Abstract
We examined the possibility that whole cell currents are involved and possibly trigger the response of mammalian cells to heat shock. Heat-sensitive cells from a radiation-induced fibrosarcoma (RIF-1) and heat-resistant variants (TR-4, TR-5) were heated at 45 degrees C for 3-30 min. We observed induction of voltage-dependent currents after heating in the heat-resistant cells. These currents decayed to nonmeasurable levels over a period of 6 h. In RIF-1 cells, however, voltage-dependent currents were detectable during heating only; these currents then decayed rapidly. Tetraethylammonium (TEA) cations blocked the currents; changing the concentration of extracellular K+ modified the current-voltage (I-V) relationship. These currents, therefore, resulted from the activation of voltage-dependent K+ channels. Addition of TEA during heating sensitized TR-4 cells to heat but had no effect on the heat response of the RIF-1 cells. Continuous exposure of the RIF-1 cells to 2% (vol/vol) dimethyl sulfoxide (DMSO) for 7 days induced the expression of additional functional, voltage-dependent K+ channels; these gave rise to currents that were measurable after heating. In parallel, these cells became heat resistant. Addition of TEA to DMSO-treated cells blocked channels and returned the heat response almost to the pre-DMSO levels. Our data show a correlation between heat resistance and expression of K+ channels. Because resistance to heat very likely relates to the heat shock response, our data suggest that activation of channels may be a very early event in initiation of the heat shock response.
Full text
PDF
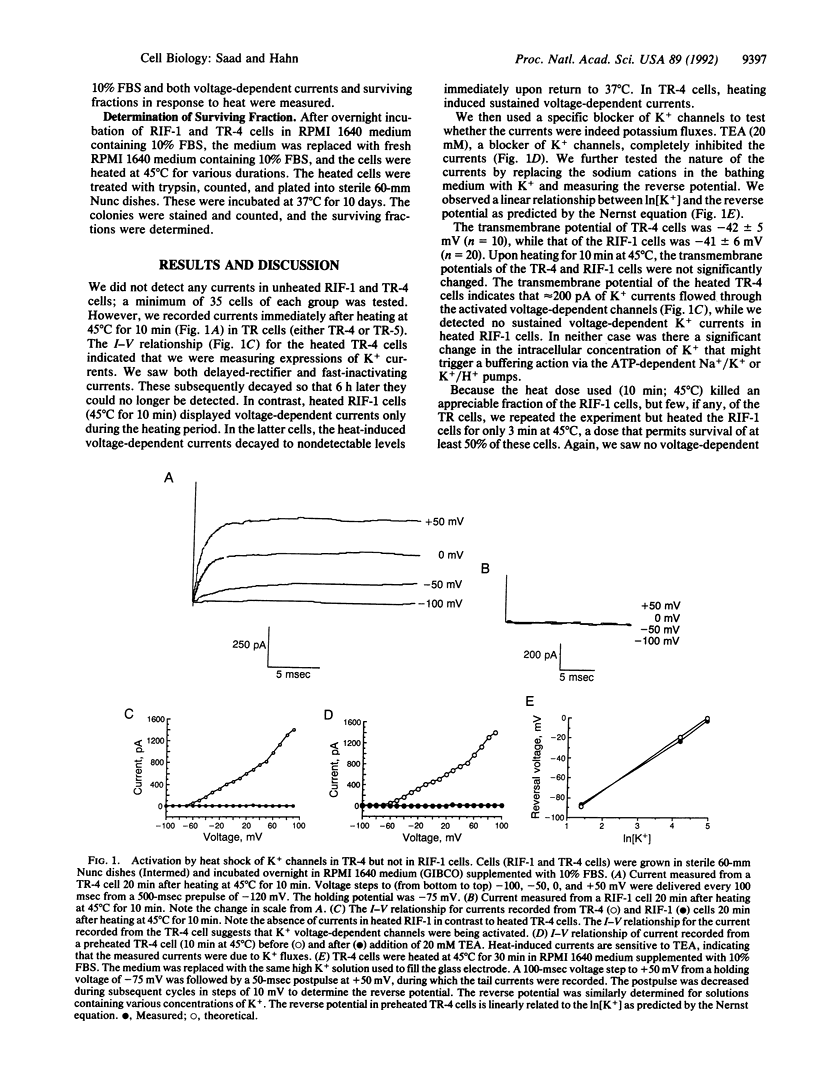
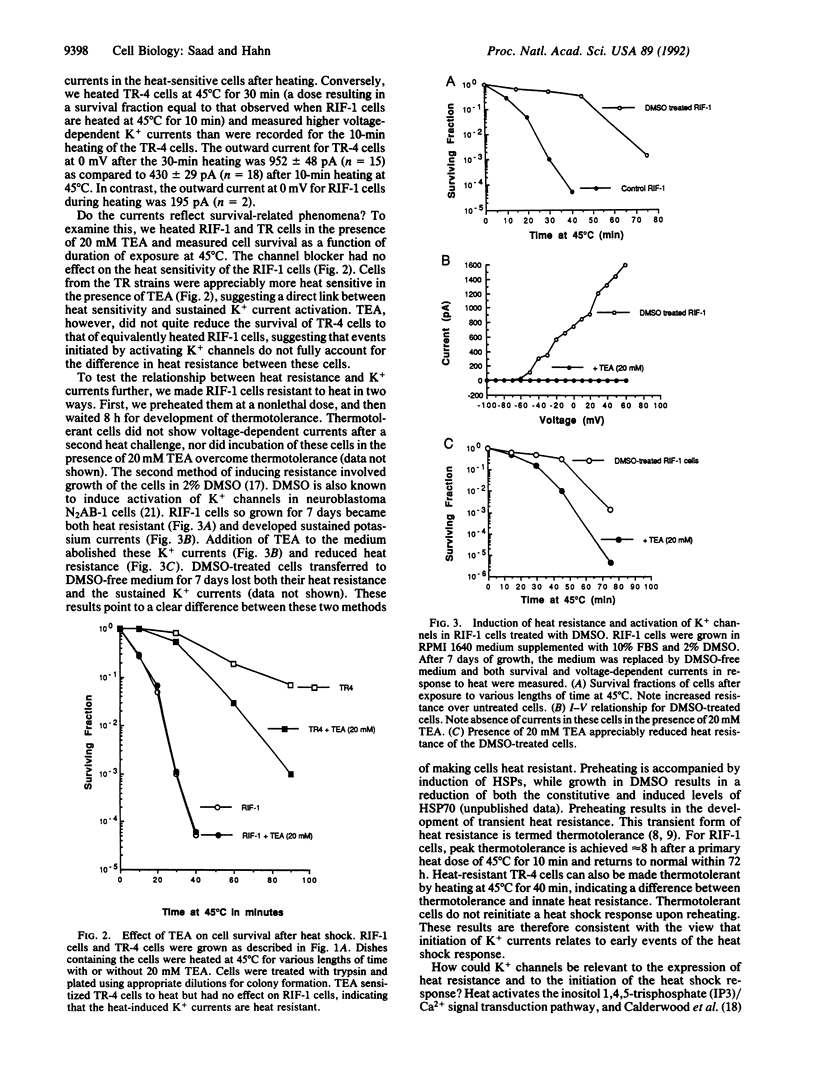

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. L., Van Kersen I., Kraft P. E., Hahn G. M. Biochemical analysis of heat-resistant mouse tumor cell strains: a new member of the HSP70 family. Mol Cell Biol. 1989 Aug;9(8):3509–3516. doi: 10.1128/mcb.9.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Calderwood S. K., Stevenson M. A., Hahn G. M. Effects of heat on cell calcium and inositol lipid metabolism. Radiat Res. 1988 Mar;113(3):414–425. [PubMed] [Google Scholar]
