Abstract
Maternally inherited Wolbachia bacteria are being introduced into vector mosquito populations, with the goal of reducing the transmission of diseases such as dengue fever. The infection dynamics of Wolbachia depends upon the ability of Wolbachia to manipulate host reproduction as well as any fitness costs imposed upon the host. Some vector mosquito species are opportunistic blood feeders, utilizing both human and nonhuman vertebrate hosts, and the effects of bloodmeal source on Wolbachia phenotype is not well understood. Here we transfer wMelPop Wolbachia from Drosophila melanogaster (Meigen) into wild-type Aedes albopictus (Skuse) and characterize the resulting triple infection by examining for an effect of human and mouse blood on the Wolbachia infection persistence and phenotypes. When provided with human blood, the triple Wolbachia infection was persistent, with high maternal inheritance and relatively little fecundity cost, and a pattern of imperfect unidirectional cytoplasmic incompatibility was observed in mating experiments between wild-type and triply infected individuals. With mouse blood, reduced female fecundity and low maternal inheritance were observed in wMelPop-infected females, which affected the typical pattern of unidirectional CI. Our findings indicate the interactive effects of Wolbachia infection and blood source drive distinct shifts in the Wolbachia–host symbiotic association.
Keywords: population replacement, fitness cost, symbiosis, host nutrition, vector control
Wolbachia are maternally inherited, intracellular bacteria that infect a variety of arthropods and nematodes and are responsible for diverse impacts on host reproduction, including male killing, parthenogenesis, feminization, and cytoplasmic incompatibility (CI; Jeong and Suh 2008, Werren et al. 2008). The effects on host reproduction can be beneficial to Wolbachia, providing infected hosts with a reproductive advantage, which can promote the spread of Wolbachia. In mosquitoes, Wolbachia can induce CI, which causes a decrease or absence of brood hatch when infected males mate with females that are uninfected or infected with a different Wolbachia type (Sinkins 2004). Individuals can be infected with one or more Wolbachia strains (i.e., superinfections), and as an example of unidirectional CI, double-infected females can be incompatible with triple-infected males, producing no or reduced progeny, while the reciprocal cross is compatible producing normal progeny (Fu et al. 2010, Zhang et al. 2015).
There is considerable interest in using Wolbachia-based strategies to control insect vectors of disease. Two of the strategies are 1) Wolbachia-based incompatible insect technique (IIT), analogous to the sterile insect technique and 2) population replacement, which replaces natural populations with Wolbachia-infected individuals that express a desired phenotype (O'Neill et al. 1997, Werren 1997). As an example of the latter, Wolbachia infections associated with a life-shortening phenotype (wMelPop) have been suggested for use in a strategy to control disease transmission by modifying the age structure of vector populations (Min and Benzer 1997, McMeniman et al. 2009). Furthermore, Wolbachia has been shown to interfere with pathogen transmission, including dengue, chikungunya, yellow fever, malaria, and filarial nematodes (Hedges et al. 2008; Kambris et al. 2009; Moreira et al. 2009b; Bian et al. 2010, 2013; Walker et al. 2011). In open release trials, a wMel-infected Aedes aegypti (L.) type has been successfully established in two local residential areas in northern Australia (Hoffmann et al. 2011, 2014; Walker et al. 2011). Relative to the wMel Wolbachia type, the wMelPop infections are predicted to strongly reduce pathogen transmission because of a higher degree of pathogen inhibition (Chrostek et al. 2013, Ferguson et al. 2015). Furthermore, the wMelPop type shows a stronger life-shortening phenotype in adults (McMeniman et al. 2009, Suh et al. 2009, Yeap et al. 2011) and egg stages (i.e., reduced egg longevity; McMeniman and O'Neill 2010, Yeap et al. 2011).
The Asian tiger mosquito, Ae. albopictus (Skuse), is indigenous to Southeast Asia and is a globally invasive species that has expanded into Africa, Europe, and the Americas (Gratz 2004). Ae. albopictus is an efficient vector of zoonotic and human pathogens including multiple arboviruses and filarial species (Francy et al. 1990, Rai 1991, Moore and Mitchell 1997, Cancrini et al. 2003, Gratz 2004). Ae. albopictus has been implicated as a vector of the chikungunya virus, which is currently epidemic throughout much of the western hemisphere (Enserink 2006, Josseran et al. 2006, Bonilauri et al. 2008, Simon et al. 2008). Ae. albopictus is naturally infected with Wolbachia. Surveys of Ae. albopictus populations suggest that individuals are consistently infected with two Wolbachia types, wAlbA and wAlbB, throughout their geographical distributions (Sinkins et al. 1995, Zhou et al. 1998, Armbruster et al. 2003). In order to drive Wolbachia into wild-type Ae. albopictus populations via unidirectional CI, it is hypothesized that a mosquito strain that is triple infected with Wolbachia would be required, such that unidirectional CI occurs in crosses with wild-type Ae. albopictus (Fu et al. 2010, Zhang et al. 2015).
