Abstract
The invasion and range expansion of Aedes albopictus (Skuse) in North America represents an outstanding opportunity to study processes of invasion, range expansion, and climatic adaptation. Furthermore, knowledge obtained from such research is relevant to developing novel strategies to control this important vector species. Substantial evidence indicates that the photoperiodic diapause response is an important adaptation to climatic variation across the range of Ae. albopictus in North America. Photoperiodic diapause is a key determinant of abundance in both space and time, and the timing of entry into and exit out of diapause strongly affects seasonal population dynamics and thus the potential for arbovirus transmission. Emerging genomic technologies are making it possible to develop high-resolution, genome-wide genetic markers that can be used for genetic mapping of traits relevant to disease transmission and phylogeographic studies to elucidate invasion history. Recent work using next-generation sequencing technologies (e.g., RNA-seq), combined with physiological experiments, has provided extensive insight into the transcriptional basis of the diapause response in Ae. albopictus. Applying this knowledge to identify novel targets for vector control represents an important future challenge. Finally, recent studies have begun to identify traits other than diapause that are affected by photoperiodism. Extending this work to identify additional traits influenced by photoperiod should produce important insights into the seasonal biology of Ae. albopictus.
Keywords: Aedes albopictus, diapause, photoperiodism, climate, adaptation
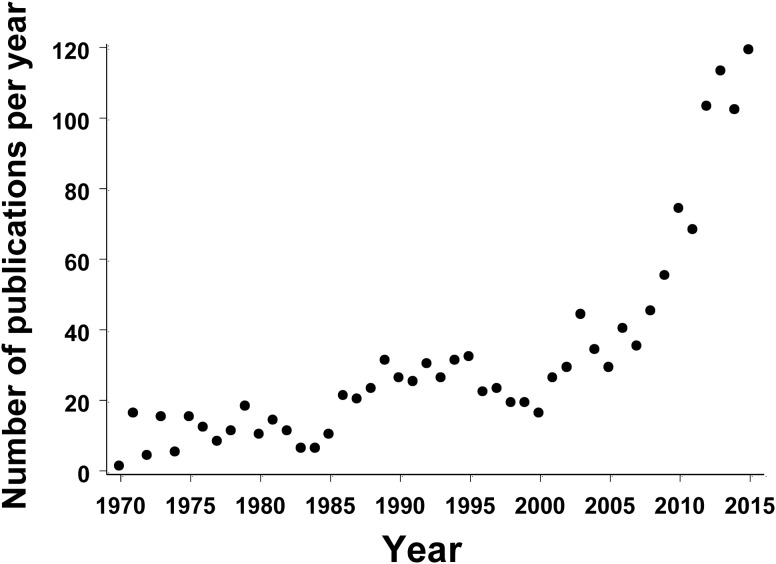
The initial discovery of Aedes albopictus (Skuse) in North America occurred on June 2, 1983, when a single adult specimen was collected at a cemetery in Memphis, TN (Reiter and Darsie 1984). On August 2, 1985, D. Sprenger and T. Wuithirangyagool of the Harris County Mosquito Control District discovered breeding populations of Ae. albopictus at several tire dumps throughout Harris County, TX (Sprenger and Wuithiranyagool 1986). Within several years of the discovery in Texas, Ae. albopictus expanded its range across ∼12 ° of latitude, extending almost to its current northern limit (Moore 1999). This rapid range expansion was likely facilitated by human-aided long-distance transport of artificial containers holding eggs and larvae (Lounibos 2002, Medley et al. 2015). Since its establishment in 1985, Ae. albopictus has become one of the most abundant container-breeding mosquitoes in many suburban and urban areas of eastern North America (Farajollahi and Nelder 2009, Dowling et al. 2013, LaDeau et al. 2013, Unlu et al. 2013). Aedes albopictus is a significant biting pest (Halasa et al. 2012, Unlu et al. 2013), and local infestations can impact human behaviors such as time spent on outdoor activities (Worobey et al. 2013, Halasa et al. 2014). Furthermore, this aggressive human-biting mosquito is capable of transmitting at least 22 arboviruses, including dengue (DENV) and chikungunya (CHIKV) viruses (Gratz 2004). As a result, the establishment of Ae. albopictus in eastern North America caused immediate public health concern (Knudsen 1986, Moore 1986). More recently, these concerns have been exacerbated by the northward spread of DENV into Florida and Texas and the widespread introduction of CHIKV into the Caribbean (CDC 2007, 2014). Thus, the invasion and range expansion of Ae. albopictus in eastern North American has attracted the attention of a wide range of researchers, and the number of published studies examining various aspects of the biology of this mosquito has increased dramatically from 1985–2015 (Fig. 1) .
Fig. 1.
Number of publications with “Aedes albopictus” in the title between 1970 and 2015 based on Pubmed search at http://www.ncbi.nlm.nih.gov/pubmed, accessed January 3, 2016. Note that this search is a conservative estimate of the number of studies concerning Aedes albopictus.
Photoperiodic diapause is a crucial mechanism of overwinter survivorship in a wide range of temperate insects (Tauber et al. 1986, Danks 1987). In its ancestral Asian range, Ae. albopictus has an unusually widespread latitudinal distribution, including tropical populations that do not undergo photoperiodic diapause and temperate populations that do undergo photoperiodic diapause (Hawley 1988). In the photoperiodic diapause response of temperate populations, the pupal and adult females are photosensitive; when exposed to short (autumnal) day lengths, females produce diapause eggs that undergo developmental arrest as a pharate larvae inside the chorion of the egg (Wang 1966, Mori et al. 1981). Diapause is a hormonally controlled developmental arrest that is programmed by a token stimulus in advance of the onset of unfavorable environmental conditions and is not immediately terminated in response to favorable conditions (Denlinger 2002, Denlinger and Armbruster 2014). Thus, diapause is a phenotypically plastic response that provides an adaptive mechanism for the temporal coordination of growth, development, and dormancy in a seasonal environment.
Because the diapause response is a fundamental requirement for overwinter survival of temperate populations, the development of chemical or genetic strategies to disrupt diapause could be an effective mechanism to reduce populations. Genetic approaches to vector control are becoming increasingly feasible (Harris et al. 2012, McGraw and O’Neill 2013), and it is possible that diapause-disrupting genetic constructs could be effectively spread by released males during early-spring and mid-summer when there is no requirement for diapause, but then would have a lethal effect when winter arrived. Additionally, because the diapause response involves the modulation of a wide variety of fundamental physiological processes including stress resistance, energy storage, and the hormonal control of development (see below), studies of the molecular regulation of diapause are likely to identify a wide range of novel targets for genetic or chemical vector control strategies.
When the North American invasion by Ae. albopictus occurred, researchers quickly recognized that the photoperiodic diapause response was a crucial ecophysiological adaptation that would have important implications for the establishment, spread, and thus potential for disease transmission by this mosquito in North America (Hawley et al. 1987). Photoperiodic diapause is a key determinant of abundance in both space and time. For example, nondiapausing strains cannot become permanently established in temperate locations, and the timing of entry into and exit out of diapause strongly affects seasonal population dynamics (Bradshaw et al. 2004). In turn, temporal (e.g., seasonal) changes in population density, stage-structure, and other components of population dynamics can have a strong impact on the vectorial capacity of a population (Black and Moore 2005).
The robust photoperiodic diapause response and cold hardiness characteristics of eggs from six North American populations of Ae. albopictus collected shortly after 1985 provided strong evidence that the invasion of North America was from a temperate origin. Tire shipping records implicated northern Japan as the likely source (Hawley et al. 1987). In the years immediately following the 1985 invasion, G. Craig, K. Rai, W. Hawley, W.C. Black IV, and colleagues at the University of Notre Dame made rapid progress in elucidating the population genetics, ecophysiology, and ecology of North American populations of Ae. albopictus (e.g., Black et al. 1988a, b; Hawley et al. 1987, 1989; Rai 1991). This early body of work was extremely important in determining the characteristics that enabled Ae. albopictus to establish and spread in North America, and also provides an unusually rich “baseline” against which to compare contemporary populations and study invasion dynamics over time (e.g., Urbanski et al. 2012).
In part due to the substantial body of work conducted shortly after the invasion of North America, Ae. albopictus has emerged as a particularly useful model system for studying photoperiodic diapause. Numerous studies have investigated the field ecology of photoperiodic diapause in North America (Hawley et al. 1989, Hanson and Craig 1994, Hanson and Craig 1995a) and in Japan (Mori et al. 1981). These studies have provided important insights into the overwintering capability of Ae. albopictus in both its native Asian range and the invasive North American range. Furthermore, C. Pumpuni’s 1989 doctoral thesis from the University of Notre Dame contains many detailed studies of geographic variation in the diapause response among North American populations and describes many important aspects of diapause induction, including the effects of food, temperature, and photoperiod (Pumpuni 1989). More recently, the rapid development of next-generation sequencing technologies has made it possible to perform detailed investigations of the molecular physiology of the photoperiodic diapause response in Ae. albopictus (Huang et al. 2015; Poelchau et al. 2011; Poelchau et al. 2013a, b). The two recently published Ae. albopictus genome sequences should further facilitate efforts to identify the genetic basis of photoperiodic diapause and other important traits in Ae. albopictus (Chen et al. 2015, Dritsou et al. 2015).
