Abstract
Evolutionary trajectories are often biased by developmental and historical factors. However, environmental factors can also impose constraints on the evolutionary trajectories of organisms leading to convergence of morphology in similar ecological contexts. The physical properties of water impose strong constraints on aquatic feeding animals by generating pressure waves that can alert prey and potentially push them away from the mouth. These hydrodynamic constraints have resulted in the independent evolution of suction feeding in most groups of secondarily aquatic tetrapods. Despite the fact that snakes cannot use suction, they have invaded the aquatic milieu many times independently. Here, we test whether the aquatic environment has constrained head shape evolution in snakes and whether shape converges on that predicted by biomechanical models. To do so, we used three-dimensional geometric morphometrics and comparative, phylogenetically informed analyses on a large sample of aquatic snake species. Our results show that aquatic snakes partially conform to our predictions and have a narrower anterior part of the head and dorsally positioned eyes and nostrils. This morphology is observed, irrespective of the phylogenetic relationships among species, suggesting that the aquatic environment does indeed drive the evolution of head shape in snakes, thus biasing the evolutionary trajectory of this group of animals.
Keywords: aquatic snakes, head morphology, hydrodynamics, constraints, convergence, geometric morphometrics
1. Introduction
Physical constraints imposed by the environment play an important role in the evolution of species. Evolution can be predictable when the constraints caused by the physical environment are strong [1]. When different species are faced with similar constraints, convergence in morphology or behaviour is predicted [2,3]. The study of convergence can help us understand whether and how the constraints imposed by the physical environment drive phenotypic diversification. The physical properties of water induce strong constraints on physiology, anatomy and behaviour resulting in a suite of adaptations in animals that have secondarily invaded the aquatic environment [4–6]. In spite of these hydrodynamic constraints, numerous species with diverse phylogenetic backgrounds have invaded aquatic habitats. The range of phenotypic responses in vertebrates is, however, limited by functional and structural constraints, leading to convergence as is observed for underwater locomotion [7–9] or feeding [4].
Underwater prey capture is extremely challenging. Indeed, any movement through water is resisted by the drag and inertial forces acting on the body of the animal. These forces are greater than in air because of the greater density and viscosity of the fluid. When animals attempt to catch prey underwater, the forward motion of the strike will involve the generation of a pressure wave that has two main adverse effects: it tends to push the prey away from the predator [10], and may trigger the escape response of the prey. Indeed, very fast escape responses, called C-starts or S-starts in fishes, can be triggered by chemical cues emitted by prey or predators [11,12] or by physical cues such as water displacement [13–15]. Both the diffusion of chemical compounds and physical cues highly depend on water displacement and consequently, predators have to limit the amount of water that they displace when chasing or attacking a prey.
To circumvent these constraints, aquatic predators have developed strategies such as suction feeding that help compensate for the displacement of water by the predator. This behaviour involves an expansion of the bucco-pharyngeal cavity owing to the displacement of the hyobranchial apparatus (i.e. the apparatus that supports the tongue in terrestrial tetrapods). Thus, a low-pressure zone is created inside the mouth of the predator that drags the surrounding water and prey into the mouth. This is one of the most widespread aquatic prey capture strategies in vertebrates [16–20]. Snakes, however, have a reduced hyoid apparatus because of the specialization of their tongue for chemoreception [21,22] and consequently are not able to expand their bucco-pharyngeal cavity. Despite this limitation, a secondary return to an aquatic lifestyle has occurred independently in many snake genera [23]. Moreover, numerous species of snakes are proficient in the capture of elusive aquatic prey and some species have become entirely piscivorous [24–28]. As drag is highly dependent on the shape of the head, and impairs the swimming or targeting efficiency of the predator [4,10], the ‘ideal’ aquatic snake should have a slender, streamlined, narrow and long head [4,29,30]. However, prey capture is not the only selective pressure acting on head morphology in snakes. Indeed, snakes use their head not only to capture prey, but also to handle and swallow them. Prey handling and swallowing prey are performed by means of a ‘pterygoid walk’ [31,32] which is more efficient in snakes with wider heads and longer quadrates [29]. Thus, the ‘ideal’ morphology for an aquatic snake is probably determined by the trade-off between a streamlined head that is still able to swallow large or bulky prey efficiently.
Previous studies that have compared head shape in snakes have mainly focused on skull bones or scalation and/or used linear measurements to quantify morphology [23,30,33–37]. In this study, we test the hypothesis that the physical constraints related to underwater prey capture constrain head shape evolution in aquatic snakes. We hypothesize that the head shape of snakes that are able to capture elusive prey underwater has converged to an ‘optimal’ shape. We predict that these snakes will present narrower and longer heads compared with snakes that do not capture prey under water. Our predictions follow previous work on aquatic snakes [29,37], but here we provide a large and diverse sample of aquatic snakes in order to test the generality of these predictions in snakes that capture elusive aquatic prey. We use three-dimensional geometric morphometric approaches [38,39] to quantify the shape of the entire head as the hydrodynamics of movement under water will probably impact the overall shape of the head. We include species representing all families of snakes in which aquatic prey capture has evolved. These species are compared with closely related species of snakes that do not eat aquatic prey, within an explicit phylogenetic framework. Finally, we describe the head shape associated with species that capture elusive prey under water and compare it with a priori predictions based on previous studies [4,29,30,37] and biomechanical models [1].
