Abstract
Bipedalism is a key adaptation that shaped human evolution, yet the timing and nature of its evolution remain unclear. Here we use new experimentally based approaches to investigate the locomotor mechanics preserved by the famous Pliocene hominin footprints from Laetoli, Tanzania. We conducted footprint formation experiments with habitually barefoot humans and with chimpanzees to quantitatively compare their footprints to those preserved at Laetoli. Our results show that the Laetoli footprints are morphologically distinct from those of both chimpanzees and habitually barefoot modern humans. By analysing biomechanical data that were collected during the human experiments we, for the first time, directly link differences between the Laetoli and modern human footprints to specific biomechanical variables. We find that the Laetoli hominin probably used a more flexed limb posture at foot strike than modern humans when walking bipedally. The Laetoli footprints provide a clear snapshot of an early hominin bipedal gait that probably involved a limb posture that was slightly but significantly different from our own, and these data support the hypothesis that important evolutionary changes to hominin bipedalism occurred within the past 3.66 Myr.
Keywords: palaeoanthropology, locomotion, biomechanics, Laetoli
1. Background
Bipedalism has long been recognized as one of the primary adaptations that shaped the course of human evolution [1,2]. However, the evolutionary history of hominin bipedalism itself has been the subject of long-standing debate [3]. Some hypotheses have suggested that a human-like form of bipedalism has existed in our clade since the mid-Pliocene [4], while others have argued that the bipedal locomotion of early hominins differed from our own, and that a human-like bipedal gait did not arise until more recently [5–7]. Understanding when and how human-like bipedalism first emerged is important, as this behaviour is certain to have had significant and broad evolutionary consequences.
Discovered in 1977, the ca 3.66 Ma hominin footprints at Laetoli, Tanzania, provided what is still today the earliest indisputable evidence of bipedalism in the human fossil record [8]. These trackways are widely considered to have been made by Australopithecus afarensis, the only hominin taxon recognized from contemporaneous fossil deposits at Laetoli, although some have suggested that they were produced by members of a different hominin taxon that has not yet been discovered [9]. The post-cranial anatomy of A. afarensis is well known, due to a number of fossil discoveries at Hadar, Ethiopia. Yet the Hadar fossils have elicited conflicting conclusions and persistent debates over whether or not the anatomy of A. afarensis offers the earliest evidence of a human-like form of bipedalism [3–6]. Continued analyses of A. afarensis skeletal morphology are unlikely to resolve these debates because of difficulties, uncertainties and long-standing disagreements over how to deduce habitual locomotor patterns from fossils that are rare and fragmentary, and that lack modern functional analogues [3].
The Laetoli footprints circumvent many issues associated with the indirect interpretation of habitual function from skeletal material, because they are direct records of the bipedal locomotion of 3.66 Ma hominins. Therefore, they offer hope for understanding the mechanics of Pliocene hominin locomotion and whether a human-like bipedal gait may have emerged by 3.66 Ma. However, past analyses of the Laetoli tracks have also reached conflicting conclusions [6,9–17]. Some of these disagreements stem from differences in qualitative interpretations [6,9,10,12,16], yet differing results have also been obtained from quantitative approaches [11,13–15,17]. It is notable that most previous quantitative analyses of the Laetoli footprints have entirely lacked data on chimpanzee footprints (or the footprints of any other non-human ape) and thus they have lacked direct evidence to support arguments that the Laetoli tracks could [13] or could not [11,14,15] reflect a bipedal gait that in some ways resembled that of modern African great apes. A study by Kullmer et al. [17] compared a single Laetoli hominin footprint with three modern human tracks and one Gorilla gorilla footprint, and they found that the Laetoli track was distinct from the one formed during normal human bipedal walking and also from the track that the gorilla formed when walking bipedally. Yet the conclusions of this study are limited by the small sample sizes. One study [15] compared plantar pressure records from non-human apes with the Laetoli footprints, although it has since been demonstrated by those same researchers [18] and others [19,20] that pressure distributions are only weakly related to footprint shapes, calling into question the extent to which non-human ape pressure distributions may reflect their footprint topographies. Even more important than the absence of relevant comparative footprint samples though has been the limited knowledge of how specific biomechanical variables are expressed in, and can be inferred from, footprint morphologies. One previous analysis [14] used biomechanical experiments that focused on how one biomechanical variable—lower limb flexion—influences footprint shape. This was accomplished by having humans make tracks under two conditions—when walking normally and when walking with flexed hips and knees. The goal of the experiments was to distinguish these two human experimental conditions and compare them with the Laetoli tracks; it was concluded that the Laetoli print profiles were not compatible with a gait on flexed hind limbs. Aside from this analysis, other studies have taken experimental approaches to analyse the Laetoli footprints, but none have shown directly how biomechanical variables can influence variation in footprint shapes. Ultimately, without the appropriate data to thoroughly understand whether and how certain biomechanical variables drive variation in footprint shape, it is difficult, if not impossible, to confidently derive functional patterns from comparative analyses of fossil hominin footprints.
The approach of the study presented here moves beyond these issues and builds upon previous work on the Laetoli footprints in three important ways. For one, we have conducted the first controlled experimental study of modern chimpanzees creating footprints while walking bipedally, which provides a critical reference sample for evaluating competing hypotheses regarding the functional patterns recorded by the Laetoli footprints. Second, our recent experimental work has involved a thorough evaluation of how biomechanical variables influence the shapes of footprints among habitually barefoot humans [21]. These experiments have provided a robust dataset that offers the basis for us to quantitatively understand in the present study the likely biomechanical causes of particular patterns of footprint shape variation. Third, our comparisons of footprint morphologies and biomechanical inferences are constructed using a novel and quantitatively robust analytical approach. This experiment-based examination provides a new opportunity to quantitatively compare the 3.66 Ma Laetoli footprints with those of modern humans, and to develop new hypotheses about the gait patterns used by the hominins who created them. In doing so, we address important questions about the evolution of hominin bipedalism using the only known direct evidence of Pliocene hominin locomotion.