In this review, I summarize the substantial body of literature on the ecology, evolution, and molecular physiology of photoperiodic diapause in Ae. albopictus. I also briefly discuss population genetic studies relevant to diapause and the establishment of Ae. albopictus in North America. Finally, I consider evidence of photoperiodism affecting other life-history traits. Throughout, I suggest topics for future research related to the diapause response of Ae. albopictus.
Diapause Ecology of Ae. albopictus
Diapause is an alternative developmental pathway that progresses through three eco-physiological phases: prediapause, diapause, and postdiapause (Kostal 2006). During prediapause the individual is sensitive to the presence of the environmental cue (sensitive stage). In response to the appropriate cue, the individual initiates preparation for entry into diapause (preparative stage). Upon entry into diapause, metabolism is reduced, stress responses are elevated, and development is arrested. Finally, after diapause termination, during postdiapause, development is immediately resumed or the individual is competent to resume development upon exposure to favorable conditions (postdiapause quiescence).
In the hibernal (autumnal) photoperiodic diapause of many temperate insects, photoperiod (day length) is utilized as a token cue that induces the diapause program (Tauber et al. 1986). The ubiquity of photoperiod in inducing a wide range of seasonal responses such as migration and reproduction, in addition to diapause, reflects the fact that photoperiod is a highly accurate indicator of the timing of seasonal change (Bradshaw and Holzapfel 2007a). The essence of photoperiodic responses, including photoperiodic diapause, is that they allow organisms to physiologically and behaviorally anticipate seasonal changes and adjust their life history in advance of changing environmental conditions. Recent work in our lab indicates that in the Washington, DC area, females begin producing diapause eggs in the first week in September (P. Armbruster, personal observation). As a result, the diapausing pharate larvae inside the chorion of the eggs must tolerate the warm days of late summer and early autumn before cold winter temperatures arrive. This late summer and early fall warm period will likely impose significant physiological stress because metabolism is reduced but not entirely shut down during this period, and the diapausing pharate larvae cannot eat or drink to replenish nutrients and water.
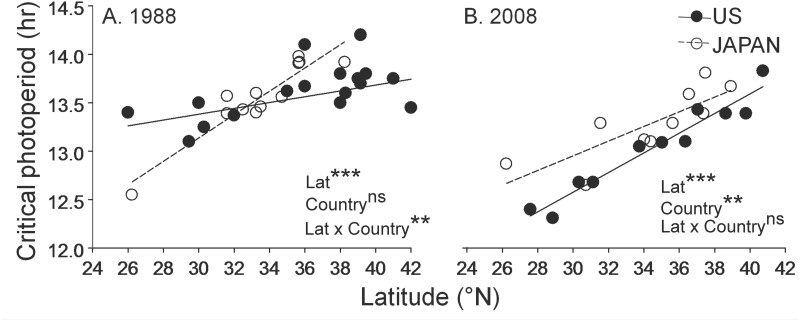
The “switching point” for photoperiodic diapause induction is quantified as the day length which induces diapause in 50% of a population and is referred to as the “critical photoperiod” (CPP). In a wide range of organisms from the northern hemisphere, CPP of geographically separated populations increases from south to north, and the adaptive significance of this pattern is well understood (Tauber et al. 1986). In the northern hemisphere, the length of the growing season declines as one moves north, and the optimal timing of entry into diapause occurs earlier in the year due to the early arrival of winter at northern relative to southern latitudes. Shortly after the 1985 establishment of Ae. albopictus in North America, Focks et al. (1994) measured the CPP of populations collected from across a range of 26 °N to 38 °N–42 °N in both the United States and Japan (Fig. 2A). In these experiments, CPP was measured under standardized laboratory conditions (i.e., a “common garden”), and therefore differences among populations reflect genetic (= evolved) differences among populations. These data will be discussed further below, but one important point to note here is that populations from the southern region of the U.S. distribution tended to exhibit more northern (longer) CPP values that those predicted by the regression between CPP and latitude of Japanese populations. Thus, the data in Fig. 2A imply an origin of 32 °N or farther north for the source of the U.S. invasion. This suggests that the rapid spread of Ae. albopictus from its initial site of discovery in Houston, TX, to more northern regions of the United States was likely facilitated by a “northern adapted” CPP of the invasive population. The gulf coast states (Louisiana, Alabama, Mississippi, and Florida) experienced record low winter temperatures in the 1980s, and these conditions likely caused dramatic reductions of Aedes aegypti (L.), thereby potentially facilitating the spread of Ae. albopictus throughout this region. The more gradual spread of Ae. albopictus from Jacksonville, FL, where it was discovered as early as 1986, south along the Florida peninsula to Miami, FL (O'Meara et al. 1995), may have been at least in part due to mismatched phenology of an ancestral “northern adapted” CPP, although other ecological factors, such as larval competition with Ae. aegypti (Juliano and Lounibos 2005), also likely contributed to the more gradual southward spread.
Fig. 2.
Effect of latitude and region (US = •, Japan = ○) in 1988 (A) and 2008 (B). 1988 data from Pumpuni (1989) and Focks et al. (1994). Results of ANCOVA in lower right of each panel indicate effects of Country, Latitude (Lat), and Country-by-Latitude interaction; ***, P < 0.001; *, P < 0.05; ns, P > 0.05. Modified from Urbanski et al. (2012) with permission from the University of Chicago Press.
It is important to note that CPP as measured under laboratory conditions may or may not reflect the CPP of a specific population expressed under field conditions. For example, Bean et al. (2012) measured the CPP of four geographically distinct populations of the beetle, Diorhabda carinulata (Desbrochers), and found that fluctuating temperatures (15–35 °C) decreased CPP by ∼30–45 min relative to constant (25 °C) temperature conditions. In Ae. albopictus, Pumpuni et al. (1992) measured the photoperiodic response of 14 North American populations in which pupae and adults (the photosensitive stage) were reared at constant temperatures of 21 °C, 26 °C, and 29 °C. In most populations, diapause incidence at any given photoperiod tended to be higher at 21 °C than at 26 °C, and was drastically reduced or abolished at 29 °C. An important implication of these results is that researchers studying any aspect of diapause in Ae. albopictus under laboratory conditions should be careful to rear pupae and adults at 21 °C in order to optimally induce the production of diapause eggs. For a population from Indianapolis exposed to unambiguous short day length at 21 °C, Pumpuni (1989) determined 7.5 d to be the required day number for production of ∼50% diapause eggs. These considerations emphasize that it is essential to empirically verify that the experimental conditions used in any given study actually do induce diapause.
In addition to the effect of temperature, Pumpuni et al. (1992) also demonstrated that a suboptimal larval diet led to slightly increased diapause incidence and a slightly longer CPP relative a near-optimal larval diet. Taken together, the results demonstrating effects of temperature and larval nutrition suggest that lab estimates of CPP performed under constant temperature conditions with larvae reared on a near optimal laboratory diet will likely not reflect CPP expressed under field conditions. Studies measuring the CPP of natural populations by collecting eggs from wild females (e.g., Lacour et al. 2015) nicely complement the current body of literature examining CPP under laboratory conditions. Performing such studies to examine differences in CPP among field populations from different habitat types (e.g., urban vs. rural) or over multiple years to document changes associated with ongoing global change would be especially valuable.
As part of a series of studies investigating the overwintering success of Ae. albopictus in the mid-western United States, Hawley et al. (1989) demonstrated that diapause eggs exhibited increased overwinter survivorship relative to nondiapause eggs at three field sites—South Bend, IN; Indianapolis, IN; and Evansville, IL. This increased overwinter survivorship of diapause eggs during the mid-western winter is presumably due to the increased cold tolerance of diapause eggs (Hanson and Craig 1994), as well as enhanced desiccation resistance (Sota and Mogi 1992, Urbanski et al. 2010) and increased lipid content (Reynolds et al. 2012). Interestingly, Hanson and Craig (1995b) showed that although cold acclimation and diapause independently decreased the lower lethal temperature of eggs from −8 °C to −12 °C, these temperatures are still substantially above the supercooling point of ∼−27 °C, which was not affected by either diapause or cold acclimation (Hanson and Craig 1995b). These results imply that the mechanistic cause of cold temperature-induced mortality is not freezing of tissue, an event that occurs at the supercooling point, but rather some other factor, such as membrane damage or condensation, metabolic disruption, or protein inactivation (Hanson and Craig 1995b).