- Campbell S. D., Kruuv J., Lepock J. R. Characterization and radiation response of a heat-resistant variant of V79 cells. Radiat Res. 1983 Jan;93(1):51–61. [PubMed] [Google Scholar]
- Carper S. W., Duffy J. J., Gerner E. W. Heat shock proteins in thermotolerance and other cellular processes. Cancer Res. 1987 Oct 15;47(20):5249–5255. [PubMed] [Google Scholar]
- Fisher G. A., Anderson R. L., Hahn G. M. Glucocorticoid-induced heat resistance in mammalian cells. J Cell Physiol. 1986 Jul;128(1):127–132. doi: 10.1002/jcp.1041280119. [DOI] [PubMed] [Google Scholar]
- Gerner E. W., Boone R., Connor W. G., Hicks J. A., Boone M. L. A transient thermotolerant survival response produced by single thermal doses in HeLa cells. Cancer Res. 1976 Mar;36(3):1035–1040. [PubMed] [Google Scholar]
- Hahn G. M., Shiu E. C., West B., Goldstein L., Li G. C. Mechanistic implications of the induction of thermotolerance in Chinese hamster cells by organic solvents. Cancer Res. 1985 Sep;45(9):4138–4143. [PubMed] [Google Scholar]
- Hahn G. M., van Kersen I. Isolation and initial characterization of thermoresistant RIF tumor cell strains. Cancer Res. 1988 Apr 1;48(7):1803–1807. [PubMed] [Google Scholar]
- Hallberg R. L. No heat shock protein synthesis is required for induced thermostabilization of translational machinery. Mol Cell Biol. 1986 Jun;6(6):2267–2270. doi: 10.1128/mcb.6.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harris M. Temperature-resistant variants in clonal populations of pig kidney cells. Exp Cell Res. 1967 May;46(2):301–314. doi: 10.1016/0014-4827(67)90068-7. [DOI] [PubMed] [Google Scholar]
- Henle K. J., Leeper D. B. Effects of hyperthermia (45 degrees) on macromolecular synthesis in Chinese hamster ovary cells. Cancer Res. 1979 Jul;39(7 Pt 1):2665–2674. [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Acetylcholine activation of K+ channels in cell-free membrane of atrial cells. Am J Physiol. 1986 Sep;251(3 Pt 2):H681–H684. doi: 10.1152/ajpheart.1986.251.3.H681. [DOI] [PubMed] [Google Scholar]
