Abstract
Adaptive radiations have been hypothesized to contribute broadly to the diversity of organisms. Models of adaptive radiation predict that ecological opportunity and ecological release, the availability of empty ecological niches and the response by adapting lineages to occupy them, respectively, drive patterns of phenotypic and lineage diversification. Adaptive radiations driven by ‘ecological opportunity’ are well established in island systems; it is less clear if ecological opportunity influences continent-wide diversification. We use Neotropical cichlid fishes to test if variation in rates of functional evolution is consistent with changing ecological opportunity. Across a functional morphological axis associated with ram–suction feeding traits, evolutionary rates declined through time as lineages diversified in South America. Evolutionary rates of ram–suction functional morphology also appear to have accelerated as cichlids colonized Central America and encountered renewed opportunity. Our results suggest that ecological opportunity may play an important role in shaping patterns of morphological diversity of even broadly distributed lineages like Neotropical cichlids.
Keywords: morphology, macroevolution, phylogenetics, adaptive radiation, freshwater fish, Cichlidae
1. Introduction
Adaptive radiation is often defined as the rapid divergence of lineages adapting to different ecological niches [1–5]. Classic examples of adaptive radiation include predominantly island-based systems, such as Greater Antilles Anoles [5] and African Rift Lake cichlids [6,7]. However, it has also been postulated that adaptive radiation may operate on a broader geographical, temporal and phylogenetic scale, and contributed substantially to the diversity of species and biological forms worldwide [1,8,9]. Ecological opportunity, the availability of ecological niches within an environment [2,9], is probably a key factor modulating the tempo and mode of diversification within adaptive radiations and perhaps continental scale diversity distributions [3–5,9–11]. Yoder et al. [12] define ecological release as the response (in trait variation, niche width, habitat use, diversification rates, etc.) to new ecological opportunity, and suggest ecological release may drive adaptive radiation [13–15]. Early diversification of lineages and phenotypes is generally interpreted as the result of adaptive radiation in the presence of ecological opportunity [3,4,16]. Nonetheless, disagreement exists in the current literature about the ubiquity of such diversification patterns [17]. In this context, testing for patterns of changing ecological opportunity by examining morphological evolution across varying time and spatial scales, functional complexes and their associated environments becomes essential to our understanding of adaptive radiation as a potential broad scale process.
Neotropical cichlids (subfamily Cichlinae) comprise one of the most species-rich families of freshwater fishes in South and Central America. Cichlinae is estimated to comprise over 600 species within seven tribes [18], with the greater species diversity distributed among the tribes Geophagini, Heroini and Cichlasomatini (electronic supplementary material, figure S1). The largely riverine Cichlinae initially diversified within South America and subsequently heroine lineages colonized Central America, in one or two major dispersal events [19,20], with two non-heroine species having more recently expanded into southern Central America. Secondary freshwater fishes such as cichlids and poecillids are believed to have colonized Central America as early as the Eocene to Late Palaeocene (approx. 30–50 Ma), earlier than presumed Isthmian dispersal of primary freshwater lineages from South America [19,21,22]. Cichlids and poecillids dominate Mesoamerican freshwater communities, in contrast with the much higher diversity of ostariophysan fishes, largely characiformes and siluriformes, in South America [23,24]. This difference among continents has led many to suggest that secondary freshwater fishes such as cichlids and poecillids filled an ‘Ostariophysan vacuum’, with the comparatively depauperate ecosystems of Central America providing new and remarkable opportunities for diversification among these early colonists [21,25–28].
Neotropical cichlids vary dramatically in ecology and morphology and also show parallel adaptations between South and Central American lineages [22,28,29]. Moreover, specialized feeding behaviours common within the major South American cichlid tribe Geophagini (e.g. piscivory, substrate-sifting) and South American ostariophysans (e.g. detritivory), though rare among the species-poor South American heroines, are found among many heroine lineages, such as Parachromis, Astatheros (=Cribroheros) and Paraneetroplus, respectively, in Central America [18,28]. A release from competition with other South American cichlids (especially the species-rich diverse geophagines) and possibly major South American lineages in Ostariophysi may have influenced lineage and phenotypic diversification in Central American heroines after colonization [18,20,27,28].
In contrast to the relatively recent and geographically restricted African Rift Lake cichlid adaptive radiations [7,30–36], Neotropical cichlids provide a system in which to examine whether ecological opportunity and release drive diversification on broader geographical and temporal scales [6,22,37] as well as intrinsically different aquatic ecosystems (i.e. lakes versus rivers). Furthermore, comparative analysis of parallel trajectories of evolution in separate continents, times and ecological contexts may reveal the signal of ecological opportunity and release in the functional evolution of independent cichlid radiations. We used the evolution of feeding functional morphology in Neotropical cichlids [29] to test two key predictions of the ecological theory of adaptive radiation [3,12]: (i) functional diversification should decrease through time in both South American and Central American cichlids, as a result of decreasing ecological opportunity, and (ii) evolutionary rates should increase following the colonization of Central America, as a result of ecological release. This expands on previous analyses of cichlid morphological diversification [22], while incorporating functional traits with direct associations with feeding biomechanics [29]. We find that declining functional diversification in South American cichlids is consistent with a continental adaptive radiation, whereas the subsequent colonization of new habitats was associated with an acceleration of evolutionary rates in Central American lineages.
2. Methods
(a). Neotropical cichlid phylogenetics and functional morphospace
We analysed patterns of evolution in a previously published functional morphospace of Neotropical cichlids [29]. This morphospace was derived from phylogenetic principal components analysis (pPCA) of 10 morphological and biomechanical variables from 75 species representing all major lineages of Cichlinae [29], which were selected to represent as many tribes, genera and ecological variation as possible given specimen availability. These variables included two oral jaw muscle masses (1, adductor mandibulae and 2, sternohyoideus mass); 3, the mass of the lower pharyngeal jaw (fifth ceratobranchial); 4, maximum jaw protrusion distance; 5–6, lower and upper jaw opening and closing mechanical advantages; 7, bite occlusion patterns (quadrate offset); 8–9 oral jaw and hyoid four bar lever transmission coefficients and 10, an index of suction feeding capability [29,38], and see the electronic supplementary material, table S1 for additional details. Data were collected from one to six specimens (housed at or on loan to the Royal Ontario Museum) per species (median = 3; electronic supplementary material, table S6). All size-dependent variables were log-transformed and phylogenetically size-corrected via regression on log body mass [29,39]. Two critical axes of variation were identified using a phylogenetic parallel analysis [29,40] (those axes with eigenvalues higher than randomly generated data), which corresponded primarily (pPC1) to ram–suction feeding morphology (lower jaw lever mechanics and suction index) and secondarily (pPC2) to biting/crushing characteristics (mass of oral jaw muscles and mass of the lower pharyngeal jaw), and see the electronic supplementary material, table S2.
