Abstract
We report an unusual cause of gastrointestinal infection occurring in a 1-year-old infant patient who was brought to a public hospital in Kuala Lumpur, Malaysia. Larvae passed out in the patient’s feces were confirmed by DNA barcoding as belonging to the species, Lasioderma serricorne (F.), known as the cigarette beetle. We postulate that the larvae were acquired from contaminated food and were responsible for gastrointestinal symptoms in the patient. To our knowledge, this the first report of human canthariasis caused by larvae of L. serricorne.
Keywords: canthariasis, DNA barcoding, Lasioderma serricorne, Malaysia, pediatric
Infection of the gastrointestinal tract is common in infancy (Purssell 2009) and viruses (rotavirus, norovirus, and enteric adenoviruses) account for the majority of cases (Iturriza-Gómara et al. 2008). Bacterial infections (including Salmonella and Campylobacter spp. infections) are significantly less common (Davies et al. 2001). Occasionally, insects of the order Diptera have been reported to cause gastrointestinal infections (referred to as intestinal myiasis) in children (Kandi et al. 2013, Francesconi and Lupi 2012). Infestation by beetle larvae is termed canthariasis and even rarer. Enteric infestation by the cigarette beetle, Lasioderma serricorne (F.), has never been reported. To date, only two cases of canthariasis in infants attributed to ingestion of dermestid beetle larvae have been reported by Okumura (1967). Lasioderma serricorne is a cosmopolitan pest of stored tobacco (Ashworth 1993). Lasioderma serricorne also infests a wide range of other stored commodities, such as grains, rice, pasta, and beans, and is of considerable economic importance (Blanc et al. 2006).
Case Report
A 1-year-old baby girl was brought to the Pediatric Emergency Department in November 2015 with a 3-day history of fever and one episode of passing multiple larvae in her stool (Fig. 1). In the last 2 d, she also had frequent bowel movements of 4 to 5 times a day. The stool consistency was normal and it was not associated with blood or mucus. Her oral intake was good and she had no vomiting. However, she was slightly irritable and the parents were unsure why the child was crying on and off, especially at night. There was no history of respiratory or urinary tract infections.
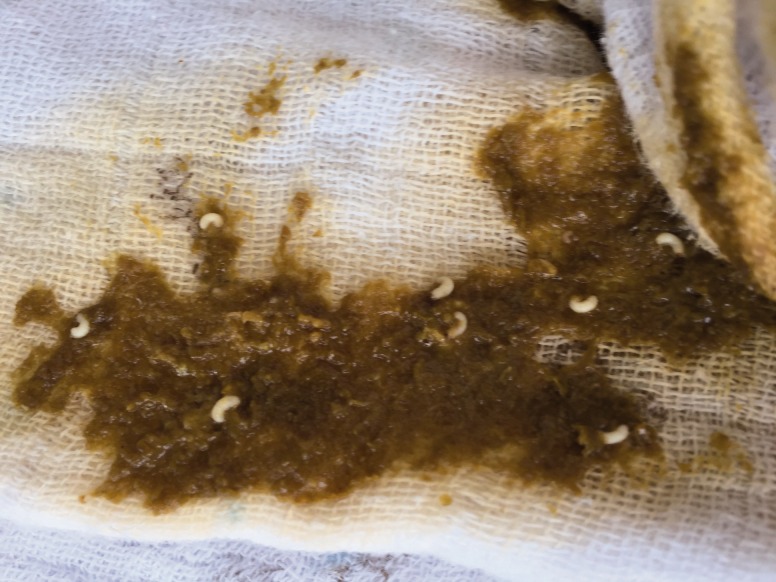
Fig. 1.
Macroscopic examination of the stool sample showing multiple, white-colored, scarab-like-shaped larvae.
Upon examination, the child weighed 9.0 kg and was febrile with a body temperature of 39.6°C. Blood pressure and heart rate were 86/60 mmHg and 130/min, respectively. General physical and systemic examinations were unremarkable. As larvae were found in the patient’s stool, the child was given a single dose of syrup albendazole 200 mg as a treatment for parasitic infection, to be repeated in 2 weeks, and syrup paracetamol 125 mg four times a day to treat fever. At the 1-week follow-up appointment, it was reported that the child continued to pass larvae until day 4 of illness. Her fever subsided by day 5 and she recovered without further complications.