2. Material and methods
(a). Specimens
Three-dimensional scans of the heads of 419 snakes were obtained using a high-resolution surface scanner: a Stereoscan3D Breuckmann white light fringe StereoSCAN3D with a camera resolution of 1.4 megapixels, available at the Museum National d'Histoire Naturelle, Paris. The specimens came from different collections; the collections of the Museum National d'Histoire Naturelle, the Field Museum of Natural History, the American Museum of Natural History, the California Academy of Sciences, the personal collection of Anthony Herrel and the personal collection of Antoine Fouquet. Only specimens with a well-preserved head and closed mouth were scanned. At least five specimens per species were used in this study where possible (see the electronic supplementary material, S1).
We included 83 species of snakes in total. We considered as ‘aquatic’ species of snakes that consume elusive aquatic prey (e.g. fishes, amphibians, crustaceans, etc.) and as ‘non-aquatic’ those that do not eat aquatic prey (see the electronic supplementary material, S1 for references on the diet). We tried to choose at least one aquatic species among each family of snakes in which a return to an aquatic lifestyle has occurred. Non-aquatic species were chosen to be phylogenetically close to the aquatic species in our analysis [40]. In total, we compared 62 aquatic species with 21 species that do not feed on elusive aquatic prey (electronic supplementary material, S1). The phylogenetic tree of Pyron [40] was pruned in Mesquite v. 3.03 [41] to only keep the species included in our dataset (electronic supplementary material, S2).
(b). Geometric morphometrics
To quantify shape variation between species, we recorded the three-dimensional coordinates of 10 landmarks and six curves (figure 1), using the ‘Landmark’ software package [42]. These landmarks include both anatomical landmarks and maxima of curvature (electronic supplementary material, S3). To assess the repeatability of the landmark positioning, we placed the set of landmarks 10 times on three specimens of the same species and checked if the variability between specimens was higher than the variability related to the landmark positioning (electronic supplementary material, S4).
Figure 1.
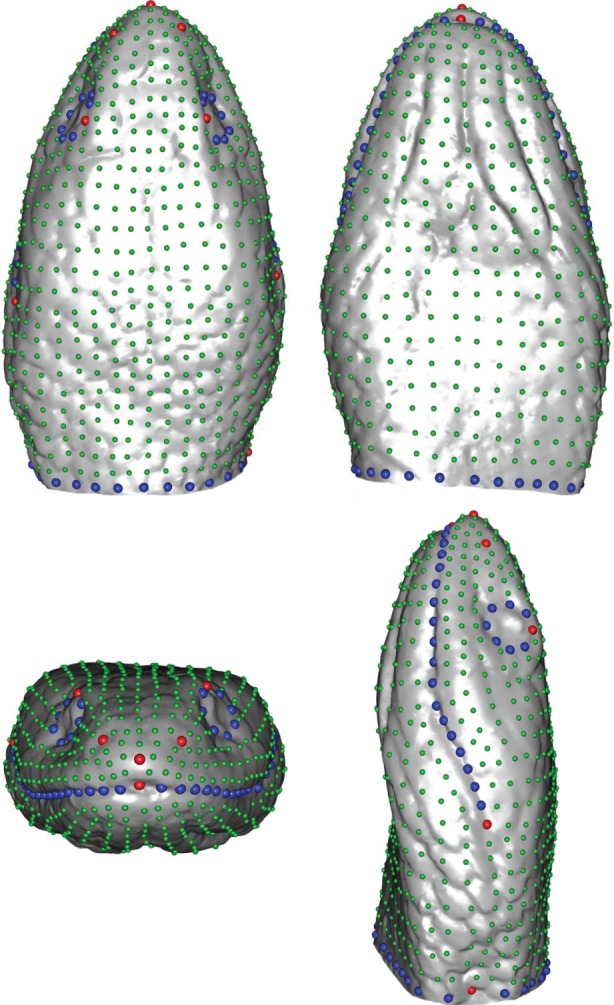
Template used for the geometric morphometric analyses. Anatomical landmarks are indicated in red, semi-landmarks on curves in blue and surface semi-landmarks in green. (Online version in colour.)
To obtain an accurate description of the head shape, we created a template consisting of 921 landmarks including 10 anatomical landmarks, 74 sliding-landmarks on curves and 837 sliding-landmarks on the surface of the head (figure 1) [38]. This template was positioned based on the anatomical landmarks and curves for each specimen. Next, semi-landmarks were projected onto the surface of the specimen and allowed to slide while minimizing the bending energy between the template and the specimen [38,43]. The sliding procedure was performed, using the Morpho package [44] in R [45]. After sliding, all landmarks were rendered symmetrical, a Procrustes superimposition was run [46] and an average head shape per species was calculated in MorphoJ [47]. A principal component analysis (PCA) was then run using the Rmorph library [48] to evaluate the overall shape variation in the dataset. The first 11 principal components (PCs) accounting for more than 95% of the shape variability were extracted and used for further analyses (table 1).
Table 1.