The wMelPop Wolbachia infection has been shown to impose fitness costs on multiple life history traits of host individuals (Moreira et al. 2009a; Turley et al. 2009; McMeniman and O'Neill 2010; Yeap et al. 2011, 2014; Suh and Dobson 2013; Ross et al. 2014), and theoretical studies suggest the invasion of the Wolbachia infection can be hindered by fitness costs (Hoffmann et al. 1990, Crain et al. 2011, Yeap et al. 2011). Particularly, introduction of wMelPop into aposymbiotic Ae. albopictus resulted in pathogenic Wolbachia infection (Suh et al. 2009), indicating wMelPop may not be useful for a population replacement strategy, because the associated fitness costs impair Wolbachia invasion. However, a recent study revealed that reductions in egg hatch and clutch size associated with wMelPop infection in Ae. aegypti can be significantly ameliorated when females are provided with human blood as compared with nonhuman blood (McMeniman et al. 2011).
In this study, the wMelPop infection was introduced into a naturally superinfected Ae. albopictus strain. Experiments were performed to examine the maternal inheritance rates, fecundity, CI, and evidence for the life-shortening phenotype in wMelPop-infected mosquito lines. The effects of human versus mouse blood were examined, comparing the maternal inheritance of the triple infection and relative fitness. Here, we report that utilization of human bloodmeal by mosquito host facilitates establishment of a pathogenic Wolbachia infection in Ae. albopictus with increased maternal inheritance of the Wolbachia that resulted in reduced fitness cost to a host. The results are discussed in relation to the use of Wolbachia in controlling vector mosquitoes.
Materials and Methods
Insect Strains
Microinjection experiments used wMelPop-infected colony of Drosophila melanogaster (Meigen) (w1118), wild-type Aedes albopictus (IH; infected with wAlbA and wAlbB), and an aposymbiotic Ae. albopictus strain (UjuTet, UT) that had been artificially generated by tetracycline treatment of the IH strain to remove the Wolbachia infection (Xi et al. 2005). Rearing conditions were as previously described (Dobson et al. 2001). In brief, all maintenance and experiments were conducted at 28 ± 2°C, 75 ± 10% relative humidity (RH), and a photoperiod of 18:6 (L:D) h. Eggs were submerged in a mixture of fish food (TetraMin Tropical Tablets, Tetra, Germany) in 400 ml of water. Larvae were given fish food ad libitum and adults were transferred into 30- by 30- by 30-cm cages with constant access to a 10% sucrose solution. The females were blood fed with an artificial feeder using freshly collected, unexpired human blood purchased from a blood bank (Kentucky Blood Center, Lexington, KY) or an anesthetized mouse (A3336-01; PHS Assurance).
Microinjection
Injection techniques for embryonic transinfection of mosquito and Drosophila were as previously described (Xi et al. 2006). Injection needles were pulled from quartz microcapillary puller (QF 100-70-7.5; Sutter Instrument Co., Novato, CA) by using a P2000 (Sutter Instrument Co., Novato, CA). wMelPop-infected cytoplasm was withdrawn from the posterior pole of donor w1118 embryos and injected into the posterior pole of IH embryos by using an IM300 microinjector (Narishige Scientific, Tokyo, Japan). Injected embryos were transferred onto wet filter paper, incubated at 27 ± 2°C and 75 ± 10% RH for 5 d, and then submerged in deoxygenated water. Resulting larvae (G0) were reared using standard conditions (Dobson et al. 2001), and pupae were isolated to produce virgins females. Eclosing females were mated with UT (i.e., uninfected) males, blood fed, allowed to oviposit, and then PCR assayed to determine their Wolbachia infection status. Females failing to produce eggs were not tested. The resulting strain was designated as “YFU.”
Multiplex PCR Amplification
DNA was extracted from adult mosquitoes as described previously (Brelsfoard et al. 2008). Three primer sets were used to detect triple Wolbachia infections; wMelPop (IS5F/IS5R; McMeniman et al. 2008), wAlbA (328F/691R) and wAlbB (183F/691R) (Zhou et al. 1998). PCR amplification was performed in a 12.5 -μl reaction volume, using a Qiagen multiplex PCR kit, following manufacturer’s instructions (1 μl DNA template; 4 μl H2O; 200 μM 1.25 μl primer mix; 6.25 μl Master Mix; Qiagen, Valencia, CA). An MJ Research PTC-200 Thermal Cycler (Bio-Rad Laboratories, Hercules, CA) was used to perform 35 cycles of 94°C for 30 s, 57°C for 90 s, and 72°C for 60 s. Template quality was confirmed in samples failing to amplify Wolbachia DNA by using 12 S mitochondrial primers as previously described (O'Neill et al. 1992).