All analyses described below were based on a previously published, time-calibrated multi-locus molecular phylogeny of 154 Neotropical cichlid species and several old-world cichlid outgroup taxa, with three fossil calibrations [18,22]. Both the maximum clade credibility tree (MCC) and a distribution of 1000 randomly sampled chronograms from the posterior distribution of the Markov chain Monte Carlo (MCMC) divergence time analysis (and taken from the supplementary materials of [22]) were used to quantify uncertainty associated with branch-length and topological variation. Comparative analyses were based on the chronograms scaled to relative time (total length = 1, henceforth ‘relative time-trees’), to make parameter estimates comparable across trees.
It is important to note that there remains considerable variation among the published divergence times for Neotropical cichlids, and this phylogeny represents the older extreme of dates available [22,37,41]. However, while the divergence times of Neotropical cichlids are controversial [22,37,41] and likely to vary with increasing molecular, taxonomic and fossil sampling, the Lopez-Fernandez et al. phylogeny [22] remains the most comprehensively sampled multi-locus phylogeny across Cichlinae. Additionally, the phylogenetic placement of two species (Cichlasoma salvini (now Trichromis salvini) and Australoheros facetus) may have been biased by sequence errors in previous studies [18,19,22]. All MCMC and significance tests were repeated with these species excluded with negligible impact on the results (electronic supplementary material, tables S4 and S5, and see BAMM results).
(b). South and Central American biogeography
Cichlid biogeography was reconstructed with stochastic character mapping [42,43], using the ‘make.simmap’ function in the R package ‘phytools’ [44], implementing an asymmetric transition rate matrix (‘make.simmap’ option: model = ‘ARD’), since the colonization of Central America from South America and vice versa may have occurred at different rates [20]. We simulated a stochastic character history on each of 1000 posterior distribution relative time-trees [45].
(c). Node height tests
The ‘node height test’ (NHT) determines whether an significant correlation exists between the absolute value of the standardized independent contrast at each node (a proxy for the rate of evolution [46,47]) and its age. NHTs were carried out using robust regression analysis [48], using functions ‘lmrob’ (R package ‘robustbase’) and ‘pic’ (R package ‘ape’), to test for decreasing rates of morphological evolution in South and Central American cichlids. We modified the NHT by also including a discrete variable for geographical region (South America versus Central America) based on the stochastic character reconstructions for each node (see above). We tested for the significance of an interaction term (time:region) using robust deviance analysis with function ‘anova.lmrob’ in R package ‘robustbase’ (pPC1: χ2 = 0.0932, p = 0.76; pPC2: χ2 = 8.97, p = 0.00274). We determined the significance of each of the regression coefficients [48], by simulating 1000 character histories based on a null, constant-rate model of trait evolution, with or without selective constraint, on both the relative-time MCC and each of the 1000 posterior relative time-trees (electronic supplementary material, tables S3 and S4). Branch lengths were transformed by α (the strength of selective constraint) where an adaptive peak (or multi-peak, with South and Central American biogeography as a mapped character) model was preferred prior to the calculation of independent contrasts [49].
(d). Disparity through time analyses
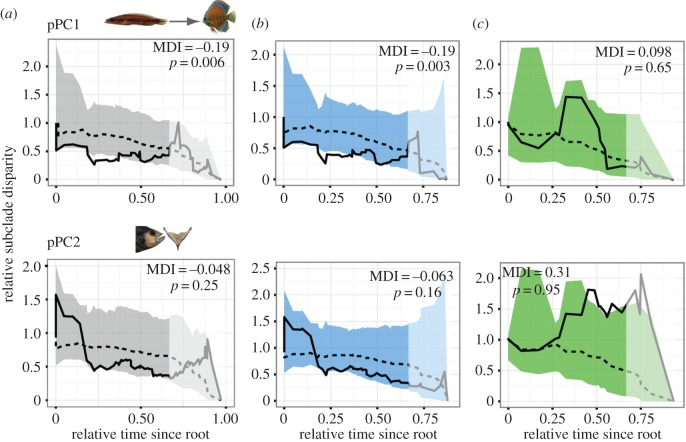
Disparity-through-time (DTT) analyses illustrate how morphological disparity has been partitioned through the evolutionary history of a clade. DTT calculates the average morphological disparity (here the average squared pairwise distance [50,51]) at various points in time, as a fraction of the total disparity of the clade (electronic supplementary material, figure S3). Patterns of phenotypic evolution can be quantified using the morphological disparity index (MDI), calculated as the area between the observed and simulated median disparity curves. Negative MDIs indicate that morphological disparity is more strongly partitioned between subclades than expected (electronic supplementary material, figure S3), an expectation under decreasing ecological opportunity. MDI values and their probability of occurring under different evolutionary expectations were calculated for 1000 simulated character histories for all relative time-trees, based on the preferred constant-rate model of morphological evolution for each axis (i.e. Brownian motion (BM), Ornstein-Uhlenbeck (OU) or multi-peak OU; electronic supplementary material, table S3) [16,22,48,52–54]. The significance of each MDI curve was assessed as the frequency of simulated character histories with lower MDI values. The last third of each time-tree was truncated prior to all calculations to account for incomplete taxonomic sampling [51]. South American species that descended from Central American lineages (electronic supplementary material, figure S4) were excluded from DTT analyses of South American cichlids owing to the inability of DTT to account for internal nodes occurring in Central America.
(e). Bayesian analysis of macroevolutionary mixtures
We used Bayesian analysis of macroevolutionary mixtures to estimate shifts in the rate of phenotypic evolution on pPC1 and pPC2 on the MCC phylogeny, using the program BAMM [55] and associated R package, BAMMtools [56]. As BAMM calculates rates under BM assumptions and both PC axes showed strong evidence for selective constraint (electronic supplementary material, table S3), the MCC phylogeny branch lengths were transformed by α (selective constraint under OU models; electronic supplementary material, table S3), as appropriate to each pPC axis, prior to analysis. Four reversible-jump MCMC chains were run for 10 000 000 generations, sampling every 10 000, with a 10% burn in. We used the default expected number of rate shifts (rate shift parameters = 1, see the electronic supplementary material, figure S10 for the prior and posterior distribution of rate shifts per pPC axis) and used R function ‘setBAMMpriors’ (R package ‘BAMMtools’) [56] to set the prior distributions for ‘betaInitPrior’ (BM rate) and ‘betaInitRootPrior’ (BM rate change) parameters. We used the function ‘credibleShiftSet’ (R package ‘BAMMtools’) to determine the 95% credible set of estimated rate shifts and the most frequently supported rate shift configuration.