- Landry J., Chrétien P., Lambert H., Hickey E., Weber L. A. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989 Jul;109(1):7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo A., Li G. C. Heat-resistant variants of Chinese hamster fibroblasts altered in expression of heat shock protein. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8029–8033. doi: 10.1073/pnas.82.23.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. C., Li L. G., Liu Y. K., Mak J. Y., Chen L. L., Lee W. M. Thermal response of rat fibroblasts stably transfected with the human 70-kDa heat shock protein-encoding gene. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1681–1685. doi: 10.1073/pnas.88.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade P., Dettbarn C., Volpe P., Alderson B., Otero A. S. Direct inhibition of inositol-1,4,5-trisphosphate-induced Ca2+ release from brain microsomes by K+ channel blockers. Mol Pharmacol. 1989 Oct;36(4):664–672. [PubMed] [Google Scholar]
- Raaphorst G. P., Romano S. L., Mitchell J. B., Bedford J. S., Dewey W. C. Intrinsic differences in heat and/or X-ray sensitivity of seven mammalian cell lines cultured and treated under identical conditions. Cancer Res. 1979 Feb;39(2 Pt 1):396–401. [PubMed] [Google Scholar]
- Ramsay N. A mutant in a major heat shock protein of Escherichia coli continues to show inducible thermotolerance. Mol Gen Genet. 1988 Feb;211(2):332–334. doi: 10.1007/BF00330612. [DOI] [PubMed] [Google Scholar]
- Smith-Maxwell C. J., Eatock R. A., Begenisich T. Induction of K-channel expression in a neuroblastoma cell line. J Neurobiol. 1991 Jun;22(4):327–341. doi: 10.1002/neu.480220403. [DOI] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- VanBogelen R. A., Acton M. A., Neidhardt F. C. Induction of the heat shock regulon does not produce thermotolerance in Escherichia coli. Genes Dev. 1987 Aug;1(6):525–531. doi: 10.1101/gad.1.6.525. [DOI] [PubMed] [Google Scholar]
- Yatani A., Birnbaumer L., Brown A. M. Direct coupling of the somatostatin receptor to potassium channels by a G protein. Metabolism. 1990 Sep;39(9 Suppl 2):91–95. doi: 10.1016/0026-0495(90)90220-7. [DOI] [PubMed] [Google Scholar]