Materials and Methods
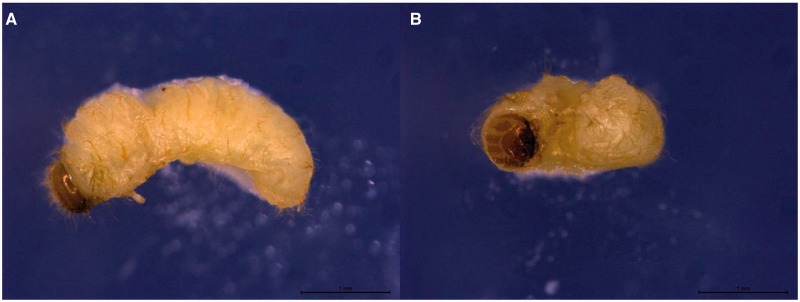
The collected larvae were sent to the Department of Parasitology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia, for identification. Four larval specimens were examined under a stereomicroscope (Leica), revealing approximately 3.5-mm, yellowish-white, hairy, full-grown larvae, with visible legs and light brown-colored head (Fig. 2).
Fig. 2.
Stereomicroscope examination of cigarette beetle larva, L. serricorne (A, lateral view and B, ventral view), which revealed a C-shaped, yellowish-white larva, thinly covered with fine brown hairs with visible legs. The light brown-colored head is evenly rounded dorsally.
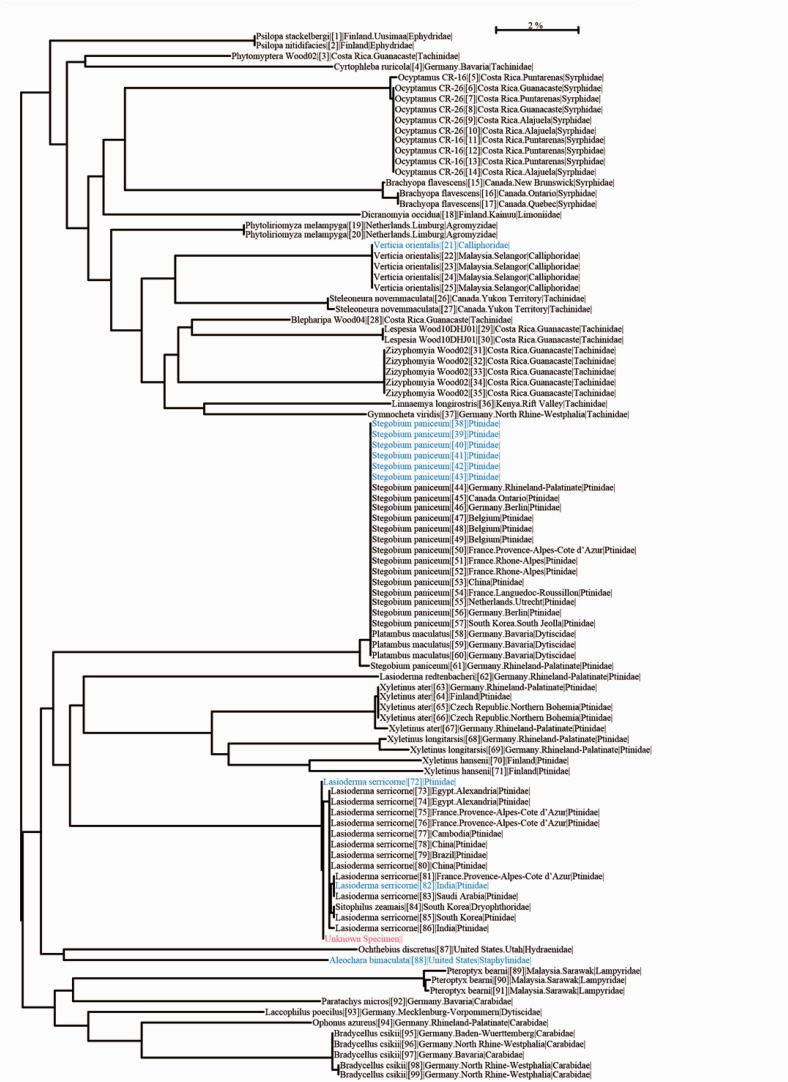
DNA barcoding (Ratnasingham and Hebert 2013) was used to obtain a species identification for the specimen. DNA was extracted from the whole-larva specimen using a NucleoSpin Tissue kit (Macherey-Nagel, Germany), and a fragment of cytochrome c oxidase subunit 1 (COI) mitochondrial DNA (mtDNA) gene was amplified using the “Lep” primer combinations via polymerase chain reaction (PCR; Wilson 2012). Cycle sequencing was performed bi-directionally using the PCR primers. The DNA barcode from the specimen shared 99.85% similarity with the closest-matching DNA barcode in the Barcode Of Life Datasystems (BOLD; Ratnasingham and Hebert 2007; BOLD ID: CICRP097-15) and nested within other DNA barcodes named L. serricorne on a BOLD identification tree (Fig. 3). Therefore, using a strict tree-based assignment model (Wilson et al. 2011), we concluded that this specimen is the larval stage of L. serricorne.