3. Results
(a). Ecological opportunity and evolutionary rates
NHT showed a significant decrease in rates of ram–suction morphological evolution (pPC1) through time on the MCC relative time-tree of all 75 Neotropical cichlids (figure 2; electronic supplementary material, table S4 and figure S7), consistent with the hypothesis of decreasing ecological opportunity. Furthermore, NHTs showed a significant increase in rates of ram–suction morphological evolution following the invasion of Central America (figure 2; electronic supplementary material, table S4 and figure S7), resulting in an initial rate of diversification in Central America that was similar to the initial rate observed in South America (figure 2). These significant results were also supported across a Bayesian posterior distribution of 1000 relative time trees (electronic supplementary material, figure S3 and tables S3–S5).
Figure 2.
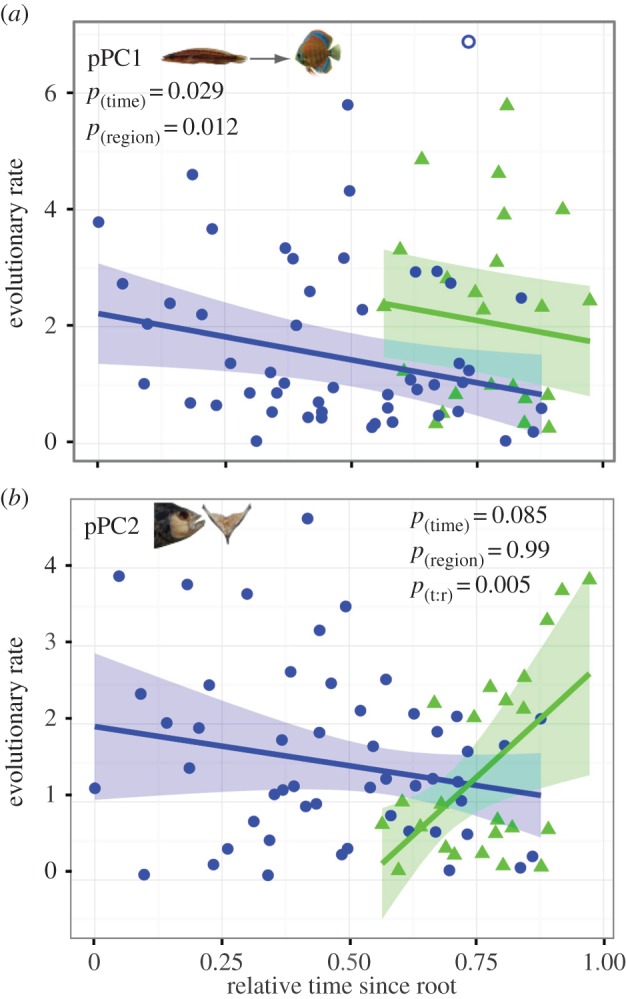
Changes in evolutionary rates of ram–suction morphology in South and Central America ((a) pPC1 and (b) pPC2) on the MCC relative time-tree (electronic supplementary material, figure S1) of 75 species of Neotropical cichlids. Evolutionary rate estimates (absolute value of standardized independent contrasts) through time for South American (blue, circles) and Central American (green, triangles) taxa, including the regression line (shaded region = 95% CI) from the NHT. Outliers are given by an open circle. (Online version in colour.)
Trends in evolutionary rates through time differed between South and Central American cichlids in biting/crushing morphology (pPC2; figure 2; electronic supplementary material, table S4 and figure S4). Based on the NHT of PC2, rates of evolution varied in the opposite direction of our predictions; rates decreased in South America, although this was followed by a sharp increase in rates within the Central American radiation (figure 2). The apparent trend of increasing rates of evolution through time in Central America appears to have been related to two non-overlapping clusters of rate values, those above a rate estimate of approximately 2 and those below approximately 1 (figure 2). High rate estimates in Central American heroines were placed on each of the two main clades. The majority of high rates occurred between Amphilophine taxa and two occurred within the Herichthyines; one between the two species of Herichthys and one at the divergence of a secondarily South American species (Cichlasoma festae, recently revised to Mesoheros festae; electronic supplementary material, figures S1 and S5).
(b). Adaptive partitioning of morphospace
DTT analysis of PC1 (ram–suction/biting morphology) showed low subclade disparities even as early as the divergence of Cichlini + Retroculini from all other Neotropical cichlids (figure 3; electronic supplementary material, figure S1), indicating earlier morphological divergence than expected under a null evolutionary process (electronic supplementary material, table S3). Subclade disparity was particularly low during the time period corresponding to divergence of Geophagini + Chaetobranchini and of Cichlasomatini + Heroini, indicating particularly strong segregation of early lineages in morphological diversity. This significant result was reflected across nearly all of a distribution of 1000 relative time-trees (electronic supplementary material, figure S8 and table S5). Across South American cichlids, DTT analyses resulted in a strongly negative MDI, a significant result supported over nearly all relative time-trees (electronic supplementary material, table S5). Comparatively, Central American heroines exhibited a slightly positive MDI (figure 3) that did not differ significantly from expectations under an OU model of evolution (electronic supplementary material, table S5). DTT analysis of pPC2 did not depart from expectations under a constant rate, two-peak model of evolution (figure 3; electronic supplementary material, table S5 and figure S8).
Figure 3.
DTT analysis of pPC1 and pPC2 of Neotropical cichlid feeding functional morphology. DTT plots generated based on the MCC relative time-tree (electronic supplementary material, figure S1). Solid black lines, observed DTT curve; dashed lines, median simulated DTT curve; shaded region, 95% range of simulated DTT curves. Lighter region shows the area excluded from MDI calculations to account for incomplete taxonomic sampling. (a) Cichlinae, (b) primary South American taxa, (c) Central American taxa. (Online version in colour.)
(c). Bayesian discrete-rate shift analyses
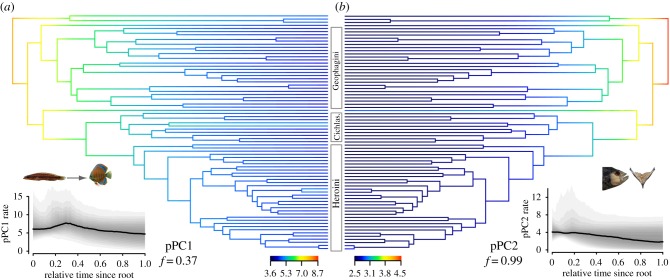
BAMM analyses of Cichlinae functional morphology on the MCC phylogeny supported a model of decreasing rates of diversification through time for both pPC1 and pPC2 (figure 4, frequencies 0.41 and 0.99, respectively, when two species were excluded). The posterior distribution of pPC1 differed from the prior distribution, while pPC2 showed very similar prior and posterior distributions (electronic supplementary material, figure S10).