Another important result from the study of Hawley et al. (1989) was the finding that diapause eggs of three northern populations (Indiana) had higher survivorship than diapause eggs of three southern populations (Texas, Florida, and New Orleans) at two Indiana field sites (South Bend and Evansville) under common garden conditions. These results imply that physiological processes either during diapause preparation or the development arrest stage of diapause have apparently evolved in response to selection imposed by longer and colder winters in more northern regions of the North American range of Ae. albopictus. This apparent evolution of traits expressed during diapause is important because it contradicts the canonical ecological perspective of diapause as “escape in time” (Slobodkin 1963). In fact, it is well established that diapause is a physiologically and metabolically active state (Denlinger 2002). However, virtually no work has been performed on this important issue of geographic variation in selection acting on traits expressed during diapause. This question has important implications for understanding factors that determine northern range limits and how Ae. albopictus and other organisms that undergo hibernal diapause will respond to global climate change. For example, it is likely that for many organisms, warmer winter temperatures will lead to decreased snow cover that will increase exposure to more severe winter temperatures and also potentially an increased frequency of freeze-thaw cycles (Bale and Hayward 2010).
In another study of diapause under field conditions, Hanson and Craig (1994) found that diapause eggs of a temperate strain of Ae. albopictus exhibited relatively high overwinter survivorship of 78% at a South Bend Indiana field site in 1990–1991, but no eggs survived the winter of 1989–1990. Interestingly, these dramatic differences in overwinter survivorship cannot be explained by differences in the average or minimum winter temperature, since 1990–1991 was actually colder than 1989–1990 (Hanson and Craig 1994). One possible factor contributing to these results is that conditions during the late-summer and early-fall stages of hibernal diapause, when the eggs are in diapause but temperatures are still relatively warm, can affect overwintering success. This issue will likely become increasingly relevant to anticipating how organisms respond to global climate warming, as extended growing seasons (e.g., later winters) will increase the length of the autumnal warm period experienced by diapause eggs and thereby potentially impose physiological stress during this period unless corresponding changes in CPP evolve rapidly.
It has recently been shown that exposure of eggs to the juvenile hormone analog pyripoxyfen promotes diapause termination (Suman et al. 2015), but we still know relatively little about the factors that terminate diapause under natural conditions. Thus, studies investigating mechanisms of diapause termination under both laboratory and field conditions should be a high priority. The issue of diapause termination has import ecological consequences because the vernal timing of larval development, mediated at least in part by diapause termination, is expected to have substantial impacts on larval competitive interactions. For example, Aedes japonicus (Theobald) is another invasive mosquito in North America that is commonly found in tires and other container habitats frequently occupied by Ae. albopictus. In their native Asian range, Ae. japonicus has a more northern distribution than Ae. albopictus, and at several field sites in Washington D.C. and New Jersey, Ae. japonicus larvae are more abundant in the early spring relative to late summer (P. Armbruster, personal observation). These results suggest that earlier diapause termination of Ae. japonicus relative to Ae. albopictus may allow the former to grow to larger size in the spring relative to the latter, providing a size-based competitive advantage. This possibility is important to consider because most studies of larval competition between Ae. albopictus and other North American container-breeding mosquitoes initiate experiments with even aged first-instar cohorts. However, these experiments do not reflect potential size asymmetries that may occur early in the growing season due to differences in the timing of diapause termination.
Diapause Evolution of Ae. albopictus
The seasonal timing of diapause induction, as measured by CPP, has undergone rapid adaptive evolution during the range expansion of Ae. albopictus in North America, indicating the crucial importance of diapause timing to climatic adaptation in temperate regions (Urbanski et al. 2012). As noted above, populations collected from across a range of ∼14 ° of latitude in North America between 1985–1988 exhibited a weak relationship between CPP and latitude with a slope that was shallower than among Japanese populations (Fig. 2A). However, populations collected from across a similar range from North America and Japan 20 yr later exhibited parallel slopes of CPP vs. latitude (Fig. 2B), with a significantly higher r2 for U.S. populations in the 2008 relative to 1988 collection (Urbanski et al. 2012). The parallel slopes of CPP vs. latitude in Japan and the United States in 2008 strongly implies an adaptive evolutionary response in U.S. populations, because although the interaction of genetic drift and secondary contact with gene flow can give rise to a correlation of a trait with latitude (i.e., a “cline”), parallel trait variation in two geographically distinct regions is a strong indication that natural selection is driving trait evolution (Partridge and French 1996, Gilchrist et al. 2004).
In contrast to the results described above, studies examining a wide variety of life-history traits not related to diapause in Ae. albopictus consistently demonstrate genetic differentiation of traits, but no consistent geographic trend implying climatic adaptation. The traits considered included those that have been implicated in climatic adaptation in other arthropods, such as body size, egg size, and wing length (Urbanski et al. 2012), as well as preadult development time and growth rate (Armbruster and Conn 2006), adult survivorship, reproductive output (Leisnham et al. 2008), and larval competitive ability (Leisnham et al. 2009). The lack of evidence for involvement of this broad range of life-history traits in climatic adaptation emphasizes the importance of the seasonal timing of diapause induction (CPP), and potentially other traits related to diapause (e.g., Hawley et al. 1989, Lounibos et al. 2003), as the major adaptation to climatic variation across the North American range of Ae. albopictus. Identifying additional traits contributing to climatic adaptation, such as traits related to thermal performance or traits expressed during diapause, represents an important future goal.
Diapause incidence, which describes the proportion of diapause eggs produced under unambiguous short-day conditions (e.g., photoperiod of 10:14 [L:D] h), has also evolved rapidly among North American populations since the 1985 invasion. As noted above, data from Pumpuni (1989) and Hawley et al. (1987) demonstrate that the colonization of North America was by a temperate, diapausing strain. However, Lounibos et al. (2003) measured under standardized laboratory conditions the diapause incidence of populations collected in 1998–2000 from between Miami, FL (25 °N), and St. Louis, IL (38 °N), and found that Jacksonville, FL (30 °N), appeared to denote a breakpoint in the diapause incidence of populations. Populations north of Jacksonville exhibited a nearly 100% diapause incidence. However, diapause incidence decreased with decreasing latitude from Jacksonville (∼90% diapause incidence) south to Miami, FL (∼65% diapause incidence). Subsequent studies indicate a further reduction of diapause incidence in Florida populations from 1998–2000 to 2005–2008 (Lounibos et al. 2011, Urbanski et al. 2012). Two hypotheses have been proposed to explain these results. The first hypothesis is that due to a more permissive winter environment in Florida, populations in Florida have evolved decreased diapause incidence as a derived trait in response to natural selection against diapause. The second hypothesis is that populations in Florida have experienced admixture between temperate, diapausing populations and nondiapausing populations of tropical origin that independently invaded the United States. As discussed below, resolving these alternatives is one of several important questions that can be addressed by developing high-resolution molecular markers to study the historical phylogeography of Ae. albopictus.
Population Genetics and Establishment of Ae. albopictus in North America
Population genetics work conducted shortly after the 1985 invasion of North America relied mostly on allozyme markers. A discriminate analysis of allozyme variation among 57 populations from North America, Japan, Malaysia, Borneo, India, and the People’s Republic of China strongly supported a Japanese origin of the North American populations (Kambhampati et al. 1991), consistent with the inference from ecological and tire-shipping data discussed earlier. North American populations of Ae. albopictus collected shortly after the 1985 invasion had levels of allozyme variability similar to Asian populations, suggesting the founding population size was not sufficiently low to erode allozyme variation (Black et al. 1988a,b; Kambhampati et al. 1990; Kambhampati et al. 1991). These studies also found high levels of local population structure (i.e., genetic differences) among North American populations, which was consistent over a 3-yr period (Kambhampati et al. 1990, 1991). Similar high population structure was also found in native (peninsular Malaysia and Borneo) populations (Black et al. 1988b). These studies therefore suggest that high levels of local population structure are not simply a consequence of invasion and range expansion in North America. These results are also consistent with studies of geographic variation in life-history traits and diapause characteristics among North American populations discussed above, implying that limited gene flow between populations facilitates evolutionary divergence among populations due to either genetic drift or natural selection.
More recent population genetic and phylogeographic studies have relied on analysis of mitochondrial DNA (mtDNA) sequences. Birungi and Munstermann (2002) utilized single-strand conformation polymorphism (SSCP) screening and direct sequencing to survey variation at a 405-bp fragment of the mitochondrial ND5 gene among populations from North America, Brazil, Madagascar, Malaysia, Indonesia, and Japan. Results of this study indicated shared haplotypes between Asian and North American populations, consistent with a Japanese origin of the North American invasion by Ae. albopictus. It was not possible to make strong inferences regarding whether multiple invasions from unique Asian sources have occurred in North American because of the low level of genetic variability detected in this study. The analysis of Birungi and Munstermann (2002) also revealed that North American and Brazilian populations did not share any ND5 haplotypes, indicating a unique origin and lack of contemporary gene flow between these regions. In addition to ND5, analysis of mtDNA gene sequences including Cytb and COI has also been used to examine the phylogeography of Ae. albopictus (Mousson et al. 2005, Usmani-Brown et al. 2009, Kamgang et al. 2011, Porretta et al. 2012, Beebe et al. 2013). Additionally, a recent study analyzing COI mtDNA variation strongly supports an Asian origin of Ae. albopictus populations established sometime after 2001 in Los Angeles county (Zhong et al. 2013). A common theme from all of these analyses is that Ae. albopictus displays a relatively low level of mitochondrial DNA variability. This result is likely due to the infection of Ae. albopictus by two strains of the intracellular parasite Wolbachia, which can cause cytoplasmic incompatibility, leading to “cytoplasmic sweeps” that homogenize mtDNA and thereby complicate the use of mitochondrial markers to infer population history (Armbruster et al. 2003).