2. Material and Methods
(a). Human footprint experiments
The experiments that produced the human comparative dataset were conducted in the field at Ileret, Kenya, with 41 habitually barefoot and minimally shod modern humans (15 adult males, 14 adult females, 10 juvenile males, 2 juvenile females) producing a total of 490 footprints, of which 245 were used in this analysis (see below). These experiments followed procedures approved by the George Washington University's Institutional Review Board, and the experimental set-up and procedures have been published in detail elsewhere [20–22]. Briefly, each subject produced footprints in at least 12 trials by walking and running at various speeds across a trackway containing a patch of hydrated sediment that was taken directly from a layer containing 1.5 Ma fossil hominin footprints. Although this sediment differs typologically from the natrocarbonatite ash that preserves the Laetoli hominin prints, there is no evidence to suggest that the slight difference in their grain sizes (the Laetoli ash was closest in size to fine-grained sand [23] while the experimental sediment was a mixture of clayey silt and sand [13]) would influence gross footprint morphology. Furthermore, the experimentally produced and fossil footprints were of similar depths and preserved similar levels of anatomical detail, suggesting that the strength of the two substrates and their cohesive properties are comparable.
In each trial, videos were recorded in a lateral view, and these were later digitized for two-dimensional kinematic analysis. The footprints produced in each trial were photographed, with scale, from a variety of angles and orientations such that high-resolution, scaled three-dimensional models could be rendered using photogrammetry software (PhotoModeler Scanner 2013, Eos Systems Inc., Vancouver, British Columbia, Canada and Agisoft Photoscan, Agisoft LLC, St Petersburg, Russia). Each three-dimensional model was imported into Geomagic Studio 14 (3D Systems, Inc., Rock Hill, SC, USA), and a plane of best fit through the undisturbed ground surface was set as the X–Y plane in coordinate space. Point coordinates were then extracted for each of 14 functionally relevant locations across each footprint that could be consistently and reliably identified (the impressions beneath the medial and lateral heel, medial and lateral midfoot, all metatarsal heads and all toes). All depth measurements were rescaled by unity-based normalization (scaled to the range [0,1]) such that they quantified footprint topography independent of the overall depth of a given footprint.
(b). Chimpanzee footprint experiments
A set of experiments analogous to the human footprint experiments were performed with two chimpanzees in the Primate Locomotion Laboratory at Stony Brook University, in accordance with the policies of the Stony Brook University Institutional Animal Care and Use Committee. Chimpanzees were trained through positive reinforcement to walk bipedally at their preferred speeds across a trackway containing a sample of the same sediment used in the human experiments. Substrate conditions (compaction and hydration levels) were adjusted such that the chimpanzees produced footprints of similar depths to those created in the human experiments, which were in turn similar to the Laetoli prints (average chimpanzee print depth = 1.07 cm, average human print depth = 1.09 cm, average Laetoli print depth = 1.57 cm). After each trial, the footprints they created were photographed in a way that allowed for three-dimensional models to be rendered using the same methods described above. Chimpanzee footprints made during bipedal walking (n1 = 24 and n2 = 21 for the two individuals) were also scaled and oriented, and depths at the same 14 functionally relevant points on each print were measured and rescaled by unity-based normalization, following the same procedures described for the human experiments. Kinematic analyses were not conducted in this particular set of chimpanzee experiments, due to logistical constraints and because bipedal hind limb kinematics of these two chimpanzees are already known from other studies [24].
(c). Laetoli footprint data collection
Because the Laetoli hominin footprints remain buried for conservation purposes, all data were collected from first-generation casts prepared during the site's initial excavation [25] and currently stored at the National Museums of Kenya. Casts of every footprint were not available, but they were available for many of the better-preserved prints from the southern portions of the footprint trackways [26]. The morphology of each was quantified following the same procedure as for the experimentally produced footprints.
As conservative an approach as possible was taken with regard to inclusion of fossil footprints for comparative analysis. The G2/3 trackway was excluded, because it represents at least two sets of superimposed footprints [27], and while new methods have been applied to extract a G3 mean track shape [28], it remains unknown how making a print onto an existing print influences the topography. Thus, this extracted shape is unfortunately not comparable with our chimpanzee and human experimental prints. Within the G1 trackway, footprints were excluded from analyses if their morphology was known to have been damaged in any way by other animals (G1-26, -36, -39) [26], by geological processes (G1-28, -30, -31, -38) [26] or potentially by excavation procedures (G1-37) [12]. This left a total sample of five footprints from the Laetoli G1 trackway (G1-25, -27, -33, -34, -35) which are, according to all published accounts, best preserved and therefore should provide the most accurate records of the locomotor pattern of the Pliocene hominin that produced them.
(d). Statistical analysis
Before directly comparing the morphologies of the Laetoli footprints with those produced by modern humans, an appropriate subset of the human comparative sample had to be selected. The human experimental prints were made at a variety of walking and running speeds, but it is only reasonable to compare those made during locomotor events that were dynamically similar [29] to the event represented by the Laetoli G1 trackway. In such a case, one could reasonably expect similar patterns of external foot function to be represented by the modern human and Laetoli hominin footprints, if indeed their locomotor styles were similar. Using human experimental data, predictions of dimensionless speed (Froude number) were generated for the Laetoli G1 trackway, assuming both human-like and chimpanzee-like footprint : leg length ratios (electronic supplementary material, note S1). These estimates ranged from 0.062 to 0.606. Because bipeds generally transition from a walking to a running gait at Froude numbers close to 0.5 [30,31], it was considered most probable that the Laetoli prints were produced by a walking gait. Therefore, the human comparative dataset was restricted to footprints formed at walking speeds. This left a final sample size of 245 footprints produced by 41 different individuals.
For any human subject who created multiple footprints that met the criteria for inclusion in the comparative sample, footprint coordinates were averaged to produce a mean footprint. The subject averages were averaged themselves to generate an overall mean human footprint that was evenly weighted across subjects. Subject averages were also used to create a between-subject covariance matrix for depth at each of the 14 regions across the footprint.
Quantitative comparison of the Laetoli hominin and modern human footprint morphologies relied only upon z-axis coordinates, in other words the depth profile across the footprints. This approach was chosen because it is already known that the orientation of the hallux in the Laetoli footprints differs from that seen in the footprints of modern people [13] and the consideration of such anatomical variations could obscure purely kinematic or kinetic signals. Because the Laetoli G1 sample consisted of five footprints, a resampling procedure was followed in which five walking footprints were sampled from any one subject in the modern human comparative dataset, and their z-coordinates were averaged to generate a mean walking footprint for that individual. The Mahalanobis distance was then calculated between that sampled mean and the mean footprint from the remainder of the human comparative sample (including all subjects except for the one that was randomly sampled), using the human between-subject covariance matrix. This procedure was repeated for 10 000 iterations (subjects were sampled with replacement, the footprints of a sampled subject were sampled without replacement) to generate a distribution of Mahalanobis distances expected to be sampled from a larger population of modern human footprints.
