Abstract
Socially isolated individuals face elevated rates of illness and death. Conventional measures of social connectedness reflect an individual's perceived network and can be subject to bias and variation in reporting. In this study of a large human social network, we find that greater indegree, a sociocentric measure of friendship and familial ties identified by a subject's social connections rather than by the subject, predicts significantly lower concentrations of fibrinogen (a biomarker of inflammation and cardiac risk), after adjusting for demographics, education, medical history and known predictors of cardiac risk. The association between fibrinogen and social isolation, as measured by low indegree, is comparable to the effect of smoking, and greater than that of low education, a conventional measure of socioeconomic disadvantage. By contrast, outdegree, which reflects an individual's perceived connectedness, displays a significantly weaker association with fibrinogen concentrations.
Keywords: social networks, social epidemiology, fibrinogen, social hierarchy, stress response
1. Introduction
Many species exhibit social hierarchies, and individuals' positions within them can profoundly affect their lives and health [1]. Socially isolated humans face particularly elevated risks of illness and death [2–5]. Isolated individuals have higher rates of behavioural risk factors such as smoking, drinking to excess and overeating [6,7], but in aggregate such risk factors probably account only for a minority of the relationship between social position and health [8].
Chief among the health risks of social isolation are ischaemic heart disease and stroke [9]. These conditions, the two leading causes of death worldwide [10], result respectively from inadequate blood supply to the heart and brain. When the vascular endothelium is damaged by trauma, the exposure of subendothelial collagen and tissue factor triggers a cascade of reactions terminating in the conversion by thrombin of fibrinogen to fibrin, the principal constituent of clots (electronic supplementary material, figure S1) [11]. Though haemostasis is essential for survival, excessive generation of fibrin leads to thrombosis, the blockage of blood vessels that constitutes the final pathway of ischaemic heart disease and stroke.
Risk of thrombosis, in turn, is governed in part by stress. The mammalian stress response famously mobilizes bodily resources for ‘fight or flight,’ for instance by raising an animal's heart rate and blood pressure in order to divert energy to active skeletal muscle. The stress response also increases hepatic synthesis of fibrinogen, which promotes quicker cessation of bleeding when trauma is incurred [12,13]. But the stress reaction, while lifesaving in the acute phase, can become pathologic when chronic. If blood pressure remains elevated beyond a period of increased muscular demand, atherosclerosis ensues; if the immune system is persistently stimulated, chronic inflammation may arise; and if coagulation factors are upregulated to clot wounds that never occur, pathologic thrombosis can result [14].
Indeed, elevated fibrinogen levels predict future ischaemic heart disease and stroke [15,16], and, as early as 1985, fibrinogen was identified as a possible link between disadvantaged social position and risk of cardiac death [17]. Fibrinogen has been linked to low occupational status, education and sense of control [18], as well as to perceptions of being socially undermined [19], and to self-reported loneliness and isolation [20].
Extant studies have assessed ‘social position’ with measures such as income and educational attainment, or with social relationships reported by the individuals themselves [21]. But if the social structures associated with stress are indeed phenomena that transcend the actions and perceptions of individuals alone [22], then a ‘sociocentric’ measure of social position, which maps the entire network and reflects social ties that may not be the same ties identified by the subject herself, may furnish a more ecologically valid measure of the relationship between social position and health. There is a meaningful difference, for instance, between the number of social contacts named by a subject (outdegree), and the number of contacts who themselves name that subject as a contact when queried (indegree). Outdegree is known to the subject, and reflects her perceived position in her network. Indegree, by contrast, is a sociocentric property that reflects the sum of all declared relationships incident on an individual, and can be reliably known only by assessing the whole network. While outdegree is likely to reflect the perceived support and status captured by conventional measures of social position, indegree may furnish a more accurate measure of the support and resources received. To our knowledge, prior work has not explored the relationship between a sociocentric measure of connectedness and a physiological marker of stress.
Here, we report on the relationship between degree centrality and levels of fibrinogen in a large human social network. We use whole network data for thousands of individuals to calculate the indegree and outdegree centrality of all individuals in the network. We adjust for education, demographics, medical history and known predictors of hypercoagulability to assess the relationship between social connectedness and fibrinogen, a principal biomarker of chronic stress, hypercoagulability and eventual cardiac death.
2. Material and methods
The Framingham Heart Study (FHS) is a community-based observational cohort study initiated in 1948. Detailed study design and selection criteria are described elsewhere [23]. The Framingham Offspring Study (FOS) began in 1971 with the recruitment of 5124 men and women who were offspring of the original FHS participants, or these offspring's spouses.
