Abstract
Avian eggs are at risk of microbial infection prior to and during incubation. A large number of defence mechanisms have evolved in response to the severe costs imposed by these infections. The eggshell's cuticle is an important component of antimicrobial defence, and its role in preventing contamination by microorganisms in domestic chickens is well known. Nanometer-scale cuticular spheres that reduce microbial attachment and penetration have recently been identified on eggs of several wild avian species. However, whether these spheres have evolved specifically for antimicrobial defence is unknown. Here, we use comparative data on eggshell cuticular structure and nesting ecology to test the hypothesis that birds nesting in habitats with higher risk of infection (e.g. wetter and warmer) are more likely to evolve cuticular nanospheres on their eggshells than those nesting in less risky habitats. We found that nanostructuring, present in 54 of 296 analysed species, is the ancestral condition of avian eggshells and has been retained more often in taxa that nest in humid infection-prone environments, suggesting that they serve critical roles in antimicrobial egg defence.
Keywords: eggshell cuticle, nest type, microbial infection, nest humidity
1. Background
Avian eggs are a model system to investigate environmental effects on biological diversity because they are critical for embryo survival, are laid in a broad range of ecological conditions and display wide phenotypic diversity. For example, variation in nest location and microclimate, parental incubation patterns and risk of predation have led to the evolution of different shapes, colours and structural components of eggs (e.g. [1–4]).
From the onset of laying, eggs are constantly at risk of infection by microbes [5,6] and certain environments are known to be particularly risky. For example, infection risk increases when ambient or nest humidity is high [7–10], nests are located in close proximity to water (e.g. floating nests, [11]), or eggs are incubated in cavities or buried in highly humid mounds of vegetation [12]. Numerous defence mechanisms have evolved in response to the severe fitness costs imposed by microbial infection. Some of those include parental behaviours that may sanitize the nesting environment, like preventing the accumulation of water in the nest [13,14], the inoculation of eggs and nesting materials with antimicrobial components from uropygial waxes [15] or the use of fresh plants containing aromatic compounds as nest liners [16].
Egg architecture is probably the best example of an adaptive response to environmental threats, including microbes. The eggshell, for example, functions as a series of resistance barriers adapted to specific nesting conditions, physiological demands by the embryo, and the types of microorganisms prevalent in nests and parents [17]. Eggshell structure is a labile trait that can rapidly change in response to environmental pressures. For example, after colonizing new environments with high ambient humidity, house finch (Carpodacus mexicanus) populations increased eggshell thickness and decreased eggshell porosity in less than 30 generations [18].
In many species, the eggshell is sealed by the cuticle, a layer of highly variable thickness, structure and composition among bird taxa; this cuticle can, however, be absent in entire lineages like parrots, pigeons and petrels [19]. Some eggshell cuticles are composed of nanometer-scale spheres (figure 1) of vaterite (CaCO3; e.g. guira cuckoo, Guira guira and smooth-billed ani, Crotophaga ani [20]) or hydroxyapatite (Ca10(PO4)6(OH)2; e.g. greater flamingo, Phoenicopterus roseus and Australian brush-turkey, Alectura lathami [21,22], domestic chicken [23]).
Figure 1.
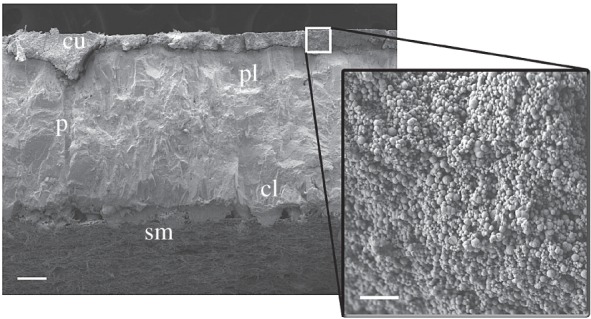
Cross-sectional scanning electron micrographs (SEMs) of a greater flamingo (Phoenicopterus roseus) eggshell showing the cuticular nanospheres (inset) covering the palisade (pl) layer and plugging the pores (p) of the shell. cu, cuticle; cl, cone layer; sm, shell membrane. Scale bars 100 µm and 5 µm in main image and inset, respectively.
The cuticle serves several important functions, including termination of eggshell formation [23], modulation of ultraviolet wavelengths that could cause harm to the embryo [24,25] and signalling function [26,27]. The specific role of the cuticle in preventing microbial infection was hypothesized four decades ago [17] but has only been experimentally demonstrated in domestic chickens [28] and more recently in the nanosphere cuticles of a megapode species [22]. Moreover, cuticles with nanospheres are effective at waterproofing eggs by capping the shell pores while allowing the diffusion of respiratory gasses (particularly important when nests are inundated [29,30]). Nanospheres may thus help protect embryos from microbial contamination by conferring, first, a hydrophobic surface that prevents accumulation of water on the egg's surface [22,31], as biofilm formation cannot proceed in the absence of water [32], and second, a rough surface that decreases bacterial attachment [33]. Different types of cuticles may also protect the egg from infection by blocking the pores used by bacteria to penetrate into the egg contents [34], but inorganic, mineralized nanospheres might be less prone to microbial digestion and degradation [8]. In particular, it has been proposed that nanosphere cuticles serve an antimicrobial function for species that nest in environments with high risk of flooding [30,33] or of microbial infection. However, this hypothesis has not been tested in a comparative context.