Fecundity, CI, Adult Life Span, and Egg Longevity
For the fecundity and CI assays, G11 and G12 of YFU and IH strain were used for cross experiments. Larvae were reared under optimal conditions (i.e., low larval density and liver powder provided ad libitum) to ensure large size adults. Newly emerged virgins (10 females and 10 males) were placed in individual cages to set up four cross types (YFU♀ × YFU♂, YFU♀ × IH♂, IH♀ × YFU♂, IH♀ × IH♂) with five replicates per treatment and provided with a constant supply of 10% sucrose solution. In order to examine effects of blood sources on relative clutch size and egg hatch rate of individual cross types, human blood (CPDA for anticoagulant; freshly collected, unexpired blood was purchased from Kentucky Blood Center [Lexington, KY]) using a Hemotek membrane feeder (Discovery Workshops, Accrington, United Kingdom) or an anesthetized mouse (A3336-01; PHS Assurance) was provided for blood feeding, as these are commonly used blood sources for general mosquito maintenance in laboratories. Adults were allowed to feed to repletion. An oviposition cup lined with wet paper (Anchor Paper Company, St. Paul, MN) was continuously available, with weekly exchanges. Eggs from the first batch were hatched after 5 d of maturation, and the resulting egg number and arcsine transformed hatch rates were used in data analyses. Two-way analysis of variance (ANOVA) was used to examine the effects of blood source and cross type on egg number and egg hatch rate (JMP 8.0.1; SAS Institute, Cary, NC). Egg number data were log transformed to meet assumptions of ANOVA (normality, homogeneity of variance, etc.). After examining any interaction effects among main variables of interest, post hoc Tukey honest significance difference (HSD) test was conducted on least square means of egg number or egg hatch (JMP 8.0.1; SAS Institute).
To examine the effect of wMelPop infection on the adult life span, rearing of larvae and preparation of adult cages were as described above (10 females × 10 males per cage; five replicates). Dead mosquitoes were collected twice per day until all individuals in a cage died. The survivorship of females and males was compared separately by using a Kaplan–Meier log-rank test (SPSS 17.0; SAS Institute).
To examine the effects of wMelPop infection on embryo longevity, eggs were collected by providing human blood (Blood center, Lexington, KY) via Hemotek blood feeding tools (Discovery Workshops), and the egg paper was subdivided into five groups to be hatched at five different time points. Eggs were matured by holding for 3 d at 100% RH and dried for 2 d. Eggs were stored at 28 ± 2°C and 75 ± 10% RH and hatched 5, 8, 16, 30, and 51 d postoviposition for 3 d by adding a pinch of liver-powder solution. Statistical analysis tested effect of time on egg hatch rate using multiple linear regression model and egg hatches were compared between cross types (JMP 8.0.1; SAS Institute).
Results
Embryonic Microinjection and Maternal Inheritance
Cytoplasm from wMelPop-infected Drosophila embryos was microinjected into naturally superinfected Ae. albopictus embryos as previously described (Xi et al. 2006). The resulting infected females (G0) were used to establish isofemale lines (Table 1). During the first six generations, three isofemale lines from Experiment 2, 3, and 4 (Table 1) were out-crossed with uninfected Ae. albopictus (UT) males, selecting for infected progeny, in an attempt to obtain a stable infection. These three lines were then combined to establish a population out-crossing with UT males until G15. At G16, outcrossing of the YFU strain ended, and the YFU females were mated with YFU males. At G20, the YFU line was subdivided further, resulting in four lines, which were selected for triple Wolbachia infection at every generation by collecting progeny from ∼10 isofemale lines from infected females (i.e., PCR confirmed after collecting eggs) to evaluate the effect of blood and paternal type on maternal inheritance of triple infection.
Table 1.
Survival and infection status of Ae. albopictus microinjected with wMelPop Wolbachia
| Expt. | % Hatch rate (larvae/injected eggs) | % Pupation (pupae/larvae) | % Eclosion (adult/pupae) | G0 infection status (% infected) |
|
|---|---|---|---|---|---|
| Female (infected/total tested) | Male (infected/total tested) | ||||
| 1 | 1 (1/109) | 100 (1/1) | 100 (1/1) | NA (0/0) | NA (0/0) |
| 2 | 11 (9/82) | 100 (9/9) | 67 (6/9) | 100 (2/2) | 100 (4/4) |
| 3 | 8 (9/119) | 89 (8/9) | 100 (8/8) | 0 (0/4) | 67 (2/3) |
| 4 | 4 (5/120) | 100 (5/5) | 100 (5/5) | 67 (2/3) | 50 (1/2) |
| 5 | 0 (0/107) | NA (0/0) | NA (0/0) | NA (0/0) | NA (0/0) |
NA, not applicable.
In the absence of selection, continued PCR monitoring demonstrated the frequency of triple infection to be maintained in a majority of YFU individuals in a closed population (96.5 ± 4.7%; mean ± 95% confidential interval; n = 9) for 13 generations (Supp. Table 1 [online only]).