Figure 4.
Results of BAMM analysis of functional morphology ((a) pPC1 and (b) pPC2) on the MCC phylogeny. Branches are coloured by evolutionary rates estimated under the highest posterior probability (given below each tree) evolutionary rate regime. Inset figures show the BAMM median evolutionary rates from the posterior distribution of BAMM calculated through time, with 95% CI as series of shaded polygons (5% intervals). (Online version in colour.)
Of those shift configurations with elevated per-branch posterior probabilities (prior to posterior odds ratio more than 5), the most frequently supported model in both cases included no internal rate shifts (figure 4), although rate shifts occurred within the 95% credible set for pPC1. Shifts were most common among the South American heroines, especially on branches associated with Symphysodon (model frequency = 0.28, branch-specific marginal odds ratio = 50.9 for Symphysodon, electronic supplementary material, figure S11).
4. Discussion
Under a model of adaptive radiation, ecological opportunity is a limiting factor on the evolution of lineages and adaptive phenotypic traits [1,2,4,5,8,9]. The pPC1 of functional morphology in Neotropical cichlids (figure 1) was previously found to represent a gradient from elongate-bodied fishes with fast oral jaw biomechanics (ram-feeders) to tall-bodied, suction-feeding fishes with high force transmission ([29] and see figure 1c; electronic supplementary material, figure S2). Axes of ram–suction morphology and associated body elongation are ecologically important to fish radiations occurring at varying spatial and temporal scales [33,57]. Along this axis (pPC1), NHT and BAMM analysis showed a decrease in rates of morphological evolution (pPC1) through time (figures 2 and 4), and DTT analyses show early morphological partitioning (figure 3), consistent with a pattern of decreasing ecological opportunity. In combination, DTT, BAMM and NHT analyses indicate that adaptive evolution drove a clade-specific distribution of feeding traits in South American cichlids along the ram–suction gradient of feeding functional morphology.
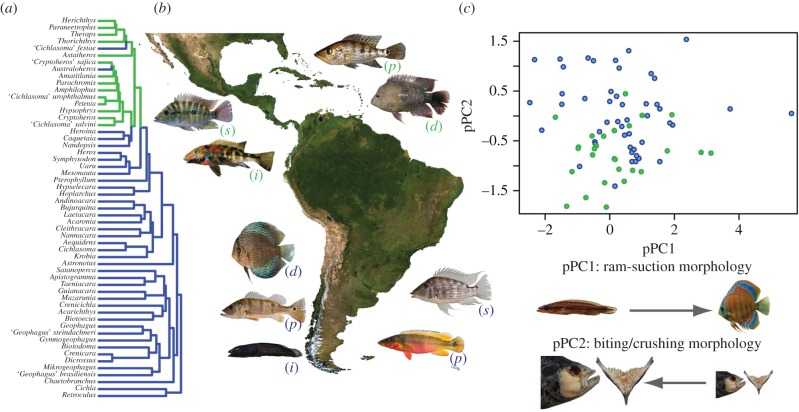
Figure 1.
Diversity and distribution of Neotropical cichlids in South (dark grey/blue) and Central America (light grey/green). (a) Phylogeny of genera represented in this study [18,22]. (b) Examples of convergent ecomorphs (p, piscivore; d, detritivore/algivore; s, substrate sifter; i, rheophilic invertivore) in Neotropical cichlids (clockwise from top = Petenia, Herichthys, Satanoperca, Crenicichla, Teleocichla, Cichla, Symphysodon, Theraps and Astatheros). (c) Phylogenetic PC scores of functional morphology in 75 species of Neotropical cichlid [29]. Morphological variation associated with the positive (right) and negative (left) extremes of each axis illustrated below. Image credits to: Jessica Arbour, Hernán López-Fernández, Michael Tobler. (Online version in colour.)
By contrast, while Central American cichlids show a decrease in rates (NHT; figure 2), no comparable decline in functional disparity is observed (figure 3). This difference between regions suggest that either, South and Central American cichlids evolved under fundamentally different regimes, or that our methods or sampling are not sufficient to detect declining morphological differentiation in Central America. For example, changes to selection on other aspects of the feeding apparatus (see discussion of pPC2 below) following the invasion of Central America may have permitted a longer period of rapid evolution on pPC1. Moreover, while South American cichlids diversified alongside a multitude of ostariophysan clades [58–60], ostariophysans were slow to colonize and diversify in Central America [27]. The absence of both other competing cichlids (particularly Geophagini [22], and see below) and ostariophysans may have allowed newly arrived heroine cichlids in Central American to diversify into niches not readily available to their South American counterparts [28,61]. Additionally, limited inter- or intraspecific sampling could result in incomplete representation of Central American cichlid functional disparity or a bias in evolutionary rate estimates in more recent nodes [5].
Patterns of functional diversification shown here are complementary to previous analyses supporting diversity-dependent models of lineage accumulation in Neotropical cichlids [22]. An analysis of ecomorphological evolution in Cichlinae using simple linear morphometric measurements showed a negative MDI in Geophagini alone, but not in Cichlinae as a whole [22]. Contrastingly, analysis of functionally explicit traits indicated that all South American lineages (i.e. additionally tribes Cichlini, Astronotini, Retroculini, Chaetobranchini, Cichlasomatini and some Heroini) contributed to a pattern of decreasing rates of functional evolution and finer partitioning of morphospace through time in the major clades of South American cichlids, alongside Geophagini [38]. Altogether, current evidence supports an early adaptive radiation of cichlids in South America, but support for an equivalent radiation in Central America remains ambiguous.