The goal of this study was to integrate data on eggshell structure (presence of cuticular nanospheres) and nesting ecology of a wide range of birds to test the hypothesis that the evolution of eggshell cuticles is associated with nesting ecologies that increase the risk of nest flooding or egg microbial infection. In particular, we predict that cuticles with nanospheres will evolve more often in species living in humid and hot environments. We also expect that cuticular spheres will be more prevalent in species nesting on the ground or in mounds, where soil bacteria are abundant, and less prevalent in species that use fresh vegetation to line their nests, as this material can sanitize nests and thereby decrease the risk of egg infection.
2. Material and methods
(a). Eggshell structure
We gathered data on cuticular nanospheres from species descriptions and photographs in [19] (N = 296) and corroborated them (when samples were available) by scanning electron microscopy (SEM) of shell fragments (N = 56 species; see the electronic supplementary material for details). We created a binary variable for shell structure by noting the presence or absence of nanospheres (electronic supplementary material, figure S1) on the cuticle for each species. Nanospheres can be composed of calcium carbonate, calcium phosphate or a mixture of the two [20–23]. Chemical data for most species is not available, but the similar developmental pathways and overlap (even in the same egg) of the two chemistries [23] suggests that they are homologous and we treat them as such.
(b). Environmental variables and nesting ecology
We used the database of Handbook of the birds of the world alive [35] and species monographs to gather information on nesting ecology for all species in our study. For each species we recorded (i) the type of nest used (ground, platform, tree cup, cavity or mound), (ii) the presence or absence of nest lining, (iii) the presence or absence of green plant material (e.g. fresh leaves, herb sprigs) in the nest (hereafter greenery), (iv) the general proximity of the nest to water (e.g. greater than 10 m from water body or above water), and the timing and geographical location of breeding. The use of greenery in the nest was categorized following the description in [16], which designates greenery as non-constituent fresh green plant material added following nest construction. We also used historical records (from 2000 to 2012) for each location using World Weather Online (http://www.worldweatheronline.com) and calculated the mean across all localities (minimum two locations) for a given species for two climatic variables: (v) mean ambient temperature, and (vi) humidity (precipitation in millimetres) during the months that incubation takes place. In cases where a wide range of breeding locations for a single species were presented, we used the mean across these locations.
(c). Phylogeny
For the comparative analyses, we used the complete Bayesian species-level avian phylogeny by Jetz et al. [36], built based on both genetic and taxonomic information and the higher-order relationship backbone from Hackett et al. [37]. This tree was constructed using genetic information for about two-thirds of avian species, with the missing species added taxonomically, based on a heterogeneous birth–death model, and represents the current most complete reconstruction of the avian tree of life. Of the 296 species considered in our study, only 16 (5.4%) lacked molecular phylogenetic data and were grafted taxonomically to the genetic tree. To account for any biases that could potentially result from including those species in our analyses we repeated our analyses using the tree containing only species for which genetic data are available and present those confirmatory results in the electronic supplementary material.
We conducted analyses on the maximum clade credibility (MCC) tree, and in order to account for phylogenetic uncertainty and minimize conflicts resulting from species added taxonomically, we repeated our analyses across a subset of 100 trees randomly sampled from the posterior distribution. To facilitate model optimization and interpretation of parameters, trees were transformed to have a root-to-tip distance of 1 before comparative analyses.
(d). Ancestral reconstruction and phylogenetic signal of eggshell structure
To reconstruct the ancestral character states of eggshell structure (the presence or absence of cuticular nanospheres), we performed maximum-likelihood (ML) reconstructions in R using the ‘ace’, or ancestral character estimation, subroutine of the APE package. We tested whether a model considering equal or different transition rates between gain and loss of cuticular nanospheres, under a continuous-time Markov chain, had the best fit to our data and tree using a likelihood ratio test [38–40]. The best model was then used to reconstruct ancestral states based on the ML at each node integrating over all other states of all other nodes weighted by their probabilities (i.e. marginal reconstruction [41]). When considering tests across the sampled trees from the posterior distribution, not all nodes will be present in all trees, and therefore, only the likelihood ratio test results and the probability of each state at the root are reported.
Because our dependent variable is scored as a binary character, we measured phylogenetic signal strength for eggshell structure using Fritz and Purvis' D test for binary data [42], as implemented in the function ‘phylo.d’ in the CAPER package [43]. This D-statistic is based on the sum of changes in node values along the branches of a tree, with values close to zero being closer to what would be expected under a threshold model for a quantitative trait evolving under Brownian motion [42]. We assessed the significance of the estimate of D through permutation tests (1000 permutations), based on the probability of obtaining the observed value of D under a random distribution of tip states (no phylogenetic signal) as well as from the Brownian evolution of an underlying threshold trait.