The rate of maternal inheritance observed in YFU females was dependent on the blood type (LR-χ2 = 6.0, df = 1, P < 0.0001; Table 2, Supp. Table 2 [online only]). All of the YFU females fed human blood were consistently triple infected, regardless the Wolbachia infection type in the male mate, i.e., UT or YFU male (Supp. Table 2 [online only]). In contrast, when YFU females were fed mouse blood, a reduced maternal inheritance of the triple infection was observed (Supp. Table 2 [online only]). Particularly, mouse-fed YFU females that were mated to YFU males were observed to lose the wMelPop infection within four generations, despite ongoing selection for the triple infection (Supp. Table 2 [online only]). The wAlbB infection was retained at 100% for all individuals, regardless the blood source or male infection type (Supp. Table 2 [online only]). In one line at G21, loss of the wAlbA infection was observed from a mouse-fed line that was out-crossed with UT (Supp. Table 2 [online only]).
Table 2.
Generalized linear model analysis to evaluate the effect of blood (human vs. mouse) and paternal type (UT vs. YFU) on maternal inheritance of triple Wolbachia infection (wMelPop, wAlbA, and wAlbB) in Ae. albopictus (binomial distribution with Logit link; bias-adjusted estimate; JMP 8.0.1; SAS Institute) for five consecutive generations (see detailed information in Supp. Table 2 [online only])
| Source of variation | df | L – R χ2 | P |
|---|---|---|---|
| Paternal type | 1 | 0.23 | 0.63 |
| Blood type | 1 | 43.4 | <0.0001 |
| Blood type × paternal type | 1 | 0.05 | 0.82 |
To observe for paternal inheritance of the wMelPop infection, PCR assays were conducted on six pools of larvae resulting from IH females crossed with YFU males. No Wolbachia infected progeny were observed (Supp. Fig. 1 [online only]), consistent with the previously reported absence of wMelPop paternal inheritance (Hoffmann and Turelli 1988, McGraw et al. 2001, Suh et al. 2009).
Clutch Size and Egg Hatch
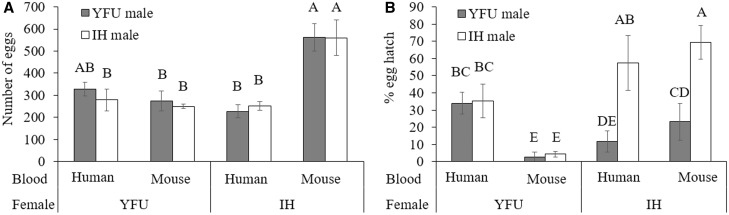
Post hoc Tukey HSD analyses were conducted to compare egg number and egg hatch across all groups, due to a significant interaction effect between blood type and cross type on egg number and egg hatch (Supp. Table 3 [online only], Fig. 1) . The number of eggs produced by YFU females remained relatively constant, regardless of blood type and the Wolbachia type present in her mate (Fig. 1A). IH females fed human blood produced egg numbers similar to that of YFU females, regardless of whether they were mated to IH or YFU males (Fig. 1A). In contrast, when IH females were fed mouse blood, they produced significantly more eggs relative to those fed human blood, and this higher clutch size occurred regardless of whether the IH females were mated with IH or YFU males (Fig. 1A).
Fig. 1.
Clutch size (A) and egg hatch (B) resulting from crosses between YFU (wMelPop-infected IH) and IH (double-infected Ae. albopictus), providing human or mouse blood. Different letters indicate significant differences at P = 0.05. Error bar = SEM for (A) and 95% confidential interval for (B) (n = 4 ∼ 5).
Lower egg hatch was observed for IH females mated to YFU males, relative to those IH mated to IH males (Fig. 1B). This is consistent with expectations for cytoplasmic incompatibility and was observed for both human- and mouse-fed females. In contrast, in crosses of YFU females, the type of Wolbachia infection in the male mates was not observed to affect the hatch rate (Fig. 1B).
In crosses of IH females, no difference in egg hatch rates was associated with blood type (Fig. 1B). This was true for IH females mated with either of the IH or YFU male types (Fig. 1B). In contrast, egg hatch resulting from crosses of YFU females was affected by blood type (Fig. 1B). Specifically, YFU females fed human blood resulted in greater egg hatch relative to those fed mouse blood (Fig. 1B). The male mate type was not observed to affect egg hatch rates resulting from YFU crosses (Fig. 1B).
Adult Life Span
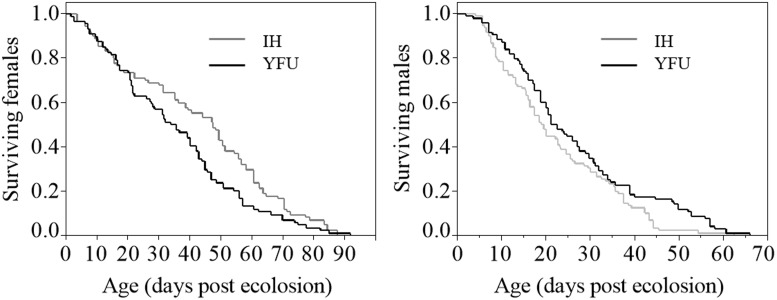
Life span was significantly reduced in YFU females compared with IH females (Kaplan–Meier log rank comparisons; χ2 = 4.84, P = 0.028; Fig. 2). Mean female life span of YFU and IH were 36 ± 2.3 (mean ± SEM) and 43 ± 2.6 d, respectively. In contrast, the life span of YFU males was higher than that observed for IH males (χ2 = 4.19, P = 0.041; Fig. 2). Mean male life spans of YFU and IH were 27 ± 1.6 and 22 ± 1.4 d, respectively.
