Abstract
Climate-induced ocean warming and acidification may render marine organisms more vulnerable to infectious diseases. We investigated the effects of warming and acidification on the immune response of the sea urchin Heliocidaris erythrogramma. Sea urchins were gradually introduced to four combinations of temperature and pHNIST (17°C/pH 8.15, 17°C/pH 7.6, 23°C/pH 8.15 and 23°C/pH 7.6) and then held in temperature–pH treatments for 1, 15 or 30 days to determine if the immune response would adjust to stressors over time. Coelomocyte concentration and type, phagocytic capacity and bactericidal activity were measured on day 1, 15 and 30 with different sea urchins used each time. At each time point, the coelomic fluid of individuals exposed to increased temperature and acidification had the lowest coelomocyte concentrations, exhibited lower phagocytic capacities and was least effective at inhibiting bacterial growth of the pathogen Vibrio anguillarum. Over time, increased temperature alleviated the negative effects of acidification on phagocytic activity. Our results demonstrate the importance of incorporating acclimation time to multiple stressors when assessing potential responses to future ocean conditions and indicate that the immune response of H. erythrogramma may be compromised under near-future ocean warming and acidification.
Keywords: disease, climate change, innate immunity, echinoderm, Heliocidaris
1. Introduction
Climate-induced changes to ocean temperature and pH are occurring rapidly [1]. One serious concern is the effect of increased temperatures and acidification on organismal stress levels and on the incidence and severity of disease. In marine invertebrates, climate change is altering the interactions between hosts and their pathogens [2] resulting in increased incidence of pathogen outbreaks, altered disease transmission due to shifts in species distributions and changes in host immune systems [2]. Elevated temperatures have been implicated in a number of devastating disease outbreaks, including the spread of white-band coral disease in the Caribbean [3], withering syndrome in Californian red abalone [4], protozoan infections in oysters [5], bacterial infections in shallow Mediterranean sponges [6] and the recent outbreak of sea star wasting disease along the Pacific coast of North America [7,8].
The impacts of disease and mortality in marine ecosystems are particularly important when the affected species are ecosystem engineers such as sea urchins [9]. For sea urchins, recent outbreaks of disease and resultant mortality appear to be strongly influenced by increased temperature [9–12]. Elevated seawater temperature in the eastern Atlantic has been correlated with outbreaks of sea urchin disease, most probably caused by the bacteria Vibrio alginolyticus, which reduced local sea urchin populations by up to 65% [9]. In the Canary Islands, a large die off in Paracentrotus lividus due to bald sea urchin disease where necrotic lesions of tissue develop on the test may have been triggered by elevated temperatures and low wave heights [11]. In eastern Australia, ocean warming is correlated with increased incidence of a similar disease characterized by dark test lesions, spine loss and mortality in Heliocidaris erythrogramma and Holopneustes purpurascens [12]. This disease occurs in many sea urchin species and is associated with aggressive bacterial infections by marine pathogens such as Vibrio anguillarum [13] which can result in significant mortality in sea urchin populations [14]. The slow recovery of Diadema antillarum following the mass mortality in the early 1980s may be due to compromised immunity [15]. Massive sea urchin die-offs may cause shifts in alternative stable states, e.g. from sea urchin barrens to kelp forests [16] and from live coral reefs to macroalgal-dominated reefs [17], as well as influencing the productivity, health and distribution of seagrass beds [18]. Therefore, disease outbreaks in sea urchins can have major ecological implications.
Sea urchins possess a relatively sophisticated innate immune system which mediates their non-specific response to marine pathogens [19]. Coelomocytes, common cells found in the open circulatory system are the primary ‘effectors’ of this innate immunity [20,21] and include amoebocytes (red and white), vibratile cells and phagocytes [20]. The immune functions of coelomocytes are thought to include antibacterial activity, clotting, oxygen transport, chemotaxis and phagocytosis [19]. Red amoebocyte levels are suggested to be a bioindicator of abiotic stress such as from increased temperature or pollution [22,23].
Studies of the impact of ocean acidification during short-term (7-day) exposure to pH 7.7 report an increase in the total coelomocyte count in a sea urchin (Strongylocentrotus droebachiensis) and in a sea star (Leptasterias polaris) [24], but a decrease in the phagocyte count in the sea star Asterias rubens during short- (7-day) and long (six-month) exposures [24]. With regard to warming (2–10°C above ambient), coelomocyte adhesion, spreading and phagocytic capacity in the sea urchins Lytechinus variegatus, Sterechinus neumayeri and Echinometra lucunter are compromised [23,25]. However, in an ocean simultaneously warming and decreasing in pH, it is important to investigate both stressors because they can have additive, synergistic or antagonistic effects [26,27].
We investigated the effects of increased temperature and acidification on the immune response of the sea urchin H. erythrogramma. The sea urchins were gradually conditioned with incremental change in these factors over 30 days and were then held in their new environment for up to 30 days and their immune response parameters were determined. Heliocidaris erythrogramma is ecologically important as it is the most abundant sea urchin species in eastern Australia [28], a global warming hot spot [29] where surface seawater temperatures (SST) are increasing three to four times the global average [30]. This rapid warming is correlated with increased disease in H. erythrogramma [12]. By the year 2100, SST in this region are estimated to increase by up to 4°C, while pH is estimated to decline by up to 0.5 units (scenario RCP 8.5) [1]. In addition to these changes, the H. erythrogramma used in this study experience temperature increases during day time low neap tides of 2–3°C and 4–7°C in winter and summer, respectively, and night time decreases in pH from a mean pHNIST of 8.19 to pHNIST 7.9 (M Byrne 2010–2014, unpublished data). These fluctuations are predicted to become more extreme under near-future conditions [31]. Several studies on the response of H. erythrogramma to combined warming and acidification indicate that larvae, juveniles and adults are resilient to moderate changes in these stressors and that warming (approx. more than 4°C above ambient) is the more important stressor with significant negative impacts [31–33]. A recent study revealed that warming and acidification had a negative additive effect on the metabolism of juvenile and adult H. erythrogramma acclimated to near-future conditions indicating that near-future climate change will incur a substantial energetic cost [34]. Here we investigate if near-future warming and acidification also impairs immune function, a key feature of sea urchin health that remains to be assessed.
The purpose of this experiment was to characterize the acute immune response of H. erythrogramma on day 1 after reaching a new warming and acidification environment following gradual introduction of these stressors and how this changed over longer acclimation periods (15 and 30 days). The latter was important to determine if the immune system would adjust its response to the new environment over time to maintain its defensive function. We assessed immune function by measuring coelomocyte concentration, types of coelomocytes (including the suitability of red amoebocytes as a stress indicator) and phagocytic activity. Additionally, we assessed the bactericidal activity of coelomic fluid against V. anguillarum, a common marine pathogen that is the primary cause of bald sea urchin disease in H. erythrogramma and many other sea urchin species [12,13].