Increased genomic resources are making it increasingly feasible to develop high-resolution, genome-wide genetic markers for conducting population genetic analyses on Ae. albopictus (Manni et al. 2015). Furthermore, over the past 10 yr, a rich body of theory and analytical methods have been developed that integrate population genetics, landscape ecology, high-resolution geographic information, and spatial statistics (Manel and Holderegger 2013). Thus, we are currently at a stage where there is enormous potential to make significant advances in our understanding the population dynamics and invasion history of Ae. albopictus both in the United States and across the globe. A recent study analyzing variation at nine microsatellite loci among populations from North America demonstrated the importance of human-aided movement along highways to long-distance dispersal dynamics and is an excellent example of the powerful inferences that can be derived from emerging landscape genetic approaches (Medley et al. 2015). These types of landscape genetic analyses, potentially combined with mechanistic physiological models that specifically account for traits such as diapause, will be extremely valuable for anticipating how the distribution of Ae. albopictus may respond to ongoing rapid global change (Chown et al. 2010, Gotelli and Stanton-Geddes 2015). They will also be useful for determining the source of new invasions and thus predicting the potential for establishment and local spread as Ae. albopictus continues to expand it distribution. Single nucleotide polymorphisms are known to be abundant in the Ae. albopictus genome (Sutherland et al. 2011), and will also be powerful genome-wide markers for both these types of landscape genetic analyses as well as genetic mapping experiments to identify the genetic basis of traits related to diapause, other aspects of geographic adaptation, and disease transmission.
Molecular Physiology of Diapause in Ae. albopictus
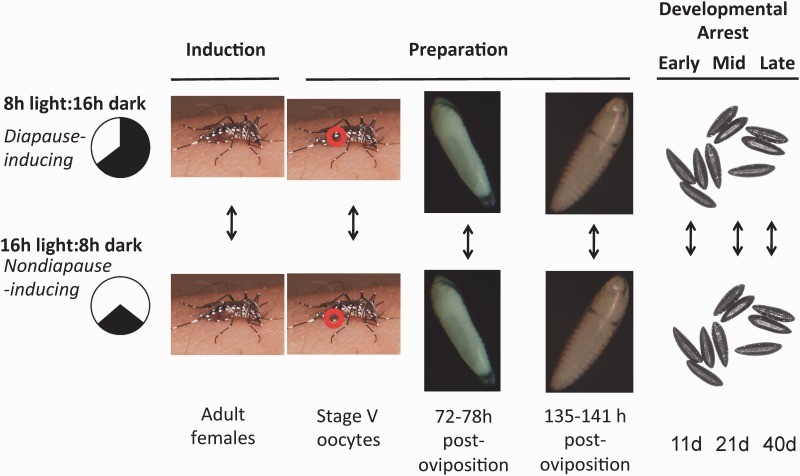
Despite the widespread appreciation of the fundamental importance of photoperiodic diapause to climatic adaptation both in Ae. albopictus and other insects, the molecular basis of this crucial adaptation remains largely unresolved. However, over the past 10 yr, both Ae. albopictus and Culex pipiens (L.) have emerged as productive model systems to address this question (Denlinger and Armbruster 2014, 2016). As emphasized earlier, diapause is not simply a developmental and metabolic shutdown, but rather a complex and dynamic physiological response that represents an alternative developmental pathway composed of a highly coordinated set of behavioral, morphological, and physiological changes (Denlinger 2002). Most of the work on the molecular basis of diapause in Ae. albopictus has used transcriptome sequencing (RNA-seq) to quantify global patterns of gene expression under diapause and nondiapause conditions at carefully defined physiological stages across the trajectory from diapause induction to diapause termination (Fig. 3). These transcriptome sequencing studies in Ae. albopictus have produced a comprehensive transcriptome assembly composed of 14,077 nonredundant gene models, with median gene model coverage of 73% that can be accessed in a user-friendly format (including BLAST searches) at http://albopictusexpression.org. Combined with physiological experiments, these studies have been productive, in part, because interpretation of large-scale transcriptome sequencing data sets is enhanced by a large body of literature on diapause physiology (Tauber et al. 1986; Hahn and Denlinger 2011; Denlinger and Armbruster 2014, 2016).
Fig. 3.
Experimental design of RNAseq studies to identify the transcriptional basis of diapause in Ae. albopictus during the stages of diapause induction, preparation, and developmental arrest (Huang et al. 2015; Poelchau et al. 2011, 2013a, 2013b). The transcriptomes of mosquitoes reared under diapause-inducing and nondiapause inducing photoperiods are compared at different developmental stages (adult females, stage V oocytes, embryos 72–78 h post oviposition, embryos 135–141 h post oviposition, and eggs 11, 21, and 40 d post oviposition).
At the adult stage, during diapause induction (Fig. 3), increased expression under diapause relative to nondiapause conditions of the two circadian clock genes timeless (tim) and cryptochrome1 (cry1) implicates involvement of the circadian clock in photoperiodic time measurement (Huang et al. 2015). Intriguingly, the CRY1 protein is responsible for the light-sensitive breakdown of the TIM protein, and TIM has been implicated in the photoperiodic time measurement of other insects (Bradshaw and Holzapfel 2007b, Meuti and Denlinger 2013, Denlinger and Armbruster 2016). Detailed functional genomics studies will be required to further investigate potential mechanisms linking the circadian clock to photoperiodic diapause induction, but the results described above suggest that breakdown products of the TIM protein might serve as components of an “interval timer” in Ae. albopictus (Huang et al. 2015). Also at the adult stage during diapause induction, differential expression of genes in two amino acid metabolism pathways (valine, leucine, isoleucine degradation and beta-alanine metabolism) under diapause conditions in adults are conspicuous and similar to results from the pupal diapause of the flesh fly, Sarcophaga crassipalpis (Macquart) (Michaud and Denlinger 2007), although the functional significance of these changes in amino acid metabolism are not clear (Huang et al. 2015). Finally at the adult stage, increased provisioning to the offspring is suggested by increased expression of the vitellogenin synthesis gene (PVG1), and fatty acid synthase, which encodes a protein involved in lipid accumulation.
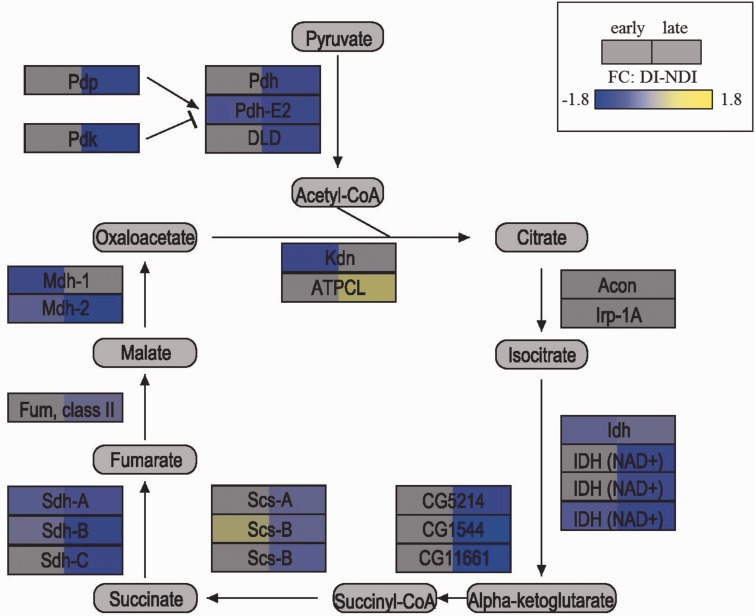
During embryological development, at the stage of diapause preparation, several genes involved in lipid metabolism are differentially expressed under diapause relative to nondiapause conditions. Similar to Aedes atropalpus (Coquillett) (Linley and Craig, 1994), diapause eggs of Ae. albopictus are larger than nondiapause eggs (Reynolds et al. 2012). Diapause eggs of Ae. albopictus also exhibit increased lipid content relative to nondiapause eggs, likely due in part due to increased maternal provisioning discussed above, but also as a result of enhanced lipid conservation during embryological development due to decreased expression of genes involved in lipid catabolism, including lipase 2, lipase 3, and lipase 4 (Reynolds et al. 2012). Lipid storage is also enhanced during diapause preparation, as indicated by elevated expression of lipid storage droplet protein 2 (Reynolds et al. 2012). There are also diapause-associated shifts during embryological development in the expression of genes involved in endocrine signaling, cell-cycle regulation, and a variety of metabolic processes. Prominent metabolism-related changes include decreased expression under diapause conditions of genes encoding proteins in the citric acid cycle (Fig. 4). These results nicely illustrate that the diapause program can involve highly coordinated transcriptional regulation across a complex metabolic pathway. Another metabolism-related change is the increased expression of pepck, a gene which encodes a protein controlling the rate-limiting step of gluconeogenesis that has been implicated in the diapause response of other insects (Poelchau et al. 2013a). Finally, the increased desiccation resistance of diapause eggs relative to nondiapause eggs is associated with increased abundance of a fatty acyl coA elongase (face) transcript under diapause conditions, both in mature oocytes before oviposition (Urbanski et al. 2010) and during embryological development (Reynolds et al. 2012). This increased abundance of the face transcript corresponds with an increase in quantities of egg surface hydrocarbons that presumably function to minimize water loss of eggs (Urbanski et al. 2010). These results thus provide a likely mechanistic explanation for the observation that diapause eggs have higher survivorship than nondiapause eggs under warm, dry conditions (Sota and Mogi 1992, Lounibos et al. 2011).