Fig. 2.
Adult life span of YFU (wMelPop-infected IH; black line) and IH (double-infected Ae. albopictus; gray line).
Egg Survivorship
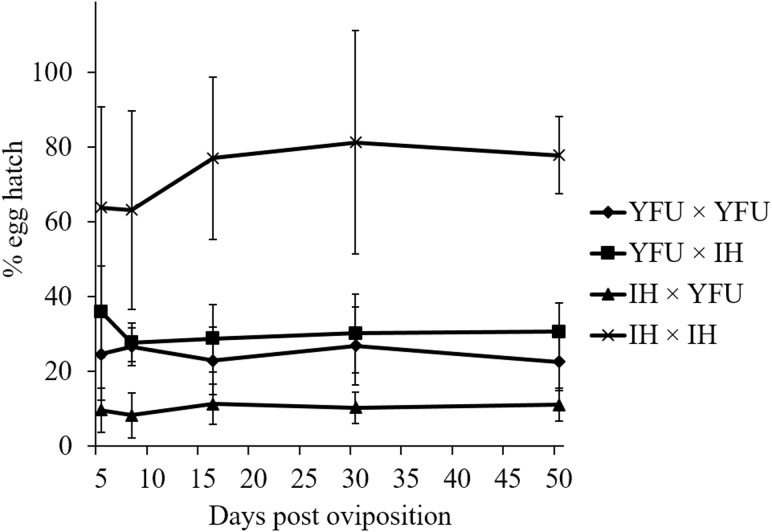
The egg hatch rate remained constant over time for all cross types during a 51-d study when eggs were produced by utilizing human blood: YFU♀ × YFU♂ (R2 = 0.0027, F1, 23 = 0.062, P = 0.81), YFU♀ × IH♂ (R2 = 0.0015, F1, 23 = 0.035, P = 0.85), IH♀ × YFU♂ (R2 = 0.023, F1, 23 = 0.55, P = 0.47), IH♀ × IH♂ (R2 = 0.021, F1, 18 = 0.39, P = 0.54; Fig. 3). The highest hatch rates resulted from compatible crosses (IH♀ × IH♂). Intermediate hatch rates were observed in crosses involving YFU females (YFU♀ × YFU♂ and YFU♀ × IH♂). The lowest hatch rates were observed in the incompatible cross (IH♀ × YFU♂; ANOVA with Tukey post hoc; F3, 16 = 236.19, P < 0.0001). Progeny that emerged from crosses of YFU females were sampled at days 5 and 51, and all were triple infected (n = 20, data not shown).
Fig. 3.
Longevity of eggs resulting from crosses between YFU (wMelPop Wolbachia-infected IH) and IH (double-infected Ae. albopictus) (♀ × ♂). Error bar = 95% confidential interval (n = 4 ∼ 5).
Discussion
Here, an unusual interaction of Wolbachia phenotype and host bloodmeal type was observed in an artificially constructed Wolbachia triple infection. When provided with mouse blood, the triple infection with wMelPop is best described as “pathogenic,” with reduced maternal inheritance and low fecundity (i.e., clutch size and egg hatch), which is consistent with a prior example of a single wMelPop infection in Ae. albopictus (Suh et al. 2009). In contrast, when fed with human blood, the wMelPop infection is relatively commensal, i.e., relatively little effect on egg number (Fig. 1A), although the infection cost on egg hatch remains high compared to human blood-fed IH lines (Fig. 1B).
Consistent with expectations for Wolbachia-induced CI, the egg hatch resulting from crosses of naturally infected females and YFU males was reduced, relative to that resulting from crosses between naturally infected individuals. The level of CI induced by the triple infection was similar to that of the single wMelPop infection in Ae. albopictus, which showed a 77% reduction in egg hatch (i.e., imperfect CI), relative to compatible crosses (Suh et al. 2009). The egg hatch resulting from compatible crosses of YFU females was lower than that resulting from compatible IH crosses, suggesting a fitness cost associated with the triple infection, relative to the natural infection.
Maternal inheritance failure of the wMelPop infection was observed in mouse-fed populations regardless of the paternal Wolbachia type. Because host nutritional factors have been shown to affect Wolbachia titer in the Drosophila germline (Serbus et al. 2015), future experiments might examine for an effect of blood type on Wolbachia density in YFU oocytes, which could in turn affect maternal inheritance rates in Ae. albopictus.