Given that ancestral state reconstructions can be influenced by incomplete sampling if sampling is biased with regards to the trait of interest and that sampling such detailed morphological data for a higher proportion of species would be impractical, we used a simulation approach to (i) estimate the overall distribution of the occurrence of nanosphere cuticles across the avian tree of life and (ii) the effects of incomplete sampling on ancestral state reconstructions. Following [44], we estimated the proportion of species per genus by sampling unobserved species from a binomial distribution, with the probability that a species has nanospheres defined by the proportion of species with that trait in the genus. This sampling approach was chosen given the phylogenetic signal in the trait (see below) and the strong bimodality in the presence and absence of nanospheres within genera, which is also robust at higher taxonomic levels (electronic supplementary material, figure S2). Genera with no sampled species were informed by the proportions of species with nanospheres among genera within families and orders, such sampling is progressively more uncertain when less information is available for closely related taxa [44]. Only families sampled were included in the simulations. We performed 1000 simulations using the R package traitfill [44, available at https://github.com/traitecoevo/traitfill] to estimate the proportion of species with nanospheres within each genera, which were then averaged and used to simulate data on the occurrence of the trait at the species level, and finally used to reconstruct ancestral states for 1000 simulations of the trait.
(e). Phylogenetic generalized linear models
We conducted phylogenetic generalized linear models to evaluate whether the evolution of nanospheres on cuticles is related to nest type, the use of nest lining, the use of green plants, proximity to water, and ambient humidity or temperature during incubation. These regressions were conducted in R using the ‘phyloglm’ subroutine of the PHYLOLM package [45]. This method estimates the phylogenetic correlation parameter α for each regression model, where α governs the rate at which phylogenetic correlations among species are lost [46].
We fitted full generalized linear models with binomial error and logit link function considering the presence or absence of nanospheres on cuticles as the dependent variable and multiple nesting ecology variables as independent terms. Model selection was performed under an Akaike information criteria (AIC) framework based on a priori select models [47]. Because of significant correlations between humidity and temperature, and humidity and proximity to water, we avoided entering them simultaneously in the models. We evaluated a total of 27 models (see the electronic supplementary material, table S1 for full list of candidate models) in the candidate set, which included feasible linear combinations of our response variables. When more than one candidate model showed explanatory ability (indicated by an AIC value that differed in less than or equal to 2 from the best model), we model-averaged parameter estimates and the associated variances from the 95% confidence set of candidate models [47]. To account for phylogenetic uncertainty, analyses were conducted across the subset of 100 trees, with model-averaged estimates (and their uncertainty) combined across trees. Binary and continuous variables were standardized to a common scale by centring binary variables and standardizing continuous variables on 2 s.d. [48,49].
3. Results
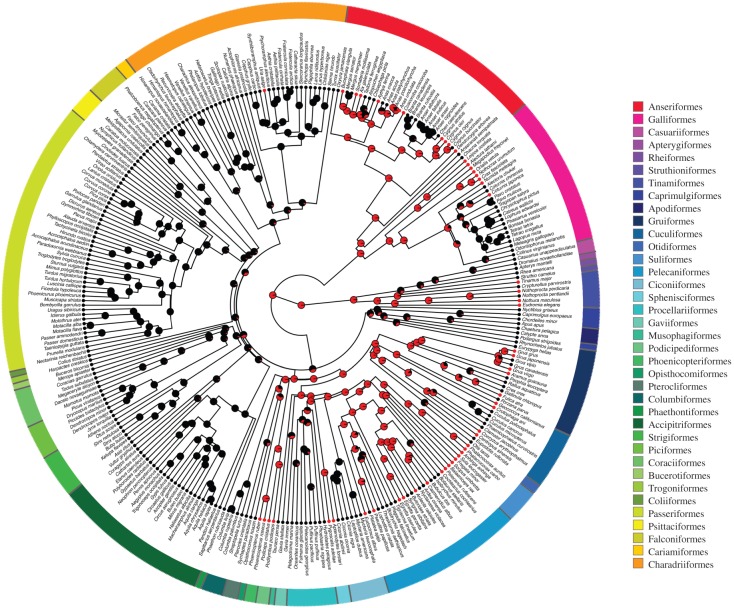
We recorded the presence of cuticle in 91% (N = 269 species) of all the species included in the study. However, the cuticle was composed of nanospheres in only 54 species (20%). Some clades (e.g. Gruiformes) were characterized by a mixture of species with and without spheres, whereas in most clades either all species had spheres (e.g. Spenisciformes and Pelecaniformes) or none had spheres (e.g. Accipitriformes, Passeriformes). As a result, the presence of cuticular spheres exhibited significant phylogenetic signal, and not significantly different from the distribution expected under a Brownian threshold model (Fritz and Purvis' D, MCC tree: D =−0.15, p(D=1) < 0.001, p(D=0) = 0.749; posterior sample mean D =−0.12, 95% quantiles: −0.18, −0.06, all p(D=1) < 0.001 and p(D=0) > 0.05). A two-rate model best described the evolution of cuticular spheres (likelihood ratio test; MCC tree: LR = 38.7, p < 0.001; posterior sample: mean LR = 40.13, 95% quantiles = 35.70, 44.46, all p < 0.001), with the rate of losses of cuticular spheres being about 30× greater than gains. As a result, the most recent common ancestor of all birds was inferred to have had eggshells with cuticular nanospheres, with these features being repeatedly lost across several bird clades (figure 2). This result was not changed when we used the phylogenies containing only species for which genetic data are available (electronic supplementary material, figure S3).