Ecological release is associated with increased trait variation resulting from changes in the rate or mode of diversification following, for example, the invasion of a new habitat or the development of a key innovation [12]. NHT of ram–suction morphology (pPC1) showed a significantly increased rate of evolution within the Central American lineages, consistent with ecological release from competing South American cichlids and other South American fish lineages. The estimated initial rate of diversification in South and Central America were similar (figure 2), suggesting that the forces driving diversification (on pPC1) are similar between the two radiations. Conversely, BAMM analyses showed no rate shift among basal Central American lineages (figure 4). Discrete-shift analyses like BAMM may have reduced power to detect evolutionary regimes changes occurring over time or progressively across a number of lineages (see BAMM documentation 3.1 at bamm-project.org). The potential complexity of exchanges between Central and South American cichlid lineages (e.g. multiple invasions of Central America, or the secondary movement of heroines back into South America [19]; electronic supplementary material, figure S4) may obscure a pattern of rate change in discrete-shift analyses, compared to methods such as DTT and NHT. Additionally, the impact of selective constraint and multiple adaptive optima (PC2, which may have been strongly affected by the prior distribution; electronic supplementary material, figure S10, and see [29]) on the estimation of discrete shifts in BAMM analyses has not been studied, and may impact the power to detect macroevolutionary regime shifts.
Functional morphological space overlapped between South and Central American cichlids (figure 1) [29]. Previous analyses also observed convergence in dietary specialization and ecomorphology among cichlid taxa from South and Central America [28], and parallels in multivariate ecomorphological divergence between Central American heroines and South American cichlids [22]. Lineage diversification analyses further identified an increase in net diversification rate within Heroini compared with other Neotropical cichlids, possibly associated with increased speciation following ecological release [12,37]. While ecological release is probably an important determinant in island radiations [1,12,62], we find that, in Neotropical cichlids, release from competition also may have facilitated diversification at a broader geographical scale, contributing to the diversity of forms present in modern Central American lineages.
Evolution on pPC2 showed only weak support for a pattern of decreasing ecological opportunity in Neotropical cichlids; while BAMM showed decreasing evolutionary rates (figure 4), DTT showed no support for such a pattern (across Cichlinae, South or Central American lineages) and NHT suggested only a decrease in South America (figure 2). However, analysis of evolutionary patterns along this axis may have been complicated by differing selective constraints among the South and Central American lineages (electronic supplementary material, table S3). The adaptive peak of Central American taxa favoured larger oral jaw muscles and larger lower pharyngeal jaws (figure 1; electronic supplementary material, table S3). At least some feeding specializations that may require a greater reliance on biting or crushing (such as detritivory, molluscivory, etc.) are more common within the Central American clade and more frequently occupied by non-cichlid fish families in South America [28,63]. Likewise, NHT identified a significant association between patterns in evolutionary rates and geographical region, with rates decreasing in South America, while initially low rates in Central America subsequently increased through time (figure 2). It is possible that this apparent trend of increasing rates in Central America is related to a higher incidence of trophic polymorphism and phenotypic plasticity, which have been observed in both oral and pharyngeal traits in each of these Central American clades [64–67]. Additionally, in at least some amphilophine taxa (e.g. within the genus Amphilophus), the colonization of crater-lake systems has been associated with sympatric divergence and trophic differentiation [68,69]. Nevertheless, these incipient crater-lake adaptive radiations are probably Pleistocene in age [70] and thus have occurred within time frames orders of magnitude younger than any current estimate for the age of Central American cichlid lineages analysed herein.
5. Conclusion
Ecological opportunity has probably been an important factor in the functional evolution of Neotropical cichlids, but its influence has varied geographically. First, we found evidence for an adaptive radiation within the South American cichlids on a multivariate axis of feeding functional morphology (pPC1); functional diversification on pPC1 in South America declined consistently with predictions of changing ecological opportunity. Second, we did not find strong evidence for an independent adaptive radiation in Central America. Nevertheless, Central American cichlids showed increased rates of evolution under NHT, parallel morphological adaptations to those of South American lineages [22,28,29], and increased lineage diversification rates [37], suggesting ecological release from competing fish lineages in South America. Our results add to a growing body of evidence supporting a role for ecological opportunity [11] and adaptive radiations in shaping highly diverse continental assemblies.
Examination of both traditional morphometrics and functionally informed traits suggest that early processes contributed substantially to the distribution of the modern morphological disparity of South American cichlids. Morphological adaptations associated with a number of important ecological roles within this clade were similarly likely to have been established comparatively early in the South American cichlid radiation [22,71]. However, placing these early macroevolutionary events into the context of the historical biogeography of this clade is complicated by the complex hydrological history of South American river systems (e.g. [23,72]). Similarly, while our understanding of the palaeontological context for Cichlinae evolution has been greatly expanded even within the last 10 years [73,74], the early fossil record for this group is limited and geographically restricted. Furthermore, it remains unclear whether mechanisms that drive adaptive radiations of cichlids in lake environments may be at play in similar ways in continent-wide riverine systems. For example, increasing evidence points towards a role for environmental factors such as depth or area [75–77], as well as intrinsic factors such as sexual selection and genomic rearrangements [78,79] as important in the African Great Lakes radiations, but none of these variables has been explored in Neotropical riverine cichlids. Further research into the genetic mechanisms underlying the patterns of morphological disparity in cichlids as well as the historical (biogeographic and palaeontological) context of cichlid evolution will be critical to forming a complete understanding of adaptive radiations within riverine and lacustrine cichlid radiations.
Supplementary Material
Acknowledgements
We are grateful to the members of J.H.A.'s doctoral supervisory committee, P. C. Wainwright (UC Davis), D. Jackson and N. Lovejoy (University of Toronto), and the members of her defense committee, A. Summers (University of Washington) and D. Evans (Royal Ontario Museum). We would also like to acknowledge the support of the ROM Ichthyology curators and staff, E. Holm, M. Burridge, M. Zur and D. Stacey for assistance with the collections. We thank J. Armbruster and D. Werneke (Auburn University Museum), M. Sabaj-Perez and J. Lundberg (Academy of Natural Sciences of Drexel University), R. Reis and C. Lucena (Museu de Zoologia da PUCRS) and R. Rodíles-Hernández (El Colegio de La Frontera Sur) for access to specimens under their care. K. Ilves, F. Hauser, S. Steele, D. Larson and V. Astudillo-Clavijo provided helpful comments on an earlier draft of this manuscript.
Data accessibility
All phylogenies used in this manuscript are available from López-Fernández et al. [22] (http://dx.doi.org/10.5061/dryad.34621) and all trait data are available from Arbour and López-Fernández [29] (http://dx.doi.org/10.5061/dryad.j04r6).
Authors' contributions
J.H.A. collected and analysed data, summarized results and drafted the manuscript. J.H.A. and H.L.F. designed the paper and produced the final version of the paper.
Competing interests
We declare no competing interests.
Funding
The Natural Sciences and Engineering Research Council of Canada funded this research through a Discovery Grant to H.L.F. and a Canada Graduate Scholarship to J.H.A. H.L.F. received additional funding from the Royal Ontario Museum.