The egg viability in wMelPop-infected females was significantly reduced when fed with mouse blood (Fig. 1B), which may be due to an effect of the wMelPop infection on females and their eggs. Prior studies of the wMelPop single infection showed a high infection density, which is associated with embryonic mortality in Ae. albopictus (Suh et al. 2009). The results reported here are consistent also with a report showing reduced egg number and egg hatch, which was associated with competition for amino acids between the mosquito host and Wolbachia, resulting in insufficient provisioning of amino acids during oocyte or embryonic development (Caragata et al. 2014). Nutritional variations have been suggested to be responsible for the fecundity phenotypes observed with wMelPop infections (McMeniman et al. 2011), suggesting a need for investigating on nutrient provisioning in the YFU strain.
Here, the effect of the wMelPop infection on Ae. albopictus longevity ranged from beneficial to detrimental. A reduced adult life span was consistently observed in wMelPop-infected females (YFU) compared with naturally infected females. This observation is similar to a prior study of the single wMelPop infection (Suh et al. 2009) while the life-shortening effect seemed to be relatively reduced comparing to prior studies of wMelPop-CLA (wMelPop cell line adapted) infection in Ae. aegypti (McMeniman et al. 2009, Yeap et al. 2011). YFU males, in contrast, experienced an increased life span, relative to naturally infected IH males (Fig. 2). Future work should examine for the potential role of differential immature competition levels in affecting adult longevity. Also, repeating the longevity assay using tetracycline-treated YFU lines should be helpful to exclude any effect of genetic drift on adult longevity resulting from the microinjection experiments. No effect on embryonic survival was observed of the wMelPop infection (Fig. 3). These observations differ from prior studies in which wMelPop-CLA Wolbachia were observed to reduce embryonic survival in Ae. aegypti (McMeniman et al. 2009, Yeap et al. 2011). Such differential phenotypic effect could be due to genotypic differences in Wolbachia strains (wMelPop vs. wMelPop-CLA) and different mosquito species, resulting in differential density and tissue tropism of wMelPop in Ae. albopictus relative to artificial infections in Ae. aegypti. Considering these Wolbachia strains and mosquito species are closely related to each other, our results observed here reinforce the unpredictability in heterologous transfer of Wolbachia as previously reported (Suh et al. 2009).
Here, we observed the Wolbachia maternal inheritance rate and fecundity of YFU females to be dependent on bloodmeal type. The results suggest that the YFU strain may not be ideally suited for a population replacement strategy. Specifically, wMelPop-infected females that feed on nonhuman bloodmeals can be less successful, and Ae. albopictus females tend to be relatively opportunistic blood feeders (Savage et al. 1993, Niebylski et al. 1994, Delatte et al. 2010).
Supplementary Material
Acknowledgments
We thank S. O’Neill and C. McMeniman for providing the w1118 D. melanogaster strain, which was used as donor material for transinfections. We appreciate Dr. R. Jason Pitts for reviewing and providing helpful comments on the manuscript. This research was supported by grants from the National Institutes of Health (AI-067434) and the Bill and Melinda Gates Foundation (44190). The information reported in this paper (No. 16-08-013) is part of a project of the Kentucky Agricultural Experiment Station and is published with the approval of the Director.
References Cited
- Armbruster P., Damsky W. E., Giordano R., Birungi J., Munstermann L. E., Conn J. E. 2003. Infection of new- and old-world Aedes albopictus (diptera: culicidae) by the intracellular parasite Wolbachia: Implications for host mitochondrial DNA evolution. J. Med. Entomol. 40: 356–360. [DOI] [PubMed] [Google Scholar]
- Bian G. W., Xu Y., Lu P., Xie Y., Xi Z. Y. 2010. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 6: e1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian G. W., Joshi D., Dong Y. M., Lu P., Zhou G. L., Pan X. L., Xu Y., Dimopoulos G., Xi Z. Y. 2013. Wolbachia invades Anopheles stephensi populations and induces refractoriness to plasmodium infection. Science 340: 748–751. [DOI] [PubMed] [Google Scholar]
- Bonilauri P., Bellini R., Calzolari M., Angeflni R., Venturi L., Fallacara F., Cordioli P., Angelini P., Venturolli C., Merialdi G., Dottori M. 2008. Chikungunya virus in Aedes albopictus, Italy. Emerg. Infect. Dis. 14: 852–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelsfoard C. L., Sechan Y., Dobson S. L. 2008. Interspecific hybridization yields strategy for south pacific filariasis vector elimination. PLoS Negl. Trop. Dis. 2: e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancrini G., Romi R., Gabrielli S., Toma L., Di Paolo M., Scaramozzino P. 2003. First finding of Dirofilaria repens in a natural population of Aedes albopictus. Med. Vet. Entomol. 17: 448–451. [DOI] [PubMed] [Google Scholar]