The coordinates of the Laetoli G1 footprint sample were averaged, and the Mahalanobis distance from the Laetoli G1 mean to the human mean footprint was calculated using the human between-subject covariance matrix. In other words, the Mahalanobis distance between the Laetoli G1 mean footprint and the mean modern human footprint was calculated as if human and A. afarensis footprints were subject to the same patterns of covariance (which would be expected if their patterns of external foot function were similar). The probability was then calculated by sampling a distance at least that large from the resampled modern human dataset.
A slightly different resampling procedure was used to determine how different the footprints of the two chimpanzees were from the human sample. In each iteration, one of the two individuals was selected at random, and five footprints created by that individual were randomly selected, without replacement. The average topography of those five footprints was calculated, and the Mahalanobis distance between the average sampled chimpanzee footprint and the mean human footprint was measured using the human between-subject covariance matrix. This process was repeated 10 000 times to generate a distribution of distances that random chimpanzee footprint samples fell from the average human print. The 95% limits of that distribution were then determined.
Next, between-group principal components (bgPC) analysis [32] was used to quantify the axis of topographic variation that best separated the Laetoli and modern human samples. The aim of this approach is similar to that of a linear discriminant analysis but the method is more appropriate mathematically for cases where the number of variables is high relative to the number of observations [32] (as was the case for the Laetoli footprint sample). Scores along the bgPC axis were extracted for all human and Laetoli footprints. A linear mixed-effects modelling approach was used to evaluate biomechanical effects of variation along the bgPC axis. Using human experimental data [21], bgPC score was designated as the single response variable; hip, knee and ankle angles at both strike and toeoff, and relative medial forefoot pressure (mean peak pressure beneath the foot's transverse axis—first and second metatarsal heads—minus the mean peak pressure beneath the foot's oblique axis—second through fifth metatarsal heads—measured by a plantar pressure pad in the stride prior to footprint creation) were considered as possible explanatory variables due to their prominence in debates over the mechanics of A. afarensis bipedalism [6]. These explanatory variables were all z-standardized prior to model construction. Subject identity was included as a random effect on the intercept.
The process of model selection followed published recommendations [33]. Initially, a model that included all potential explanatory variables was fitted using a maximum-likelihood approach. The summary of the model fit was examined, and the variable with the least significant independent effect was removed. If the removal of this variable had a non-significant effect on the overall model fit (evaluated using ANOVA) and its removal resulted in a decrease in the Akaike information criterion (AIC), it would be kept out of the final best-fit model. The AIC represents a measure of overall model fit that incorporates a balanced weight against the number of explanatory variables included as a way to prevent overfitting of the model to the data at hand. This process was repeated iteratively until it led to a final model that included only variables whose removal would adversely affect overall model fit (in terms of AIC). At this point, walking speed (Froude number) was considered for inclusion as a potential confounding variable in the final best-fit model. Speed had not been included in the initial model because an analysis of variance inflation factors revealed that speed was, unsurprisingly, highly correlated with other biomechanical variables. When speed was added to the final best-fit model, we found that its addition did not improve overall model fit (evaluated using ANOVA), and it resulted in an increase to the AIC. As such, speed was not included in the final best-fit model.
All quantitative analyses utilized custom-written scripts in the R programming language and environment [34]. The bgPC analysis utilized functions available in the ‘Morpho’ package [35].
3. Results and Discussion
The three-dimensional shapes of the Laetoli hominin footprints were quantified and compared with the shapes of a large (n = 245) sample of footprints produced by 41 habitually barefoot modern humans walking at similar speeds and in substrates of similar compliance (figure 1; electronic supplementary material, notes S1 and S2). The topography of the Laetoli footprints was found to be significantly different from the human comparative sample. A resampling procedure showed that the probability of sampling a set of five human footprints with a mean topography as far from the overall human mean (in terms of Mahalanobis distance) as the mean from the five well-preserved footprints within the Laetoli G1 trackway was 0 (figure 2). However, the Laetoli tracks are not as different from those of modern humans as are the prints of modern chimpanzees. The mean topographies from random samples of five chimpanzee footprints were far more distant than were the Laetoli tracks from the average human footprint (95% of chimpanzee samples had D2 distances between 332 and 707, compared with a D2 distance of 109 for the mean Laetoli print).
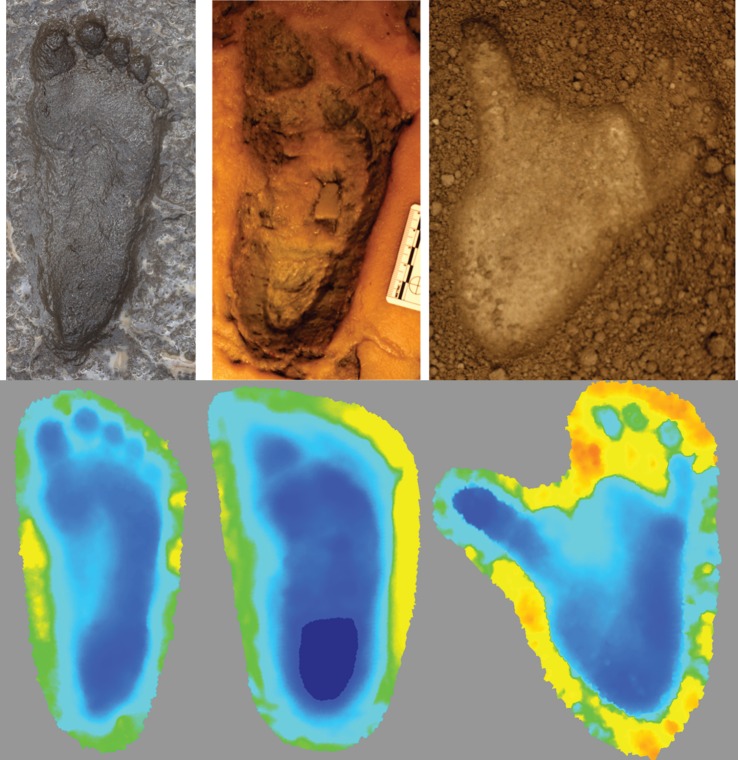
Figure 1.