In each generation of the study, participants underwent routine medical histories and physical examinations, laboratory studies of cardiovascular risk factors and anthropometric measurements every 3–8 years. Recent waves of the study have included collection of a panel of biomarkers including fibrinogen. Here, we use data from Wave 5 of the FOS, which ran from 23 January 1991 to 29 June 1995, and which included fibrinogen values for the greatest number of individuals (n = 3568).
We construct a network of friendship and familial ties using administrative data, as previously described [7,24–26]. To best match the social network data to the dates of fibrinogen measurement, we include only ties initiated prior to 29 June 1995, and terminated after 23 January 1991 (the end and start dates of Wave 5 of the study, respectively). We include ties of the following types: friend, spouse, sibling, parent, child, cousin and other relative. We weight all ties equally. For all individuals with fibrinogen observations, we calculate total indegree (i.e. incoming nominations of all permitted types), friendship indegree, sibship size and total outdegree.
We first characterize fibrinogen as a function of basic demographic and health variables: age, sex, body mass index (BMI), smoking status, income and educational attainment. We then examine the bivariate relationships between fibrinogen levels and indegree, and fibrinogen and outdegree.
As biomarker levels can be partially heritable, the inclusion of familial ties in the network could induce clustering on fibrinogen levels, and thus correlated observations, for reasons unrelated to individuals' overall social connectedness. To mitigate the potential effects of such familial clustering, we assess for assortativity on fibrinogen [27], and include as a control in our statistical models the mean fibrinogen level of each individual's direct social contacts in the network.
We estimate a series of linear models to characterize the relationship between indegree and fibrinogen. We regress fibrinogen levels on indegree, with and without loge transformations of both variables, and adjusting for age, sex, mean fibrinogen level of social contacts, educational attainment, self-reported smoking, use of cholesterol-lowering, antihypertensive and antidepressant medications, and medical risk factors including BMI, systolic and diastolic blood pressure, and levels of total and HDL cholesterol. We estimate additional models with indegree measures restricted to friendship or sibship ties. Finally, we estimate analogous models regressing fibrinogen on outdegree, a conventional egocentric measure of social connectedness.
3. Results
The social network at Wave 5 contains 22 633 individuals, connected via 45 286 friendship and familial ties. In total, 66.0% of individuals (n = 14 942) are connected in the single largest component. Indegree values (number of incoming friendship and familial nominations) range from 0 to 28, with a median value of 4 (electronic supplementary material, figure S2). Outdegree (number of outgoing friendship and familial nominations) ranges from 2 to 32, with a median value of 10.
