Abstract
To characterize behavioral deficits in pre-adolescent offspring exposed in utero to Benzo(a)pyrene [B(a)P], timed-pregnant Long Evans Hooded rats were treated with B(a)P (150, 300, 600, and 1200 µg/kg BW) or peanut oil (vehicle) on E14, 15, 16, and 17. Following birth, during the pre-weaning period, B(a)P metabolites were examined in plasma and whole brain or cerebral cortex from exposed and control offspring. Tissue concentrations of B(a)P metabolites were (1) dose-dependent and (2) followed a time-dependence for elimination with ∼60% reduction by PND5 in the 1200 µg/kg BW experimental group. Spatial discrimination-reversal learning was utilized to evaluate potential behavioral neurotoxicity in P40–P60 offspring. Late-adolescent offspring exposed in utero to 600 and 1200 µg/kg BW were indistinguishable from their control counterparts for ability to acquire an original discrimination (OD) and reach criterion. However, a dose-dependent effect of in utero B(a)P-exposure was evident upon a discrimination reversal as exposed offspring perseverated on the previously correct response. This newly characterized behavioral deficit phenotype for the first reversal was not apparent in either the (1) OD or (2) subsequent reversal sessions relative to the respective control offspring. Furthermore, the expression of activity related-cytoskeletal-associated protein (Arc), an experience-dependent cortical protein marker known to be up-regulated in response to acquisition of a novel behavior, was greater in B(a)P-exposed offspring included in the spatial discrimination cohort versus home cage controls. Collectively, these findings support the hypothesis that in utero exposure to B(a)P during critical windows of development representing peak periods of neurogenesis results in behavioral deficits in later life.
Keywords: polycyclic aromatic hydrocarbon, benzo(a)pyrene, susceptibility exposure paradigm, B(a)P-metabolites, Spatial Discrimination Reversal Task, Activity-Related Cytoskeletal (Arc)-associated protein
Gestational exposure to environmental contaminants may result in increased adverse health outcomes, often affecting operant voluntary behavior (Koger et al., 2005; Weiss and Landrigan, 2000; Wormley et al., 2004a). One such environmental contaminant, Benzo(a)pyrene, [B(a)P] a combustion product frequently encountered in urban settings, is a member of the polycyclic aromatic hydrocarbon (PAH) family and is highly lipophilic. B(a)P crosses the human placenta, exposing the fetus to the contaminant body burden of the mother, resulting in adverse health outcomes, including effects on central nervous system (CNS) development (Brown et al., 2007; Hood et al., 2006; Li et al., 2012; McCallister et al., 2008; Ramesh et al., 2001; Sheng et al., 2010; Wormley, et al., 2004a, b). Since the CNS of developing fetuses is particularly susceptible to toxicant exposure in utero, it is postulated that in utero exposure to low levels of B(a)P cause long-term deficits in both learning and memory, consistent with the Barker hypothesis (Barker, 1995, 2003). Results from previous studies in our lab strongly suggest that in utero exposure to B(a)P can modulate glutamate receptor subunit protein expression which would likely impact the mechanisms governing N-methyl-d-aspartate (NMDA)-mediated learning and memory (Brown, et al., 2007; Li, et al., 2012).
Human exposure to B(a)P occurs through the ingestion of contaminated food and water (Phillips, 1999; Ramesh et al., 2004) or the inhalation of particulates in the ambient air, such as occurred after the collapse of the World Trade Center on September 11, 2001. B(a)P partitions mainly into soil (82%) and sediment (17%) and the food chain is the dominant pathway of human exposure, accounting for about 97% of the total daily intake of B(a)P. Inhalation and consumption of contaminated water are only minor pathways of human exposure. The long-term average daily intake of B(a)P by the general population of the U.S. is estimated to be 2.2 µg per day. Cigarette smoking and indoor activities do not substantially increase human exposure to B(a)P relative to exposures to background levels of B(a)P present in the environment (Hattemer-Frey and Travis, 1991). In a prospective study of an inner-city minority population, the effects of PAH exposure, including B(a)P in pregnant women and their offspring, were tracked. Children exposed during gestation presented with a reduction in head circumference at birth and, subsequently, lower IQ and poorer cognitive functioning later in life (Perera et al., 2009, 2011). This suggests that in utero exposure to PAHs produces alterations in nervous system functioning and could be a neurological risk factor by the time these children reach school age (Perera et al., 2011). These studies are highly provocative because of their correlational nature and the suggestion of causality. The presence of multiple contaminants or other confounders makes it impossible to link the behavioral effects directly to a specific contaminant, to determine brain levels of these contaminants, to identify windows of vulnerability, or to elucidate mechanism(s) of action. Mechanistic questions can be addressed in rodent models in which experimental control over exposure is easily maintained.