2. Material and methods
(a). Collection, maintenance and experimental conditions
Heliocidaris erythrogramma (83 ± 14 mm diameter, 70.1 ± 3.2 g wet weight;  ± s.e.; n = 120) were collected at low tide (0.5–1.0 m depth) in winter (June 2014) from Chowder Bay (33°84′ S, 151°26′ E) and Little Bay (33°98′ S, 151°24′ E) New South Wales (NSW), Australia. In the weeks prior to collection the sea urchins experienced SST of 17–18°C (M Byrne 2010–2014, unpublished logger data). The sea urchins were immediately transported to the Sydney Institute of Marine Science. Six sea urchins were randomly placed in each of 20 aquaria (32 l) supplied with flow-through (400 ml min−1) filtered fresh natural seawater (FSW, 20 µm) under ambient conditions (17°C, pH 8.15, a salinity of 35) with aeration for a week while their post-collection condition was monitored. This sea urchin density was far lower than in nature to avoid potential stress from overcrowding.
± s.e.; n = 120) were collected at low tide (0.5–1.0 m depth) in winter (June 2014) from Chowder Bay (33°84′ S, 151°26′ E) and Little Bay (33°98′ S, 151°24′ E) New South Wales (NSW), Australia. In the weeks prior to collection the sea urchins experienced SST of 17–18°C (M Byrne 2010–2014, unpublished logger data). The sea urchins were immediately transported to the Sydney Institute of Marine Science. Six sea urchins were randomly placed in each of 20 aquaria (32 l) supplied with flow-through (400 ml min−1) filtered fresh natural seawater (FSW, 20 µm) under ambient conditions (17°C, pH 8.15, a salinity of 35) with aeration for a week while their post-collection condition was monitored. This sea urchin density was far lower than in nature to avoid potential stress from overcrowding.
The aquaria were randomly assigned to one of four experimental treatments, with five aquaria per treatment in a temperature-controlled room (19°C). Each aquarium contained six sea urchins. Over a 30-day conditioning period, the temperature and pH of the header tanks supplying the designated aquaria were adjusted every 3 days (+1°C, −0.1 pH unit) until target temperature and pH levels in the experimental treatments (17°C/pH 7.6, 23°C/pH 8.15 and 23°C/pH 7.6) were reached. The ambient aquaria (17°C/pH 8.15) were not changed and were used as the control conditions, while the other three treatments represented exposure to near-future conditions of temperature and/or pH [35]. On each adjustment the change was gradual because it took most of the day for the conditions in the holding aquaria to reach the new set values. Seawater temperature was controlled with EHEIM Jager heaters (75 W) placed in header tanks and seawater pH was manipulated by bubbling CO2 and air through a large air stone into header tanks until the desired levels were reached. All header and experimental tanks had tightly fitting plastic lids, to prevent the outgassing of CO2 during the experiment and dissolved oxygen levels in all tanks were maintained at more than 95%. Throughout the study the sea urchins were held under a 12 L : 12 D cycle and fed an excess of Sargassum sp. once per week. The tanks were cleaned every third day to remove faeces and uneaten algae. This siphoning exercise resulted in removal of one-third of the experimental water on each occasion which was gradually refilled by the water from the header tanks.
Seawater temperatures and pH were measured in each experimental aquarium once per day using a 6391A-LabQuest 2 Vernier Temperature Probe and Tris-Compatible Flat pH Sensor. The probe was calibrated with NIST (high precision) buffers pH 4 and 10 (ProSciTech, Kirwan, Qld, Australia). Seawater temperatures and pH determined once the target values were reached are presented in electronic supplementary material, table S1. Water samples were fixed with mercuric chloride and total alkalinity (AT) was determined by potentiometric titration (907 Titrando, Metrohm) using certified reference standards. pCO2, ΩCa and ΩAr were calculated with CO2SYS [36] using data for salinity, temperature, pHNIST and average AT of source water and the Mehrbach et al. (1973) dissociation constants refitted by Dickson & Millero [37] (electronic supplementary material, table S2).
(b). Experimental design
All immune response parameters were measured on days 1, 15 and 30 following the gradual (30-day) exposure to experimental temperature and pH levels. At each time point (1, 15 and 30 days), 2 ml of coelomic fluid was withdrawn using a glass syringe equipped with an 18-gauge needle from each of two randomly selected sea urchins per aquarium. This was done rapidly, one urchin at a time with prompt analysis of the coelomic fluid to reduce the potential impact of handling on the immune parameters. Each syringe contained 2 ml of an anticoagulant solution [38] to prevent clotting of the coelomic fluid. Coelomic fluid removed from the first individual in each aquarium (n = 5 per treatment) was used to quantify the number and type of coelomocytes, as well as bactericidal activity of coelomic fluid against the bacterium V. anguillarum. The coelomic fluid from the second individual in each aquarium (n = 5 per treatment) was used to quantify the phagocytic capacity of coelomocytes. Those individuals were removed from the aquaria so they would not be resampled during later time points. Therefore, the number of sea urchins in each aquarium decreased over the course of the experiment, but as the initial density was very low and the conditions were flow-through, with renewal of one-third of the water every 3 days, we do not anticipate that this would affect our results. Importantly, each replicate had identical treatment.
(c). Coelomocyte counts, phagocytosis and bactericidal activity
After each experimental exposure period, the number and type of coelomocytes (per unit volume coelomic fluid) was quantified by adding 100 µl of coelomic fluid to a haemocytometer and counting at least 100 coelomocytes. Counts were performed in triplicate for each sea urchin and the mean value was used as the datum for analysis.
The phagocytic capacity of coelomocytes was determined by adding 100 µl of coelomic fluid to a glass slide, allowing coelomocytes to spread and adhere for 1 h while being held at room temperature (20°C), and then exposing them to 100 µl of a yeast solution (Saccharomyces cerevisiae) for an additional hour. The yeast solution was diluted with sterile seawater until it contained approximately 10 cells per phagocytic coelomocyte. The coelomocytes were then examined with phase microscopy and phagocytic capacity calculated as:
After each experimental exposure period, the bactericidal activity of coelomic fluid against a common marine pathogen, V. anguillarum cultured in Difco marine broth 2216 at 20°C was determined. After 24 h, the bacterial solution was serially diluted to approximately 4000 colony forming bacteria ml−1 [39] and 0.1 ml of the diluted bacterial suspension was added to 1.9 ml of coelomic fluid containing coelomocytes. Controls consisted of 0.1 ml of bacterial suspension added to 1.9 ml of control solutions (sterile seawater or sterile marine broth; n = 5 each) and were used to verify bacterial viability.
The bacterial suspensions and coelomic fluid or control solutions were incubated at 20°C and 0.1 ml subsamples were spread plated on marine agar plates (75% Difco marine broth 2216 + 25% Difco Bactoagar) at 0, 24 and 48 h. After incubation for 24 h at 20°C, the bacterial colonies on each plate were counted and used to calculate the bacterial survival index as:
Subsamples plated at time zero were compared with 0.1 ml subsamples plated from the stock bacterial solution and the bacterial survival index equation was modified to:
A survival index value of 100 indicated bacterial counts in the subsamples (bacterial suspension and coelomic fluid) were equal to bacterial counts in the subsamples at time zero (i.e. no bacterial growth or clearance occurred). An index value of greater than 100 indicated an increase in bacterial numbers while a value less than 100 indicated bacterial clearance or decrease from the coelomic fluid, as indicative of bactericidal activity [39].