Figure 2.
Maximum clade credibility tree [30] pruned to the 296 species across 36 orders included in this study, indicating the presence (shown in red) or absence (black) of nanospheres on eggshell cuticles. ML ancestral character reconstructions of reproductive mode are shown along the tree. The presence of cuticular spheres probabilities on internal nodes were estimated using maximum-likelihood under the two-rates model.
Simulations suggested that species with cuticular nanospheres were oversampled in our dataset (observed proportion of species with nanospheres: 18.24%, estimated: mean 06.69%, 95% quantiles 06.02–07.51%). Nonetheless, reconstructions taking this into account still supported the presence of cuticular nanospheres in the ancestor of all birds (p(spheres) at root = 0.99, 95% quantiles 0.998–0.999).
Species breeding in very humid environments tended to have shell cuticles with spheres. Consistent with an association between nesting ecology and the evolution of eggshell structure, ambient humidity, the use of nest lining and green plants were included in the two best models (those with ΔAIC ≤ 2; mean WAIC = 0.65 and 0.22, respectively) from the phylogenetic generalized linear models tested (electronic supplementary material, table S1). Cuticular nanospheres on eggshells were associated with increased precipitation during the egg laying and incubation periods, however, after accounting for model and phylogenetic uncertainty there was little evidence that the presence of cuticle spheres is affected by the incorporation of nest lining (table 1).
Table 1.
Comparison of phylogenetic generalized linear models explaining the presence of nanospheres on cuticles of eggshells of 296 bird species used in the study. Lower ΔAIC scores (AICmodel (i) – AICbest model) indicate better models. Only models that contain 99% of the evidence weight are shown. Bold numbers denote the top-ranked models (those with ΔAIC ≤ 2). See electronic supplementary material, table S1 for all model results. The proportion of trees in which each model was preferred is presented. Effect sizes standardized on 2 s.d., following Gelman [49].
| variable | estimate | unconditional s.e. | 95% CI | relative importance |
|---|---|---|---|---|
| (intercept) | −3.40 | 1.20 | (−5.75, −1.05) | |
| humidity | 1.78 | 0.64 | (0.53, 3.04) | 1 |
| lining | −0.80 | 0.63 | (−2.03, 0.44) | 0.793 |
| green plants | −0.17 | 0.45 | (−1.05, 0.71) | 0.266 |
| nesta | 0.023 | |||
| platform | 0.00 | 0.16 | (−0.32, 0.32) | |
| tree cup | 0.07 | 0.23 | (−0.37, 0.51) | |
| cavity | −0.08 | 0.27 | (−0.61, 0.44) | |
| mound | 0.19 | 0.59 | (−0.96, 1.34) | |
| near waterb | 0 | |||
| temperatureb | 0 |
aGround nesting was the reference category.
bVariables not included in the 95% confidence set of candidate models.
We expected that elevated ambient temperature would be associated with the presence of cuticular spheres because of its potential role in increasing the risk of egg microbial infection. However, we found that the evolution of spheres was not associated with ambient temperature or with nest type (table 1). Results and patterns were statistically similar when data were analysed using the phylogenies excluding species for which molecular data were not available (electronic supplementary material, table S2).
4. Discussion
The structure of avian eggshells has evolved in part as a response to ambient conditions during incubation [21] and plays an important role in the adaptation of birds to various environments [50–52]. Only one previous study has investigated the relationship between life-history parameters and the role of eggshell conductance within a comparative framework [53]. Here we report for the first time an association between the nanostructure of eggshells and nesting ecology in birds. Eggs of species that nest in more humid and wet environments are more likely to have cuticular nanospheres. This is most likely because humid environments increase the risk of flooding of eggs and/or microbial infection [10,21]. Therefore, these data suggest that nanospheres are selected for protection against occlusion of pores and microbial or other environmental threats. Importantly, cuticular spheres appear to be ancestral, but have been lost numerous times and are entirely absent in large, derived clades like Passeriformes. Thus, extant bird species with cuticular spheres have likely not gained them, but rather have not lost them.
In wet environments, the eggshell pore canal can become flooded and impede exchange of vital gasses through the shell, impairing embryonic growth and potentially leading to asphyxiation [21]. In addition, bacterial transport with liquid water or water vapour through the pores is a mechanism for microbial infection [17]. Aside from its documented antimicrobial effects [22], cuticle nanospheres can also plug the eggshell pores [22,54–56], preventing flooding and trans-shell movement of bacteria. Thus, nanospheres may represent a structural adaptation for both their antimicrobial and anti-flooding benefits, as evidenced by their frequent association in our data with wet and flood-prone environments.