Fig. 4.
Diagram of the citric acid (TCA) cycle, adapted from Ragland et al. (2010), illustrating gene expression changes between diapause (DI) and nondiapause (NDI) photoperiods for early (72–78 h) and late (135–141 h) stage embryos. Enzymes are depicted as rectangles, and metabolites as rounded rectangles. Significant fold-change values are depicted as shades of blue or yellow as indicated in the legend in the upper right; nonsignificant fold-change values are grey. Data from Poelchau et al. (2013b).
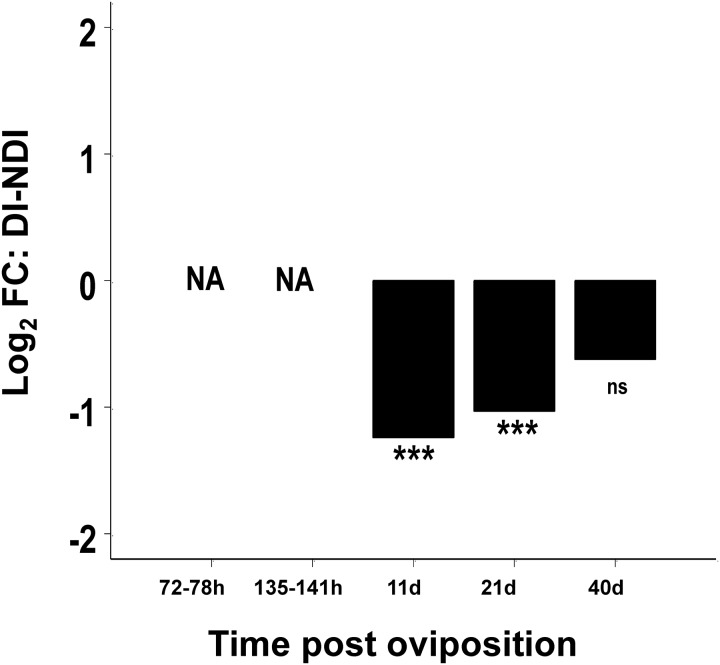
During the development arrest stage of diapause, a relatively large number of genes are differentially expressed at 11 d post oviposition (pov). However, this differential expression dissipates over time so that by 40 d pov, when many eggs are susceptible to a hatching stimulus, relatively few genes are differentially expressed (Poelchau et al. 2013b). Thus, at the transcriptional level, diapause appears to dissipate over time, gradually converging on the nondiapause (quiescent) state. Differential expression of genes involved in carbohydrate, lipid, and amino acid metabolism represents the major distinction between diapause and nondiapause (quiescent) eggs. Similar to results from embryos, pepck expression is higher under diapause relative to nondiapause conditions, implying increased utilization of the gluconeogenesis pathway and a shift toward anaerobic respiration during diapause. Altered amino acid metabolism is reflected by mostly down regulation of genes involved in glutamine, glycine, and serine metabolic pathways (Poelchau et al. 2013b). Amino acids are thought to contribute to both desiccation and cold tolerance during diapause (Michaud and Denlinger 2007). A preliminary metabolomics analysis of diapause eggs indicated substantially elevated levels of amino acids under diapause conditions (leucine, serine, threonine, tyrosine, lysine, and proline; P. Armbruster, personal observation), suggesting that the transcriptional changes detected in the eggs may contribute to increased amino acid concentrations due to decreased degradation. In terms of lipid metabolism, the expression of both lipases and hydrolases is increased under diapause conditions at 11, 20, and 40 d pov, implying that lipids are being utilized as an energy source as early as 11 d pov and throughout the time course of diapause. Finally, an intriguing pattern of differential expression was detected for a member of the cytochrome p450 family, cyp18a1. The cytochrome P450 family includes proteins with a diverse range of functions including steroid biosynthesis. The putative ortholog of Ae. albopictus cyp18a1 in Drosophila melanogaster (Meigen) encodes an enzyme that inactivates steroid hormones and loss-of-function mutations cause an extended final larval instar (Guittard et al. 2011). This loss-of-function phenotype in D. melanogaster is strikingly similar to the pharate larval developmental arrest of diapause in Ae. albopictus. The D. melanogaster cyp18a1 is also homologous to the Ceanorhabditis elegans (Maupas) gene daf-9, which regulates dauer formation, larval growth, and longevity (Gerisch et al. 2001). Dauer formation in C. elegans is a type of dormancy that is similar to insect diapause at both the physiological and molecular level (Sim and Denlinger 2008), and in C. elegans daf-9 loss-of-function mutants form constitutive dauer larvae (Gerisch et al. 2001, Jia et al. 2002). As illustrated in Fig. 5, cyp18a1 exhibits lower expression under diapause relative to nondiapause conditions at 11 d and 21 d pov, two time points at which the developmental arrest of diapause is firmly established. However, the lack of differential expression of cyp18a1 at 40 d pov is intriguing because at this stage many eggs have terminated diapause and regained hatching competence. Therefore, cyp18a1 represents a promising candidate for regulation of developmental arrest during diapause due to both its diapause-associated pattern of differential expression and the diapause-like phenotype induced by knockout of cyp18a1 orthologues in D. melanogaster and C. elegans.
Fig. 5.
Log2 fold change (FC) of the Ae. albopictus cyp18a1 orthologue under diapause (DI) vs. nondiapause (NDI) conditions at early embryological development (72–78 h), late embryological development (135–141 h), and 11 d, 21 d, and 40 d post oviposition. *** indicates P < 0.001, ns indicates P > 0.05. Data from Poelchau et al. (2013a, b).
Taken together, results of transcriptome sequencing across the trajectory of diapause in Ae. albopictus lead to a number of general conclusions. First, diapause involves primarily quantitative rather than qualitative changes in transcription. Thousands of genes are differentially expressed under diapause vs. nondiapause conditions but few genes are uniquely expressed under either condition (Poelchau et al. 2013a,b; Huang et al. 2015). Several genes identified as differentially expressed as part of the diapause program in Ae. albopictus, such as pepck and fatty acid synthase, have also been implicated in the diapause response of other insects, implying a set of conserved genes that have been repeatedly acted on by natural selection during the independent evolution of diapause in diverse insect lineages (Poelchau et al. 2013a, Denlinger and Armbruster 2014, Huang et al. 2015). Finally, these results indicate that the altered regulation of fundamental physiological pathways that occurs during diapause can be used to identify genes underlying important ecological and stress resistance traits that may provide useful targets for vector control, even in species that don’t undergo diapause. For example, the face transcript described above is expressed in Ae. albopictus under nondiapause conditions, and the inferred amino acid sequence is 96% identical to the Ae. aegypti orthologue, suggesting that the Ae. aegypti orthologue may also be involved in mediating egg desiccation resistance, even though Ae. aegypti does not undergo diapause.
Other Traits Affected by Photoperiodism in Ae. albopictus
Photoperiodism refers to the ability of organisms to use day length (photoperiod) as an anticipatory cue to initiate developmental, physiological, and behavioral responses in advance of seasonally changing environmental conditions. Seasonal environmental variation has a pervasive effect on the growth, survival, and reproduction of temperate organisms and day length is a highly accurate indicator of seasonality in temperate environments. Thus, it is not surprising that a wide range of traits in a broad range of taxa are affected by photoperiodism (Bradshaw and Holzapfel 2007a). Although photoperiodic diapause has received by far the most attention in Ae. albopictus, recent studies have begun to investigate other traits affected by photoperiodism.
Oviposition behavior of adult females is a crucial fitness trait in container-breeding mosquitoes, and in a New Jersey population of Ae. albopictus, oviposition behavior appears to change in response to photoperiod and temperature levels (Fonseca et al. 2015). Under a long-day photoperiod (15:9, L:D) at 27 °C, gravid females provided with multiple oviposition containers prefer to lay eggs in open containers with leaf infusion. However, presumably to avoid containers that already contain eggs, females will eventually shift egg laying to containers that are covered and/or do not contain leaf infusion. Under a short-day photoperiod at 21 °C, females also prefer to oviposit in open containers with leaf infusion, but females continue to oviposit in these containers even as they become saturated with eggs, in some cases ovipositing eggs on top of previously laid eggs (Fonseca et al. 2015). Overwinter egg mortality is likely to reduce the impact of intraspecific competition the following spring when eggs hatch, suggesting that this shift in oviposition behavior between summer and fall conditions may reflect a change in the relative importance of avoiding intraspecific competition versus increasing the likelihood that offspring hatch in containers with high nutrient levels. These results are also potentially relevant to mosquito control efforts because they imply that strategies to target specific containers for larviciding can change on a seasonal basis (Fonseca et al. 2015). The oviposition behavior of Ae. atropalpus also changes in response to photoperiod. Under long-day conditions, females often lay eggs on the water surface, but in response to short-day lengths, females shift to laying eggs on solid surfaces (Means 1979).