References
- 1.Simpson GG. 1953. The major features of evolution. New York, NY: Columbia University Press. [Google Scholar]
- 2.Schluter D. 2000. Ecology of adaptive radiations. New York, NY: Oxford University Press. [Google Scholar]
- 3.Gavrilets S, Losos JB. 2009. Adaptive radiation: contrasting theory with data. Science 323, 732–737. ( 10.1126/science.1157966) [DOI] [PubMed] [Google Scholar]
- 4.Glor RE. 2010. Phylogenetic insights on adaptive radiation. Annu. Rev. Ecol. Evol. Syst. 41, 251–270. ( 10.1146/annurev.ecolsys.39.110707.173447) [DOI] [Google Scholar]
- 5.Mahler DL, Revell LJ, Glor RE, Losos JB. 2010. Ecological opportunity and the rate of morphological evolution in the diversification of Greater Antillean anoles. Evolution 64, 2731–2745. ( 10.1111/j.1558-5646.2010.01026.x) [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T, Koblmüller S. 2011. The adaptive radiation of cichlid fish in Lake Tanganyika: a morphological perspective. Int. J. Evol. Biol. 2011, 1–14. ( 10.4061/2011/620754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seehausen O. 2006. African cichlid fish: a model system in adaptive radiation research. Proc. R. Soc. B 273, 1987–1998. ( 10.1098/rspb.2006.3539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simpson GG. 1944. Tempo and mode in evolution. New York, NY: Columbia University Press. [Google Scholar]
- 9.Losos JB. 2010. Adaptive radiation, ecological opportunity, and evolutionary determinism. Am. Nat. 175, 623–639. ( 10.1086/652433) [DOI] [PubMed] [Google Scholar]
- 10.Schluter D. 1996. Ecological causes of adaptive radiation. Am. Nat. 148, S40–S64. ( 10.1086/285901) [DOI] [Google Scholar]
- 11.Schluter D. 2016. Speciation, ecological opportunity, and latitude. Am. Nat. 187, 1–18. ( 10.1086/684193) [DOI] [PubMed] [Google Scholar]
- 12.Yoder JB, et al. 2010. Ecological opportunity and the origin of adaptive radiations. J. Evol. Biol. 23, 1581–1596. ( 10.1111/j.1420-9101.2010.02029.x) [DOI] [PubMed] [Google Scholar]
- 13.Losos JB, De Queiroz K. 1997. Evolutionary consequences of ecological release in Caribbean Anolis lizards. Biol. J. Linn. Soc. 61, 459–483. ( 10.1111/j.1095-8312.1997.tb01802.x) [DOI] [Google Scholar]
- 14.Des Roches S, Robertson JM, Harmon LJ, Rosenblum EB. 2011. Ecological release in White Sands lizards. Ecol. Evol. 1, 571–578. ( 10.1002/ece3.50) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolnick DI, Ingram T, Stutz WE, Snowberg LK, Lau OL, Paull JS. 2010. Ecological release from interspecific competition leads to decoupled changes in population and individual niche width. Proc. R. Soc. B 277, 1789–1797. ( 10.1098/rspb.2010.0018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slater GJ, Price SA, Santini F, Alfaro ME. 2010. Diversity versus disparity and the radiation of modern cetaceans. Proc. R. Soc. B 277, 3097–3104. ( 10.1098/rspb.2010.0408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harmon LJ, et al. 2010. Early bursts of body size and shape evolution are rare in comparative data. Evolution 64, 2385–2396. ( 10.1111/j.1558-5646.2010.01025.x) [DOI] [PubMed] [Google Scholar]
- 18.López-Fernández H, Winemiller KO, Honeycutt RL. 2010. Multilocus phylogeny and rapid radiations in Neotropical cichlid fishes (Perciformes: Cichlidae: Cichlinae). Mol. Phylogenet. Evol. 55, 1070–1086. ( 10.1016/j.ympev.2010.02.020) [DOI] [PubMed] [Google Scholar]
- 19.Říčan O, Piálek L, Zardoya R, Doadrio I, Zrzavý J. 2013. Biogeography of the Mesoamerican Cichlidae (Teleostei: Heroini): colonization through the GAARlandia land bridge and early diversification. J. Biogeogr. 40, 579–593. ( 10.1111/jbi.12023) [DOI] [Google Scholar]
- 20.Hulsey CD, Hollingsworth PR, Fordyce JA. 2010. Temporal diversification of Central American cichlids. BMC Evol. Biol. 10, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tagliacollo VA, Duke-Sylvester SM, Matamoros WA, Chakrabarty P, Albert JS. In press Coordinated dispersal and pre-Isthmian assembly of the Central American ichthyofauna. Syst. Biol. ( 10.1093/sysbio/syv064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.López-Fernández H, Arbour JH, Winemiller KO, Honeycutt RL. 2013. Testing for ancient adaptive radiations in Neotropical cichlid fishes. Evolution 67, 1321–1337. ( 10.1111/evo.12038) [DOI] [PubMed] [Google Scholar]
- 23.Albert J, Reis RE. 2011. Historical biogeography of neotropical freshwater fishes. Berkeley, CA: University of California Press. [Google Scholar]
- 24.Matamoros WA, Kreiser BR, Schaefer JF. 2012. A delineation of nuclear Middle America biogeographical provinces based on river basin faunistic similarities. Rev. Fish Biol. Fish. 22, 351–365. ( 10.1007/s11160-011-9232-8) [DOI] [Google Scholar]
- 25.Myers GS. 1966. Derivation of the freshwater fish fauna of Central America. Copeia1 4, 766–773. ( 10.2307/1441405) [DOI] [Google Scholar]
- 26.Smith SA, Bermingham E. 2005. The biogeography of lower Mesoamerican freshwater fishes. J. Biogeogr. 32, 1835–1854. ( 10.1111/j.1365-2699.2005.01317.x) [DOI] [Google Scholar]
- 27.Matamoros WA, McMahan CD, Chakrabarty P, Albert JS, Schaefer JF. 2015. Derivation of the freshwater fish fauna of Central America revisited: Myers's hypothesis in the twenty-first century. Cladistics 31, 177–188. ( 10.1111/cla.12081) [DOI] [PubMed] [Google Scholar]
- 28.Winemiller KO, Kelso-Winemiller LC, Brenkert AL. 1995. Ecomorphological diversification and convergence in fluvial cichlid fishes. Environ. Biol. Fishes 44, 235–261. ( 10.1007/BF00005919) [DOI] [Google Scholar]
- 29.Arbour JH, López-Fernández H. 2014. Adaptive landscape and functional diversity of Neotropical cichlids: implications for the ecology and evolution of Cichlinae (Cichlidae; Cichliformes). J. Evol. Biol. 27, 2431–2442. ( 10.1111/jeb.12486) [DOI] [PubMed] [Google Scholar]