(d). Statistical analyses
Prior to statistical analysis normality and homogeneity of the data were confirmed using Shapiro–Wilk's and Bartlett's tests for equality of variances, respectively. Coelomocyte concentration, red amoebocyte concentration and phagocytic activity were analysed using a three-way analysis of variance (ANOVA) with time (1, 15 and 30 days), temperature (17°C and 23°C) and pH (7.6 and 8.15) as fixed factors. Treatments that differed were compared using Tukey's post hoc test. The types of coelomocytes comprising the coelomic fluid were analysed at each time point (1, 15 and 30 days) using the R vegan package, Adonis function with temperature and pH as fixed factors and the similarity percentage (SIMPER) analysis.
The data for bactericidal activity of coelomic fluid on day 1, 15 and 30 in three incubation times with V. anguillarum (0, 24 and 48 h) were not homogeneous. However, as the ANOVA is relatively robust to violations of homogeneity [40], these data were analysed using a three-way ANOVA with time, temperature and pH as fixed factors and Tukey's post hoc test. GraphPad Prism 6 and R software [41] were used to conduct all statistical analyses and p < 0.05 was considered significant.
3. Results
(a). Coelomocyte concentration
Control sea urchins maintained in ambient treatment conditions (17°C/pH 8.15) had coelomocyte concentrations between 3.07 and 5.67 × 106 cells ml−1 in their coelomic fluid (figure 1). Time (F2,48 = 4.62; p = 0.015; D30 > D1) and temperature (F1,48 = 33.62; p < 0.0001; 17°C > 23°C) but not pH had a significant negative effect on coelomocyte concentrations (figure 1; table 1). Increased temperature alone (days 15 and 30) and both stressors in combination (days 1, 15 and 30) lowered the coelomocyte concentration by approximately one-third (figure 1).
Figure 1.
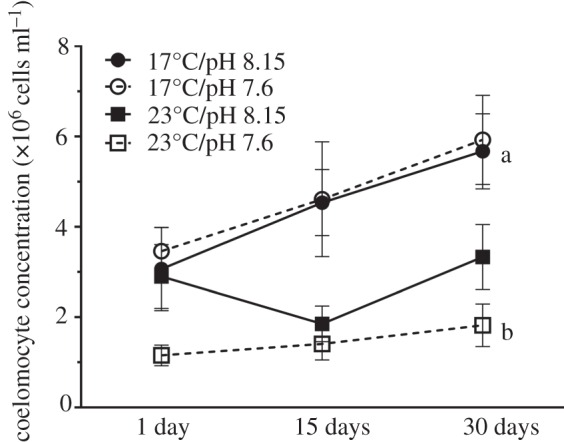
Coelomocyte concentration (×106 cells ml−1;  ± s.e.) in coelomic fluid collected from Heliocidaris erythrogramma that were exposed to temperature and pH treatments for 1, 15 and 30 days (n = 5 per treatment) following a 30-day conditioning period. Letters indicate significant differences between the control (17°C/pH 8.15) and experimental treatments within each time point.
± s.e.) in coelomic fluid collected from Heliocidaris erythrogramma that were exposed to temperature and pH treatments for 1, 15 and 30 days (n = 5 per treatment) following a 30-day conditioning period. Letters indicate significant differences between the control (17°C/pH 8.15) and experimental treatments within each time point.
Table 1.
Three-way ANOVA of the effects of time, temperature and pH on the coelomocyte concentration of Heliocidaris erythrogramma exposed to temperature and pH treatments for 1, 15 and 30 days (n = 5 per treatment). Significant factors (p < 0.05) are indicated in italics.
| measurement | factor | d.f. | MS | F | p-value | Tukey's HSD |
|---|---|---|---|---|---|---|
| coelomocyte concentration | time | 2 | 1.26 × 1013 | 4.62 | 0.015 | D30 > D1 |
| temperature | 1 | 9.13 × 1013 | 33.62 | <0.0001 | 17°C > 23°C | |
| pH | 1 | 3.70 × 1012 | 1.36 | 0.249 | ||
| time : temperature | 2 | 5.78 × 1012 | 2.13 | 0.130 | ||
| time : pH | 2 | 3.68 × 1011 | 0.14 | 0.874 | ||
| temperature : pH | 1 | 8.17 × 1012 | 3.01 | 0.089 | ||
| time : temperature : pH | 2 | 9.00 × 1011 | 0.33 | 0.720 | D30 17°C/pH 8.15 > D30 23°C/pH 7.6 | |
| residuals | 48 | 2.72 × 1012 |
The two stressor treatments had the greatest effect on coelomocyte concentration. After 30 days in experimental conditions, post hoc comparisons indicated that the coelomic fluid of sea urchins exposed to increased temperature and acidification contained significantly fewer coelomocytes than that from sea urchins in the other treatments (Tukey's HSD; p = 0.025; figure 1; table 1). The negative effect of the two stressors may have been additive, but the significant effect of temperature (table 1) indicates that the negative effect was largely driven by warming.
(b). Cell types
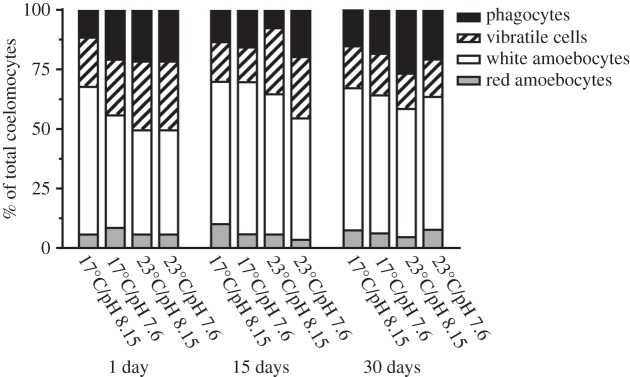
The coelomic fluid contained amoebocytes (red and white), vibratile cells and phagocytes (petalloid and dendritic; electronic supplementary material, figure S1). White amoebocytes were the most common cell type in control sea urchins (43.7–63.9% of the cells), and this was fairly consistent at each time point (figure 2).
Figure 2.
Percentage of red amoebocytes, white amoebocytes, vibratile cells and phagocytes (dendritic and petalloid) in the total coelomocyte counts in coelomic fluid collected from Heliocidaris erythrogramma. Sea urchins were exposed to temperature and pH treatments for 1, 15 and 30 days (n = 5 per treatment).
On day 1, only pH had a significant effect on the cell types in the coelomic fluid (Adonis, F1,16 = 5.087, R2 = 0.202, p = 0.018), with the largest dissimilarity (22.39%) observed between the 23°C/pH 8.15 and 23°C/pH 7.6 treatments (electronic supplementary material, table S3). Within all treatments on day 1, white amoebocytes and phagocytes contributed the most to the dissimilarity between treatments. On day 15, only temperature had a significant effect on the coelomocyte populations (Adonis, F1,16 = 6.016, R2 = 0.235, p = 0.003) with the largest dissimilarity (19.36%) observed between the 17°C/pH 7.6 and 23°C/pH 7.6 treatments (electronic supplementary material, table S4). By day 30, temperature (Adonis, F1,16 = 13.252, R2 = 0.247, p = 0.002) and pH (Adonis, F1,16 = 12.220, R2 = 0.228, p = 0.001) had a significant effect on the types of coelomocytes in the coelomic fluid, with the largest dissimilarity (48.27%) observed between the 17°C/pH 8.15 and 23°C/pH 7.6 treatments (electronic supplementary material, table S5). The reduction in vibratile cells was the largest contributor to this dissimilarity (45.59%; electronic supplementary material, table S5). In addition, at day 30, there was an interactive effect between temperature and pH (Adonis, F1,16 = 12.097, R2 = 0.253, p = 0.002).