Maintaining optimal gas exchange during incubation is extremely important for embryo survival [57]. The rate at which oxygen, CO2 and water vapour are exchanged is determined largely by shell conductance and nest humidity [58]. In certain species, the cuticle is a highly conducible layer that has negligible effects on gas exchange [59]. In other species, the cuticle can modulate shell conductance [60]. These differing patterns might depend on the cuticle's ultrastructure (e.g. presence of fissures; [60]) or potentially on its chemistry (e.g. lipid content). Nests in high altitude, and dry or humid environments can impose important challenges to gas exchange, so we expect that selection would act to modify conductance of eggshells to match the requirements imposed by environment. For example, nanospheres on the thick cuticles of Adelie penguin (Pygoscelis adeliae) eggs significantly reduce the rate of water loss that would otherwise be detrimental in the arid Antarctic environment [61].
In more humid environments, however, higher shell conductance may be beneficial to sustain optimal water loss [59]. Deeming [60] suggested that in certain conditions, for example, when eggs have low conductance (e.g. at high altitude or dry environments), the absence of a cuticle would be beneficial to embryonic survival. Thus, the presence of cuticles with nanospheres could impose an effective resistance to gas diffusion and represent a fitness cost to the maintenance of this eggshell trait. Additionally, hydroxyapatite or vaterite nanoparticles greatly increase fracture toughness of ceramics [62] by effectively deflecting crack propagation [63]. This suggests that nanospheres on cuticles might increase eggshell toughness and potentially hinder the hatching process for embryos with otherwise brittle eggshells [64]. However, testing these two hypotheses will require further investigation. Thus, a thick cuticle may lower conductance and gas exchange, or increase eggshell toughness, costs that may be balanced by its other positive effects in certain situations.
Relaxed selection due to lowered infection in less humid environments, or selection for greater conductivity in more water-restricted environments, may also explain the repeated loss of eggshell cuticular spheres. Passerines, for example, may lack nanospheres in part because of their tendency to nest higher in trees or in suspended vegetation, and thus further away from water. Indeed, no passerines are known to use floating nests or ground mounds of vegetation. By nesting above ground and with lower risk of flooding or water accumulation, selection on antimicrobial properties may have been reduced, leading to the loss of nanospheres. In addition, the use of sanitizing greenery in nests (e.g. Accipitriformes, Falconiformes) suggests that antimicrobial or antiparasitic [65] defence is still important for other groups that have lost nanospheres, and given the low evolutionary rates to regain the presence of cuticle nanospheres, these groups may have evolved alternative solutions to this problem.
In addition to their many other roles, eggshells may also help prevent harmful ultraviolet wavelengths from reaching the embryo [24,25]. This effect could be particularly important in open cup nests that are exposed to more sunlight. The cuticle appears to absorb ultraviolet wavelengths [25], and indeed the nanospheres on the thick cuticle found in megapode eggs absorb UV light [25]. However, UV protection is unlikely to be ecologically significant in megapode eggs, as these birds bury their eggs in mounds. Moreover, we found no relationship between nest type and the presence of nanospheres, suggesting that relative UV exposure is not a significant selective pressure. Egg colours may also be sexually selected (e.g. [26]) or important for egg recognition in brood parasitized birds (e.g. [66]). However, more research is needed on how the cuticle itself [27], and whether the nanospheres, in particular, affect coloration, especially in the context of communication and signalling functions, before we consider these potential effects.
From a developmental perspective, the loss of nanospheres may result from a pH shift in the intrauterine milieu during egg development (i.e. less alkaline or having specific molecules that can act as inhibitors of mineralization [67]). The presence of organic molecules, including amino acids, peptides and proteins, promote and control crystallization of vaterite [68] and hydroxyapatite spheres [69]. The uterine conditions of birds other than chickens during egg development are largely unknown, meaning that considerable work will have to be done to test this hypothesis.
In conclusion, we have shown that a largely unstudied, but critical, aspect of avian eggshell morphology evolved early and was subsequently lost in many groups. The association between the presence of nanospheres and moisture suggests that these patterns may be explained by the need to protect the egg against flooding and microbial invasion. Future work should focus on testing the effects of nanospheres on conductance and gas exchange, as well as the uterine conditions needed for their development and deposition.
Supplementary Material
Acknowledgements
We thank Juan A. Amat, Erpur Hansen, Roxana Torres, Sigríður Hanna Sigurðardóttir, Walter Piper and Patti Diehl for providing shell samples specimens. We are grateful to the Shawkey lab and three anonymous reviewers who provided useful comments on previous versions of the manuscript.
Data accessibility
Original data available from the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.56gn0).
Authors' contributions
L.D.A. conceived the study and collected the data; R.M. and L.D.A. analysed the data; and all authors contributed to writing.
Competing interests
We have no competing interests.
Funding
This study was funded by a Human Frontier Science Program Young Investigator's grant RGY-0083 to M.D.S. and M.E.H. and AFOSR FA9550-13-1-0222 to M.D.S. This work was partially supported by a Junior Fellow award from the Simons Foundation to R.M.
