3-5 | (4–6) | Leukopenia, Neutropenia | ||
| 3-6 | (3–5) | Diarrhea, Abdominal pain | ||
(1) A fever of 38 °C or higher accompanied by grade 3 neutropenia.
(2) Grade 3 or higher thrombocytopenia.
(3) Grade 3 or higher nonhematologic toxicity (excluding anorexia and nausea/vomiting),
(4) If the dose of S-1 was decreased or the number of days of treatment was less than two thirds of that required by the protocol because of adverse events (i.e., 13 days or less).
(5) If treatment with irinotecan on days 8, 22, or 29 was skipped or the dose was decreased because of adverse events.
(6) If the resumption of chemotherapy on day 22 had to be delayed for 1 week or longer because of adverse events.
(7) If irradiation had to be discontinued for 1 week or longer because of adverse events.
Types of DLT (Definition)
Rules for the dose-escalation scheme: If DLT occurred in at least 3 of 6 patients at a given dose, that dose level was defined as the MTD. According to this definition, dose level 3 was the MTD. However, after the dose was increased from level 1 to level 2, only 3 patients had received level 2. Therefore, 3 additional patients were given level 2. All 3 of these patients had DLT at level 2, and level 2, at which DLT developed in 3 of the 6 patients, was designated as the MTD, and dose level 1 was the RD.
Adverse events developing between the start of treatment and day 28 after the completion of chemoradiotherapy were evaluated (Table 3). Grade 3 or higher adverse events were leukopenia in 3 patients (16.7%), neutropenia in 3 (16.7%), thrombocytopenia in 1 (5.6%), anorexia in 1 (5.6%), and diarrhea in 2 (11.1%). At level 1, the only grade 3 or higher adverse event was diarrhea in 1 patient. At level 2, diarrhea occurred in 2 patients. No patients had grade 3 diarrhea. At level 3, diarrhea occurred in 3 patients. One patient had grade 3 diarrhea. There were no grade 4 adverse events.
Table 3.
Adverse events.
| Adverse events (CTCAE, ver. 3.0) | Total (N = 18) |
Level 1 (N = 6) |
Level 2 (N = 6) |
Level 3 (N = 6) |
||||
|---|---|---|---|---|---|---|---|---|
| Any Grade | G3 | Any Grade | G3 | Any Grade | G3 | Any Grade | G3 | |
| Laboratory findings | ||||||||
| Leukopenia | 17 | 3 | 6 | 5 | 2 | 6 | 1 | |
| Neutropenia | 7 | 3 | 2 | 2 | 2 | 3 | 1 | |
| Thrombocytopenia | 6 | 1 | 2 | 2 | 1 | 2 | ||
| Hemoglobin | 3 | 1 | 2 | |||||
| Total bilirubin | 3 | 3 | ||||||
| Creatinine | 1 | 1 | ||||||
| AST (GOT) | 2 | 1 | 1 | |||||
| ALT (GPT) | 2 | 1 | 1 | |||||
| Clinical findings | ||||||||
| Anorexia | 4 | 1 | 1 | 2 | 1 | 1 | ||
| Radiation-associated dermatitis | 2 | 2 | ||||||
| Diarrhea | 8 | 2 | 3 | 1 | 2 | 3 | 1 | |
| Nausea | 2 | 1 | 1 | |||||
| Vomiting | 2 | 1 | 1 | |||||
| Fatigue | 2 | 2 | ||||||
| Stomatitis/pharyngitis | 1 | 1 | ||||||
| Anal pain | 5 | 3 | 1 | 1 | ||||
| Hyperpigmentation | 1 | 1 | ||||||
| Hiccoughs | 1 | 1 | ||||||
| Alopecia | 1 | 1 | ||||||
Postoperative complications were liver dysfunction in 1 patient at level 1 and bleeding, acute intestinal ischemia, perineal wound infection, and intestinal obstruction in 1 patient each at level 3. The patient with acute intestinal ischemia subsequently reported to have died of treatment-related causes.