Temperature had a significant effect on the concentration of red amoebocytes, with fewer cells in the 23°C treatments (F1,53 = 4.78; p = 0.033; 17°C > 23°C; figure 2; table 2). This difference, however, was small with 6–11% red amoebocytes in the 17°C treatments and 4–8% in the 23°C treatments.
Table 2.
Three-way ANOVA of the effects of time, temperature and pH on the red amoebocyte concentration of Heliocidaris erythrogramma that were exposed to temperature and pH treatments for 1, 15 and 30 days (n = 5 per treatment). Significant factors (p < 0.05) are indicated in italics.
| measurement | factor | d.f. | MS | F | p-value | Tukey's HSD |
|---|---|---|---|---|---|---|
| red coelomocyte concentration | time | 1 | 0.15 | 0.02 | 0.903 | |
| temperature | 1 | 49.8 | 4.78 | 0.033 | 17°C > 23°C | |
| pH | 1 | 1.48 | 0.14 | 0.707 | ||
| time : temperature | 1 | 1.32 | 0.13 | 0.723 | ||
| time : pH | 1 | 0.46 | 0.04 | 0.834 | ||
| temperature : pH | 1 | 5.22 | 0.50 | 0.482 | ||
| time : temperature : pH | 1 | 30.46 | 2.93 | 0.093 | ||
| residuals | 53 | 10.41 |
(c). Phagocytic activity
There was a significant effect of time (F2,48 = 9.23; p = 0.0004; D15 = D30 > D1) and pH (F2,48 = 46.79; p < 0.0001; 8.15 > 7.6) on phagocytic activity and an interactive effect between time and temperature (F2,48 = 13.73; p < 0.0001; D1/17°C > D1/23°C; D15/17°C > D15/23°C; D30/23°C > D30/17°C), and between temperature and pH (F1,48 = 76.33; p < 0.0001; 17°C/8.15 > 17°C/7.6 = 23°C/8.15 = 23°C/7.6; table 3). Thus, the effects of the stressors on phagocytic activity were complex.
Table 3.
Three-way ANOVA of the effects of time, temperature and pH on the phagocytic activity of coelomocytes collected from Heliocidaris erythrogramma exposed to temperature and pH treatments for 1, 15 and 30 days (n = 5 per treatment). Significant factors (p < 0.05) are indicated in italics.
| measurement | factor | d.f. | MS | F | p-value | Tukey's HSD |
|---|---|---|---|---|---|---|
| phagocytic activity | time | 2 | 325 | 9.23 | 0.0004 | D15 = D30 > D1; |
| temperature | 1 | 8.3 | 0.24 | 0.629 | ||
| pH | 1 | 1647.3 | 46.79 | <?0.0001 | 8.15 > 7.6 | |
| time : temperature | 2 | 483.5 | 13.73 | <?0.0001 | D1/17°C > D1/23°C; D15/17°C > D15/23°C; D30/23°C > D30/17°C | |
| time : pH | 2 | 36.7 | 1.04 | 0.360 | ||
| temperature : pH | 1 | 2687.4 | 76.33 | <?0.0001 | 17°C/8.15 > 17°C/7.6 = 23°C/8.15 = 23°C/7.6 | |
| time : temperature : pH | 2 | 7.6 | 0.22 | 0.807 | ||
| residuals | 48 | 35.2 |
Throughout the experiment the coelomic fluid of the sea urchins held in the control treatment had a high phagocytic activity (29–35%). The phagocytic activity of sea urchins in all the experimental treatments was 30% lower on day 1. By day 15, phagocytic activity of sea urchins in the 23°C treatments had increased but remained 50% lower than that of control sea urchins (figure 3), and after 30 days, the phagocytic activity of the coelomic fluid of sea urchins in the control and elevated temperature treatments (23°C/pH 8.15 and 23°C/pH 7.6) were similar (figure 3; table 3) indicating a recovery of phagocytic activity in the warm treatments. By contrast, phagocytic activity in the coelomic fluid of urchins from the 17°C/pH 7.6 treatment was depressed at every time point.
Figure 3.
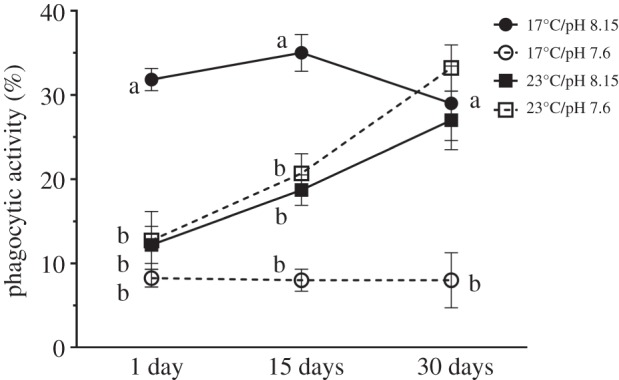
Phagocytic activity (%;  ± s.e.) of dendritic and petalloid phagocytes from Heliocidaris erythrogramma that were exposed to temperature and pH treatments for 1, 15 and 30 days (n = 5 per treatment). Letters indicate significant differences between the control (17°C/pH 8.15) and experimental treatments within each exposure period.
± s.e.) of dendritic and petalloid phagocytes from Heliocidaris erythrogramma that were exposed to temperature and pH treatments for 1, 15 and 30 days (n = 5 per treatment). Letters indicate significant differences between the control (17°C/pH 8.15) and experimental treatments within each exposure period.
(d). Bactericidal activity
Immediately after combining the bacterial suspensions of V. anguillarum with coelomic fluid (time zero), the bacterial survival index of coelomic fluid from sea urchins in all temperature and pH treatments was approximately 100, indicating that there was no immediate effect of coelomic fluid source on the bacterial suspension (figure 4a). This effect remained consistent regardless of how long the sea urchins were held in the conditions (1, 15 or 30 days), and there was no significant effect of time, temperature or pH on bacterial survival indices at time zero (table 4).
Figure 4.