There is also recent evidence that larval development time and adult body size are affected by photoperiodism in North American populations of Ae. albopictus (Yee et al. 2012). Yee et al. (2012) quantified these life-history traits in populations from New Jersey, Virginia, North Carolina, and Florida under three photoperiod conditions at 20 °C—a short-static photoperiod (10 h 28 min:13 h 32 min, L:D), a decreasing photoperiod (alternating daily decrease of 2 min to 3 min per day starting from 11 h 8 min:12 h 52 min, L:D), and a long-static photoperiod (13 h 49 min:10 h 11 min, L:D). Under both the short-static and decreasing photoperiods, females, and to a lesser extent males, exhibited shorter larval development periods and reduced adult mass relative to the long-day photoperiod. The shorter larval development time under short-day conditions potentially indicated adaptive seasonal phenotypic plasticity to ensure maturation and successful reproduction before the onset of unfavorable winter conditions, with the sex-specificity of the response reflecting differences in the reproductive constraints of males versus females (Yee et al. 2012).
Costanzo et al. (2015) used a population of Ae. albopictus from Florida to measure larval development time, adult female size, survival, life span, fecundity, and propensity to blood feed at 25 °C under three photoperiod conditions—short day length (10:14, L:D), intermediate day length (12:12, L:D), and long day length (14:10, L:D). Only the size of adult females was affected by photoperiod, and in contrast to the results described above from Yee et al. (2012), Constanzo et al. (2015) found that size increased rather than decreased under short day length conditions. The difference between the results of Costanzo et al. (2015) and those of Yee et al. (2012) is likely due at least in part to the different temperature conditions used in these experiments (25 °C vs. 20 °C). As noted above, photoperiodic effects can be highly temperature dependent (Pumpuni et al. 1992, Bean et al. 2012).
Summary Conclusions
The invasion and range expansion of Ae. albopictus in North America represents an outstanding opportunity to study processes of invasion, range expansion, and climatic adaptation. It turn, such knowledge is highly relevant to anticipating future population dynamics and range expansion in response to ongoing global climate change. Substantial evidence indicates that multiple aspects of the diapause response have contributed to climatic adaptation of Ae. albopictus across its range in North America (Hawley et al. 1989; Lounibos et al. 2003, 2011; Leisnham et al. 2011; Urbanski et al. 2012). Further studies of diapause ecology, especially studies focusing on diapause induction and termination of diapause in natural populations and geographic variation of traits expressed during diapause will be especially important. Recent work using next-generation sequencing technologies combined with physiological experiments has provided extensive insight into the transcriptional basis of the diapause response in Ae. albopictus. Applying this knowledge to identify desperately needed novel targets for vector control represents an important future challenge. The recently completed genome sequence of Ae. albopictus and rapidly developing technologies for genetic modification (e.g., CRISPR-Cas9) should make it feasible to develop such genetic approaches to disrupting the wide range of fundamental physiological processes associated with diapause in the near future. Emerging genomic technologies should also facilitate the development of high-resolution, genome-wide genetic markers that can be used for landscape-level population genetics analyses to both prospectively predict the dynamics of range expansion, and to retrospectively identify the source populations of novel invasions with a high level of precision. Such markers will also facilitate genetic mapping of traits related to invasion success, climatic adaptation, and disease transmission. Finally, identifying additional traits affected by photoperiodism will contribute to a more refined understanding of climatic adaptation, seasonal population dynamics, and potentially seasonal patterns of disease transmission.
Acknowledgments
I thank Donald Yee for his organization of, and invitation to participate in, the symposium “30 Years of Hunting the Tiger. Aedes albopictus in America: Current Perspectives and Future Challenges” at the 2015 Entomological Society of America meeting. Monica Poelchau kindly drafted Fig. 4. Comments from Zachary Batz, Austin Garner and Xin Huang, William Reisen, Donald Yee, and two anonymous reviewers improved the quality of this review. This work was supported in part by National Institutes of Health grant R15AI111328-01 to Peter Armbruster.
References Cited
- Armbruster P., Conn J. E. (2006). Geographic variation of larval growth in North American Aedes albopictus (Diptera: Culicidae). Ann. Entomol. Soc. Am. 99: 1234–1243. [Google Scholar]
- Armbruster P., Damsky W.E.J., Giordano R., Birungi J., Munstermann L. E., Conn J. E. (2003). Infection of New- and Old-World Aedes albopictus (Diptera: Culicidae) by the intracellular parasite Wolbachia: implications for host mitochondrial DNA evolution. J. Med. Entomol. 40: 356–360. [DOI] [PubMed] [Google Scholar]
- Bale J. S., Hayward S.A.L. (2010). Insect overwintering in a changing climate. J. Exp. Biol. 213: 980–994. [DOI] [PubMed] [Google Scholar]
- Bean D. W., Dalin P., Dudley T. L. (2012). Evolution of critical day length for diapause induction enables range expansion of Diorhabda carinulata, a biological control agent against tamarisk (Tamarix spp.). Evol. Appl. 5: 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe N. W., Ambrose L., Hill L. A., Davis J. B., Hapgood G., Cooper R. D., Russell R. C., Ritchie S. A., Reimer L. J., Lobo N. F., et al. (2013). Tracing the tiger: Population genetics provides valuable insights into the Aedes (Stegomyia) albopictus invasion of the Australasian region. PLoS Negl. Trop. Dis. 7: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birungi J., Munstermann L. E. (2002). Genetic structure of Aedes albopictus (Diptera: Culicidae) populations based on mitochondrial ND5 sequences: Evidence for an independant invasion into Brazil and United States. Ann. Entomol. Soc. Am. 95: 125–132. [Google Scholar]
- Black W. C., Moore C. G. (2005). Population biology as a tool to study vector-borne disease. In Marquardt W. C. (ed.), Biology of disease vectors. Elsevier Academic Press, Burlington, MA. [Google Scholar]
- Black W. C., IV, Ferrari J. A., Rai K. S., Sprenger D. (1988a). Breeding structure of a colonizing species: Aedes albopictus (Skuse) in the United States. Heredity 60: 173–181. [DOI] [PubMed] [Google Scholar]
- Black W. C., IV, Ferrari J. A., Rai K. S., Sprenger D. (1988b). Breeding structure of a colonizing species: Aedes albopictus (Skuse) in peninsular Malaysia and Borneo. Heredity 61: 439–436. [DOI] [PubMed] [Google Scholar]
- Bradshaw W. E., Holzapfel C. M. (2007a). Evolution of animal photoperiodism. Ann. Rev. Ecol. Evol. Syst. 38: 1–35. [Google Scholar]
- Bradshaw W. E., Holzapfel C. M. (2007b). Tantalizing timeless. Science 316: 1851–1852. [DOI] [PubMed] [Google Scholar]
- Bradshaw W. E., Zani P. A., Holzapfel C. M. (2004). Adaptation to temperate climates. Evolution 58: 1748–1762. [DOI] [PubMed] [Google Scholar]
- CDC. (2007). Dengue hemorrhagic fever- U.S.-Mexico border, (2005). MMWR Morb. Mortal. Wkly. Rep. 56: 785–789. [PubMed] [Google Scholar]
- CDC. (2014). Chikungunya in the Caribbean. (http://wwwnc.cdc.gov/travel/notices/watch/chikungunya-saint-martin) (accessed 14 February (2014)). [Google Scholar]
- Chen X. G., Jiang X. T., Gu J. B., Xu M., Wu Y., Deng Y. H., Zhang C., Bonizzoni M., Dermauw W., Vontas J., et al. (2015). Genome sequence of the Asian tiger mosquito, Aedes albopictus, reveals insights into its biology, genetics, and evolution. Proc. Natl. Acad. Sci. USA. 112: E5907–E5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown S. L., Hoffmann A. A., Kristensen T. N., Angilletta M. J., Stenseth N. C., Pertoldi C. (2010). Adapting to climate change: a perspective from evolutionary physiology. Clim. Res. 43: 3–15. [Google Scholar]
- Costanzo K. S., Schelble S., Jerz K., Keenan M. (2015). The effect of photoperiod on life history and blood-feeding activity in Aedes albopictus and Aedes aegypti (Diptera: Culicidae). J. Vector Ecol. 40: 164–171. [DOI] [PubMed] [Google Scholar]
- Danks H. V. (1987). Insect dormancy: An ecological perspective., Biological Survey of Canada (Terrestrial Arthropods), Ottowa, Canada. [Google Scholar]