Examples of human, Laetoli hominin and chimpanzee footprints. These randomly selected footprints demonstrate the ‘stereotypical’ morphologies of, from left to right, human, Laetoli hominin and chimpanzee footprints. The human and chimpanzee prints were produced experimentally in this study. The Laetoli footprint shown here is G1-25. The top row includes standard photographs of these footprints while the bottom row shows depth-coloured maps of the same tracks pictured above, to emphasize their three-dimensional topographies. In the depth-coloured maps, dark blue corresponds to areas of greatest depth and depths become shallower along the gradient from blue to green to yellow to orange. In the absence of colour, areas with darker shading are deeper. Important morphological comparisons described in the main text include the similarity of proportional toe depths (toe relative to heel depths) of the Laetoli and chimpanzee footprints and their distinction from proportional toe depths of modern human footprints, and the intermediate depth of the medial midfoot region in the Laetoli tracks. Images are not set to common scale. (Online version in colour.)
Figure 2.
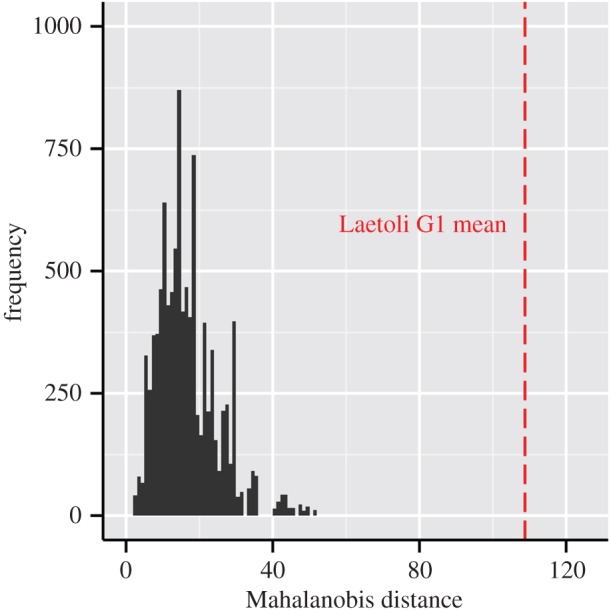
Comparisons of Laetoli hominin and modern human footprints. Histogram of Mahalanobis distances between the mean modern human footprint and the means of 10 000 random samples of human footprints equivalent in number (n = 5) to the Laetoli G1 sample. The human footprint dataset that was resampled included a total of 245 footprints made by 41 different subjects. The vertical dashed line indicates the distance between the Laetoli and human means; the probability of a human sample falling this far from the mean is 0. The interval of Mahalanobis distances in which 95% of chimpanzee footprint samples fall—332 to 707—is not plotted. (Online version in colour.)
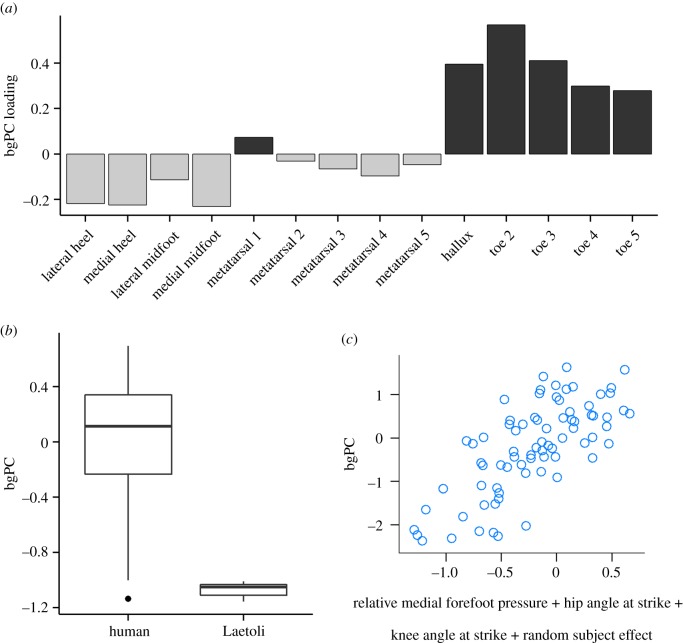
The bgPC analysis [32] was used to quantify a principal component axis of footprint topographic variation that best separated the Laetoli footprints from those of modern humans. The bgPC axis was found to explain approximately 51% of the total topographic variance among all footprints, a substantial amount of between-group (compared with within-group) variance given the relatively large size of the human footprint sample (n = 245 compared with n = 5 for the Laetoli sample). Examinations of the loadings along this bgPC axis and comparisons of the bgPC scores for the Laetoli and modern human footprints showed that the Laetoli sample is characterized by relatively deep impressions beneath the heel and midfoot compared with other parts of the foot, especially the toes (figures 1 and 3).
Figure 3.
Analysis of biomechanical effects that distinguish Laetoli G1 footprints from those of modern humans. (a) Barplot shows loadings of original footprint depth measurements on the Laetoli–modern human bgPC axis. Negative bgPC scores are probably influenced by deep heel and midfoot (especially medial midfoot) impressions. Positive bgPC scores are probably influenced by deep toe impressions. (b) Box plot showing the bgPC scores of the Laetoli G1 (n = 5) and modern human (n = 245) footprint samples. The Laetoli G1 footprints have lower bgPC scores than those of modern humans. (c) Scatterplot to visualize the overall fit of the mixed-effects model found to best explain, within the human dataset, variation along the bgPC axis (n = 78 human experimental observations with all relevant data available). The y-axis plots observed bgPC score, while the x-axis corresponds to fitted bgPC scores predicted from relative medial forefoot pressure, hip angle at foot strike, knee angle at foot strike and a random subject effect. (Online version in colour.)