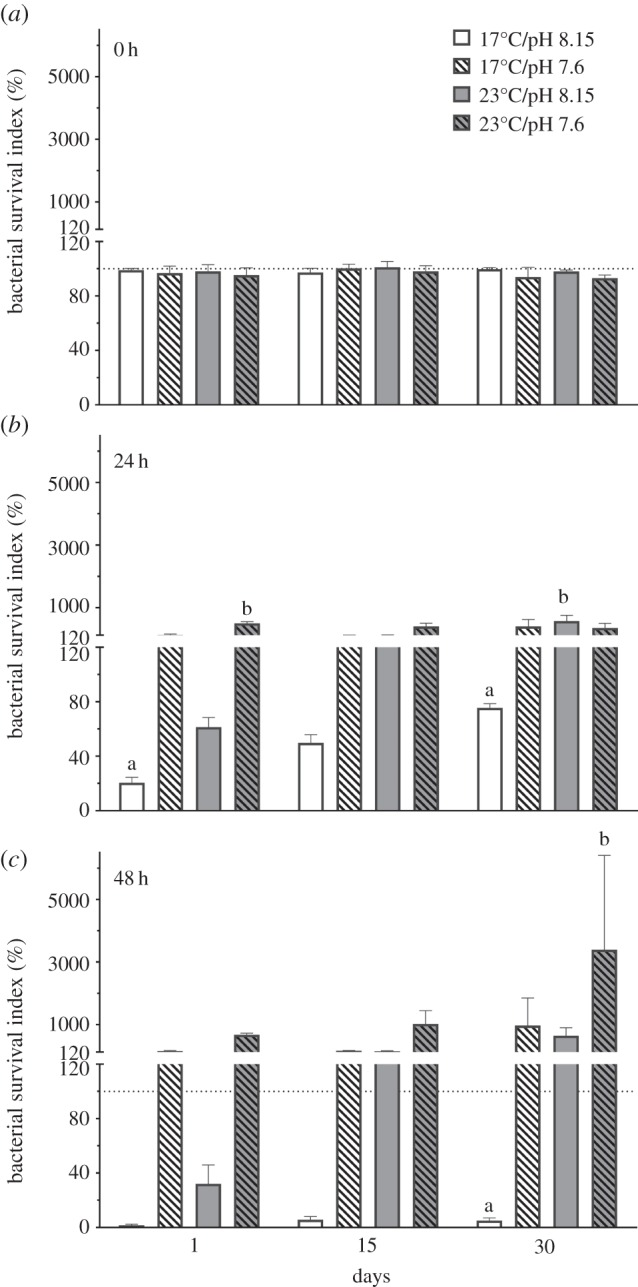
Bacterial survival indices (%;  ± s.e.) of Vibrio anguillarum exposed to coelomic fluid collected from Heliocidaris erythrogramma. Sea urchins were exposed to temperature and pH treatments for 1, 15 and 30 days and the coelomic fluid and bacterial suspensions were spread plated after (a) 0 (b) 24 and (c) 48 h (n = 5 per treatment). Letters indicate significant differences between the control (17°C/pH 8.15) and experimental treatments within each time point (1, 15 and 30 days). The dashed line indicates 100% bacterial survival. An index value of 100 indicates that bacterial counts in the subsamples (bacterial suspension and CF) were equal to bacterial counts in the subsamples at the beginning of the experiment (time zero). An index value of greater than 100 indicates bacterial growth while a value less than 100 indicates bacterial clearance due to bactericidal activity [39].
± s.e.) of Vibrio anguillarum exposed to coelomic fluid collected from Heliocidaris erythrogramma. Sea urchins were exposed to temperature and pH treatments for 1, 15 and 30 days and the coelomic fluid and bacterial suspensions were spread plated after (a) 0 (b) 24 and (c) 48 h (n = 5 per treatment). Letters indicate significant differences between the control (17°C/pH 8.15) and experimental treatments within each time point (1, 15 and 30 days). The dashed line indicates 100% bacterial survival. An index value of 100 indicates that bacterial counts in the subsamples (bacterial suspension and CF) were equal to bacterial counts in the subsamples at the beginning of the experiment (time zero). An index value of greater than 100 indicates bacterial growth while a value less than 100 indicates bacterial clearance due to bactericidal activity [39].
Table 4.
Three-way ANOVA of the effects of time, temperature and pH on the bactericidal activity of coelomocytes collected from Heliocidaris erythrogramma exposed to temperature and pH treatments for 1, 15 and 30 days. Bactericidal activity was measured after (a) 0 (b) 24 and (c) 48 h after bacterial suspensions and coelomic fluid were combined (n = 5 per treatment). Significant factors (p < 0.05) are indicated in italics.
| measurement | factor | d.f. | MS | F | p-value | Tukey's HSD |
|---|---|---|---|---|---|---|
| (a) bacterial survival—0 h | time | 2 | 31.22 | 0.50 | 0.608 | |
| temperature | 1 | 0.27 | 0.00 | 0.948 | ||
| pH | 1 | 72.6 | 1.17 | 0.285 | ||
| time : temperature | 2 | 3.32 | 0.05 | 0.948 | ||
| time : pH | 2 | 45.15 | 0.73 | 0.488 | ||
| temperature : pH | 1 | 15.00 | 0.24 | 0.625 | ||
| time : temperature : pH | 2 | 13.65 | 0.22 | 0.803 | ||
| residuals | 48 | 62.06 | ||||
| (b) bacterial survival—24 h | time | 2 | 206 552 | 5.93 | 0.005 | D30 > D1 = D15 |
| temperature | 1 | 622 405 | 17.87 | <0.0001 | 23°C > 17°C | |
| pH | 1 | 420 509 | 12.07 | 0.001 | 7.6 > 8.15 | |
| time : temperature | 2 | 3443 | 0.10 | 0.906 | ||
| time : pH | 2 | 58 958 | 1.69 | 0.195 | ||
| temperature : pH | 1 | 23 | 0.00 | 0.98 | ||
| time : temperature : pH | 2 | 276 014 | 7.92 | 0.001 | D1 23°C/7.6 > D1 17°C/8.15; D30 23°C/8.15 > D30 17°C/8.15 | |
| residuals | 48 | 34 835 | ||||
| (c) bacterial survival—48 h | time | 2 | 6 473 404 | 6.18 | 0.004 | D30 > D1; D30 > D15 |
| temperature | 1 | 8 836 611 | 8.44 | 0.006 | 23°C > 17°C | |
| pH | 1 | 12 514 493 | 11.95 | 0.001 | 7.6 > 8.15 | |
| time : temperature | 2 | 2 243 521 | 2.14 | 0.129 | ||
| time : pH | 2 | 3 225 822 | 3.08 | 0.055 | ||
| temperature : pH | 1 | 3 630 960 | 3.47 | 0.069 | ||
| time : temperature : pH | 2 | 566 451 | 0.54 | 0.586 | D30 23°C/7.6 > D30 17°C/8.15 | |
| residuals | 48 | 1 047 356 |
After the bacterial suspensions and coelomic fluid were incubated for 24 h, there was a significant effect of time (F2,48 = 5.93; p = 0.005; D30 > D1 = D15), temperature (F1,48 = 17.87; p < 0.0001; 23°C > 17°C) and pH (F1,48 = 12.07; p = 0.001; 7.6 > 8.15) on bacterial survival indices, as well as a significant interaction between time, temperature and pH (F1,48 = 7.92; p = 0.001; D1 23°C/7.6 > D30 17°C/8.15; D30 23°C/8.15 > D30 17°C/8.15; table 4b). The coelomic fluid from sea urchins held in ambient conditions for 1, 15 and 30 days had bacterial survival indices less than 100, indicating that the coelomic fluid was relatively effective at bacterial clearance (figure 4b). However, the coelomic fluid of sea urchins exposed to reduced pH for 1 day and elevated temperatures or reduced pH for longer periods (15 and 30 days) had bacterial survival indices greater than 100 and, therefore, was less effective at inhibiting bacterial growth (figure 4b and table 4).