- 30.Kocher TD. 2004. Adaptive evolution and explosive speciation: the cichlid fish model. Nat. Rev. Genet. 5, 288–298. ( 10.1038/nrg1316) [DOI] [PubMed] [Google Scholar]
- 31.Kornfield I, Smith PF. 2000. African cichlid fishes: model systems for evolutionary biology. Annu. Rev. Ecol. Syst. 31, 163–196. ( 10.1146/annurev.ecolsys.31.1.163) [DOI] [Google Scholar]
- 32.Stiassny MLJ. 1991. Phylogenetic intrarelationships of the family Cichlidae: an overview. In Cichlid fishes: behaviour, ecology and evolution (ed. Keenleyside MHA.), pp. 1–35. London, UK: Chapman Hall. [Google Scholar]
- 33.Cooper WJ, Parsons K, McIntyre A, Kern B, McGee-Moore A, Albertson RC. 2010. Bentho-pelagic divergence of cichlid feeding architecture was prodigious and consistent during multiple adaptive radiations within African rift-lakes. PLoS ONE 5, e9551 ( 10.1371/journal.pone.0009551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Streelman JT, Danley PD. 2003. The stages of vertebrate evolutionary radiation. Trends Ecol. Evol. 18, 126–131. ( 10.1016/S0169-5347(02)00036-8) [DOI] [Google Scholar]
- 35.Salzburger W, Mack T, Verheyen E, Meyer A. 2005. Out of Tanganyika: genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes. BMC Evol. Biol. 5, 17 ( 10.1186/1471-2148-5-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sturmbauer C. 1998. Explosive speciation in cichlid fishes of the African Great Lakes : a dynamic model of adaptive radiation. J. Fish Biol. 53, 18–36. ( 10.1111/j.1095-8649.1998.tb01015.x) [DOI] [Google Scholar]
- 37.McMahan CD, Chakrabarty P, Sparks JS, Smith WL, Davis MP. 2013. Temporal patterns of diversification across global cichlid biodiversity (Acanthomorpha: Cichlidae). PLoS ONE 8, e71162 ( 10.1371/journal.pone.0071162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arbour JH, López-Fernández H. 2013. Ecological variation in South American geophagine cichlids arose during an early burst of adaptive morphological and functional evolution. Proc. R. Soc. B 280, 20130849 ( 10.1098/rspb.2013.0849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Revell LJ. 2009. Size-correction and principal components for interspecific comparative studies. Evolution 63, 3258–3268. ( 10.1111/j.1558-5646.2009.00804.x) [DOI] [PubMed] [Google Scholar]
- 40.Horn JL. 1965. A rationale and test for the number of factors in factor analysis. Psychometrika 32, 179–185. ( 10.1007/BF02289447) [DOI] [PubMed] [Google Scholar]
- 41.Friedman M, et al. 2013. Molecular and fossil evidence place the origin of cichlid fishes long after Gondwanan rifting. Proc. R. Soc. B 280, 20131733 ( 10.1098/rspb.2013.1733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huelsenbeck JP, Nielsen R, Bollback JP. 2003. Stochastic mapping of morphological characters. Syst. Biol. 52, 131–158. ( 10.1080/10635150390192780) [DOI] [PubMed] [Google Scholar]
- 43.Springer MS, Meredith RW, Janecka JE, Murphy WJ. 2011. The historical biogeography of Mammalia. Phil. Trans. R. Soc. B 366, 2478–2502. ( 10.1098/rstb.2011.0023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 45.Collar DC, Schulte JA II, Losos JB. 2011. Evolution of extreme body size disparity in monitor lizards (Varanus). Evolution 65, 2664–2680. ( 10.1111/j.1558-5646.2011.01335.x) [DOI] [PubMed] [Google Scholar]
- 46.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15. ( 10.1086/284325) [DOI] [Google Scholar]
- 47.McPeek MA. 1995. Testing hypotheses about evolutionary change on single branches of a phylogeny using evolutionary contrasts. Am. Nat. 45, 686–703. ( 10.1086/285763) [DOI] [Google Scholar]
- 48.Slater GJ, Pennell MW. 2014. Robust regression and posterior predictive simulation increase power to detect early bursts of trait evolution. Syst. Biol. 63, 293–308. ( 10.1093/sysbio/syt066) [DOI] [PubMed] [Google Scholar]
- 49.Garland T, Harvey PH, Ives AR. 1992. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 41, 18–32. ( 10.1093/sysbio/41.1.18) [DOI] [Google Scholar]
- 50.Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. 2008. GEIGER: investigating evolutionary radiations. Bioinformatics 24, 129–131. ( 10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- 51.Harmon LJ, Schulte JA, Larson A, Losos JB. 2003. Tempo and mode of evolutionary radiation in iguanian lizards. Science 301, 961–964. ( 10.1126/science.1084786) [DOI] [PubMed] [Google Scholar]
- 52.Brown JM. 2014. Predictive approaches to assessing the fit of evolutionary models. Syst. Biol. 63, 289–292. ( 10.1093/sysbio/syu009) [DOI] [PubMed] [Google Scholar]
- 53.Derryberry EP, Claramunt S, Derryberry G, Chesser RT, Cracraft J, Aleixo A, Pérez-Emán J, Remsen JV Jr, Brumfield RT. 2011. Lineage diversification and morphological evolution in a large-scale continental radiation: the Neotropical ovenbirds and woodcreepers (Aves: Furnariidae). Evolution 65, 2973–2986. ( 10.1111/j.1558-5646.2011.01374.x) [DOI] [PubMed] [Google Scholar]
- 54.Colombo M, Damerau M, Hanel R, Salzburger W, Matschiner M. 2015. Diversity and disparity through time in the adaptive radiation of Antarctic notothenioid fishes. J. Evol. Biol. 28, 376–394. ( 10.1111/jeb.12570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rabosky DL. 2014. Automatic detection of key innovations, rate shifts, and diversity-dependence on phylogenetic trees. PLoS ONE 9, e89543 ( 10.1371/journal.pone.0089543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rabosky DL, Grundler M, Anderson C, Title P, Shi JJ, Brown JW, Huang H, Larson JG. 2014. BAMMtools : an R package for the analysis of evolutionary dynamics on phylogenetic trees. Methods Ecol. Evol. 5, 701–707. ( 10.1111/2041-210X.12199) [DOI] [Google Scholar]