A linear mixed-effects modelling approach was used to examine within the experimental modern human dataset whether the pattern of footprint variation described by this bgPC axis could be explained by variation in any specific kinematic or kinetic variables (thus testing whether any specific kinematic or kinetic differences may have been likely to have characterized the gait of the Laetoli hominins compared with modern humans). Ultimately, our best-fit model (determined using the AIC) included three fixed effects: flexion of the knee joint at foot strike, flexion of the hip joint at foot strike and the relative pressure on the medial compared with the lateral forefoot (figure 3c). Knee flexion had an independently significant effect (p = 0.0368) while the independent effects of hip angle at foot strike and relative medial forefoot pressure approached independent significance at p = 0.0565 and p = 0.0827, respectively (table 1). The addition of speed (quantified as Froude number) as a potential explanatory variable had a non-significant effect on the overall model fit and increased the value of AIC; it was thus not included in the best-fit model. Therefore, it is unlikely that the differences between the Laetoli and modern human footprints are influenced by differences in walking speed. Instead, the model-fitting results demonstrate that variation along the bgPC axis is most directly influenced by knee flexion at foot strike, with greater amounts of flexion leading to lower values of bgPC scores.
Table 1.
Summary of mixed-effects model describing biomechanical effects on bgPC axis. All fixed effects listed below were included in the overall best-fit model that described variation along the Laetoli–modern human bgPC axis. The p-values describe the significance of the independent effects, given the presence of the other effects in the model.
| value | s.e. | p-value | |
|---|---|---|---|
| intercept | −0.2375 | 0.1328 | 0.0820 |
| relative medial forefoot pressure | −0.2188 | 0.1227 | 0.0827 |
| hip angle at foot strike | −0.3022 | 0.1534 | 0.0565 |
| knee angle at foot strike | 0.3332 | 0.1538 | 0.0368 |
The Laetoli footprints, which are morphologically distinct from those of modern humans (figure 2), show lower bgPC scores than the footprints of modern humans (figure 3b), and therefore point to a bipedal gait that involved a more flexed lower limb posture at foot strike than is typically observed in modern humans. This direct evidence of a bipedal gait that involved relatively more flexed lower limbs concurs with certain inferences derived from Australopithecus skeletal fossils. It has been suggested that the pelvic morphology of A.L. 288-1 (i.e. ‘Lucy’), an A. afarensis partial skeleton from Hadar, Ethiopia, would have affected the orientation of the lesser gluteal muscles in a way that would have compromised balance and stability if that individual were to have walked bipedally with an extended hip joint like humans do [6]. Based on this and other aspects of post-cranial anatomy, those authors proposed that the A.L. 288-1 individual, and other members of A. afarensis, may have instead accommodated such an anatomy by walking bipedally like modern chimpanzees, with a bent-hip–bent-knee (BHBK) gait.
Two recent analyses of the Laetoli footprints have specifically hypothesized that they are inconsistent with a degree of knee and/or hip flexion that exceeds what is seen during modern human bipedalism. One analysis used forward dynamics computer simulations based on human muscle activity data to generate hypothetical pressure distributions beneath a lower limb and foot modelled based on skeletal dimensions of A. afarensis [15]. Based on dissimilarities between these hypothetical pressure distributions and the topography of the Laetoli footprints, the authors concluded that a BHBK gait could not have created the Laetoli prints. A second study showed that the shapes of the Laetoli footprints were significantly different from those of footprints created by humans walking with a strongly BHBK gait [14]. Both of these studies based their interpretations of the Laetoli tracks on the observation that the BHBK footprints in their simulations and experiments were relatively deep under the forefoot and shallow under the heel [14,15]. Here, we directly studied experimentally produced chimpanzee footprints and found that this pattern does not hold. Chimpanzees walking with BHBK bipedal gaits produced footprints with deep heel and generally shallow forefoot impressions (although the impression for the distal hallucal phalanx is relatively deep; figures 1 and 4). Using a post hoc pairwise t-test, with Bonferroni correction, we found that proportional toe depths of chimpanzee footprints and the Laetoli G1 footprints (calculated in the same manner as in previous analyses [14]) were significantly shallower than those observed in modern human footprints (p = 0.039 and p = 0.037, respectively). Meanwhile, the proportional toe depths of the Laetoli G1 footprints did not differ significantly from those of chimpanzee footprints (p = 0.366). The two chimpanzees studied in these experiments walk bipedally with a more flexed lower limb posture at foot strike than modern humans (approx. 45° of hip flexion and 25° of knee flexion [24]). As such, the relatively shallow toe depths of the Laetoli G1 footprints are in fact compatible with a form of bipedalism involving a more flexed limb posture than is observed in the normal gait of modern humans.
Figure 4.
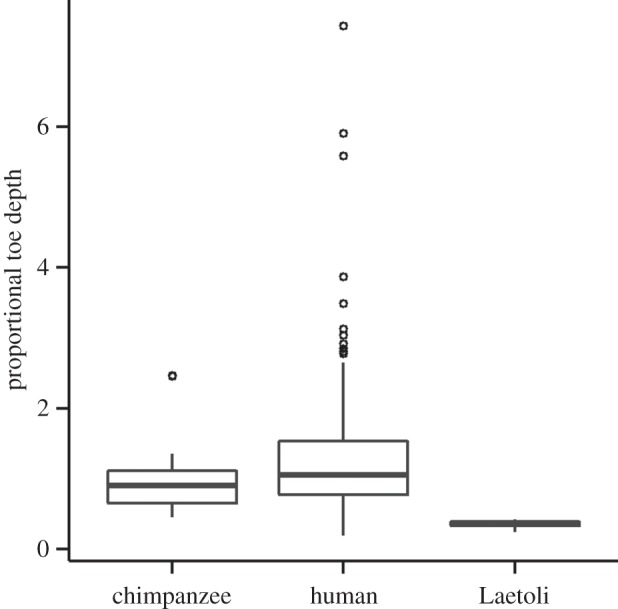
Comparison of proportional toe depths of chimpanzee, human and Laetoli G1 footprints. Proportional toe depth was calculated as maximum depth in the toe region divided by maximum depth in the heel region, following an earlier published study [14]. To aid in visualization of the box plots, three extreme outlying human data points are not included (those proportional toe depths are equal to 10.34, 10.79 and 25.03). Post hoc pairwise t-tests (with Bonferroni correction) show that footprints produced by two chimpanzees (n1 = 24 and n2 = 21) and the Laetoli G1 hominin (n = 5) have significantly shallower proportional toe depths than do the footprints of 41 modern humans (n = 245). The chimpanzee and Laetoli G1 samples do not significantly differ from each other. This result demonstrates that, contrary to previous hypotheses [14,15], the Laetoli G1 footprints are compatible with a more flexed limb posture than is typical of modern humans.