After 48 h incubation of the coelomocytes with the pathogen, there was a significant effect of time (F2,48 = 6.18; p = 0.004; D30 > D1; D30 > D15), temperature (F1,48 = 8.44; p = 0.006 23°C > 17°C) and pH (F1,48 = 2.14; p = 0.001; 7.6 > 8.15) on bacterial survival indices (table 4). Similar to the results obtained at 24 h, the coelomic fluid from sea urchins held in ambient conditions was relatively effective at bacterial clearance (figure 4c). By contrast, the bacterial survival indices from sea urchins held in all experimental treatments increased over each time point (1, 15 and 30 days), indicating that exposure to a single stressor (temperature or pH) or multiple stressors gradually decreased the ability of the sea urchins to clear bacteria from their coelomic fluid. At each time point, coelomic fluid from sea urchins exposed to both stressors was the least effective at inhibiting bacterial growth, and after 30 days, the bacterial survival index from sea urchins held in 23°C/pH 7.6 was significantly higher than from sea urchins held in ambient conditions (figure 4c and table 4).
4. Discussion
In this first study of the effects of acclimation to near-future ocean warming and acidification on the sea urchin immune response we show that these stressors had a negative effect on immune function resulting in an altered coelomocyte profile and lower defense against the pathogen V. anguillarum. The coelomocyte concentrations and profile seen here for H. erythrogramma from ambient conditions were similar to that determined for other sea urchin species, with some variation between taxa [24,42,43]. Thus, in the non-stressed situation the data for H. erythrogramma are typical. By contrast, sea urchins simultaneously exposed to warming and acidification for 15 and 30 days had reduced coelomocyte concentrations. Previous studies of the immune response of echinoderms to acidification as a single stressor have shown varying responses. Similar to H. erythrogramma, exposure to reduced pH for one week or six months decreased the total coelomocyte concentrations in the coelomic fluid of the sea star, A. rubens [24]. By contrast, in the sea star L. polaris short-term (5–7 days) exposure to reduced pH resulted in an increase in total coelomocyte concentrations [42].
The coelomic fluid has an ionic composition similar to seawater; however, the pH is generally 0.5–1.5 pH units lower than that of seawater, which is most likely due to slow CO2 diffusion rates from tissues and a build up of acidic metabolites [44]. As osmoconformers, some sea urchin species have relatively poor ionic regulatory capacity, and as ambient seawater pH decreases, the pH of their coelomic fluid also decreases [44,45]. Other studies show that several sea urchin species are relatively effective at maintaining the pH of their coelomic fluid under future ocean acidification conditions [46], as is also the case for H. erythrogramma [47]. However, no studies have investigated the physiological costs of regulating the pH of the coelomic fluid, and the associated energy trade-offs, which may limit the amount of energy available for physiological processes such as the immune response. A recent study with H. erythrogramma acclimated to combined warming and acidification at the same levels used here showed that these stressors exerted an additive effect to increase metabolism indicating significant energetic costs [34]. Although the physiological mechanisms underlying the depression of the immune response seen here is not known, it may involve the diversion of energetic resources away from maintenance of innate immunity.
For H. erythrogramma, innate immune functions were affected by increased temperature and acidification. The largest change in the types of coelomocytes making up the coelomic fluid (48% dissimilarity) was observed between sea urchins held for 30 days at ambient conditions and those in elevated temperatures and reduced pH. This suggests that longer exposures to near-future conditions had a greater impact on coelomocyte types than shorter exposures as the sea urchins respond over time to the new environment. The physiological implications of a changing coelomocyte profile are not understood. However, we hypothesize that the reduction of flagellated vibratile cells observed in sea urchins held for 30 days in elevated temperatures and reduced pH may decrease the ability of the coelomic fluid to circulate waste materials and nutrients.
The concentration of the red amoebocytes in H. erythrogramma differed among treatments with a decrease in these cells with warming. This contrasts with L. variegatus where an increase in these cells is proposed to be an indicator of stress [22], including increased temperature [25]. Acidification did not have a significant effect on red amoebocyte concentrations as also observed for S. droebachiensis [42]. Phagocytes usually comprise the highest proportion of cell types in sea urchin coelomic fluid [19], whereas white amoebocytes were most abundant in H. erythrogramma in both control and experimental treatments. Future studies are needed to determine if this immune cell profile is characteristic for this species.
At each of the three time points examined, the phagocytic activity of coelomocytes from H. erythrogramma exposed to ambient temperature and reduced pH was lower than that for control sea urchins. In echinoderms, phagocytosis, digestion and degradation of foreign material such as bacteria by coelomocytes is considered to be the ‘first line’ of defence against pathogens [43]. Reduced phagocytic activity has also been documented in response to acidification as a single stressor in other marine invertebrates. After a four-month exposure to reduced seawater pH, haemocyte concentrations in the lobster Nephrops norvegicus were reduced by almost 50% and the phagocytic capacity of the remaining haemocytes was inhibited by 60% [48]. A single-stressor experiment investigating the effects of increased temperature on the phagocytic activity of coelomocytes from the sea urchin S. neumayeri indicated that ocean warming may increase phagocytic activity [23]. Similarly, our results suggest that increased temperature may alleviate the negative effects of reduced seawater pH on phagocytic activity.
Measuring the susceptibility of an organism to a pathogen provides the most predictive assessment of immune function [49]. In this study, H. erythrogramma exposed to both stressors over 30 days were the least effective at inhibiting growth of V. anguillarum. Similarly, reduced seawater pH decreased antibacterial activity in the mussel Mytilus edulis while elevated temperature increased antibacterial activity [49]. Our findings suggest that in H. erythrogramma, exposure to combined warming and acidification over 30 days had a greater reduction in antibacterial activity than those exposed over a similar period to a single stressor. This combined effect is expressed in a 1000-fold reduction in the ability to inhibit bacterial growth. As the coastal habitat of H. erythrogramma increases in temperature with an increase SST to 25–26°C and acidification to pHNIST 7.6–7.7 [31], and as fluctuations in its shallow water habitats become more extreme, this species may exhibit characteristics of a compromised immune system.
The recovery of coelomocyte concentration and phagocytic activity over 30 days in the single stressor warm treatment indicates a capacity for H. erythrogramma to adjust immune function following acclimation to increased temperature. This is an important consideration because increased coastal air and sea temperatures are the most important contemporary climate change stressors in the region [50]. Although our regime of 30 days of change followed by 30 days of response is very different to the more gradual change projected over coming decades, our acclimation approach indicated scope for phenotypic adjustment to warming. It is not known what the influence of a more gradual change (months to years) to lower pH would be or how this would be influenced by diurnal fluctuations. Equally noteworthy was the variability we observed in the ability of sea urchins exposed to the same conditions to inhibit bacterial growth, suggesting that some individuals may be more resilient and so may be better preadapted to withstand elevated temperature and reduced pH than others. Natural selection over the much longer timescale on which climate change is occurring may allow further adaptation to the temperature and pH conditions used here. Transgenerational experiments may provide an indication whether this variability in immune function or resilience can be passed on to progeny to allow H. erythrogramma to adapt or acclimate to the pathogenic challenges of near-future changes in the ocean environment. In summary, the differences between the immune response on day 1 and day 30 in experimental conditions show the importance of gradual introduction of stressors followed by an acclimation period to avoid acute responses which are likely to be less reflective of future ocean conditions. The recovery of some immune functions with time indicates that this recovery might have been enhanced with a longer acclimation time and suggests that some sea urchins may be able to recover their immune response, although metabolic studies indicate that this would entail an energetic cost [34].
Supplementary Material
Acknowledgements
We thank Joshua Aldridge, Dr Nicholas Carey, Steve Doo, Dr Diane McDougald, Amanda Scholes and the staff at the Sydney Institute of Marine Sciences for logistical help, as well as Drs Robert Angus and Cole Easson for help with statistics.