- Caragata E. P., Rances E., O'Neill S. L., McGraw E. A. 2014. Competition for amino acids between Wolbachia and the mosquito host, Aedes aegypti. Microb. Ecol. 67: 205–218. [DOI] [PubMed] [Google Scholar]
- Chrostek E., Marialva M.S.P., Esteves S. S., Weinert L. A., Martinez J., Jiggins F. M., Teixeira L. 2013. Wolbachia variants induce differential protection to viruses in Drosophila melanogaster a phenotypic and phylogenomic analysis. PloS Genetics 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain P. R., Mains J. W., Suh E., Huang Y. X., Crowley P. H., Dobson S. L. 2011. Wolbachia infections that reduce immature insect survival: Predicted impacts on population replacement. BMC Evol. Biol. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte H., Desvars A., Bouetard A., Bord S., Gimonneau G., Vourc'h G., Fontenille D. 2010. Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Reunion. Vector Borne Zoonotic Dis. 10: 249–258. [DOI] [PubMed] [Google Scholar]
- Dobson S. L., Marsland E. J., Rattanadechakul W. 2001. Wolbachia-induced cytoplasmic incompatibility in single-and superinfected Aedes albopictus (Diptera : Culicidae). J. Med. Entomol. 38: 382–387. [DOI] [PubMed] [Google Scholar]
- Enserink M. 2006. Infectious diseases - massive outbreak draws fresh attention to little-known virus. Science 311: 1085–1085. [DOI] [PubMed] [Google Scholar]
- Ferguson N. M., Kien D.T.H., Clapham H., Aguas R., Trung V. T., Chau T.N.B., Popovici J., Ryan P. A., O'Neill S. L., McGraw E. A., et al. 2015. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci. Trans. Med. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francy D. B., Karabatsos N., Wesson D. M., Moore C. G., Lazuick J. S., Niebylski M. L., Tsai T. F., Craig G. B. 1990. A new arbovirus from Aedes albopictus, an asian mosquito established in the United States. Science 250: 1738–1740. [DOI] [PubMed] [Google Scholar]
- Fu Y. Q., Gavotte L., Mercer D. R., Dobson S. L. 2010. Artificial triple Wolbachia infection in Aedes albopictus yields a new pattern of unidirectional cytoplasmic Incompatibility. Appl. Environ. Microbiol. 76: 5887–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz N. G. 2004. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 18: 215–227. [DOI] [PubMed] [Google Scholar]
- Hedges L. M., Brownlie J. C., O'Neill S. L., Johnson K. N. 2008. Wolbachia and virus protection in Insects. Science 322: 702–702. [DOI] [PubMed] [Google Scholar]
- Hoffmann A. A., Turelli M. 1988. Unidirectional incompatibility in Drosophila simulans: inheritance, geographic variation and fitness effects. Genetics 119: 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A. A., Turelli M., Harshman L. G. 1990. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics 126: 933–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A. A., Iturbe-Ormaetxe I., Callahan A. G., Phillips B., Billington K., Axford J. K., Montgomery B., Turley A. P., O'Neill S. L. 2014. Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PLoS Negl. Trop. Dis. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A. A., Montgomery B. L., Popovici J., Iturbe-Ormaetxe I., Johnson P. H., Muzzi F., Greenfield M., Durkan M., Leong Y. S., Dong Y., et al. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476: 454–U107. [DOI] [PubMed] [Google Scholar]
- Jeong G., Suh E. 2008. Wolbachia-induced reproductive anomalies and their future applications. Entomol. Res. 38: 41–48. [Google Scholar]
- Josseran L., Paquet C., Zehgnoun A., Caillere N., Le Tertre A., Solet J. L., Ledrans M. 2006. Chikungunya disease outbreak, Reunion Island. Emerg. Infect. Dis. 12: 1994–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambris Z., Cook P. E., Phuc H. K., Sinkins S. P. 2009. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326: 134–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw E. A., Merritt D. J., Droller J. N., O'Neill S. L. 2001. Wolbachia-mediated sperm modification is dependent on the host genotype in Drosophila. Proc. Biol. Sci. 268: 2565–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeniman C. J., O'Neill S. L. 2010. A virulent Wolbachia infection decreases the viability of the dengue vector Aedes aegypti during periods of embryonic quiescence. PLoS Negl. Trop. Dis. 4: e748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeniman C. J., Lane R. V., Cass B. N., Fong A.W.C., Sidhu M., Wang Y. F., O'Neill S. L. 2009. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323: 141–144. [DOI] [PubMed] [Google Scholar]
- McMeniman C. J., Hughes G. L., O'Neill S. L. 2011. A Wolbachia symbiont in Aedes aegypti disrupts mosquito egg development to a greater extent when mosquitoes feed on nonhuman versus human blood. J. Med. Entomol. 48: 76–84. [DOI] [PubMed] [Google Scholar]