We do not infer a chimpanzee-like limb posture from the Laetoli footprints but rather one that simply involved slightly more knee and hip flexion at touchdown than is typical of modern humans. But even small differences in limb posture could have had important evolutionary implications. If the Laetoli G1 footprints were made by A. afarensis, and members of that taxon typically used a bipedal gait that involved relatively more flexed limbs, then A. afarensis probably would have experienced somewhat higher bipedal locomotor costs compared with modern humans [36–38] despite having similarly long lower limbs [39]. A higher cost of bipedal locomotion relative to modern humans could have had important adaptive consequences for A. afarensis, affecting mobility, home range size and many other aspects of interactions with the environment. This may have been a necessary trade-off, however, if some degree of arboreal locomotion still played an important adaptive role for A. afarensis [6,37,38].
Beyond the results from linear mixed-effects models, direct comparisons of medial midfoot morphology in footprints produced by chimpanzees and habitually barefoot modern humans appear to directly reflect anatomical differences known to exist between human and chimpanzee feet. Specifically, chimpanzees leave deeper impressions than humans beneath the medial midfoot due to their lack of a medial longitudinal arch (figures 1 and 5), and perhaps to some extent due to the mobility of their midfoot during locomotion (i.e. the ‘midfoot’ or ‘midtarsal’ break), contrary to the relative stiffness of the human midfoot [40,41]. The medial midfoot depth of the Laetoli footprints reflects an intermediate longitudinal arch height, being deeper in this region than all but outlying modern human footprints, but also shallower than is seen in most chimpanzee tracks (figures 1 and 5). Similar conclusions have been drawn from past qualitative examinations of the Laetoli footprints [6,16]. Some modern humans may have a relatively flexible midfoot [42–44], but this mobility is typically observed on the lateral side of the foot. It could be more likely that the differences between the Laetoli G1 and modern human medial midfoot impressions are related to a difference in the structure of the medial longitudinal arch. Analyses of the skeletal morphology of the A. afarensis foot have found that the lateral midfoot is quite human-like [45], but the medial midfoot differs from that of modern humans. The shape of the A. afarensis navicular has even been suggested by some to imply an ape-like level of medial midfoot flexibility [46]. Our analysis of the Laetoli footprints does not necessarily imply a mobile medial midfoot, but at the very least suggests that the structure of the medial longitudinal arch of A. afarensis differed from that seen in modern humans.
Figure 5.
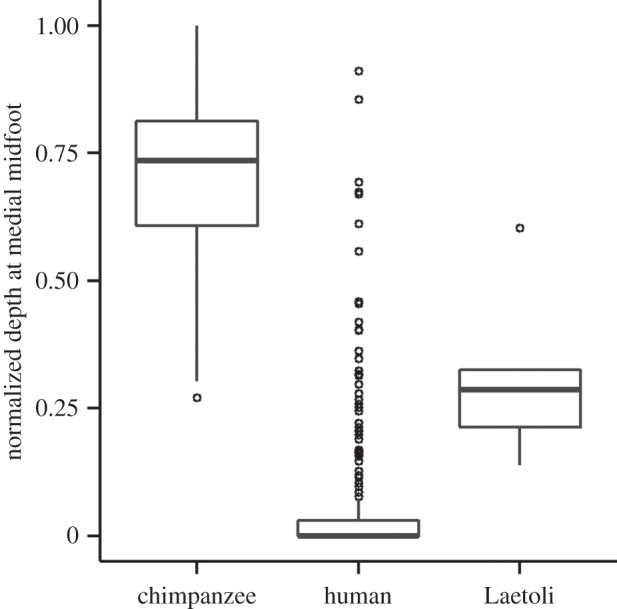
Comparison of medial midfoot depth in chimpanzee, human and Laetoli G1 footprints. Box plot shows the normalized medial midfoot depths of footprints produced by two chimpanzees (n1 = 24 and n2 = 21), 41 modern humans (n = 245) and the Laetoli G1 hominin (n = 5). Post hoc pairwise t-tests, with Bonferroni correction, show that the Laetoli medial midfoot impressions are significantly deeper than humans' (p = 0.001) but still significantly shallower than chimpanzees' (p < 0.0001). There are several outliers in the human sample, due to highly variable midfoot impressions within a few human subjects.
In sum, the functional implications of the Laetoli tracks are consistent with previous interpretations of distinctive anatomy in Australopithecus and provide an emerging picture, based on direct records of locomotor behaviour, of a form of bipedalism in early hominins that differed from that of modern humans. We acknowledge that there are limitations to the conclusions that can be drawn from our experimental approach (electronic supplementary material, note S3). But, based on our data, it appears that relative to the specific pattern of gait seen in modern humans today, the Laetoli footprints show evidence of a distinct gait that involved relatively greater limb flexion at touchdown and potentially a less arched foot. These differences were almost certainly not as dramatic as those that distinguish the bipedal gaits of modern humans and modern chimpanzees, but nonetheless they may have had critical wide-ranging effects on the palaeobiology of the Laetoli hominins.
Ultimately, these results support the hypothesis that the evolution of hominin bipedalism was a process [47] during which slightly but significantly different gait kinematics, kinetics and morphology evolved in different hominin taxa. Regardless of the environmental and evolutionary circumstances that may have surrounded the Laetoli trackmakers, direct evidence from the Laetoli footprints suggests that the Pliocene hominins at Laetoli (probably but not certainly A. afarensis) employed a form of bipedalism that was well developed but not equivalent to that seen in modern humans today. While the post-cranial anatomy required for a well-developed bipedal gait may have emerged at an earlier date and persisted for a long time [7], it remains to be seen when, how and why the specific biomechanics of modern human bipedalism evolved.
Supplementary Material
Acknowledgements
We thank Emma Mbua for providing access to the original casts of the Laetoli footprints at the National Museums of Kenya, and Susan Larson and Kristin Fuehrer for their assistance with chimpanzee footprint experiments. We also thank Matt Tocheri, Bernard Wood and Roshna Wunderlich for comments that improved earlier versions of this manuscript.