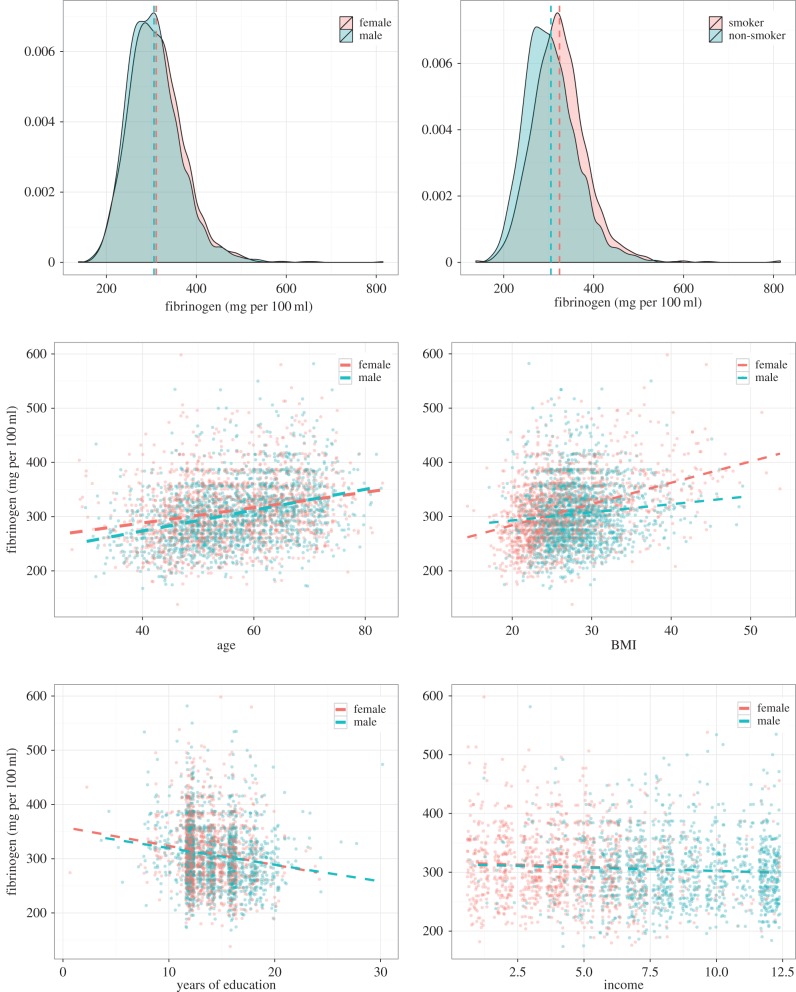
A total of 3568 individuals had fibrinogen measurements taken during the study period. Fibrinogen values range from 138 to 815 mg per 100 ml, with a median value of 303 mg per 100 ml (figure 1). Fibrinogen values are greater for women (two-sample t-test, p = 0.01) and smokers (p < 0.0001). Fibrinogen is positively correlated with age (r = 0.27, p < 0.0001) and BMI (r = 0.25, p < 0.0001), and negatively with years of formal education (r = −0.15, p < 0.0001) and with annual income in FOS Wave 3 (1983–1987, r = −0.07, p = 0.0004).
Figure 1.
Relationship of fibrinogen to sex, age, BMI, smoking status, income and education. In total, 3568 individuals in Wave 5 of the Framingham Offspring Study have fibrinogen measurements. Fibrinogen values range from 138 to 815 mg per 100 ml, with a median value of 303 mg per 100 ml. Fibrinogen values are greater for women (two-sample t-test, p = 0.01) and smokers (p < 0.0001). Fibrinogen is positively correlated with age (r = 0.27, p < 0.0001) and BMI (r = 0.25, p < 0.0001), and negatively with years of formal education (r = −0.15, p < 0.0001) and with unadjusted annual income (in USD$1000) in FOS Wave 3 (1983–1987, r = −0.07, p = 0.0004).
Fibrinogen levels are clustered in the network, with an assortativity coefficient [27] of 0.14. To account for correlated fibrinogen values across familial ties, we calculate the mean fibrinogen value of an individual's social contacts. A total of 87.8% (3134/3568) of individuals with fibrinogen measurements have social contacts who also have fibrinogen measured. As expected, an individual's fibrinogen level is correlated with the mean fibrinogen level of her social contacts (r = 0.19, p < 0.0001).
Individuals nominated more often as friends and family members (i.e. with higher indegree) have lower levels of fibrinogen (figure 2; r = −0.14, p < 0.0001). By contrast, outdegree (the number of social contacts named by a subject) is more weakly associated with the subject's fibrinogen level (electronic supplementary material, figure S3, r = −0.04, p = 0.007). The difference in correlations is significant at p < 0.0001 (Fisher r-to-z transformation [28]).
Figure 2.
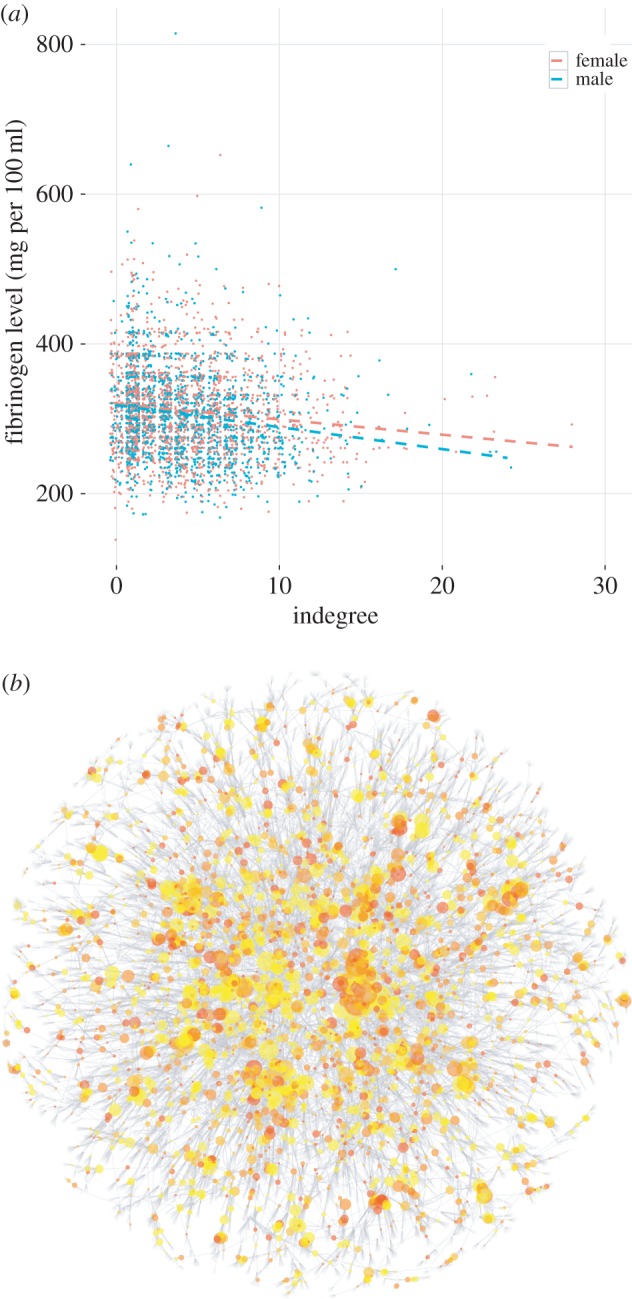
Socially isolated individuals exhibit higher levels of the clotting factor fibrinogen. Panel (a) shows the fibrinogen (mg per 100 ml) and indegree values for Wave 5 of the Framingham Offspring Study (n = 3568 with fibrinogen measurements). Individuals nominated more often as friends and family members have lower levels of the clotting factor fibrinogen (r = −0.14, p < 0.0001). Points are jittered horizontally to better depict the density of the data around discrete values of indegree. Panel (b) shows the largest connected subcomponent of the social network, containing 66.0% of the individuals in the whole network. Of the 14 942 individuals in the largest component, 2451 (16.4%) have fibrinogen measurements. Redder colouring indicates higher fibrinogen levels, and yellower colouring indicates lower fibrinogen levels. Nodes in the component without fibrinogen measurements (used for calculation of centrality measures and for layout of the graph) are depicted minimally in pale grey. Node size is proportional to indegree. The network is laid out using the Fruchterman–Reingold algorithm, tuned for greater repulsion between nodes. Low-fibrinogen (yellower) individuals tend to have higher indegree (larger diameter) and to be globally or locally central, whereas a preponderance of high-fibrinogen (redder) individuals have lower indegree (smaller diameter) and are relatively more isolated in the social network.
We estimate a series of linear models regressing fibrinogen levels on indegree, adjusting for age, sex, years of education (a conventional measure of social position), and a host of cardiovascular risk factors and related medication use (electronic supplementary material, table S1). In a minimal model adjusting for age, sex and education, the relationship between indegree and fibrinogen is such that the most highly connected people in the network are predicted to have 0.96 s.d. lower fibrinogen values than the least connected (p < 0.0001, Model 1a). Adjusting for cardiac risk factors and medication use attenuates the relationship between indegree and fibrinogen by 2% (Model 1b). When accounting additionally for the mean fibrinogen levels of an individual's social contacts, the relationship between indegree and fibrinogen is attenuated further by 6%, remaining highly significant (p < 0.0001, Model 2a).
When including only friendship ties in the calculation of indegree, individuals with the most friend nominations are predicted to have 0.40 s.d. lower fibrinogen than those with none (p < 0.0001, Model 2b). When including only sibship ties, individuals from the largest sibships are predicted to have 0.45 s.d. lower fibrinogen than those from the smallest (p < 0.0001, Model 2c). Thus, it appears that friendship and sibship contribute additively to the relationship between connectedness and fibrinogen levels.
We estimate models in which we loge-transform values of indegree, fibrinogen or both. In all cases, the estimated relationship between indegree and fibrinogen, net of all controls, is negative and highly significant (p < 0.0001). When re-transforming the coefficient estimates, their substantive magnitudes, measured in terms of standard deviations of fibrinogen, are attenuated from Model 2a by 21% when loge-transforming both indegree and fibrinogen (Model 3a), or indegree alone. Loge-transformation of fibrinogen alone (Model 3b) has no effect compared with the primary, untransformed Model 2a.