- Denlinger D. L. (2002). Regulation of diapause. Ann. Rev. Entomol. 47: 93–122. [DOI] [PubMed] [Google Scholar]
- Denlinger D. L., Armbruster P. (2014). Mosquito diapause. Ann. Rev. Entomol. 59: 73–93. [DOI] [PubMed] [Google Scholar]
- Denlinger D. L., Armbruster P. (2016). The physiology of mosquito diapause, In Raikhel A. S. (ed.), Advances in insect phsyiology. Elsevier, New York, NY. [Google Scholar]
- Dowling Z., Ladeau S. L., Armbruster P., Biehler D., Leisnham P. T. (2013). Socioeconomic status affect mosquito (Diptera:Culicidae) larval habitat type availability and infestation level. J. Med. Entomol. 50: 764–772. [DOI] [PubMed] [Google Scholar]
- Dritsou V., Topalis P., Windbichler N., Simoni A., Hall A., Lawson D., Hinsley M., Hughes D., Napolioni V., Crucianelli F., et al. (2015). A draft genome sequence of an invasive mosquito: an Italian Aedes albopictus. Path. Global Health 109: 207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajollahi A., Nelder M. P. (2009). Changes in Aedes albopictus (Diptera: Culicidae) populations in New Jersey and implications for arbovirus transmission. J. Med. Entomol. 46: 1220–1224. [DOI] [PubMed] [Google Scholar]
- Focks D. A., Linda S. B., Craig G.B.J., Hawley W. A., Pumpuni C. B. (1994). Aedes albopictus (Diptera: Culicidae): A statistical model of the role of temeperature, photoperiod, and geography in the induction of egg diapause. J. Med. Entomol. 31: 278–286. [DOI] [PubMed] [Google Scholar]
- Fonseca D. M., Kaplan L. R., Heiry R. A., Strickman D. (2015). Density-dependent oviposition by female Aedes albopictus (Diptera: Culicidae) spreads eggs among containers during the summer but accumulates them in the fall. J. Med. Entomol. 52: 705–712. [DOI] [PubMed] [Google Scholar]
- Gerisch B., Weitzel C., Kober-Eisermann C., Rottiers V., Antebi A. (2001). A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Dev. Cell 1: 841–851. [DOI] [PubMed] [Google Scholar]
- Gilchrist G. W., Huey R. B., Balanya J., Pascual M., Serra L. (2004). A time series of evolution in action: A latitudinal cline in wing size in South American Drosophila subobscura. Evolution 58: 768–780. [DOI] [PubMed] [Google Scholar]
- Gotelli N. J., Stanton-Geddes J. (2015). Climate change, genetic markers and species distribution modelling. J. Biogeog. 42: 1577–1585. [Google Scholar]
- Gratz N. (2004). Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 18: 215–227. [DOI] [PubMed] [Google Scholar]
- Guittard E., Blais C., Maria A., Parvy J. P., Pasricha S., Lumb C., Lafont R., Daborn P. J., Dauphin-Villemant C. (2011). CYP18A1, a key enzyme of Drosophila steroid hormone inactivation, is essential for metamorphosis. Dev. Biol. 349: 35–45. [DOI] [PubMed] [Google Scholar]
- Hahn D. A., Denlinger D. L. (2011). Energetics of insect diapause. Ann. Rev. Entomol. 56: 103–121. [DOI] [PubMed] [Google Scholar]
- Halasa Y. A., Shepard D. S., Wittenberg E., Fonseca D. M., Farajollahi A., Healy A., Gaugler R., Strickman D., Clark G. G. (2012). Willingness-to-pay for an area-wide integrated pest management program to control the Asian tiger mosquito in New Jersey. J. Am. Mosq. Control Assoc. 28: 225–236. [DOI] [PubMed] [Google Scholar]
- Halasa Y. A., Shepard D. S., Fonseca D. M., Farajollahi A., Healy S., Gaugler R., Bartlett-Healy K., Strickman D. A., Clark G. G. (2014). Quantifying the impact of mosquitoes on quality of life and enjoyment of yard and porch activities in New Jersey. PLoS ONE 9: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson S. M., Craig G. B. (1994). Cold-acclimation, diapause, and geographic origin affect cold-hardiness in eggs of Aedes albopictus (Diptera, Culicidae). J. Med. Entomol. 31: 192–201. [DOI] [PubMed] [Google Scholar]
- Hanson S. M., Craig G.B.J. (1995a). Aedes albopictus (Diptera:Culicidae) eggs: Field survivorship during northern Indiana winters. J. Med. Entomol. 32: 599–604. [DOI] [PubMed] [Google Scholar]
- Hanson S. M., Craig G.B.J. (1995b). Relationship between cold hardiness and supercooling point in Aedes albopictus eggs. J. Am. Mosq. Control Assoc. 11: 35–38. [PubMed] [Google Scholar]
- Harris A. F., McKemey A. R., Nimmo D., Curtis Z., Black I., Morgan S. A., Oviedo M. N., Lacroix R., Naish N., Morrison N. I., et al. (2012). Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotech. 30: 828–830. [DOI] [PubMed] [Google Scholar]
- Hawley W. A. (1988). The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. 4: 1–39. [PubMed] [Google Scholar]
- Hawley W. A., Reiter P., Copeland R. S., Pumpuni C. B., Craig G. B., Jr. (1987). Aedes albopictus in North America: Probable introduction in used tires from northern Asia. Science 236: 1114–1116. [DOI] [PubMed] [Google Scholar]
- Hawley W. A., Pumpuni C. B., Brady R. H., Craig G. B. (1989). Overwintering survival of Aedes albopictus (Diptera, Culicidae) eggs in Indiana. J. Med. Entomol. 26: 122–129. [DOI] [PubMed] [Google Scholar]
- Huang X., Poelchau M. F., Armbruster P. (2015). Global transcriptional dynamics of diapause induction in non-blood-fed and blood-fed Aedes albopictus. PLoS Negl. Trop. Dis. 9: e0003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia K. L., Albert P. S., Riddle D. L. (2002). DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development 129: 221–231. [DOI] [PubMed] [Google Scholar]
- Juliano S. A., Lounibos L. P. (2005). Ecology of invasive mosquitoes: Effects on resident species and human health. Ecology Lett. 8: 558–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambhampati S., Black W. C., IV, Rai K. S., Sprenger D. (1990). Temporal variation in genetic structure of a colonizing species: Aedes albopictus in the United States. Heredity 64: 281–287. [DOI] [PubMed] [Google Scholar]
- Kambhampati S., Black W. C., IV, Rai K. S. (1991). Geographic origin of the U.S. and Brazilian Aedes albopictus inferred from allozyme analysis. Heredity 67: 85–94. [DOI] [PubMed] [Google Scholar]
- Kamgang B., Brengues C., Fontenille D., Njiokou F., Simard F., Paupy C. (2011). Genetic structure of the tiger mosquito, Aedes albopictus, in Cameroon (Central Africa). PLoS ONE 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen A. B. (1986). The significance of the introduction of Aedes albopictus into the southeastern United States with implications for the Caribbean, and perspectives of the Pan-American health organization. J. Am. Mosq. Control Assoc. 2: 420–423. [PubMed] [Google Scholar]
- Kostal V. (2006). Eco-physiological phases of insect diapause. J. Insect Physiol. 52: 113–127. [DOI] [PubMed] [Google Scholar]
- Lacour G., Chanaud L., L'Ambert G., Hance T. (2015). Seasonal synchronization of diapuse phases in Aedes albopictus (Diptera:Culicidae). PLoS ONE 10: e0145311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDeau S. L., Leisnham P. T., Biehler D., Bodner D. (2013). Higher mosquito production in low-income neighborhoods of Baltimore and Washington, DC: Understanding ecological drivers and mosquito-borne disease risk in temperate cities. Intl. J. Environ. Res. Public Health 10: 1505–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham P. T., Sala L. M., Juliano S. A. (2008). Geographic variation in adult survival and reproductive tactics of the mosquito Aedes albopictus. J. Med. Entomol. 45: 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham P. T., Lounibos L. P., O'Meara G. F., Juliano S. A. (2009). Interpopulation divergence in competitive interactions of the mosquito Aedes albopictus. Ecology 90: 2405–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham P. T., Towler L., Juliano S. A. (2011). Geographic variation of photoperiodic diapause but not adult survival or reproduction of the invasive mosquito Aedes albopictus (Diptera: Culicidae) in North America. Ann. Entomol. Soc. Am. 104: 1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley J. R., Craig G. B., Jr. (1994). Morphology of long- and short-day eggs of Aedes atropalpus and A. epactius (Diptera: Culicidae). J. Med. Entomol. 31: 855–867. [DOI] [PubMed] [Google Scholar]
- Lounibos L. P. (2002). Invasions by insect vectors of human disease. Ann. Rev. Entomol. 47: 233–266. [DOI] [PubMed] [Google Scholar]
- Lounibos L. P., Escher R. L., Lourenco-de-Oliveira R. (2003). Asymmetric evolution of photoperiodic diapause in temperate and tropical invasive populations of Aedes albopictus (Diptera:Culicidae). Ann. Entomol. Soc. Am. 96: 512–518. [Google Scholar]