Results of the statistical analyses performed on the first 11 principal components. (Statistical significance highlighted in italics.)
| proportion of variance (%) |
univariate K statistics |
phylogenetic ANOVA |
||||
|---|---|---|---|---|---|---|
| proportion | cumulative proportion | K | p-value | F1,81 | p-value | |
| PC1 | 49.3 | 49.3 | 0.37 | 0.0009 | 3.23 | 0.1 |
| PC2 | 13.7 | 63.0 | 0.34 | 0.0009 | 30.62 | 0.0009 |
| PC3 | 7.3 | 70.3 | 0.41 | 0.0009 | 0.64 | 0.5 |
| PC4 | 6.6 | 76.9 | 0.28 | 0.001 | 0.46 | 0.5 |
| PC5 | 5.6 | 82.6 | 0.24 | 0.1 | 4.82 | 0.06 |
| PC6 | 4.0 | 86.6 | 0.25 | 0.04 | 0.001 | 0.9 |
| PC7 | 3.0 | 89.7 | 0.30 | 0.3 | 0.15 | 0.7 |
| PC8 | 1.8 | 91.5 | 0.26 | 0.02 | 0.43 | 0.5 |
| PC9 | 1.5 | 93.0 | 1.45 | 0.0009 | 8.25 | 0.02 |
| PC10 | 1.1 | 94.2 | 0.37 | 0.004 | 1.03 | 0.4 |
| PC11 | 0.8 | 95.0 | 0.30 | 0.0009 | 2.88 | 0.1 |
(c). Analyses
We first assessed whether a phylogenetic signal was present in the dataset, using the multivariate version of the K-statistic [39,49]. This test was performed using the ‘geomorph’ library [50] in R. Next, the univariate K-statistic was calculated to test for phylogenetic signal in the first 11 PC axes using the ‘picante’ library in R [51]. Given that a significant phylogenetic signal was detected, a phylogenetic MANOVA was performed on the first 11 PC axes to test for differences in shape between species that capture elusive aquatic prey and those that do not. Subsequently, we ran phylogenetic ANOVAs to evaluate which axes contributed to the observed differences in head shape. To evaluate whether size impacted the results, we ran a MANCOVA with the log10-transformed centroid size as a covariate. Finally, a linear discriminant analysis (LDA) was performed. We extracted the shapes associated with species that capture elusive aquatic prey and those that do not (figure 2). Reclassification rates using a leave-one-out cross validation were then calculated. To relate the observed shape differences to differences in hydrodynamics, we opened the jaws of the shapes extracted from the LDA in silico (Blender 2.75). The gape angle was set at 70° for both models based on in vivo video recordings of snakes striking [1,52,53]. Next, we measured the size of the jaws as well as the projected frontal surface area (area of the mouth facing the current) to assess the drag associated with both shapes during prey capture (table 2). Finally, we also measured the projected frontal surface area for identical gape distance (table 2). All statistical analyses were performed using R [45]. The significance level of all statistical tests was set at 5%.
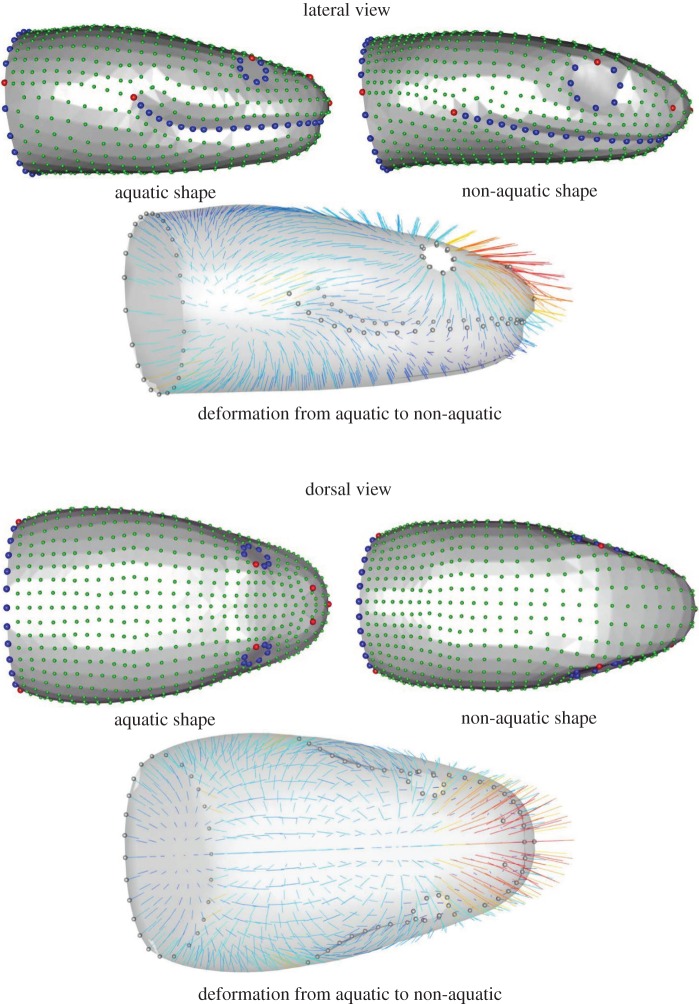
Figure 2.
Results of the linear discriminant analysis illustrating the head shapes associated with species capturing elusive aquatic prey on the left and the non-aquatic ones on the right. Anatomical landmarks are indicated in red, semi-landmarks on curves in blue and surface semi-landmarks in green. Vectors are coloured by deformation intensity from dark blue to red and from the aquatic to the non-aquatic shape. (Online version in colour.)