The antitumor effectiveness of preoperative chemoradiotherapy was evaluated in all 18 patients. Down-staging was observed in 10 patients (55.6%). Down-staging of T stage was confirmed in 9 patients (50%) and down-staging of N stage in 7 (38.9%). The pCR rate was 27.8%, and the R0 rate was 100% (Table 4).
Table 4.
Tumor response.
| PatientsNo. | Level | Before treatment | → | After treatment | Down Stage | pCR (Grade 3) | R0 resection |
|---|---|---|---|---|---|---|---|
| 1-1 | 1 | T3N1M0 | T2N0M0 | ✓ | ✓ | ||
| 1-2 | 1 | T3N0M0 | T3N0M0 | ✓ | |||
| 1-3 | 1 | T3N0M0 | T3N1M0 | ✓ | |||
| 1-4 | 1 | T3N1M0 | T3N0M0 | ✓ | ✓ | ||
| 1-5 | 1 | T3N0M0 | T0N0M0 | ✓ | ✓ | ✓ | |
| 1-6 | 1 | T3N0M0 | T2N0M0 | ✓ | ✓ | ||
| 2-1 | 2 | T4N1M0 | T3N1M0 | ✓ | |||
| 2-2 | 2 | T3N1M0 | T0N0M0 | ✓ | ✓ | ✓ | |
| 2-3 | 2 | T3N1M0 | T3N2M0 | ✓ | |||
| 2-4 | 2 | T3N1M0 | T3N0M0 | ✓ | ✓ | ||
| 2-5 | 2 | T3N1M0 | T0N0M0 | ✓ | ✓ | ✓ | |
| 2-6 | 2 | T4N2M0 | T2N0M0 | ✓ | ✓ | ||
| 3-1 | 3 | T3N0M0 | T3N1M0 | ✓ | |||
| 3-2 | 3 | T3N1M0 | T3N2M0 | ✓ | |||
| 3-3 | 3 | T3N1M0 | T4N2M0 | ✓ | |||
| 3-4 | 3 | T3N1M0 | T0N0M0 | ✓ | ✓ | ✓ | |
| 3-5 | 3 | T3N0M0 | T3N0M0 | ✓ | |||
| 3-6 | 3 | T3N0M0 | T0N0M0 | ✓ | ✓ | ✓ |
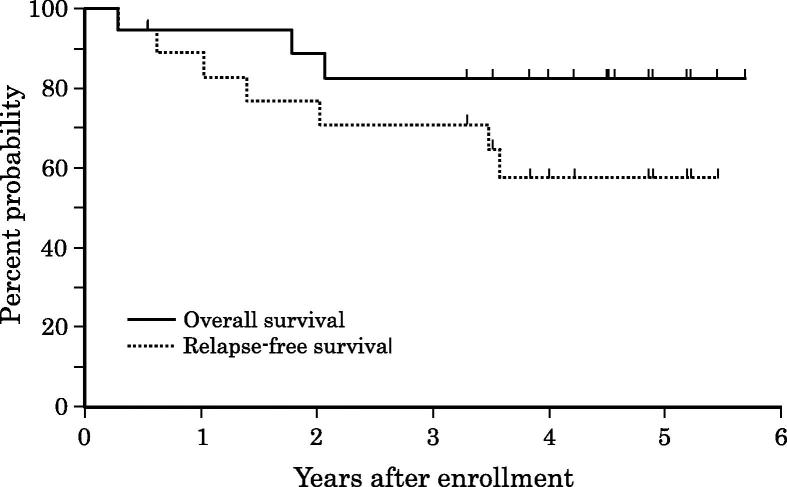
Fig. 2 shows the OS and RFS. As of June 2015, 3 patients (16.7%) died, among whom 2 patients died of recurrence of primary cancer, and 1 died of sepsis and necrosis of the small intestine. Six patients (33.3%) had metastases to the lung (5 patients, 27.8%) or distant lymph nodes (1 patient, 5.6%). After a median follow-up of 54.4 months, the 3-year and 5-year OS rates were both 82.6%, and the 3-year and 5-year RFS rates were 70.8% and 57.2%, respectively.