Ethics
All human subjects who participated in these experiments gave their informed consent to participate in accordance with procedures approved by The George Washington University's Institutional Review Board. All chimpanzee experiments were conducted in accordance with the policies of the Stony Brook University Institutional Animal Care and Use Committee
Data accessibility
Data related to this manuscript have been deposited with Dryad: http://dx.doi.org/10.5061/dryad.rt0t0. For access to additional data and/or scripts, contact K.G.H. (kevin.g.hatala@gmail.com).
Authors' contributions
K.G.H. and B.G.R. conceived and designed the study. All authors collected the data. K.G.H. analysed the data. All authors interpreted the data and wrote the paper.
Competing interests
We have no competing interests.
Funding
K.G.H. was supported by the Leakey Foundation, the National Science Foundation (BCS-1232522, BCS-1128170, DGE-080163, SMA-1409612), the Wenner-Gren Foundation and the George Washington University's Research Enhancement Fund. B.D. was supported by the National Science Foundation (BCS-0935321). B.G.R. was supported by the National Science Foundation (BCS-1232522, BCS-1128170, BCS-1515054) and the George Washington University's Research Enhancement Fund.
References
- 1.Lamarck JBPA. 1809. Philosophie zoologique. Paris, France: Museum d'Histoire Naturelle. [Google Scholar]
- 2.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: J. Murray. [Google Scholar]
- 3.Ward CV. 2002. Interpreting the posture and locomotion of Australopithecus afarensis: where do we stand? Am. J. Phys. Anthropol. 119, 185–215. ( 10.1002/ajpa.10185) [DOI] [PubMed] [Google Scholar]
- 4.Latimer B, Lovejoy CO. 1989. The calcaneus of Australopithecus afarensis and its implications for the evolution of bipedality. Am. J. Phys. Anthropol. 78, 369–386. ( 10.1002/ajpa.1330780306) [DOI] [PubMed] [Google Scholar]
- 5.Jungers WL. 1982. Lucy's limbs: skeletal allometry and locomotion in Australopithecus afarensis. Nature 297, 676–678. ( 10.1038/297676a0) [DOI] [Google Scholar]
- 6.Stern JT, Susman RL. 1983. The locomotor anatomy of Australopithecus afarensis. Am. J. Phys. Anthropol. 60, 279–317. ( 10.1002/ajpa.1330600302) [DOI] [PubMed] [Google Scholar]
- 7.Richmond BG, Jungers WL. 2008. Orrorin tugenensis femoral morphology and the evolution of hominin bipedalism. Science 319, 1662–1665. ( 10.1126/science.1154197) [DOI] [PubMed] [Google Scholar]
- 8.Leakey MD, Hay RL. 1979. Pliocene footprints in the Laetolil beds at Laetoli, northern Tanzania. Nature 278, 317–323. ( 10.1038/278317a0) [DOI] [Google Scholar]
- 9.Tuttle RH, Webb DM, Baksh M. 1991. Laetoli toes and Australopithecus afarensis. Hum. Evol. 6, 193–200. ( 10.1007/BF02438142) [DOI] [Google Scholar]
- 10.Day MH, Wickens EH. 1980. Laetoli Pliocene hominid footprints and bipedalism. Nature 286, 385–387. ( 10.1038/286385a0) [DOI] [Google Scholar]
- 11.Tuttle RH. 1987. Kinesiological inferences and evolutionary implications from Laetoli bipedal trails G-1, G-2/3, and A. In Laetoli: a Pliocene site in northern Tanzania (eds Leakey MD, Harris JM), pp. 503–520. Oxford, UK: Clarendon Press. [Google Scholar]
- 12.White TD, Suwa G. 1987. Hominid footprints at Laetoli: facts and interpretations. Am. J. Phys. Anthropol. 72, 485–514. ( 10.1002/ajpa.1330720409) [DOI] [PubMed] [Google Scholar]
- 13.Bennett MR, et al. 2009. Early hominin foot morphology based on 1.5-million-year-old footprints from Ileret, Kenya. Science 323, 1197–1201. ( 10.1126/science.1168132) [DOI] [PubMed] [Google Scholar]
- 14.Raichlen DA, Gordon AD, Harcourt-Smith WE, Foster AD, Haas WR. 2010. Laetoli footprints preserve earliest direct evidence of human-like bipedal biomechanics. PLoS ONE 5, e9769 ( 10.1371/journal.pone.0009769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crompton RH, Pataky TC, Savage R, D'Août K, Bennett MR, Day MH, Bates K, Morse S, Sellers WI. 2011. Human-like external function of the foot, and fully upright gait, confirmed in the 3.66 million year old Laetoli hominin footprints by topographic statistics, experimental footprint-formation and computer simulation. J. R. Soc. Interface 9, 707–719. ( 10.1098/rsif.2011.0258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meldrum DJ. 2004. Fossilized Hawaiian footprints compared with Laetoli hominid footprints. In From biped to strider: the emergence of modern human walking, running, and resource transport (eds Meldrum DJ, Hilton CE), pp. 63–83. New York, NY: Kluwer Academic/Plenum Publishers. [Google Scholar]
- 17.Kullmer O, Schrenk F, Dörrhöfer B. 2003. High-resolution 3D-image analysis of ape, hominid and human footprints. Cour. Forsch. Inst. Senckenberg 243, 85–91. [Google Scholar]
- 18.Bates KT, et al. 2013. Does footprint depth correlate with foot motion and pressure? J. R. Soc. Interface 10, 20130009 ( 10.1098/rsif.2013.0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Août K, Meert L, Van Gheluwe B, De Clercq D, Aerts P. 2010. Experimentally generated footprints in sand: analysis and consequences for the interpretation of fossil and forensic footprints. Am. J. Phys. Anthropol. 141, 515–525. [DOI] [PubMed] [Google Scholar]
- 20.Hatala KG, Dingwall HL, Wunderlich RE, Richmond BG. 2013. The relationship between plantar pressure and footprint shape. J. Hum. Evol. 65, 21–28. ( 10.1016/j.jhevol.2013.03.009) [DOI] [PubMed] [Google Scholar]