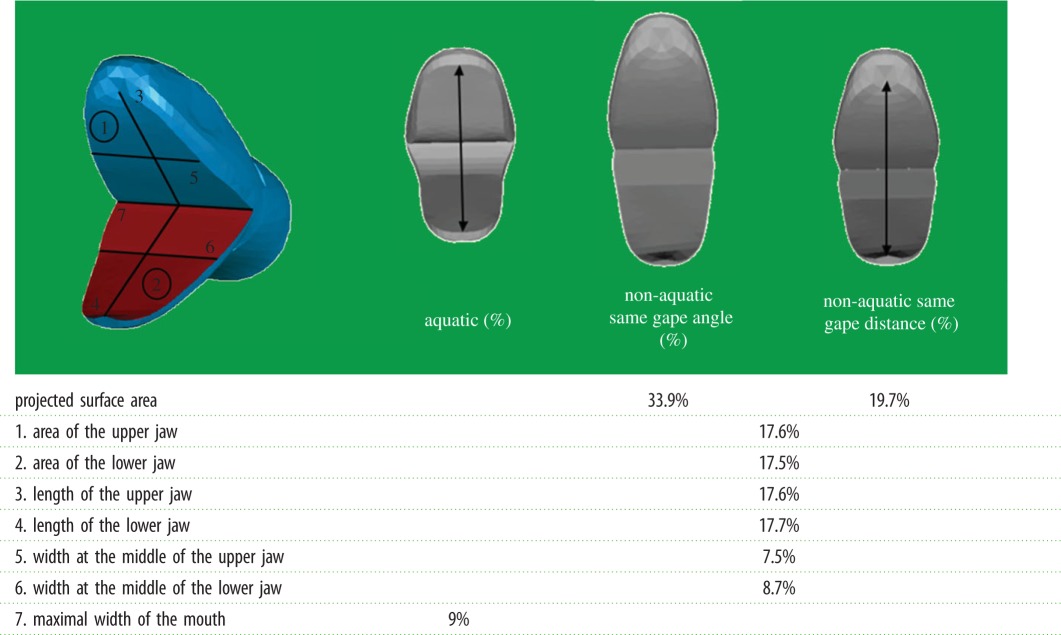
Table 2.
Quantitative comparison between the aquatic head shape and the non-aquatic one. (Measurements are indicated by number on the first scheme. The shapes associated with both ecologies are at the same scale. The non-aquatic gape was adjusted to be equal to the aquatic shape gape distance or gape angle. Values in the table indicate the percentage of extra surface or length.)
 |
3. Results
The first and the second axes of the PCA, respectively, accounted for 49.3% and 13.7% of the overall variability (table 1). We detected a phylogenetic signal in our morphological dataset (p = 0.001) with a multivariate K that was less than one (Kmult = 0.34). The univariate K-statistics are significant for the majority of the PC axes with K-values around 0.3 (table 1).
The phylogenetic MANOVA reveals significant differences between the head shapes of snakes that capture elusive aquatic prey and those that do not (Wilk's lambda = 0.47, F1,81 = 7.25, pphy= 0.0009). Phylogenetic ANOVAs run on each of the PC axes highlight a significant difference between the two groups on the axes 2 and 9 (table 1 and electronic supplementary material, S5). Both ecology and size impact the head shape of snakes (MANCOVA: ecology: F1,79 = 7.83, p < 0.001; size: F1,79 = 3.25, p = 0.001), but the interaction between both was not significant (MANCOVA: F1,79 = 0.68, p = 0.7).
(a). Shape comparisons
The following shape description is based on the LDA that allowed us to extract mean head shapes for species that capture elusive prey under water and those that do not (figure 2). The LDA shows a difference between the aquatic group and the non-aquatic one (F1,81 = 9.54, p = 0.002). The LDA reclassification rates are high (LDA: aquatic group = 89%; non-aquatic = 71%), meaning that the linear discriminant function accurately describes the differences between groups.
As the ‘non-aquatic’ group is non-homogeneous (i.e. species were selected to because they are closely related to an aquatic species or group of species only), we here focus only on differences between aquatic and non-aquatic species and the shape observed in the aquatic group. The shape associated with the ‘non-aquatic’ group is globally oblong with the head–neck transition that is clearly marked. The shape associated with snakes that capture elusive prey under water is strikingly different (figure 2). The anterior part of the head is proportionally narrower in the aquatic species whereas the posterior part is larger in comparison with the non-aquatic foragers. The height, width and length of the anterior part are lower in the aquatic snakes. The posterior part of the head is longer and the jaw is proportionally shorter in the aquatic species. Additionally, the shape of the mouth profile is more curved in the aquatic species. The eyes are proportionally smaller and more dorsally positioned in the aquatic species, whereas they are positioned on the lateral side of the head in the ‘non-aquatic’ species. Likewise, the nostrils are in a more dorsal position and closer to each other in the ‘aquatic’ species, whereas they are positioned more laterally in the ‘non-aquatic’ ones. In absolute terms and both when controlling for gape angle and gape distance, the size of the parts of the head that face the fluid flow are smaller in the aquatic group, both in terms of projected frontal surface area and linear measurements. The only feature that is greater in the aquatic group is the maximal width of the mouth which is the distance between the commissures of the mouth (figure 2 and table 2).