Glutamatergic signaling to include NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid glutamate receptor subunit proteins have been shown to be negatively modulated in response to in utero exposure to B(a)P (Brown et al., 2007; Hood et al., 2006; Li et al., 2012; McCallister et al., 2008). This negative modulation occurs at a time during the peak period of neurogenesis and synaptogenesis in the neocortex. Normal functioning of the NMDA receptor is obligatory to processes of long-term potentiation, synaptic plasticity and is thought to underlie learning and behavior changes (Paoletti et al., 2013). A protein that is important for these same processes is activity-related cytoskeleton-associated protein (Arc) (Guzowski et al., 2000; Rosi et al., 2005). Activity-regulated cytoskeletal-associated protein (Arc/Arg 3.1) is an immediate early gene induced by neural activity and critical for synaptic plasticity in critical brain regions and in the consolidation of long-term memory (Guzowski et al., 2000; Ploski et al., 2008). More recently, Arc expression in the basolateral amygdala was shown to be necessary for both the consolidation (Ploski et al., 2008), and reconsolidation (Maddox and Schafe, 2011), of Pavlovian fear conditioning. Arc expression is induced in hippocampal and parietal cortical neurons after a novel behavioral experience.
Previous studies have also confirmed the presence of Arc-associated protein in the prefrontal cortex. Zavala et al. (2008) indicated that reinstatement of extinguished cocaine-seeking behavior by response contingent cue presentations is associated with region-specific increases in mRNA of the plasticity-associated Arc gene in the cingulate cortex area 1, prelimbic, and orbitofrontal subregions of prefrontal cortex. These results suggest a cue-conditioned upregulation of Arc mRNA in these cortical brain regions. The present findings are in agreement with, and extend, previous work demonstrating an up-regulation of Arc in several limbic and cortical regions, including anterior cingulate, prelimbic, and orbitofrontal cortices following exposure to contextual cues associated with nicotine (Schiltz et al., 2005; Zavala et al., 2008) or food (Schiltz et al., 2007; Zavala et al., 2008) and following cue-induced reinstatement of extinguished heroin-seeking behavior (Koya et al., 2006; Zavala et al., 2008). Taken together, these observations suggest that B(a)P exposure might disrupt glutamatergic signaling; perhaps by altering Arc expression, and that these effects might be manifested as deficits in specific behavioral learning tasks. In a previous study, Wormley et al. (2004a) showed that in utero B(a)P-exposed offspring animals had significant deficits on an operant conditioning task; the fixed-ratio schedule of reinforcement, in which a pre-determined number of responses is required for a single reinforcer. As the ratio requirement increased from 1 to 5, 10, and then 25 responses per reinforcer, the response rates of animals exposed in utero to B(a)P-showed significant deficits in lever pressing learning behavior that did not occur in the controls (Wormley et al., 2004a). The Fixed Ratio schedule is sensitive to motor (Newland, 1995) and motivational (Baron and Derenne, 2000; Hodos, 1961; Kheramin et al., 2005; Mobini et al., 2000; Stafford et al., 1998) variables, so the increased ratio value could represent impairment in either of these domains. Here, we chose to explore the Spatial Discrimination Reversal procedure. This procedure is a marker of perseveration and difficulty in adjusting to new response requirements and is sensitive to impairment of the orbitofrontal cortex and alterations in choice (Robbins, 1996; Schultz et al., 1997, 1998). We chose to focus on the cerebral cortex and a task that requires normal functioning of cortical circuits. We also use Arc protein expression in the cerebral cortex as a cellular marker to verify appropriate induction of protein expression that is customarily associated with synaptic plasticity processes during the consolidation phases linked to the acquisition of novel behaviors.
MATERIALS AND METHODS
Power Analysis
Power analysis was used to estimate the number of offspring required for the study considering the number of litters as the statistical unit. From this analysis, 3 cohorts of randomized timed-pregnant dams between 2 experimental groups were utilized: (1) a control group (vehicle-exposed), (2) a 150 -µg/kg body weight (BW), (3) a 300 -µg/kg BW, (4) a 600 -µg/kg BW, and (5) a 1200 -µg/kg BW. Eight to 11 offspring from 3 different litters/cohorts were utilized for the behavioral analyses. Power analysis indicated that 3 repetitions from each cohort were required and it was estimated that the variance between measures from litters would be 10% of the mean response. Power analysis also indicated that 3 successful experiments in each cohort would be required to detect 20% change in any of the experimental end-points with 80% power and a type-I error rate of 5%.