In analogous models regressing fibrinogen levels on outdegree (electronic supplementary material, table S2), the relationship between outdegree and fibrinogen is such that the individuals nominating the most friends and family members are predicted to have 0.50 s.d. lower fibrinogen values than those nominating the fewest, after adjusting for age, sex, education and known cardiac risk factors (p < 0.0001, Model 4). Adjusting for the fibrinogen levels of social contacts attenuates the relationship between outdegree and fibrinogen by 9% (Model 5a). When including only friendship ties, outdegree is not significantly associated with fibrinogen (Model 5b).
4. Discussion
Many species, humans included, inhabit complex social structures of dominance and support [29,30] and individuals' positions within these structures can profoundly affect their lives and health [1]. The soluble protein fibrinogen, which permits wound healing in health and thrombosis in disease, is a marker for both coronary risk [16] as well as self-reported isolation and stress [18,20], but has not previously been studied in relation to a sociocentric measure of connectedness. Here, we report on the relationship between degree centrality and levels of fibrinogen in a large human social network. Adjusting for education, demographics, known medical predictors of hypercoagulability, and the fibrinogen levels of an individual's social contacts, a greater number of incoming friendship and familial ties predicts significantly lower levels of fibrinogen.
The magnitude of the relationship is considerable, with social isolation (i.e. low indegree) in our study population, net of controls, predicting elevations in fibrinogen comparable to the effect of smoking. In our models, an additional social connection predicts a greater decrement in fibrinogen than even an additional year of education. Educational attainment is among the most robust and predictive measures of social status as it relates to health [31,32]. Thus, the inclusion of educational attainment as a control in our models allows us to evaluate the relationship between social connectedness and fibrinogen net of socioeconomic status as conventionally defined.
Our basic descriptive analyses of fibrinogen with respect to sex, age and education are consistent with previous studies [18], and the basic direction of the indegree finding is consistent with the vast literature on the socioeconomic gradient in health [33,34]. As in previous studies, smoking accounts for some but not all of the social gradient in fibrinogen [18,35].
What, then, is added by the use of a sociocentric measure of connectedness? Research on social isolation and mortality has conventionally treated networks egocentrically, with the structure and function of networks assessed from the individual's perspective [9]. Though the power of these measures to predict health outcomes is indisputable, what the measures actually mean has been open to much debate, with ‘networks’ often invoked more as metaphor than as measurement [9,36]. In our analyses, greater social connectedness, as measured by the sociocentric measure indegree (the number of times an individual is nominated as a friend or family member by others in the network) is associated with substantially lower levels of fibrinogen. The link between social connectedness and lower fibrinogen reflects independently significant effects of friendship and familial ties, suggesting that different sources of indegree contribute additively to its protective effect. This finding is consistent with research in non-human primates, in which an aggregate measure of connectedness is more predictive of health than a single form of affiliation [37].
By contrast, outdegree (the number of social contacts named by an individual), despite its similar range to indegree in the data, is significantly less predictive of fibrinogen values, and the association vanishes when stratifying by relationship type. While outdegree signals the perceived support and status captured by conventional measures of social connectedness, indegree is more likely to reflect the social support, resources and recognition an individual actually receives. Furthermore, indegree is, by definition, less subject to individual variation in the perception and reporting of the psychosocial environment (such as potential gender effects on the self-reporting of social connections), or to cultural or socioeconomic differences in the ‘self-enhancement’ bias [38].
Social network position has also been theorized to be associated with other features of individuals, such as immunity and personality [39–41]. Being central or peripheral in the social network would, in principle, expose a person to different types of infection risk, and may therefore correlate with both immune genes and function. In this way, diverse physiological (and psychological) phenotypes may be distributed in meaningful ways across human social networks.
Though human and non-human sociality differ in obvious ways, the association between indegree and fibrinogen we observe may have biological relevance beyond humans alone. Though social dominance hierarchies are known to affect the health of their members, most notably in non-human primates [1], recent evidence has also demonstrated affiliative, non-hierarchical and non-kin relationships in primates, birds, ungulates and cetaceans [42]. Such relationships lend themselves to sociocentric network analyses of the type we deploy here [43]. Because the coagulation cascade itself is highly conserved across mammals [44], non-human friendship networks may also modulate fibrinogen and other biomarkers of coagulation and inflammation, although as non-human ties are ascertained through observation rather than nomination, determining the potential directedness of these ties (in order, for instance, to distinguish between indegree and outdegree, as we do here) may require novel ethological methods.