- 21.Hatala KG, Wunderlich RE, Dingwall HL, Richmond BG. 2016. Interpreting locomotor biomechanics from the morphology of human footprints. J. Hum. Evol. 90, 38–48. ( 10.1016/j.jhevol.2015.08.009) [DOI] [PubMed] [Google Scholar]
- 22.Dingwall HL, Hatala KG, Wunderlich RE, Richmond BG. 2013. Hominin stature, body mass, and walking speed estimates based on 1.5 million-year-old fossil footprints at Ileret, Kenya. J. Hum. Evol. 64, 556–568. ( 10.1016/j.jhevol.2013.02.004) [DOI] [PubMed] [Google Scholar]
- 23.Hay RL. 1987. Geology of the Laetoli area. In Laetoli: a Pliocene site in northern Tanzania (eds Leakey MD, Harris JM), pp. 23–61. Oxford, UK: Clarendon Press. [Google Scholar]
- 24.O'Neill MC, Lee L-F, Demes B, Thompson NE, Larson SG, Stern JT, Umberger BR. 2015. Three-dimensional kinematics of the pelvis and hind limbs in chimpanzee (Pan troglodytes) and human bipedal walking. J. Hum. Evol. 86, 32–42. ( 10.1016/j.jhevol.2015.05.012) [DOI] [PubMed] [Google Scholar]
- 25.Jones PR. 1987. Recording the hominid footprints. In Laetoli: a Pliocene site in northern Tanzania (eds Leakey MD, Harris JM), pp. 551–558. Oxford, UK: Clarendon Press. [Google Scholar]
- 26.Leakey MD. 1987. The hominid footprints: introduction. In Laetoli: a Pliocene site in northern Tanzania (eds Leakey MD, Harris JM), pp. 490–496. Oxford, UK: Clarendon Press. [Google Scholar]
- 27.Leakey MD. 1981. Tracks and tools. Phil. Trans. R. Soc. Lond. B 292, 95–102. ( 10.1098/rstb.1981.0017) [DOI] [Google Scholar]
- 28.Bennett MR, Reynolds SC, Morse SA, Budka M. 2016. Laetoli's lost tracks: 3D generated mean shape and missing footprints. Sci. Reports 6, 21916 ( 10.1038/srep21916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexander RM, Jayes AS. 1983. A dynamic similarity hypothesis for the gaits of quadrupedal mammals. J. Zool. 201, 135–152. ( 10.1111/j.1469-7998.1983.tb04266.x) [DOI] [Google Scholar]
- 30.Alexander RM. 1989. Optimization and gaits in the locomotion of vertebrates. Physiol. Rev. 69, 1199–1227. [DOI] [PubMed] [Google Scholar]
- 31.Gatesy SM, Biewener AA. 1991. Bipedal locomotion: effects of speed, size and limb posture in birds and humans. J. Zool. 224, 127–147. ( 10.1111/j.1469-7998.1991.tb04794.x) [DOI] [Google Scholar]
- 32.Mitteroecker P, Bookstein F. 2011. Linear discrimination, ordination, and the visualization of selection gradients in modern morphometrics. Evol. Biol. 38, 100–114. ( 10.1007/s11692-011-9109-8) [DOI] [Google Scholar]
- 33.Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 34.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 35.Schlager S, Jefferis G. 2015. Package 'Morpho': calculations and visualisations related to geometric morphometrics. Version 2.3.0. See http://cran.r-project.org/web/packages/Morpho/index.html.
- 36.Crompton RH, Weijie LYW, Günther M, Savage R. 1998. The mechanical effectiveness of erect and ‘bent-hip, bent-knee’ bipedal walking in Australopithecus afarensis. J. Hum. Evol. 35, 55–74. ( 10.1006/jhev.1998.0222) [DOI] [PubMed] [Google Scholar]
- 37.Stern JT. 1999. The cost of bent-knee, bent-hip bipedal gait. A reply to Crompton et al. J. Hum. Evol. 36, 567–570. ( 10.1006/jhev.1999.0290) [DOI] [PubMed] [Google Scholar]
- 38.Schmitt D. 2003. Insights into the evolution of human bipedalism from experimental studies of humans and other primates. J. Exp. Biol. 206, 1437–1448. ( 10.1242/jeb.00279) [DOI] [PubMed] [Google Scholar]
- 39.Pontzer H. 2012. Ecological energetics in early Homo. Curr. Anthropol. 53, S346–S358. ( 10.1086/667402) [DOI] [Google Scholar]
- 40.Elftman H, Manter J. 1935. Chimpanzee and human feet in bipedal walking. Am. J. Phys. Anthropol. 20, 69–79. ( 10.1002/ajpa.1330200109) [DOI] [Google Scholar]
- 41.DeSilva JM. 2010. Revisiting the ‘midtarsal’ break. Am. J. Phys. Anthropol. 141, 245–258. ( 10.1002/ajpa.21140). [DOI] [PubMed] [Google Scholar]
- 42.Lundgren P, Nester C, Liu A, Arndt A, Jones R, Stacoff A, Wolf P, Lundberg A. 2008. Invasive in vivo measurement of rear-, mid- and forefoot motion during walking. Gait Posture 28, 93–100. ( 10.1016/j.gaitpost.2007.10.009) [DOI] [PubMed] [Google Scholar]
- 43.DeSilva JM, Gill SV. 2013. Brief communication: a midtarsal (midfoot) break in the human foot. Am. J. Phys. Anthropol. 151, 495–499. ( 10.1002/ajpa.22287) [DOI] [PubMed] [Google Scholar]
- 44.Bates KT, et al. 2013. The evolution of compliance in the human lateral mid-foot. Proc. R. Soc. B 280, 20131818 ( 10.1098/rspb.2013.1818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ward CV, Kimbel WH, Johanson DC. 2011. Complete fourth metatarsal and arches in the foot of Australopithecus afarensis. Science 331, 750–753. ( 10.1126/science.1201463) [DOI] [PubMed] [Google Scholar]
- 46.Harcourt-Smith WE. 2002. Form and function in the hominoid tarsal skeleton. London, UK: University College of London. [Google Scholar]
- 47.Rose MD. 1991. The process of bipedalization in hominids. In Origine(s) de la Bipedie chez les Hominides (eds Coppens Y, Senut B), pp. 37–48. Paris, France: CNRS. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data related to this manuscript have been deposited with Dryad: http://dx.doi.org/10.5061/dryad.rt0t0. For access to additional data and/or scripts, contact K.G.H. (kevin.g.hatala@gmail.com).