- Min K. T., Benzer S. 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl. Acad. Sci. USA. 94: 10792–10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. G., Mitchell C. J. 1997. Aedes albopictus in the United States: Ten-year presence and public health implications. Emerg. Infect. Dis. 3: 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira L. A., Saig E., Turley A. P., Ribeiro J.M.C., O'Neill S. L., McGraw E. A. 2009a. Human probing behavior of Aedes aegypti when infected with a life-shortening strain of Wolbachia. PLoS Negl. Trop. Dis. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira L. A., Iturbe-Ormaetxe I., Jeffery J. A., Lu G. J., Pyke A. T., Hedges L. M., Rocha B. C., Hall-Mendelin S., Day A., Riegler M., et al. 2009b. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139: 1268–1278. [DOI] [PubMed] [Google Scholar]
- Niebylski M. L., Savage H. M., Nasci R. S., Craig G. B. 1994. Blood hosts of Aedes albopictus in the United States. J. Am. Mosq. Control. Assoc. 10: 447–450. [PubMed] [Google Scholar]
- O'Neill S. L., Giordano R., Colbert A.M.E., Karr T. L., Robertson H. M. 1992. 16s ribosomal-RNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl. Acad. Sci. USA. 89: 2699–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill S. L., Hoffmann A. A., Werren J. H. 1997. Influential passengers: Inherited microorganisms and arthropod reproduction, Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- Rai K. S. 1991. Aedes albopictus in the America. Annu. Rev. Entomol. 36: 459–484. [DOI] [PubMed] [Google Scholar]
- Ross P. A., Endersby N. M., Yeap H. L., Hoffmann A. A. 2014. Larval competition extends developmental time and decreases adult size of wMelPop Wolbachia-infected Aedes aegypti. Am. J. Trop. Med. Hyg. 91: 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage H. M., Niebylski M. L., Smith G. C., Mitchell C. J., Craig G. B. 1993. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) at a temperate North American site. J. Med. Entomol. 30: 27–34. [DOI] [PubMed] [Google Scholar]
- Serbus L. R., White P. M., Silva J. P., Rabe A., Teixeira L., Albertson R., Sullivan W. 2015. The impact of host diet on Wolbachia titer in Drosophila. PLoS Pathog. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon F., Savini H., Parola P. 2008. Chikungunya: A paradigm of emergence and globalization of vector-borne diseases. Med. Clin. North. Am. 92: 1323–1343. [DOI] [PubMed] [Google Scholar]
- Sinkins S. P. 2004. Wolbachia and cytoplasmic incompatibility in mosquitoes. Insect Biochem. Mol. Biol. 34: 723–729. [DOI] [PubMed] [Google Scholar]
- Sinkins S. P., Braig H. R., O'Neill S. L. 1995. Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proc. Biol. Sci. 261: 325–330. [DOI] [PubMed] [Google Scholar]
- Suh E., Dobson S. L. 2013. Reduced competitiveness of Wolbachia infected Aedes aegypti larvae in intra- and inter-specific immature interactions. J. Invertebr. Pathol. 114: 173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh E., Mercer D. R., Fu Y. Q., Dobson S. L. 2009. Pathogenicity of life-shortening Wolbachia in Aedes albopictus after transfer from Drosophila melanogaster. Appl. Environ. Microbiol. 75: 7783–7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley A. P., Moreira L. A., O'Neill S. L., McGraw E. A. 2009. Wolbachia infection reduces blood-feeding success in the dengue fever mosquito, Aedes aegypti. PLoS Negl. Trop. Dis. 3: e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T., Johnson P. H., Moreira L. A., Iturbe-Ormaetxe I., Frentiu F. D., McMeniman C. J., Leong Y. S., Dong Y., Axford J., Kriesner P., et al. 2011. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476: 450–U101. [DOI] [PubMed] [Google Scholar]
- Werren J. H. 1997. Biology of Wolbachia. Annu. Rev. Entomol. 42: 587–609. [DOI] [PubMed] [Google Scholar]
- Werren J. H., Baldo L., Clark M. E. 2008. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6: 741–751. [DOI] [PubMed] [Google Scholar]
- Xi Z. Y., Khoo C.C.H., Dobson S. L. 2005. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310: 326–328. [DOI] [PubMed] [Google Scholar]
- Xi Z. Y., Khoo C.C.H., Dobson S. L. 2006. Interspecific transfer of Wolbachia into the mosquito disease vector Aedes albopictus. Proc. Biol. Sci. 273: 1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap H. L., Mee P., Walker T., Weeks A. R., O'Neill S. L., Johnson P., Ritchie S. A., Richardson K. M., Doig C., Endersby N. M., et al. 2011. Dynamics of the “popcorn” Wolbachia infection in outbred Aedes aegypti informs prospects for mosquito vector control. Genetics 187: 583–U346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap H. L., Axford J. K., Popovici J., Endersby N. M., Iturbe-Ormaetxe I., Ritchie S. A., Hoffmann A. A. 2014. Assessing quality of life-shortening Wolbachia-infected Aedes aegypti mosquitoes in the field based on capture rates and morphometric assessments. Parasites Vectors 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. J., Zheng X. Y., Xi Z. Y., Bourtzis K., Gilles J.R.L. 2015. Combining the sterile insect technique with the incompatible insect technique: I-impact of Wolbachia infection on the fitness of triple- and double-infected strains of Aedes albopictus. PLoS ONE 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W. G., Rousset F., O'Neill S. 1998. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. Biol. Sci. 265: 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.