4. Discussion
We detected a significant phylogenetic signal in our dataset meaning that the head shape of the snakes in our dataset is at least partly constrained by shared ancestry. As the multivariate K was lower than one, species resemble each other less than expected, under Brownian motion evolution. One possible explanation of such a result is convergent evolution to specific environmental constraints [49]. The analyses of the overall shape variation in the dataset highlighted differences in head shape between the species that capture elusive prey under water versus those that do not. We found two PC axes (PC2 and PC9) that statistically differentiate between aquatic and non-aquatic snakes, irrespective of phylogeny. This demonstrates that the selective pressure associated with the underwater capture of elusive prey is strong enough to drive convergence in head shape across snakes despite a significant phylogenetic signal. Although snakes that capture elusive prey under water have evolved independently many times in the evolutionary history of snakes, most studies to date have focused on a single family, the natricines [1,37,52,54,55]. Our results show that convergence in head shape is independent of phylogeny and suggest that the aquatic medium has indeed constrained the evolutionary trajectory of these animals.
Snakes that capture elusive prey under water tend to have narrower heads as predicted a priori, but only for the anterior part of the head. The head is also dorsoventrally flattened in comparison with species that do not capture elusive aquatic prey. This dorsoventral compression of the head is a feature that has been suggested to be associated with an aquatic lifestyle and the need to be more streamlined in aquatic reptiles in general [4]. Proportionally, aquatic snakes have an enlarged posterior part of the head. This could reflect a solution to the trade-off between the need for a more streamlined head to circumvent the physical constraints of underwater prey capture, and the need to be able to swallow large or bulky prey. The jaw is shorter in species that capture elusive aquatic prey in contrast to results of previous studies [37]. Moreover, the mouth is more curved, which, once the mouth is opened (table 2) allows a large opening while limiting the surface area facing the flow. The reduction of both length and width of the front part of the head were predicted by Taylor [4]. Indeed, these parts of the head play a major role in prey capture in snakes. The opening of the mouth during underwater prey capture produces considerable drag that can decrease capture success [4,10,29,37]. As the hydrodynamic drag force is proportional to the surface area of the object that moves perpendicularly to the flow, a reduction in both length, width and surface area of the mouth probably decreases the drag associated with the open-mouth capture typically observed in these animals as suggested by the lower projected frontal surface area [4,10]. By contrast, the head of aquatic snakes is proportionally larger posteriorly. This may ensure an efficient ‘pterygoid walk’ in aquatic snakes despite their reduced jaw length. According to Young [29], the width of the posterior part of the head impacts the length of the lever arm which is involved in the pterygoid walk; the larger the width, the more efficient the swallowing. Moreover, this ensures a smooth head–neck transition in aquatic snakes. The jaw tips (i.e. landmarks 4 and 5) are not prominent which could possibly be an advantage during swimming as this may avoid the detachment of the flow behind the head.
As the nostrils are more dorsally positioned in aquatic snakes, this could allow them to breathe at the surface of the water while remaining submerged [56]. Likewise, the more dorsal position of the eyes could allow them to target prey or to see predators that are positioned above [57,58]. Eyes in predators generally tend to have a more frontal position to increase their binocular overlap, allowing them to better judge the distance to the prey. By contrast, species that tend to have more laterally positioned eyes have a wider visual field [59]. As most aquatic snakes rely on visual cues to detect and capture prey [54,60–63], their eyes may have moved closer together to allow a better perception of depth and distance [37].
Our results show that the head shape of snakes that capture elusive prey under water has indeed converged. Nevertheless, the shape observed does not exactly correspond to our a priori predictions. Most of previous work on this subject predicted that the hydrodynamic forces should favour an elongated snout [37], a smaller or narrower head [29] and a decrease of the overall head width [1], at least in frontal strikers. The head of the ‘aquatic’ snakes in our study is indeed proportionally narrower, but only in the anterior part. The enlargement of the posterior part of the head and the smaller size of the jaw is in contrast with the prediction of prior studies. However, simulation studies showed that an increase in head width is not likely to impair the strike speed of a snake [10]. As such, the head shape observed in aquatic snakes combines a narrow anterior part that will reduce drag, and a wide posterior head that allows an efficient prey transport. Direct measurements of the hydrodynamic forces and bow wave generation are needed, however, to test these ideas.
Interestingly, a considerable amount of variation in head shape is present among the snakes that capture elusive prey under water suggesting that multiple solutions to the same problem may exist. Snakes that capture elusive aquatic prey are known to use mostly one of two types of behaviours: frontally versus laterally directed strikes [1]. The strike behaviour greatly influences the flow of water around the head and the associated drag forces during a prey capture event [29]. Moreover, similarity in shape does not per se result in a similarity in performance [64] and the ecological relevance of variation in shape remains to be tested. The exploration of the relationship between morphology, the behaviour and the hydrodynamics of prey capture is a promising avenue to better understand how the physical environment may constrain the evolution of form in aquatic species.
Supplementary Material
Acknowledgements
We thank the herpetological collections of the Field Museum of Natural History, the American Museum of Natural History, the Museum of Comparative Zoology and the California Academy of Sciences and their respective curators Alan Resetar (FMNH), David A. Kizirian (AMNH), Jens Vindum (CAS) and Jose P. Rosado (MCZ) for their help in the choice of specimens and the loans. We especially wanted to thank the staff of the Laboratoire Reptiles et Amphibiens of the National Museum of Natural History of Paris for their help, patience and effectiveness. The ‘plate-forme de morphometrie’ of the UMS 2700 (CNRS, MNHN) is acknowledged for allowing us to use the surface scanner.