References
- 1.Martin TE. 1987. Food as a limit on breeding birds: a life-history perspective. Annu. Rev. Ecol. Evol. Syst. 18, 453–487. ( 10.1146/annurev.es.18.110187.002321) [DOI] [Google Scholar]
- 2.Smart IH. 1991. Egg-shape in birds. In Egg incubation: its effects on embryonic development in birds and reptiles (eds Deeming DC, Ferguson MWJ), pp. 101–116. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Avilés JM, et al. 2006. Environmental conditions influence egg color of reed warblers Acrocephalus scirpaceus and their parasite, the common cuckoo Cuculus canorus. Behav. Ecol. Sociobiol. 61, 475–485. ( 10.1007/s00265-006-0275-0) [DOI] [Google Scholar]
- 4.Lahti DC. 2008. Population differentiation and rapid evolution of egg color in accordance with solar radiation. Auk 125, 796–802. ( 10.1525/auk.2008.07033) [DOI] [Google Scholar]
- 5.Packard G, Packard M. 1980. Evolution of the cleidoic egg among reptilian antecedents of birds. Integr. Comp. Biol. 20, 351–362. ( 10.1093/icb/20.2.351) [DOI] [Google Scholar]
- 6.Baggott GK, Graeme-Cook K. 2002. Microbiology of natural incubation. In Avian incubation behaviour, environment, and evolution (ed. Deeming DC.), pp. 179–191. Oxford, UK: Oxford University Press. [Google Scholar]
- 7.Graves R, MacLaury D. 1962. The effect of temperature, vapour pressure, and absolute humidity on bacteria contamination of shell eggs. Poult. Sci. 41, 1219–1225. ( 10.3382/ps.0411219) [DOI] [Google Scholar]
- 8.Board RG, Loseby S, Miles VR. 1979. A note on microbial growth on hen eggshells. Br. Poult. Sci. 20, 413–420. ( 10.1080/00071667908416600) [DOI] [Google Scholar]
- 9.Cook MI, Beissinger SR, Toranzos GA, Rodriguez RA, Arendt WJ. 2003. Trans-shell infection by pathogenic micro-organisms reduces the shelf life of non-incubated bird's eggs: a constraint on the onset of incubation? Proc. R. Soc. Lond. B 270, 2233–2240. ( 10.1098/rspb.2003.2508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook MI, et al. 2005. Microbial infection affects egg viability and incubation behavior in a tropical passerine. Behav. Ecol. 16, 30–36. ( 10.1093/beheco/arh131) [DOI] [Google Scholar]
- 11.Sotherland PR, Ashen MD, Shuman RD, Tracy CR. 1984. The water balance of bird eggs incubated in water. Physiol. Zool. 57, 338–348. ( 10.1086/physzool.57.3.30163723) [DOI] [Google Scholar]
- 12.Peralta-Sánchez JM, Martín-Vivaldi M, Martín-Platero AM, Martinez-Bueno M, Onate M, Ruiz-Rodriguez M, Soler JJ. 2012. Avian life history traits influence eggshell bacterial loads: a comparative analysis. Ibis 154, 725–737. ( 10.1111/j.1474-919X.2012.01256.x) [DOI] [Google Scholar]
- 13.D'Alba L, Oborn A, Shawkey MD. 2010. Experimental evidence that keeping eggs dry is a mechanism for the antimicrobial effects of avian incubation. Naturwissenschaften 97, 1089–1095. ( 10.1007/s00114-010-0735-2) [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-De-Castañeda R, et al. 2011. Drying eggs to inhibit bacteria: incubation during laying in a cavity nesting passerine. Behav. Process. 88, 142–148. ( 10.1016/j.beproc.2011.08.012) [DOI] [PubMed] [Google Scholar]
- 15.Martín-Vivaldi M, et al. 2014. Special structures of hoopoe eggshells enhance the adhesion of symbiont-carrying uropygial secretion that increase hatching success. J. Anim. Ecol. 83, 1289–1301. ( 10.1111/1365-2656.12243) [DOI] [PubMed] [Google Scholar]
- 16.Dubiec A, Góźdź I, Mazgajski TD. 2013. Green plant material in avian nests. Avian Biol. Res. 6, 133–146. ( 10.3184/175815513X13615363233558) [DOI] [Google Scholar]
- 17.Board R, Fuller R. 1974. Non-specific antimicrobial defences of the avian egg, embryo and neonate. Biol. Rev. 49, 15–49. ( 10.1111/j.1469-185X.1974.tb01297.x) [DOI] [PubMed] [Google Scholar]
- 18.Stein LR, Badyaev AV. 2011. Evolution of eggshell structure during rapid range expansion in a passerine bird. Funct. Ecol. 25, 1215–1222. ( 10.1111/j.1365-2435.2011.01887.x) [DOI] [Google Scholar]
- 19.Mikhailov KE. 1997. Avian eggshells: an atlas of scanning electron micrographs. Hertfordshire, UK: British Ornithologists’ Club. [Google Scholar]
- 20.Sparks NHC. 1994. Shell accessory materials: structure and function. In Microbiology of the avian egg (eds Board RG, Fuller R), pp. 25–42. Cornwall, UK: Springer. [Google Scholar]