- 57.Claverie T, Wainwright PC. 2014. A morphospace for reef fishes: elongation is the dominant axis of body shape evolution. PLoS ONE 9, e112732 ( 10.1371/journal.pone.0112732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiss FE, Malabarba LR, Malabarba MC. 2012. Phylogenetic relationships of Paleotetra, a new characiform fish (Ostariophysi) with two new species from the Eocene-Oligocene of south-eastern Brazil. J. Syst. Palaeontol. 10, 73–86. ( 10.1080/14772019.2011.565082) [DOI] [Google Scholar]
- 59.Malabarba MC, Lundberg JG. 2007. A fossil loricariid catfish (Siluriformes : Loricarioidea) from the Taubaté Basin, eastern Brazil. Neotrop. Ichthyol. 5, 263–270. ( 10.1590/S1679-62252007000300005) [DOI] [Google Scholar]
- 60.López-Fernández H, Albert J. 2011. Paleogene radiations. In Historical biogeography of Neotropical freshwater fishes (ed. Reis RE.), pp. 105–117. Berkeley, CA: University of California Press. [Google Scholar]
- 61.Winemiller KO. 1991. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol. Monogr. 61, 343–365. ( 10.2307/2937046) [DOI] [Google Scholar]
- 62.Seehausen O. 2007. Evolution and ecological theory: chance, historical contingency and ecological determinism jointly determine the rate of adaptive radiation. Heredity (Edinb). 99, 361–363. ( 10.1038/sj.hdy.6801047) [DOI] [PubMed] [Google Scholar]
- 63.Hulsey CD, Roberts RJ, Lin ASP, Guldberg R, Streelman JT. 2008. Convergence in a mechanically complex phenotype: detecting structural adaptations for crushing in cichlid fish. Evolution 62, 1587–1599. ( 10.1111/j.1558-5646.2008.00384.x) [DOI] [PubMed] [Google Scholar]
- 64.Swanson BO, Gibb AC, Marks JC, Hendrickson DA. 2003. Trophic polymorphism and behavioral differences decrease intraspecific competition in a cichlid, Herichthys minckleyi. Ecology 84, 1441–1446. ( 10.1890/02-0353) [DOI] [Google Scholar]
- 65.Hulsey CD. 2006. Function of a key morphological innovation: fusion of the cichlid pharyngeal jaw. Proc. R. Soc. B 273, 669–675. ( 10.1098/rspb.2005.3375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meyer A. 1990. Ecological and evolutionary consequences of the trophic polymorphism in Cichlasoma citrinellum (Pisces: Cichlidae). Biol. J. Linn. Soc. 39, 279–299. ( 10.1111/j.1095-8312.1990.tb00517.x) [DOI] [Google Scholar]
- 67.Muschick M, Barluenga M, Salzburger W, Meyer A. 2011. Adaptive phenotypic plasticity in the Midas cichlid fish pharyngeal jaw and its relevance in adaptive radiation. BMC Evol. Biol. 11, 116 ( 10.1186/1471-2148-11-116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elmer KR, Lehtonen TK, Kautt AF, Harrod C, Meyer A. 2010. Rapid sympatric ecological differentiation of crater lake cichlid fishes within historic times. BMC Biol. 8, 60 ( 10.1186/1741-7007-8-60) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Recknagel H, Elmer K, Meyer A. 2014. Crater lake habitat predicts morphological diversity in adaptive radiations of cichlid fishes. Evolution 68, 2145–2155. ( 10.1111/evo.12412) [DOI] [PubMed] [Google Scholar]
- 70.Barluenga M, Stölting KN, Salzburger W, Muschick M, Meyer A. 2006. Sympatric speciation in Nicaraguan crater lake cichlid fish. Nature 439, 719–723. ( 10.1038/nature04325) [DOI] [PubMed] [Google Scholar]
- 71.López-Fernández H, Winemiller KO, Montaña CG, Honeycutt RL. 2012. Diet-morphology correlations in the radiation of South America geophagine cichlids (Perciformes: Cichlidae: Cichlinae). PLoS ONE 7, e33997 ( 10.1371/journal.pone.0033997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lundberg JGG, Marshall LGG, Guerrero J, Horton B, Malabarba MC, Wesselingh F. 1998. The stage for neotropical fish diversification a history of tropical south American rivers. In Phylogeny and classification of neotropical fishes (eds Reis RE, Vari RP, Lucena ZM, Lucena CAS), pp. 13–48. Porto Alegre, Brasil: Edipucrs. [Google Scholar]
- 73.Malabarba MC, Malabarba LR, López-Fernández H. 2014. On the Eocene cichlids from the Lumbrera Formation: additions and implications for the Neotropical ichthyofauna. J. Vertebr. Paleontol. 34, 49–58. ( 10.1080/02724634.2013.830021) [DOI] [Google Scholar]
- 74.Malabarba MC, Zuleta O, Papa CDEL. 2006. Proterocara argentina, a new fossil cichlid from the Lumbrera formation, Eocene of Argentina. J. Vertebr. Paleontol. 26, 267–275. ( 10.1671/0272-4634(2006)26%5B267:PAANFC%5D2.0.CO;2) [DOI] [Google Scholar]
- 75.Wagner CE, Harmon LJ, Seehausen O. 2012. Ecological opportunity and sexual selection together predict adaptive radiation. Nature 487, 366–369. ( 10.1038/nature11144) [DOI] [PubMed] [Google Scholar]
- 76.Seehausen O. 2015. Process and pattern in cichlid radiations: inferences for understanding unusually high rates of evolutionary diversification. New Phytol. 207, 304–312. ( 10.1111/nph.13450) [DOI] [PubMed] [Google Scholar]
- 77.Salzburger W, Van Bocxlaer B, Cohen AS. 2014. Ecology and evolution of the African great lakes and their faunas. Annu. Rev. Ecol. Evol. Syst. 45, 519–545. ( 10.1146/annurev-ecolsys-120213-091804) [DOI] [Google Scholar]
- 78.Seehausen O, et al. 2008. Speciation through sensory drive in cichlid fish. Nature 455, 620–627. ( 10.1038/nature07285) [DOI] [PubMed] [Google Scholar]
- 79.Brawand D, et al. 2014. The genomic substrate for adaptive radiation in African cichlid fish. Nature 513, 375–381. ( 10.1038/nature13726) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All phylogenies used in this manuscript are available from López-Fernández et al. [22] (http://dx.doi.org/10.5061/dryad.34621) and all trait data are available from Arbour and López-Fernández [29] (http://dx.doi.org/10.5061/dryad.j04r6).