Fig. 2.
Overall survival (solid line) and relapse-free survival (dotted line).
Discussion
In this phase I study of preoperative chemoradiotherapy with S-1 and irinotecan, the RD of irinotecan was determined to be 60 mg/m2 in patients with locally advanced lower rectal cancer. In patients with a genotype of UGT1A1*6/*6 or UGT1A1*28/*28 or who were heterozygous for both UGT1A1*6/*28, treatment with irinotecan has been reported to possibly cause serious adverse events, especially neutropenia. Therefore, patients with these genotypes were excluded from the present study. Although the RD of irinotecan was determined, caution is required for characteristic adverse events, such as neutropenia and diarrhea. Several clinical trials of chemoradiotherapy with S-1 and irinotecan have been performed in Japan and Korea. A phase I/II study performed by Shin et al. obtained a pCR rate of 21.0% with a regimen combining 70 mg/m2/day of S-1 with 40 mg/m2 of weekly irinotecan [15]. In a single center phase I/II study, Sato et al. obtained a pCR rate of 34.7% with a regimen of 80 mg/m2/day of S-1 plus 80 mg/m2 of irinotecan with same schedule as this study [8]. Furthermore, the 5-year local recurrence-free survival rates were 93% [16]. We obtained a pCR rate of 27.8%, which was consistent with the results of previous studies. On the other hand, in the chemoradiotherapy with fluoropyrimidine and oxaliplatin studies, pCR rate was about 20%, and observed increased toxicity with addition of oxaliplatin [17], [18].
This was a multicenter phase I study of patients who were enrolled at 7 institutions. To prevent differences in radiotherapy among hospitals, a radiotherapy committee was established to clearly define the radiation fields and doses before the study began. The radiotherapy committee was responsible for quality control and quality assurance, ensuring that radiotherapy was administered according to the study protocol. We thereby confirmed that there were no protocol violations, despite minor differences among the participating centers. We confirmed that there were no major differences in the radiation methods or fields between patients with DLT and those without DLT. The relation between radiotherapy and the occurrence of DLT in individual patients appeared to be weak. Although there was only a weak relation of radiotherapy to DLT, our radiation field was larger than that used in the single-center study performed by Sato et al., potentially increasing toxicity at level 2 of irinotecan (80 mg/m2). The larger radiation field might have led to the lower RD of irinotecan (60 mg/m2) as compared with that in single-center studies. In the study by Sato et al. (10), the dose of irinotecan was 80 mg/m2, and all grades of diarrhea occurred in 7 (10.4%) of the 67 patients. Grade 3 or higher diarrhea occurred in 3 patients (4.5%). In this study, at level 2 (dose irinotecan, 80 mg/m2), all grades of diarrhea occurred in 2 (33.3%) of the 6 patients, and no patient had grade 3 or higher diarrhea. We could not conclude that the size of the irradiation field influenced the incidence of diarrhea.
The chemoradiotherapy with S-1 and irinotecan was generally tolerable, and the pCR rate was promising. At present, a phase II study of chemoradiotherapy with S-1 plus irinotecan at the RD of 60 mg/m2 is ongoing to better define the radiation field (SAMRAI-2, UMIN000011115).
Role of the funding source
This study was supported by Non-Profit Organization, the Tokyo Cooperative Oncology Group (TCOG), with funding from Taiho Pharmaceutical Co. Ltd., Japan under the study contract. The above mentioned company had no role in the study design, data collection, data analysis, and data interpretation, or writing of the manuscript.
Ethical considerations
The study has been approved by the appropriate ethical committees of Kitasato University, Japan, and all the other institutions of the authors, in which it was performed and that subjects gave informed consent to the work.
Conflict of interest statement
All authors disclose they have no financial and personal relationships with other people or organizations that could inappropriately influence their work.
Acknowledgments
This study was supported by Non-Profit Organization, the Tokyo Cooperative Oncology Group (TCOG), with funding from Taiho Pharmaceutical Co. Ltd., Japan under the study contract. We thank Medical Network K.K. for review on English expression of this manuscript.