Ethics
Sea urchins were collected under permit from NSW Primary Industries (P00/0015-6.0) and the research organisms did not require animal ethics approval. The research methodology was approved by the Sydney Institute of Marine Sciences.
Data accessibility
All supporting datasets are deposited in Dryad: http://dx.doi.org/10.5061/dryad.9hr7t.
Authors' contributions
C.J.B., J.H., J.B.M. and M.B. conceived and designed the experiment. C.J.B. and J.H. performed the experiment. J.B.M. and M.B. contributed materials and funding. C.J.B., J.H., J.B.M. and M.B. analysed the data, drafted the manuscript and approved its publication.
Competing interests
We have no competing interests.
Funding
Funding was provided by an East Asia and Pacific Summer Institutes Fellowship from the US National Science Foundation (IIA-1414656), the Australian Academy of Sciences and the New South Wales Environmental Trust (RD/0090). This research was supported in part by grant no. ANT-1041022 from the US National Science Foundation to J.B.M., Charles Amsler and Robert Angus.
References
- 1.Rhein M, et al. 2013. Observations: ocean. In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, pp. 255–315. Cambridge: Cambridge University Press. [Google Scholar]
- 2.Harvell CD, et al. 2002. Climate warming and disease risks for terrestrial and marine biota. Science 296, 2158–2162. ( 10.1126/science.1063699) [DOI] [PubMed] [Google Scholar]
- 3.Randall CJ, van Woesik R. 2015. Contemporary white-band disease in Caribbean corals driven by climate change. Nat. Clim. Change 5, 375–379. ( 10.1038/nclimate2530) [DOI] [Google Scholar]
- 4.Moore JD, Robbins TT, Friedman CS. 2000. Withering syndrome in farmed red abalone Haliotis rufescens: thermal induction and association with a gastrointestinal Rickettsiales-like prokaryote. J. Aquat. Anim. Health 12, 26–34. ( 10.1577/1548-8667(2000)012%3C0026:WSIFRA%3E2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 5.Villalba A, Reece KS, Camino Ordás M, Casas SM, Figueras A. 2004. Perkinsosis in molluscs: a review. Aquat. Living Resour. 17, 411–432. ( 10.1051/alr:2004050) [DOI] [Google Scholar]
- 6.Cebrian E, Uriz MJ, Garrabou J, Ballesteros E. 2011. Sponge mass mortalities in a warming Mediterranean Sea: are cyanobacteria-harboring species worse off? PLoS ONE 6, e20211 ( 10.1371/journal.pone.0020211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hewson I, et al. 2014. Densovirus associated with sea-star wasting disease and mass mortality. Proc. Natl Acad. Sci. USA 111, 17 278–17 283. ( 10.1073/pnas.1416625111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bates AE, Hilton BJ, Harley CD. 2009. Effects of temperature, season and locality on wasting disease in the keystone predatory sea star Pisaster ochraceus. Dis. Aquat. Organ. 86, 245–251. ( 10.3354/dao02125) [DOI] [PubMed] [Google Scholar]
- 9.Clemente S, Lorenzo-Morales J, Mendoza J, López C, Sangil C, Alves F, Kaufmann M, Hernández JC. 2014. Sea urchin Diadema africanum mass mortality in the subtropical eastern Atlantic: role of waterborne bacteria in a warming ocean. Mar. Ecol. Prog. Ser. 506, 1–14. ( 10.3354/meps10829) [DOI] [Google Scholar]
- 10.Scheibling RE, Hennigar AW. 1997. Recurrent outbreaks of disease in sea urchins Strongylocentrotus droebachiensis in Nova Scotia: evidence for a link with large-scale meteorologic and oceanographic events. Mar. Ecol. Prog. Ser. 152, 155–165. ( 10.3354/meps152155) [DOI] [Google Scholar]
- 11.Girard D, Clemente S, Toledo-Guedes K, Brito A, Hernandez JC. 2012. A mass mortality of subtropical intertidal populations of the sea urchin Paracentrotus lividus: analysis of potential links with environmental conditions. Mar. Ecol. 33, 377–385. ( 10.1111/j.1439-0485.2011.00491.x) [DOI] [Google Scholar]
- 12.Sweet M, Bulling M, Williamson JE. 2016. New disease outbreak affects two dominant sea urchin species associated with Australian temperate reefs. Mar. Ecol. Prog. Ser. 551, 171–183. ( 10.3354/meps11750) [DOI] [Google Scholar]
- 13.Yui MA, Bayne CJ. 1983. Echinoderm immunology: bacterial clearance by the sea urchin Strongylocentrotus purpuratus. Biol. Bull. 165, 473–486. ( 10.2307/1541213) [DOI] [PubMed] [Google Scholar]
- 14.Gilles KW, Pearse JS. 1986. Disease in sea urchins Strongylocentrotus purpuratus: experimental infection and bacterial virulence. Dis. Aquat. Organ. 1, 105–114. ( 10.3354/dao001105) [DOI] [Google Scholar]
- 15.Beck G, Miller R, Ebersole J. 2014. Mass mortality and slow recovery of Diadema antillarum: could compromised immunity be a factor? Mar. Biol. 161, 1001–1013. ( 10.1007/s00227-013-2382-6) [DOI] [Google Scholar]
- 16.Filbee-Dexter K, Scheibling RE. 2014. Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar. Ecol. Prog. Ser. 495, 1–25. ( 10.3354/meps10573) [DOI] [Google Scholar]
- 17.Lessios H. 2016. The great Diadema antillarum die-off: 30 years later. Annu. Rev. Mar. Sci. 8, 267–283. ( 10.1146/annurev-marine-122414-033857) [DOI] [PubMed] [Google Scholar]
- 18.Heck KL, Valentine JF. 1995. Sea urchin herbivory: evidence for long-lasting effects in subtropical seagrass meadows. J. Exp. Mar. Biol. Ecol. 189, 205–217. ( 10.1016/0022-0981(95)00012-G) [DOI] [Google Scholar]
- 19.Smith L, Rast J, Brockton V, Terwilliger D, Nair S, Buckley K, Majeske AJ. 2006. The sea urchin immune system. Invert. Surviv. J. 3, 25–39. [Google Scholar]
- 20.Pinsino A, Matranga V. 2015. Sea urchin immune cells as sentinels of environmental stress. Dev. Comp. Immunol. 49, 198–205. ( 10.1016/j.dci.2014.11.013) [DOI] [PubMed] [Google Scholar]
- 21.Silva J. 2013. Immunology in sea urchins. In Sea urchins: biology and ecology, 3rd edn (ed Lawrence JM.), pp. 187–195. San Diego, CA: Academic Press. [Google Scholar]
- 22.Matranga V, Toia G, Bonaventura R, Müller WE. 2000. Cellular and biochemical responses to environmental and experimentally induced stress in sea urchin coelomocytes. Cell Stress Chaperones 5, 113–120. ( 10.1379/1466-1268(2000)005%3C0113:CABRTE%3E2.0.CO;2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branco PC, et al. 2012. Cellular biomarkers to elucidate global warming effects on Antarctic sea urchin Sterechinus neumayeri. Polar Biol. 35, 221–229. ( 10.1007/s00300-011-1063-5) [DOI] [Google Scholar]