- 21.Board R. 1982. Properties of avian eggshells and their adaptive value. Biol. Rev. 57, 1–28. ( 10.1111/j.1469-185X.1982.tb00362.x) [DOI] [Google Scholar]
- 22.D'Alba L, Jones DN, Badawy HT, Eliason CM, Shawkey MD. 2014. Antimicrobial properties of a nanostructured eggshell from a compost-nesting bird. J. Exp. Biol. 217, 1116–1121. ( 10.1242/jeb.098343) [DOI] [PubMed] [Google Scholar]
- 23.Dennis JE, et al. 1996. Microstructure of matrix and mineral components of eggshells from white leghorn chickens (Gallus gallus). J. Morphol. 228, 287–306. ( 10.1002/(SICI)1097-4687(199606)228) [DOI] [PubMed] [Google Scholar]
- 24.Maurer G, Portugal SJ, Hauber ME, Mikšík I, Russell DGD, Cassey P. 2015. First light for avian embryos: eggshell thickness and pigmentation mediate variation in development and UV exposure in wild bird eggs. Funct. Ecol. 29, 209–218. ( 10.1111/1365-2435.12314) [DOI] [Google Scholar]
- 25.Fecheyr-Lippens DC, et al. 2015. The cuticle modulates ultraviolet reflectance of avian eggshells. Biol. Open. 4, 753–759. ( 10.1242/bio.012211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreno J, Morales J, Lobato E, Merino S, Tomas G, Martinez-de la Puente J. 2005. Evidence for the signaling function of egg color in the pied flycatcher Ficedula hypoleuca. Behav. Ecol. 16, 931–937. ( 10.1093/beheco/ari072) [DOI] [Google Scholar]
- 27.Igic B, et al. 2015. A nanostructural basis for gloss of avian eggshells. J. R. Soc. Interface 12, 20141210 ( 10.1098/rsif.2014.1210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vadehra DV, Baker RC, Naylor HB. 1970. Role of cuticle in spoilage of chicken eggs. J. Food Sci. 35, 5–6. ( 10.1111/j.1365-2621.1970.tb12354.x) [DOI] [Google Scholar]
- 29.Board RG, Halls NA. 1973. The cuticle: a barrier to liquid and particle penetration of the shell of the hen's egg. Br. Poult. Sci 14 69–97. ( 10.1080/00071667308415999) [DOI] [Google Scholar]
- 30.Board R. 1980. The avian eggshell—a resistance network. J. Appl. Bacteriol. 48, 303–313. ( 10.1111/j.1365-2672.1980.tb01230.x) [DOI] [Google Scholar]
- 31.Carman M, et al. 2006. Engineered antifouling microtopographies—correlating wettability with cell attachment. Biofouling 22, 11–21. ( 10.1080/08927010500484854) [DOI] [PubMed] [Google Scholar]
- 32.Donlan RM. 2001. Biofilm formation: a clinically relevant microbiological process. Clin. Infect. Dis. 33.8, 1387–1392. ( 10.1086/322972) [DOI] [PubMed] [Google Scholar]
- 33.Board R, Tullett S, Perrott H. 1977. An arbitrary classification of the pore systems in avian eggshells. J. Zool. 182, 251–265. ( 10.1111/j.1469-7998.1977.tb04158.x) [DOI] [Google Scholar]
- 34.Board R, Scott V. 1980. Porosity of the avian eggshell. Am. Zool. 20, 339–349. ( 10.1093/icb/20.2.339) [DOI] [Google Scholar]
- 35.Handbook of the birds of the world alive. 2013. See http://www.hbw.com/ (accessed 20 September 2015).
- 36.Jetz W, et al. 2012. The global diversity of birds in space and time. Nature 491, 444–448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 37.Hackett SJR, et al. 2008. A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763–1768. ( 10.1126/science.1157704) [DOI] [PubMed] [Google Scholar]
- 38.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15. ( 10.1086/284325) [DOI] [Google Scholar]
- 39.Pagel M. 1994. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. Lond. B 255, 37–45. ( 10.1098/rspb.1994.0006) [DOI] [Google Scholar]
- 40.Schluter D, et al. 1997. Likelihood of ancestor states in adaptive radiation. Evolution 51, 1699–1711. ( 10.2307/2410994) [DOI] [PubMed] [Google Scholar]
- 41.Yang Z, Kumar S, Nei M. 1995. A new method of inference of ancestral nucleotide and amino acid sequences. Genetics 141, 1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fritz SA, Purvis A. 2010. Selectivity in mammalian extinction risk and threat types: a new measure of phylogenetic signal strength in binary traits. Conserv. Biol. 24, 1042–1051. ( 10.1111/j.1523-1739.2010.01455.x) [DOI] [PubMed] [Google Scholar]
- 43.Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W. 2012. Caper: comparative analyses of phylogenetics and evolution in R. R package version 5. See http://CRANR-projectorg/package=caper.