Data accessibility
PC scores for each species are available online (Dryad: http://dx.doi.org/10.5061/dryad.kd3qm) [65].
Authors' contributions
All authors helped revise and approved the manuscript. M.S. carried out the data collection, the statistical analyses, and wrote the manuscript. R.C. helped with the geometric morphometrics. R.G.D. helped with the interpretation of the result in a fluid mechanics context. A.C.F. helped with the phylogenetic comparative analysis. A.H. conceived the project and participated in the writing of the manuscript.
Competing interests
The authors declare no competing interests.
Funding
M.S. thanks the Région Ile de France for funding this research project and the doctoral school Frontières du Vivant (FdV) – Programme Bettencourt. A.C.F. thanks the Marie-Skłodowska Curie fellowship (EU project 655694 - GETAGRIP) for funding.
References
- 1.Herrel A, Vincent SE, Alfaro ME, Van Wassenbergh S, Vanhooydonck B, Irschick DJ. 2008. Morphological convergence as a consequence of extreme functional demands: examples from the feeding system of natricine snakes. J. Evol. Biol. 21, 1438–1448. ( 10.1111/j.1420-9101.2008.01552.x) [DOI] [PubMed] [Google Scholar]
- 2.Winemiller KO, Kelso-Winemiller LC, Brenkert AL. 1995. Ecological diversification and convergence in fluvial cichlid fishes. Environ. Biol. Fishes 44, 235–261. ( 10.1007/BF00005919) [DOI] [Google Scholar]
- 3.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Taylor MA. 1987. How tetrapods feed in water: a functional analysis by paradigm. Zool. J. Linn. Soc. 91, 171–195. ( 10.1111/j.1096-3642.1987.tb01727.x) [DOI] [Google Scholar]
- 5.Gans C. 1969. Comments on inertial feeding. Copeia 1969, 855–857. ( 10.2307/1441816) [DOI] [Google Scholar]
- 6.Vogel S. 1994. Life in moving fluids: the physical biology of flow. Princeton, NJ: Princeton University Press. [Google Scholar]
- 7.Videler JJ, Müller UK, Stamhuis EJ. 1999. Aquatic vertebrate locomotion: wakes from body waves. J. Exp. Biol. 202, 3423–3430. [DOI] [PubMed] [Google Scholar]
- 8.Howell AB. 1971. Aquatic mammals: their adaptations to life in the water. New York, NY: Dover Publications Inc. [Google Scholar]
- 9.Fish FE. 1993. Influence of hydrodynamic design and propulsive mode on mammalian swimming energetics. Aust. J. Zool. 42, 79–101. ( 10.1071/ZO9940079) [DOI] [Google Scholar]
- 10.Van Wassenbergh S, Brecko J, Aerts P, Stouten I, Vanheusden G, Camps A, Van Damme R, Herrel A. 2010. Hydrodynamic constraints on prey-capture performance in forward-striking snakes. J. R. Soc. Interface 7, 773–785. ( 10.1098/rsif.2009.0385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chivers DP, Jan FS. 1998. Chemical alarm signalling in aquatic predator-prey systems: a review and prospectus. Ecoscience 5, 338–352. [Google Scholar]
- 12.Feminella JW, Hawkins CP. 1994. Tailed frog tadpoles differentially alter their feeding behavior in response to non-visual cues from four predators. J. N. Am. Benthol. Soc. 13, 310–320. ( 10.2307/1467249) [DOI] [Google Scholar]
- 13.Faber DS, Fetcho JR, Korn H. 1989. Neuronal networks underlying the escape response in goldfish. General implications for motor control. Ann. N.Y. Acad. Sci. 563, 11–33. ( 10.1111/j.1749-6632.1989.tb42187.x) [DOI] [PubMed] [Google Scholar]
- 14.Zottoli SJ. 1977. Correlation of the startle reflex and Mauthner cell auditory responses in unrestrained goldfish. J. Exp. Biol. 66, 243–254. [DOI] [PubMed] [Google Scholar]
- 15.Zeddies DG, Fay RR. 2005. Development of the acoustically evoked behavioral response in zebrafish to pure tones. J. Exp. Biol. 208, 1363–1372. ( 10.1242/jeb.01534) [DOI] [PubMed] [Google Scholar]
- 16.Deban SM, Wake DB. 2000. Aquatic feeding in salamanders. In Feeding: form, function and evolution in tetrapod vertebrates (ed. Schwenk K.), pp. 65–94. San Diego, CA: Academic Press. [Google Scholar]
- 17.Wainwright PC, Sanford CP, Reilly SM, Lauder GV. 1989. Evolution of motor patterns: aquatic feeding in salamanders and ray-finned fishes. Brain Behav. Evol. 34, 329–341. ( 10.1159/000116519) [DOI] [PubMed] [Google Scholar]
- 18.Muller M, Osse JWM. 1984. Hydrodynamics of suction feeding in fish. Trans Zool. Soc. Lond. 37, 51–135. ( 10.1111/j.1096-3642.1984.tb00068.x) [DOI] [Google Scholar]