Our study has limitations. The study population of the FHS is exclusively American and predominantly white, and our findings may not generalize to other settings or groups. The FHS social network dataset also has well-known limitations, although these data are still of extremely high quality [45]. More importantly, causal inferences cannot be drawn from a cross-sectional design. Social connectedness or isolation may alter the stress response and thereby change the physiologic set point of fibrinogen and other biomarkers [46,47]. But it may also be the case that disadvantaged individuals, perhaps via early life factors [18], develop both higher fibrinogen and fewer social connections. Future work will benefit from longitudinal measurements of both network position and inflammatory biomarkers across the lifecourse.
More generally, while our study specifies social connectedness more precisely than previous work, the mechanisms through which social connectedness may mediate fibrinogen remain subject to debate. Indegree differences in fibrinogen must reflect indegree differences in the determinants of fibrinogen. Our analyses isolate a robust relationship between social connectedness and fibrinogen net of age, sex, education and conventional predictors of hypercoagulability and cardiac risk, but the specific exposures or resources through which indegree may mediate fibrinogen remain to be shown. Does the relationship between indegree and fibrinogen reflect social support, social influence, social engagement, access to social or material resources or another mechanism altogether?
The now-ubiquitous concept of the coronary ‘risk factor’ originated with the FHS [48]. But while such risk factors have overwhelmingly measured proximate behavioural and biological markers, research on the social determinants of health has identified persistent effects of socioeconomic processes difficult to reconcile with a biomedical approach. How do social phenomena like ‘status’ and ‘support’ translate to the physiologic mechanisms of disease? The causal question is complicated by the variegated and often figurative operationalizations of the concept of ‘social position’ [9]. By specifying social position and connectedness precisely, reproducibly and independently of individual biases in self-report, network analysis can help connect the ‘macro’ level of social structure to the ‘micro’ level psychobiologic mechanisms through which adverse or protective exposures become manifest in individual health and disease.
Supplementary Material
Acknowledgements
We thank Chris O'Donnell for his contributions to data collection, and Laurie Meneades for her help in data preparation.
Ethics
This study was approved by the Yale University Institutional Review Board (Protocol no. 1306012263). All FHS participants provided written informed consent.
Data accessibility
The FHS is conducted and supported by the National Heart, Lung and Blood Institute in collaboration with Boston University (Contract N01-HC-25195). The data used in this study are available on request from the FHS (https://www.framinghamheartstudy.org/researchers/application-review.php).
Authors' contributions
D.A.K., J.H.F. and N.A.C. conceived of the study. D.A.K. and N.A.C. drafted the manuscript and J.H.F. and E.J.B. contributed critical revisions. D.A.K., N.A.C., E.J.B. and J.H.F. contributed to data collection and preparation. D.A.K. performed the statistical analyses and produced the figures. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by NIH grant no. P01AG31093 and by the National Institute on Aging. D.A.K. was supported by the Canadian Institutes of Health Research. The FHS is supported by HHSN268201500001I and N01-HC 25195.
References
- 1.Sapolsky RM. 2005. The influence of social hierarchy on primate health. Science 308, 648–652. ( 10.1126/science.1106477) [DOI] [PubMed] [Google Scholar]
- 2.Berkman LF, Syme SL. 1979. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am. J. Epidemiol. 109, 186–204. [DOI] [PubMed] [Google Scholar]
- 3.House JS, Robbins C, Metzner HL. 1982. The association of social relationships and activities with mortality: prospective evidence from the Tecumsel Community Health Study. Am. J. Epidemiol. 116, 123–140. [DOI] [PubMed] [Google Scholar]