Fig. 3.
BOLD identification tree. This tree is produced by a full database sequence identification request in BOLD for the larval specimen. The sequence (designated as unknown specimen) groups closely with those from France, Egypt, India, Finland, and Saudi Arabia, and the species is nested within a cluster of Lasioderma serricorne.
The University of Malaya Medical Centre Ethics Committee (MEC Ref. No. 201312-0608) approved our research protocols involving human subjects. The parents provided written consent for this study.
Results and Discussion
Gastrointestinal infection in infants is usually self-limiting and treatable. However, severe complications can occasionally arise, particularly when the infection manifests as acute infectious enteritis, which can be fatal in children (Thapar and Sanderson 2004). In developing countries, cases of gastrointestinal infection remain high, with an estimated 1.8 billion episodes of childhood diarrhoea, and this is an important clinical problem in children, despite improvements in public health and economic status (Casburn-Jones and Farthing 2004).
The life cycle of L. serricorne is completed in 45–70 d (Retief and Nicholas 1988). Females oviposit as many as 100 eggs singly onto dried food materials and the eggs hatch in 6-8 days. The larvae undergo 4–6 larval stages before they transform into inactive pupae and emerge into fully developed adult beetles in about 7–18 d, depending on the environmental temperature and humidity (Retief and Nicholas 1988, Reed and Vinzant 1942). The adult is small, stout, oval, and brownish red in color and lives for 2–4 wk. When fully grown, both adults and larvae are 2–3 mm in length.
The present case is noteworthy, as this is the first report of canthariasis associated with the larvae of L. serricorne, which is not known to be medically important. This patient was a full-term baby weighing 3.0 kg at birth. Her developmental milestones were appropriate and she received up-to-date immunizations. The family lives in a healthy and hygienic environment with a domestic helper taking care of the patient. The working parents are well-educated and they have another healthy and asymptomatic 3-year-old daughter.
At the time of infection, the patient was no longer breast-feeding and had started eating solid food. The larvae passed out in the patient’s feces may have developed in the intestine after the baby ingested eggs from contaminated food prepared from ingredients that do not involve cooking, such as cereals and biscuits infested with cigarette beetles. The previous cases of infant canthariasis, which involved dermestid beetle larvae in the United States, reported the presence of beetle larvae in the cereal being fed to the infants (Okumura, 1967). Alternatively, the patient may have become infected by drinking contaminated infant formula. Intestinal disorder of a newborn due to consumption of milk powder contaminated with eggs and larvae of Musca domestica (Diptera) has been reported from India (Shekhawat et al. 1993). Babies, by nature, have the habit of putting things into their mouth, as was observed by the parents in the present case. It is therefore also plausible that the patient picked up contaminated materials (food or nonfood substances infested with eggs or larvae) off the floor that led to the infection. Two dogs also reside in the house, and the source of infection could be pet food. Pet food is commonly infested by L. serricorne in houses (Choe 2013). As the source of the infection was not investigated further, we can only speculate.
The risk of gastrointestinal infections in infancy can be reduced through good hygiene practices by parents and caregivers. Cigarette beetle infestations in food products can be prevented and controlled by locating and eliminating the source of infestation. Infested items can be cold-treated (16 days at 2°C, 7 days at −4°C, or 4 to 7 days at 0°C) or heat-treated (88°C for an hour, 49°C for 16 to 24 hours) to eliminate infestation of all stages of L. serricorne (Cabrera 2014). Healthcare professionals can raise awareness and provide advice on proper treatment for the underlying cause of gastrointestinal infections, particularly when the symptoms signify serious complications. To our knowledge, this report is the first of human canthariasis caused by L. serricorne.