- 19.Lauder GV, Prendergast T. 1992. Kinematics of aquatic prey capture in the snapping turtle Chelydra serpentina. J. Exp. Biol. 164, 55–78. ( 10.1016/0022-0981(92)90136-X) [DOI] [Google Scholar]
- 20.Van Damme J, Aerts P. 1997. Kinematics and functional morphology of aquatic feeding in Australian snake-necked turtles (Pleurodira; Chelodina). J. Morphol. 233, 113–125. ( 10.1002/(SICI)1097-4687(199708)233:2%3C113::AID-JMOR3%3E3.0.CO;2-7) [DOI] [PubMed] [Google Scholar]
- 21.Langebartel DA. 1968. The hyoid and its associated muscles in snakes. In Illinois biological monographs, 38. Urbana, IL: University of Illinois Press. [Google Scholar]
- 22.Schwenk K. 1994. Why snakes have forked tongues. Science 263, 1573–1577. ( 10.1126/science.263.5153.1573) [DOI] [PubMed] [Google Scholar]
- 23.Murphy JC. 2012. Marine invasions by non-sea snakes, with thoughts on terrestrial–aquatic–marine transitions. Integr. Comp. Biol. 52, 217–226. ( 10.1093/icb/ics060) [DOI] [PubMed] [Google Scholar]
- 24.Alfaro ME. 2002. Forward attack modes of aquatic feeding garter snakes. Funct. Ecol. 16, 204–215. ( 10.1046/j.1365-2435.2002.00620.x) [DOI] [Google Scholar]
- 25.Lillywhite HB. 1996. Husbandry of the little file snake, Acrochordus granulatus. Zoo Biol. 15, 315–327. ( 10.1002/(SICI)1098-2361(1996)15:3%3C315::AID-ZOO10%3E3.0.CO;2-9) [DOI] [Google Scholar]
- 26.Murphy JC. 2007. Homalopsid snakes. Evolution in the mud. Malabar, FL: Krieger Publishing Company. [Google Scholar]
- 27.Glodek GS, Voris HK. 1982. Marine snake diets : prey composition, diversity and overlap. Copeia 1982, 661–666. ( 10.2307/1444667) [DOI] [Google Scholar]
- 28.Heatwole H. 1987. Sea snakes. Kensington, Australia: New South Wales University Press in association with the Australian Institute of Biology. [Google Scholar]
- 29.Young BA. 1991. The influences of the aquatic medium on the prey capture system of snakes. J. Nat. Hist. 25, 519–531. ( 10.1080/00222939100770321) [DOI] [Google Scholar]
- 30.Savitzky AH. 1983. Coadapted character complexes among snakes: fossoriality, piscivory, and durophagy. Am. Zool. 23, 397–409. ( 10.1093/icb/23.2.397) [DOI] [Google Scholar]
- 31.Cundall D, Gans C. 1979. Feeding in watersnakes: an electromyographic study. J. Exp. Zool. 209, 189–207. ( 10.1002/jez.1402090202) [DOI] [Google Scholar]
- 32.Boltt RE, Ewer RF. 1964. The functional anatomy of the head of the puff adder, Bitis arietans. J. Morphol. 114, 83–105. ( 10.1002/jmor.1051140105) [DOI] [Google Scholar]
- 33.Vincent SE, Herrel A, Irschick DJ. 2004. Sexual dimorphism in head shape and diet in the cottonmouth snake (Agkistrodon piscivorus). J. Zool. 264, 53–59. ( 10.1017/S0952836904005503) [DOI] [Google Scholar]
- 34.Marx H, Rabb GB. 1972. Phyletic analysis of fifty characters of advanced snakes. Fieldiana (Zoology) 63, 1–321. [Google Scholar]
- 35.Camilleri C, Shine R. 1990. Sexual dimorphism and dietary divergence: differences in trophic morphology between male and female snakes. Copeia 1990, 649–658. ( 10.2307/1446430) [DOI] [Google Scholar]
- 36.Vincent SE, Dang PD, Herrel A, Kley NJ. 2006. Morphological integration and adaptation in the snake feeding system: a comparative phylogenetic study. J. Evol. Biol. 19, 1545–1554. ( 10.1111/j.1420-9101.2006.01126.x) [DOI] [PubMed] [Google Scholar]
- 37.Hibbitts TJ, Fitzgerald LA. 2005. Morphological and ecological convergence in two natricine snakes. Biol. J. Linn. Soc. 85, 363–371. ( 10.1111/j.1095-8312.2005.00493.x) [DOI] [Google Scholar]
- 38.Gunz P, Mitteroecker P. 2013. Semilandmarks: a method for quantifying curves and surfaces. Hystrix 24, 103–109. [Google Scholar]
- 39.Adams DC. 2014. A generalized K statistic for estimating phylogenetic signal from shape and other high-dimensional multivariate data. Syst. Biol. 63, 685–697. ( 10.1093/sysbio/syu030) [DOI] [PubMed] [Google Scholar]
- 40.Pyron RA, Burbrink FT, Wiens JJ. 2013. A phylogeny and revised classification of squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 13, 93 ( 10.1186/1471-2148-13-93) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maddison WP, Maddison DR.2014. Mesquite: a modular system for evolutionary analysis. Version 3.04. See http://mesquiteproject.org .