- 4.Holt-Lunstad J, Smith TB, Layton JB. 2010. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 7, e1000316 ( 10.1371/journal.pmed.1000316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang YC, Boen C, Gerken K, Li T, Schorpp K, Harris KM. 2016. Social relationships and physiological determinants of longevity across the human life span. Proc. Natl Acad. Sci. USA 113, 578–583. ( 10.1073/pnas.1511085112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch JW, Kaplan GA, Salonen JT. 1997. Why do poor people behave poorly? Variation in adult health behaviours and psychosocial characteristics by stages of the socioeconomic lifecourse. Social Sci. Med. 44, 809–819. ( 10.1016/s0277-9536(96)00191-8) [DOI] [PubMed] [Google Scholar]
- 7.Christakis NA, Fowler JH. 2008. The collective dynamics of smoking in a large social network. N. Engl. J. Med. 358, 2249–2258. ( 10.1056/NEJMsa0706154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkinson RG. 2000. Mind the gap: hierarchies, health and human evolution. London, UK: Weidenfeld & Nicholson. [Google Scholar]
- 9.Berkman LF, Glass T. 2000. Social integration, social netowrks, social support, and health. In Social epidemiology (eds Berkman LF, Kawachi I), pp. 137–173. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.Lozano R, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128. ( 10.1016/S0140-6736(12)61728-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furie B, Furie BC. 2008. Mechanisms of thrombus formation. N. Engl. J. Med. 359, 938–949. ( 10.1056/NEJMra0801082) [DOI] [PubMed] [Google Scholar]
- 12.Stuart J. 1984. The acute-phase reaction and haematological stress syndrome in vascular disease. Int. J. Microcirc. Clin. Exp. 3, 115–129. [PubMed] [Google Scholar]
- 13.Desborough JP. 2000. The stress response to trauma and surgery. Br. J. Anaesth. 85, 109–117. ( 10.1093/bja/85.1.109) [DOI] [PubMed] [Google Scholar]
- 14.Sapolsky RM. 2004. Social status and health in humans and other animals. Annu. Rev. Anthropol. 33, 393–418. ( 10.1146/annurev.anthro.33.070203.144000) [DOI] [Google Scholar]
- 15.Brunner EJ. 2000. Toward a new social biology. In Social epidemiology (eds Berkman LF, Kawachi I), pp. 306–331. Oxford, UK: Oxford University Press. [Google Scholar]
- 16.Danesh J, Collins R, Appleby P, Peto R. 1998. Association of fibrinogen, c-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 279, 1477–1482. ( 10.1001/jama.279.18.1477) [DOI] [PubMed] [Google Scholar]
- 17.Markowe HLJ, Marmot MG, Shipley MJ, Bulpitt CJ, Meade TW, Stirling Y, Vickers MV, Semmence A. 1985. Fibrinogen: a possible link between social class and coronary heart disease. Br. Med. J. (Clin. Res. Ed.) 291, 1312–1314. ( 10.2307/29521203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunner EJ, Marmot M, Canner R, Beksinska M, Davey Smith G, O'Brien J. 1996. Childhood social circumstances and psychosocial and behavioural factors as determinants of plasma fibrinogen. Lancet 347, 1008–1013. ( 10.1016/S0140-6736(96)90147-6) [DOI] [PubMed] [Google Scholar]
- 19.Davis MC, Swan PD. 1999. Association of negative and positive social ties with fibrinogen levels in young women. Health Psychol. 18, 131–139. ( 10.1037/0278-6133.18.2.131) [DOI] [PubMed] [Google Scholar]
- 20.Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. 2004. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology 29, 593–611. ( 10.1016/S0306-4530(03)00086-6) [DOI] [PubMed] [Google Scholar]
- 21.Lynch JW, Kaplan GA. 2000. Socioeconomic position. In Social epidemiology (eds Berkman LF, Kawachi I), pp. 13–35. Oxford, UK: Oxford University Press. [Google Scholar]
- 22.Chase ID, Tovey C, Spangler-Martin D, Manfredonia M. 2002. Individual differences versus social dynamics in the formation of animal dominance hierarchies. Proc. Natl Acad. Sci. USA 99, 5744–5749. ( 10.1073/pnas.082104199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. 1979. An investigation of coronary heart disease in families. Am. J. Epidemiol. 110, 281–290. [DOI] [PubMed] [Google Scholar]
- 24.Christakis NA, Fowler JH. 2007. The spread of obesity in a large social network over 32 years. N. Engl. J. Med. 357, 370–379. ( 10.1056/NEJMsa066082) [DOI] [PubMed] [Google Scholar]
- 25.Cacioppo JT, Fowler JH, Christakis NA. 2009. Alone in the crowd: the structure and spread of loneliness in a large social network. J. Pers. Soc. Psychol. 97, 977–991. ( 10.1037/a0016076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenquist JN, Murabito J, Fowler JH, Christakis NA. 2010. The spread of alcohol consumption behavior in a large social network. Ann. Intern. Med. 152, 426–433. ( 10.1059/0003-4819-152-7-201004060-00007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman MEJ. 2002. Assortative mixing in networks. Phys. Rev. Lett. 89, 208701 ( 10.1103/PhysRevLett.89.208701) [DOI] [PubMed] [Google Scholar]