References
- 1.Libutti S.K., Willett C.G., Saltz L.B. Cancer of the rectum. In: Devita V.T., Lawrence T.S., Rosenberg S.A., editors. Cancer: Principles and Practice of Oncology. 9. Williams and Wilkins; Hiladelphia, Lippincott: 2011. pp. 1127–1141. [Google Scholar]
- 2.Sauer R., Liersch T., Merkel S. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 3.National comprehensive cancer network (NCCN) clinical practice guideline in oncology-v.4.2013 (Rectal Cancer): http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.
- 4.Gérard J.P., Azria D., Gourgou-Bourgade S. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012;30:4558–4565. doi: 10.1200/JCO.2012.42.8771. [DOI] [PubMed] [Google Scholar]
- 5.Aschele C., Cionini L., Lonardi S. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773–2780. doi: 10.1200/JCO.2010.34.4911. [DOI] [PubMed] [Google Scholar]
- 6.Rodel C., Liersch T., Becker H. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679–687. doi: 10.1016/S1470-2045(12)70187-0. [DOI] [PubMed] [Google Scholar]
- 7.Rodel C., Graeven U., Fietkau R. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979–989. doi: 10.1016/S1470-2045(15)00159-X. [DOI] [PubMed] [Google Scholar]
- 8.Fukushima M., Ohshimo H., Taguchi T. Combined therapy with radiation and S-1, an oral new 5-FU prodrug, is markedly effective against non-small cell lung cancer xenografts in mice. Eur J Cancer. 2005:343. Suppl 3. [Google Scholar]
- 9.Nakata E., Fukushima M., Takai Y. S-1, an oral fluoropyrimidine, enhances radiation response of DLD-1/FU human colon cancer xenografts resistant to 5-FU. Oncol Rep. 2006;16:465–471. [PubMed] [Google Scholar]
- 10.Muro K., Boku N., Shimada Y. Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study) Lancet Oncol. 2010;11:853–860. doi: 10.1016/S1470-2045(10)70181-9. [DOI] [PubMed] [Google Scholar]
- 11.Sato T., Ozawa H., Hatate K. A Phase II trial of neoadjuvant preoperative chemoradiotherapy with S-1 plus irinotecan and radiation in patients with locally advanced rectal cancer: clinical feasibility and response rate. Int J Radiat Oncol Biol Phys. 2011;79:677–683. doi: 10.1016/j.ijrobp.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Innocenti F., Undevia S.D., Iyer L. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol. 2004;22:1382–1388. doi: 10.1200/JCO.2004.07.173. [DOI] [PubMed] [Google Scholar]
- 13.Sobin L.H., Wittekind C. 6th ed. John Wiley & Sons; Hoboken, New Jersey: 2002. TNM Classification of Malignant Tumours. [Google Scholar]
- 14.Japanese Society for Cancer of the Colon and Rectum . 7th Ed. Kanehara & Co.; Tokyo: 2006. General Rules for Clinical and Pathological Studies on Cancer of the Colon, Rectum and Anus. [Google Scholar]
- 15.Shin S.J., Kim N.K., Keum K.C. Phase II study of preoperative chemoradiotherapy (CRT) with irinotecan plus S-1 in locally advanced rectal cancer. Radiother Oncol. 2010;95:303–307. doi: 10.1016/j.radonc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T., Yamashita K., Sato T., Ema A., Naito M., Watanabe M. Neoadjuvant chemoradiation therapy using concurrent s-1 and irinotecan in rectal cancer: impact on long-term clinical outcomes and prognostic factors. Int J Radiat Oncol Biol Phys. 2014;89:547–555. doi: 10.1016/j.ijrobp.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Gérard J.P., Azria D., Gourgou-Bourgade S. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638–1644. doi: 10.1200/JCO.2009.25.8376. [DOI] [PubMed] [Google Scholar]
- 18.O’Connell M.J., Colangelo L.H., Beart R.W. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from national surgical adjuvant breast and bowel project trial R-04. J Clin Oncol. 2014;32:1927–1934. doi: 10.1200/JCO.2013.53.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]