- 42.Wiley DF, et al. 2005. Evolutionary morphing. In VIS 05. IEEE Visualization, 2005, pp. 431–438. New York, NY: IEEE Publishing. [Google Scholar]
- 43.Botton-Divet L, Houssaye A, Herrel A, Fabre A-C, Cornette R. 2015. Tools for quantitative form description; an evaluation of different software packages for semi-landmark analysis. PeerJ 3, e1417 ( 10.7717/peerj.1417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlager S.2013. Morpho: Calculations and visualizations related to geometric morphometrics, 2013. See http://sourceforge.net/projects/morpho-rpackage/ .
- 45.R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.r-project.org/. [Google Scholar]
- 46.Rohlf FJ, Slice D. 1990. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst. Zool. 39, 40–59. ( 10.2307/2992207) [DOI] [Google Scholar]
- 47.Klingenberg CP. 2011. MorphoJ: an integrated software package for geometric morphometrics. Mol. Ecol. Resour. 11, 353–357. ( 10.1111/j.1755-0998.2010.02924.x) [DOI] [PubMed] [Google Scholar]
- 48.Baylac M.2012. Rmorph: a R geometric and multivariate morphometrics library. Available from the author: baylac@mnhn.fr.
- 49.Blomberg SP, Garland TJ, Ives AR. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745. ( 10.1111/j.0014-3820.2003.tb00285.x) [DOI] [PubMed] [Google Scholar]
- 50.Adams DC, Otarola-Castillo E. 2013. Geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods Ecol. Evol. 4, 393–399. ( 10.1111/2041-210X.12035) [DOI] [Google Scholar]
- 51.Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO. 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464. ( 10.1093/bioinformatics/btq166) [DOI] [PubMed] [Google Scholar]
- 52.Bilcke J, Herrel A, Van Damme R. 2006. Correlated evolution of aquatic prey-capture strategies in European and American natricine snakes. Biol. J. Linn. Soc. 88, 73–83. ( 10.1111/j.1095-8312.2006.00608.x) [DOI] [Google Scholar]
- 53.Vincent SE, Herrel A, Irschick DJ. 2005. Comparisons of aquatic versus terrestrial predatory strikes in the pitviper, Agkistrodon piscivorus. J. Exp. Zool. Part A Comp. Exp. Biol. 303, 476–488. ( 10.1002/jez.a.179) [DOI] [PubMed] [Google Scholar]
- 54.Drummond H. 1985. The role of vision in the predatory behaviour of natricine snakes. Anim. Behav. 33, 206–215. ( 10.1016/S0003-3472(85)80134-2) [DOI] [Google Scholar]
- 55.Vincent SE, Brandley MC, Herrel A, Alfaro ME. 2009. Convergence in trophic morphology and feeding performance among piscivorous natricine snakes. J. Evol. Biol. 22, 1203–1211. ( 10.1111/j.1420-9101.2009.01739.x) [DOI] [PubMed] [Google Scholar]
- 56.Somaweera R. 2004. Sri Lankan Colubrid snakes. Sri Lanka Nat. 5, 32–46. [Google Scholar]
- 57.Trapp B, Mebert K. 2011. Upward position of eyes and nostrils of the dice snake to break the water surface? Mertensiella 18, 440. [Google Scholar]
- 58.Povel D, Van Der Kooij J. 1997. Scale sensillae of the file snake (Serpentes: Acrochordidae) and some other aquatic and burrowing snakes. Netherlands J. Zool. 47, 443–456. ( 10.1163/156854297X00111) [DOI] [Google Scholar]
- 59.Walls GL. 1942. The vertebrate eye and its adaptive radiation. Optometry and vision science. New York, NY: Hafner Publishing Company. [Google Scholar]
- 60.Schaeffel F, Mathis U. 1991. Underwater vision in semi-aquatic European snakes. Naturwissenschaften. 78, 373–375. ( 10.1007/BF01131614) [DOI] [Google Scholar]
- 61.Schaeffel F, de Queiroz A. 1990. Alternative mechanisms of enhanced underwater vision in the garter snakes Thamnophis melanogaster and T. couchii. Copeia 1990, 50–58. ( 10.2307/1445821) [DOI] [Google Scholar]
- 62.Shine R, Brown GP, Elphick MJ. 2004. Field experiments on foraging in free-ranging water snakes Enhydris polylepis (Homalopsinae). Anim. Behav. 68, 1313–1324. ( 10.1016/j.anbehav.2004.03.004) [DOI] [Google Scholar]
- 63.Brischoux F, Lillywhite HB. 2011. Light- and flotsam-dependent ‘float-and-wait’ foraging by pelagic sea snakes (Pelamis platurus). Mar. Biol. 158, 2343–2347. ( 10.1007/s00227-011-1738-z) [DOI] [Google Scholar]
- 64.Miles DB. 1994. Covariation between morphology and locomotory performance in Scleropine lizards. In Lizard ecology: historical and experimental perspectives (eds Pianka ER, Vitt LJ), pp. 207–236. Princeton, NJ: Princeton University Press. [Google Scholar]
- 65.Segall M, Cornetter R, Fabre A-C, Godoy-Diana R, Herrel A. 2016. Data from: Does aquatic foraging impact head shape evolution in snakes? Dryad Digital Repository. 10.5061/dryad.kd3qm. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
PC scores for each species are available online (Dryad: http://dx.doi.org/10.5061/dryad.kd3qm) [65].