- Lounibos L. P., Escher R. L., Nishimura N. (2011). Retention and adaptiveness of photoperiodic egg diapause in Florida populations of invasive Aedes albopictus. J. Am. Mosq. Control Assoc. 27: 433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manel S., Holderegger R. (2013). Ten years of landscape genetics. Trends Ecol. Evol. 28: 614–621. [DOI] [PubMed] [Google Scholar]
- Manni M., Gomulski L. M., Aketarawong N., Tait G., Scolari F., Somboon P., Guglielmino C. R., Malacrida A. R., Gasperi G. (2015). Molecular markers for analyses of intraspecific genetic diversity in the Asian tiger mosquito, Aedes albopictus. Parasit. Vectors 8: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw E. A., O'Neill S. L. (2013). Beyond insecticides: New thinking on an ancient problem. Nat. Rev. Microbiol. 11: 181–193. [DOI] [PubMed] [Google Scholar]
- Means R. G. (1979). Mosquitoes of New York: Part 1. The genus Aedes (Meigen) with identification keys to genera of Culicidae. Bulletin 430a, New York State Museum, Albany, NY. [Google Scholar]
- Medley K. A., Jenkins D. G., Hoffman E. A. (2015). Human-aided and natural dispersal drive gene flow across the range of an invasive mosquito. Mol. Ecol. 24: 284–295. [DOI] [PubMed] [Google Scholar]
- Meuti M. E., Denlinger D. L. (2013). Evolutionary links between circadian clocks and photoperiodic diapause in insects. Integr. Comp. Biol. 53: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M. R., Denlinger D. L. (2007). Shifts in the carbohydrate, polyol, and amino acid pools during rapid cold-hardening and diapause-associated cold-hardening in flesh flies (Sarcophaga crassipalpis): a metabolomic comparison. J. Comp. Physiol. [B] 177: 753–763. [DOI] [PubMed] [Google Scholar]
- Moore C. G. (1986). The centers for disease control's perpective of the introduction of Aedes albopictus in the United States. J. Am. Mosq. Control Assoc. 2: 416–417. [PubMed] [Google Scholar]
- Moore C. G. (1999). Aedes albopictus in the United States: Current status and prospects for further spread. J. Am. Mosq. Control Assoc. 15: 221–227. [PubMed] [Google Scholar]
- Mori A., Oda T., Wada Y. (1981). Studies on the egg diapause and overwintering of Aedes albopictus in Nagasaki. Trop. Med. 23: 79–90. [Google Scholar]
- Mousson L., Dauga C., Garrigues T., Schaffner F., Vazeille M., Failloux A. B. (2005). Phylogeography of Aedes (Stegomyia) aegypti (L.) and Aedes (Stegomyia) albopictus (Skuse) (Diptera : Culicidae) based on mitochondrial DNA variations. Genet. Res. 86: 1–11. [DOI] [PubMed] [Google Scholar]
- O'Meara G. F., Evans L. F., Gettman A. D., Cuda J. P. (1995). Spread of Aedes albopictus and decline of Ae. aegypti (Diptera:Culicidae) in Florida. J. Med. Entomol. 32: 554–562. [DOI] [PubMed] [Google Scholar]
- Partridge L., French V. (1996). Thermal evolution of ectotherm body size: Why get bigger in the cold?, pp. 265–292. In Johnston I. A., Bennett A. F. (eds.), Animals and temperature: phenotypic and evolutionary adaptation. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- Poelchau M., Reynolds J., Denlinger D., Elsik C., Armbruster P. (2011). A de novo transcriptome of the Asian tiger mosquito, Aedes albopictus, to identify candidate transcripts for diapause preparation. BMC Genomics 12: 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelchau M. F., Reynolds J. A., Elsik C. G., Denlinger D. L., Armbruster P. A. (2013a). Deep sequencing reveals complex mechanisms of diapuase preparation in the invasive mosquito, Aedes albopictus. Proc. B 280: (2013)0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelchau M. F., Reynolds J. A., Elsik C. G., Denlinger D. L., Armbruster P. (2013b). RNA-Seq reveals ealy distinctions and late convergence of gene expression between diapause and quiescence in the Asian tiger mosquito, Aedes albopictus. J. Exp. Biol. 216: 4082–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porretta D., Mastrantonio V., Bellini R., Somboon P., Urbanelli S. (2012). Glacial history of a modern invader: Phylogeography and species distribution modelling of the Asian tiger mosquito Aedes albopictus. PLoS ONE 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumpuni C. B. (1989). Factors influencing photoperiodic control of egg diapause in Aedes albopictus (Skuse). Ph.D., University of Notre Dame Notre Dame, Indiana. [Google Scholar]
- Pumpuni C. B., Knepler J., Craig G. B. (1992). Influence of temperature and larval nutrition on the diapause inducing photoperiod of Aedes albopictus. J. Am. Mosq. Control Assoc. 14: 223–227. [PubMed] [Google Scholar]
- Ragland G. J., Denlinger D. L., Hahn D. A. (2010). Mechanisms of suspended animation are revealed by transcript profiling of diapause in the flesh fly. Proc. Natl. Acad. Sci. USA 17: 14909–14914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai K. S. (1991). Aedes albopictus in the Americas. Ann. Rev. Entomol. 36: 459–484. [DOI] [PubMed] [Google Scholar]
- Reiter P., Darsie R. F. (1984). Aedes albopictus in Memphis, Tennessee (USA): An achievement of modern transportation? Mosq. News 44: 396–399. [Google Scholar]
- Reynolds J. A., Poelchau M. F., Rahman Z., Armbruster P. A., Denlinger D. L. (2012). Transcript profiling reveals mechanisms for lipid conservation during diapause in the mosquito, Aedes albopictus. J. Insect Physiol. 58: 966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C., Denlinger D. L. (2008). Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl. Acad. Sci. USA. 105: 6777–6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobodkin L. B. (1963). Growth and regulation of animal populations, Rinehart & Winston, New York, NY. [Google Scholar]
- Sota T., Mogi M. (1992). Survival time and resistance to desiccation of diapause and non-diapause eggs of temperate Aedes (Stegomyia) mosquitoes. Ent. Exp. Appl. 63: 155–161. [Google Scholar]
- Sprenger D., Wuithiranyagool T. (1986). The discovery and distribution of Aedes albopictus in Harris County, Texas. J. Am. Mosq. Control Assoc. 2: 217–219. [PubMed] [Google Scholar]
- Suman D. S., Wang Y., Gaugler R. (2015). The insect growth regulator pyriproxyfen terminates egg diapause in the Asian tiger mosquito, Aedes albopictus. PLoS ONE 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W., Mori A., Montgomery J., Fleming K. L., Anderson J. M., Valenzuala J. G., Severson D. W., Black W. C., IV (2011). A linkage map of the Asian tiger mosquito (Aedes albopictus) based on cDNA markers. J. Heredity 102: 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber M. J., Tauber C. A., Masaki S. (1986). Seasonal Adaptations of Insects, Oxford University Press, New York, NY. [Google Scholar]
- Unlu I., Farajollahi A., Strickman D., Fonseca D. M. (2013). Crouching tiger, hidden trouble: Urban sources of Aedes albopictus (Diptera: Culicidae) refractory to source-reduction. PLoS ONE 8: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski J. M., Benoit J. B., Michaud M. R., Denlinger D. L., Armbruster P. (2010). The molecular basis of increased desiccation resistance during diapause in the Asian tiger mosquito, Aedes albopictus. Proc. R. Soc. B 277: 2683–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski J., Mogi M., O'Donnell D., DeCotiis M., Toma T., Armbruster P. (2012). Rapid adaptive evolution of photoperiodic response during invasion and range expansion across a climatic gradient. Am. Nat. 179: 490–500. [DOI] [PubMed] [Google Scholar]
- Usmani-Brown S., Cohnstaedt L., Munstermann L. E. (2009). Population Genetics of Aedes albopictus (Diptera: Culicidae) invading populations, using mitochondrial nicotinamide adenine dinucleotide dehydrogenase subunit 5 sequences. Ann. Entomol. Soc. Am. 102: 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. L. (1966). Observations on the influence of photoperiod on egg diapause in Aedes albopictus Skuse. Acta Entomol. Sin. 15: 75–77. [Google Scholar]
- Worobey J., Fonseca D. M., Espinosa C., Healy S., Gaugler R. (2013). Child outdoor physical activity is reduced by prevalence of the Asian tiger mosquito, Aedes albopictus. J. Am. Mosq. Control Assoc. 29: 78–80. [DOI] [PubMed] [Google Scholar]
- Yee D. A., Juliano S. A., Vamosi S. M. (2012). Seasonal photoperiods alter developmental time and mass of an invasive mosquito, Aedes albopictus (Diptera: Culicidae), across its north-south range in the United States. J. Med. Entomol. 49: 825–832. [DOI] [PubMed] [Google Scholar]
- Zhong D. B., Lo E., Hu R. J., Metzger M. E., Cummings R., Bonizzoni M., Fujioka K. K., Sorvillo T. E., Kluh S., Healy S. P., et al. (2013). Genetic analysis of invasive Aedes albopictus populations in Los Angeles County, California and its potential public health impact. PLoS ONE 8. [DOI] [PMC free article] [PubMed] [Google Scholar]