- 24.Hernroth B, Baden S, Thorndyke M, Dupont S. 2011. Immune suppression of the echinoderm Asterias rubens (L.) following long-term ocean acidification. Aquat. Toxicol. 103, 222–224. ( 10.1016/j.aquatox.2011.03.001) [DOI] [PubMed] [Google Scholar]
- 25.Branco PC, Borges JCS, Santos MF, Junior BEJ, da Silva JRMC. 2013. The impact of rising sea temperature on innate immune parameters in the tropical subtidal sea urchin Lytechinus variegatus and the intertidal sea urchin Echinometra lucunter. Mar. Environ. Res. 92, 95–101. ( 10.1016/j.marenvres.2013.09.005) [DOI] [PubMed] [Google Scholar]
- 26.Byrne M, Przeslawski R. 2013. Multistressor impacts of warming and acidification of the ocean on marine invertebrates’ life histories. Integr. Comp. Biol. 53, 582–596. ( 10.1093/icb/ict049) [DOI] [PubMed] [Google Scholar]
- 27.Przeslawski R, Byrne M, Mellin C. 2015. A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob. Change Biol. 21, 2122–2140. ( 10.1111/gcb.12833) [DOI] [PubMed] [Google Scholar]
- 28.Keesing JK. 2013. Heliocidaris erythrogramma. In Sea urchins: biology and ecology, pp. 369–377. London, UK: Academic Press. [Google Scholar]
- 29.Hobday AJ, Pecl GT. 2014. Identification of global marine hotspots: sentinels for change and vanguards for adaptation action. Rev. Fish Biol. Fish. 24, 415–425. ( 10.1007/s11160-013-9326-6) [DOI] [Google Scholar]
- 30.Ridgway K. 2007. Long-term trend and decadal variability of the southward penetration of the East Australian Current. Geophys. Res. Lett. 34, L13613 ( 10.1029/2007GL030393) [DOI] [Google Scholar]
- 31.Wolfe K, Dworjanyn SA, Byrne M. 2013. Effects of ocean warming and acidification on survival, growth and skeletal development in the early benthic juvenile sea urchin (Heliocidaris erythrogramma). Glob. Change Biol. 19, 2698–2707. ( 10.1111/gcb.12249) [DOI] [PubMed] [Google Scholar]
- 32.Byrne M, Ho M, Selvakumaraswamy P, Nguyen HD, Dworjanyn SA, Davis AR. 2009. Temperature, but not pH, compromises sea urchin fertilization and early development under near-future climate change scenarios. Proc. R. Soc. B 276, 1883–1888. ( 10.1098/rspb.2008.1935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrne M, et al. 2010. Fertilization in a suite of coastal marine invertebrates from SE Australia is robust to near-future ocean warming and acidification. Mar. Biol. 157, 2061–2069. ( 10.1007/s00227-010-1474-9) [DOI] [Google Scholar]
- 34.Carey N, Harianto J, Byrne M. 2016. Urchins in a high CO2 world: partitioned effects of body-size, ocean warming and acidification on metabolic rate. J. Exp. Biol. 219, 1178–1186. ( 10.1242/jeb.136101) [DOI] [PubMed] [Google Scholar]
- 35.Field CB, Barros VR, Mach K, Mastrandrea M. 2014. Climate change 2014: impacts, adaptation, and vulnerability. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 36.Pierrot D, Lewis E, Wallace D. 2006. MS Excel program developed for CO2 system calculations. Oak Ridge, TN: ORNL/CDIAC-105a Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy. [Google Scholar]
- 37.Dickson AG, Sabine CL, Christian JR. 2007. Guide to best practices for ocean CO2 measurements.
- 38.Dheilly NM, Haynes PA, Bove U, Nair SV, Raftos DA. 2011. Comparative proteomic analysis of a sea urchin (Heliocidaris erythrogramma) antibacterial response revealed the involvement of apextrin and calreticulin. J. Invert. Pathol. 106, 223–229. ( 10.1016/j.jip.2010.09.008) [DOI] [PubMed] [Google Scholar]
- 39.Böttger SA, McClintock JB. 2009. The effects of chronic inorganic and organic phosphate exposure on bactericidal activity of the coelomic fluid of the sea urchin Lytechinus variegatus (Lamarck)(Echinodermata: Echinoidea). Comp. Biochem. Physiol. C 150, 39–44. ( 10.1016/j.cbpc.2009.02.002) [DOI] [PubMed] [Google Scholar]
- 40.Underwood A. 1981. Techniques of analysis of variance in experimental marine biology and ecology. Oceanogr. Mar. Biol. Annu. Rev. 19, 513–605. [Google Scholar]
- 41.R Core Team. 2013. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.r-project.org. [Google Scholar]
- 42.Dupont S, Thorndyke M. 2012. Relationship between CO2-driven changes in extracellular acid–base balance and cellular immune response in two polar echinoderm species. J. Exp. Mar. Biol. Ecol. 424, 32–37. ( 10.1016/j.jembe.2012.05.007) [DOI] [Google Scholar]
- 43.Chia F-S, Xing J. 1996. Echinoderm coelomocytes. Zool. Stud. Taipei 35, 231–254. [Google Scholar]
- 44.Catarino AI, Bauwens M, Dubois P. 2012. Acid–base balance and metabolic response of the sea urchin Paracentrotus lividus to different seawater pH and temperatures. Environ. Sci. Pollut. Res. Int. 19, 2344–2353. ( 10.1007/s11356-012-0743-1) [DOI] [PubMed] [Google Scholar]
- 45.Miles H, Widdicombe S, Spicer JI, Hall-Spencer J. 2007. Effects of anthropogenic seawater acidification on acid–base balance in the sea urchin Psammechinus miliaris. Mar. Pollut. Bull. 54, 89–96. ( 10.1016/j.marpolbul.2006.09.021) [DOI] [PubMed] [Google Scholar]
- 46.Collard M, Dery A, Dehairs F, Dubois P. 2014. Euechinoidea and Cidaroidea respond differently to ocean acidification. Comp. Biochem. Physiol. A 174, 45–55. ( 10.1016/j.cbpa.2014.04.011) [DOI] [PubMed] [Google Scholar]
- 47.Johnson R. 2015. The physiological and skeletal response of the sea urchin Heliocidaris erythrogramma to climate change stressors. BSc thesis, University of Sydney, Sydney, Australia. [Google Scholar]
- 48.Hernroth B, Skold HN, Wiklander K, Jutfelt F, Baden S. 2012. Simulated climate change causes immune suppression and protein damage in the crustacean Nephrops norvegicus. Fish Shellfish Immunol. 33, 1095–1101. ( 10.1016/j.fsi.2012.08.011) [DOI] [PubMed] [Google Scholar]
- 49.Ellis RP, Widdicombe S, Parry H, Hutchinson TH, Spicer JI. 2015. Pathogenic challenge reveals immune trade-off in mussels exposed to reduced seawater pH and increased temperature. J. Exp. Mar. Biol. Ecol. 462, 83–89. ( 10.1016/j.jembe.2014.10.015) [DOI] [Google Scholar]
- 50.Hobday AJ, Lough JM. 2011. Projected climate change in Australian marine and freshwater environments. Mar. Freshw. Res. 62, 1000–1014. ( 10.1071/MF10302) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All supporting datasets are deposited in Dryad: http://dx.doi.org/10.5061/dryad.9hr7t.