- 44.FitzJohn RG, Pennell MW, Zanne AE, Stevens PF, Tank DC, Cornwell WK. 2014. How much of the world is woody? J. Ecol. 102, 1266–1272. ( 10.1111/1365-2745.12260) [DOI] [Google Scholar]
- 45.Ho TLS, Ane C. 2014. A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst. Biol. 63, 397–408. ( 10.1093/sysbio/syu005) [DOI] [PubMed] [Google Scholar]
- 46.Ives AR, Garland T. 2010. Phylogenetic logistic regression for binary dependent variables. Syst. Biol. 59, 9–26. ( 10.1093/sysbio/syp074) [DOI] [PubMed] [Google Scholar]
- 47.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach, p. 488 New York, NY: Springer. [Google Scholar]
- 48.Grueber CE, Nakagawa S, Laws RJ, Jamieson IG. 2011. Multimodel inference in ecology and evolution: challenges and solutions. J. Evol. Biol. 24, 699–711. ( 10.1111/j.1420-9101.2010.02210.x) [DOI] [PubMed] [Google Scholar]
- 49.Gelman A. 2008. Scaling regression inputs by dividing by two standard deviations. Stat. Med. 27, 2865–2873. ( 10.1002/sim.3107) [DOI] [PubMed] [Google Scholar]
- 50.Lack D. 1968. Ecological adaptations for breeding in birds. London, UK: Chapman and Hall. [Google Scholar]
- 51.Carey C. 1983. Structure and function of avian eggs. In Current ornithology 1 (ed. Johnston RF.), pp. 69–103. New York, NY: Springer. [Google Scholar]
- 52.Zi-Kui Z. 1996. Dinosaur eggs in China: on the structure and evolution of eggshells. In Dinosaur eggs and babies (eds Carpenter K, Hirsch KF, Horner JR), pp. 184–203. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 53.Portugal SJ, Maurer G, Thomas GH, Hauber ME, Grim T, Cassey P. 2014. Nesting behaviour influences species-specific gas exchange across avian eggshells. J. Exp. Biol. 217, 3326–3332. ( 10.1242/jeb.103291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Board RG, Perrott HR. 1979. Vaterite, a constituent of the eggshells of the nonparasitic cuckoos, Guira guira and Crotophagi ani. Calcif. Tissue Int. 29, 63–69. ( 10.1007/BF02408056) [DOI] [PubMed] [Google Scholar]
- 55.Board RG, Perrott HR, Love G, Seymour RS. 1982. A novel pore system in the eggshells of the mallee fowl, Leipoa ocellata. J. Exp. Zool. 220, 131–134. ( 10.1002/jez.1402200118) [DOI] [Google Scholar]
- 56.Board RG, Perrott HR, Love G, Scott VD. 1984. The phosphate-rich cover on the eggshells of grebes (Aves: Podicipitiformes). J. Zool. 203, 329–343. ( 10.1111/j.1469-7998.1984.tb02336.x) [DOI] [Google Scholar]
- 57.Ar A, Rahn H. 1985. Pores in avian eggshells: gas conductance gas exchange and embryonic growth rate Resp. Respir. Physiol. 61, 1–20. ( 10.1016/0034-5687(85)90024-6) [DOI] [PubMed] [Google Scholar]
- 58.Ar A, Paganelli CV, Reeves RB, Greene DG, Rahn H. 1974. The avian egg: water vapor conductance shell thickness and functional pore area. Condor 76, 153–158. ( 10.2307/1366725) [DOI] [Google Scholar]
- 59.Tullett SG, Board RG. 1976. Oxygen flux across the integument of the avian egg during incubation. Br. Poult. Sci. 17, 441–450. ( 10.1080/00071667608416297) [DOI] [Google Scholar]
- 60.Deeming DC. 1987. Effect of cuticle removal on the water vapour conductance of egg shells of several species of domestic bird. Br. Poult. Sci. 28, 231–237. ( 10.1080/00071668708416957) [DOI] [Google Scholar]
- 61.Thompson MB, Goldie KN. 1990. Conductance and structure of eggs of Adelie penguins Pygoscelis adeliae and its implications for incubation. Condor 92, 304–312. ( 10.2307/1368228) [DOI] [Google Scholar]
- 62.Wang J, Shaw LL. 2009. Nanocrystalline hydroxyapatite with simultaneous enhancements in hardness and toughness. Biomaterials 30, 6565–6572. ( 10.1016/j.biomaterials.2009.08.048) [DOI] [PubMed] [Google Scholar]
- 63.Faber KT, Evans AG. 1983. Crack deflection processes. II. Experiment. Acta Metall. 31, 577–584. ( 10.1016/0001-6160(83)90047-0) [DOI] [Google Scholar]
- 64.Bond GM, Board RG, Scott VD. 1988. An account of the hatching strategies of birds. Biol. Rev. 63, 395–415. ( 10.1111/j.1469-185X.1988.tb00723.x) [DOI] [Google Scholar]
- 65.Wimberger PH. 1984. The use of green plant material in bird nests to avoid ectoparasites. Auk 101, 615–618. [Google Scholar]
- 66.Abernathy VE, Peer BD. 2015. Mechanisms of egg recognition in brown-headed cowbird hosts: the role of ultraviolet reflectance. Anim. Behav. 109, 73–79. ( 10.1016/j.anbehav.2015.08.006) [DOI] [Google Scholar]
- 67.Hunter GK, et al. 1996. Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochem. J. 317, 59–64. ( 10.1042/bj3170059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu Q. 2011. CaCO3 crystallization influenced by additives and templates. PhD thesis, The University of Michigan.
- 69.Zhai Y, Cui FZ. 2006. Recombinant human-like collagen directed growth of hydroxyapatite nanocrystals. J. Cryst. Growth 291, 202–206. ( 10.1186/s11671-015-0752-3) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data available from the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.56gn0).