Acknowledgments
This study was supported by research grants PG042-2013A, RP003D-13SUS, and RG509-13HTM from University of Malaya, Kuala Lumpur, Malaysia, and UM.C/HIR/MOHE/MED/16 from the Ministry of Higher Education, Malaysia.
References Cited
- Ashworth J. R. 1993. The biology of Lasioderma serricorne. J. Stored Prod. Res. 29: 291–303. [Google Scholar]
- Blanc M. P., Lugon-Moulin N., Panighini C., Pijnenburg H., Rossi. L. 2006. Structure of worldwide populations of Lasioderma serricorne (Coleoptera: Anobiidae) as revealed by amplified fragment length polymorphism profiles. Bull. Entomol. Res. 96: 111–116. [DOI] [PubMed] [Google Scholar]
- Cabrera B. J. 2014. Cigarette Beetle, Lasioderma serricorne (F.) (Insecta: Coleoptera: Anobiidae). UF/IFAS Extension, Ft. Lauderdale, FL. [Google Scholar]
- Casburn-Jones A. C., Farthing M.J.G. 2004. Management of infectious diarrhoea. Gut. 53: 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe D. H. 2013. Pantry pests. In Fayard M. L. (eds.), Pest Notes. UC ANR, Oakland, CA. [Google Scholar]
- Davies E. G., Elliman D.A.C., Hart C. A., Nicoll A., Rudd P. T. 2001. Manual of Childhood Infections. W.B .Saunders, London, United Kingdom. [Google Scholar]
- Francesconi F., Lupi O. 2012. Myiasis. Clin. Microbiol. Rev. 25: 79–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriza-Gómara M., Simpson R., Perault A. M., Redpath C., Lorgelly P., Joshi D., Mugford M., Hughes C. A., Dalrymple J., Desselberger U., Gray J. 2008. Structured surveillance of infantile gastroenteritis in East Anglia, UK: Incidence of infection with common viral gastroenteric pathogens. Epidemiol. Infect. 136: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandi V., Lal S. K., Akhila, Shruthi K., Sandhya H., Simar M., Pranuthi M. V., Kumar K., Anand S. D., Rao 2013. Persistent pediatric gastro-intestinal myiasis: a case report of fly larval infestation with Musca domestica with review of literature. J. Glob. Infect. Dis. 5: 114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura G. T. 1967. A report of canthariasis and allergy caused by Trogoderma (Coleoptera: Dermestidae). Cali. Vector Views 14: 19–22. [Google Scholar]
- Purssell E. 2009. Prevention and management of gastrointestinal infections in infants from a nutritional perspective. J. Fam. Health Care 19: 200–203. [PubMed] [Google Scholar]
- Ratnasingham S., Hebert. P.D.N. 2007. BOLD: The barcode of life data system ( www.barcodinglife.org). Mol. Ecol. Notes 7: 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnasingham S., Hebert. P.D.N. 2013. A DNA-based registry for all animal species: The barcode index number (BIN) system. PLoS ONE 8: e66213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed W. D., Vinzant J. P. 1942. Control of insects attacking stored tobacco and tobacco product. United States Department of Agriculture, Washington. [Google Scholar]
- Retief E., Nicholas. A. 1988. The cigarette beetle Lasioderma serricorne (F.) (Coleoptera: Anobiidae): a serious herbarium pest. Bothalia 18: 97–99. [Google Scholar]
- Shekhawat P. S., Joshi K. R., Shekhawat. R. 1993. Contaminated milk powder and intestinal myiasis. Indian. Pediatr. 30: 1138–1139. [PubMed] [Google Scholar]
- Thapar N., Sanderson. I. R. 2004. Diarrhoea in children: an interface between developing and developed countries. Lancet 363: 641–653. [DOI] [PubMed] [Google Scholar]
- Wilson J. J. 2012. DNA barcoding for insects. Method Mol. Biol. 858: 17–46. [DOI] [PubMed] [Google Scholar]
- Wilson J. J., Rougerie R., Shonfeld J., Janzen D. H., Hallwachs W., Hajibabaei M., Kitching I. J., Haxaire J., Hebert P.D.N. 2011. When species matches are unavailable are DNA barcodes correctly assigned to higher taxa? An assessment using sphingid moths. BMC Ecol. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]