- 28.Kenny DA. 1987. Testing measures of association. in statistics for the social and behavioral sciences. Boston, MA: Little, Brown. [Google Scholar]
- 29.Boehm C. 1999. Hierarchy in the forest: the evolution of egalitarian behavior. Cambridge, MA: Harvard University Press. [Google Scholar]
- 30.Krause J, James R, Franks D, Croft D. 2015. Animal social networks. Oxford, UK: Oxford University Press. [Google Scholar]
- 31.Cutler D, Deaton A, Lleras-Muney A. 2006. The determinants of mortality. J. Econ. Perspect. 20, 97–120. ( 10.1257/jep.20.3.97) [DOI] [Google Scholar]
- 32.Link BG, Phelan JC. 1995. Social conditions as fundamental causes of disease. J. Health Soc. Behav. 35, 80–94. ( 10.2307/2626958) [DOI] [PubMed] [Google Scholar]
- 33.Marmot M. 2004. Status syndrome: how social standing affects our health and longevity. London, UK: Bloomsbury. [Google Scholar]
- 34.Wilkinson RG, Pickett KE. 2008. Income inequality and socioeconomic gradients in mortality. Am. J. Public Health 98, 699–704. ( 10.2105/ajph.2007.109637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunner EJ, Marmot MG, White IR, O'Brien JR, Etherington MD, Slavin BM, Kearney EM, Davey Smith G. 1993. Gender and employment grade differences in blood cholesterol, apolipoproteins and haemostatic factors in the Whitehall II study. Atherosclerosis 102, 195–207. ( 10.1016/0021-9150(93)90162-N) [DOI] [PubMed] [Google Scholar]
- 36.Smith KP, Christakis NA. 2008. Social networks and health. Annu. Rev. Soc. 34, 405–429. ( 10.1146/annurev.soc.34.040507.134601) [DOI] [Google Scholar]
- 37.Sapolsky RM, Alberts SC, Altmann J. 1997. Hypercortisolism associated with social subordinance or social isolation among wild baboons. Arch. Gen. Psychiatry 54, 1137–1143. ( 10.1001/archpsyc.54.12.1137) [DOI] [PubMed] [Google Scholar]
- 38.Loughnan S, et al. 2011. Economic inequality is linked to biased self-perception. Psychol. Sci. 22, 1254–1258. ( 10.1177/0956797611417003) [DOI] [PubMed] [Google Scholar]
- 39.Fowler JH, Settle JE, Christakis NA. 2011. Correlated genotypes in friendship networks. Proc. Natl Acad. Sci. USA 108, 1993–1997. ( 10.1073/pnas.1011687108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christakis NA, Fowler JH. 2014. Friendship and natural selection. Proc. Natl Acad. Sci. USA 111, 10 796–10 801. ( 10.1073/pnas.1400825111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fowler JH, Dawes CT, Christakis NA. 2009. Model of genetic variation in human social networks. Proc. Natl Acad. Sci. USA 106, 1720–1724. ( 10.1073/pnas.0806746106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brent LJN, Chang SWC, Gariépy J-F, Platt ML. 2014. The neuroethology of friendship. Ann. N. Y. Acad. Sci. 1316, 1–17. ( 10.1111/nyas.12315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilby IC, Brent LJN, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, Pusey AE. 2013. Fitness benefits of coalitionary aggression in male chimpanzees. Behav. Ecol. Sociobiol. 67, 373–381. ( 10.1007/s00265-012-1457-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michelson AD. 2011. Platelets, 2nd edn London, UK: Academic Press. [Google Scholar]
- 45.Christakis NA, Fowler JH. 2013. Social contagion theory: examining dynamic social networks and human behavior. Stat. Med. 32, 556–577. ( 10.1002/sim.5408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schulkin J. 2003. Allostasis: a neural behavioral perspective. Horm. Behav. 43, 21–27. ( 10.1016/s0018-506x(02)00035-1) [DOI] [PubMed] [Google Scholar]
- 47.Sterling P, Eyer J. 1988. Allostasis: a new paradigm to explain arousal pathology. In Handbook of life stress, cognition and health (eds Fisher S, Reason J), pp. 629–649. New York, NY: John Wiley & Sons. [Google Scholar]
- 48.Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes IJ. 1961. Factors of risk in the development of coronary heart disease—six-year follow-up experience: the Framingham study. Ann. Intern. Med. 55, 33–50. ( 10.7326/0003-4819-55-1-33) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The FHS is conducted and supported by the National Heart, Lung and Blood Institute in collaboration with Boston University (Contract N01-HC-25195). The data used in this study are available on request from the FHS (https://www.framinghamheartstudy.org/researchers/application-review.php).