Animal Husbandry
Timed-pregnant Long Evans Hooded rats arrived at the Meharry Medical College animal care facility on embryonic day 11 (E11). Upon arrival, animals were housed individually in clear plastic cages with laboratory grade (heat-treated) pine shavings as bedding. Animals were quarantined for 2 days in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal care facility and were maintained in a controlled environment with a temperature at 21°C ± 2°C and relative humidity of 50% ± 10% with a 12/12 h light/dark cycle. Dams were fed commercial food (Rat Chow 5012: Purina Mills, St. Louis, Missouri). Water and food were available ad libitum. The animals were then quarantined for 24 h after delivery. On E12, the animals were weighed and their pregnancy status was verified by the weight gain of the animal each day. Rats that were not pregnant were excluded from dosing. Each pregnant dam was randomly assigned to either a control or experimental group. Daily beginning on E14 and continuing through E17, timed-pregnant dams were treated/exposed, by oral gavage in a total volume of 0.875 ml to (1) peanut oil or to (2) 150, 300, 600, or 1200 µg/kg B(a)P.
Offspring of control and B(a)P-exposed dams were maintained in standard polycarbonate cages (Lab Products, Inc., Seaford, DE). Offspring were evaluated using a Spatial Discrimination Reversal procedure from PND 40 to 60 to determine if in utero exposure resulted in later-life behavioral deficit phenotypes in offspring.
Susceptibility exposure paradigm
Over the years, we have attempted to develop a susceptibility exposure paradigm to better assess the effects of in utero exposure to environmental toxicants on certain aspects of CNS development (Brown et al., 2007; Hood et al., 2000; Li et al., 2012; McCallister et al., 2008; Ramesh et al., 2001; Sheng et al., 2010; Wormley et al., 2004a, b). Because CNS events have ‘windows of susceptibility’ during development, we reasoned that there should be a time frame when the lowest dose and shortest duration of exposure to an environmental contaminant would be expected to have a significant negative impact on brain development. We developed this susceptibility exposure paradigm based on the peak periods of neuroepithelial cell proliferation (neurogenesis) for specific brain structures. Embryonic days 14 through 17 (E14–E17) represent the range of neurogenesis from beginning to end for the cerebral cortex in the rat. E15 represents the maximal peak period of neurogenesis for the cerebral cortex in the rat. Subsequent to this peak period of neurogenesis, each neuronal cell continues to mature through a process of migration, settling to a specific location, and extending projections to a designated target site. In many cases, such as for the external germinal layer, this process of migration continues well after birth and in the human can continue for 7 months to 2 years after birth. Specifically, cortical synapses at birth are still immature and in the human the morphological characteristics of maturity are reached between 6 and 24 months after birth.
As a result, using this critical window of exposure from E14 to E17 defined as our ‘susceptibility exposure paradigm’ has allowed us to ask relevant questions pertaining to nervous system malformations that can arise from alterations of neurogenesis subsequent to environmental contaminant exposure (ie, changes in developmental expression of proteins involved in synaptic development, changes in the timing of migration and perturbation of migratory mechanisms). The translational relevance of this susceptibility exposure paradigm to the human situation is that the earliest synapses develop during the gestational period, and by 10-weeks immature synapses are present. Unintended in utero exposure of the fetus to environmental contaminants has been shown to result in alterations in neurodevelopment such as reduced head circumference and neurobehavioral deficits such as poorer outcome on selective aspects of cognitive and neuromotor functioning in offspring. We have continually utilized this susceptibility exposure paradigm in our laboratory to unmask deficits in learning behavior that seem to be in parallel with down-regulation of various aspects of glutamatergic receptor subunit expression (Brown et al., 2007; Li et al., 2012; Wormley et al., 2004a).
Acute Oral Bioavailability Sampling
Blood and whole cortex samples were collected from the male offspring (n = 6) exposed to oral doses of B(a)P (150, 300, 600, and 1200 μg/kg BW). For this analysis, offspring were killed on postnatal day (PND) 0, 3, 5, 7, 9, 11, 13, and 15. After that, blood samples were retrieved, maintained on ice, and subsequently centrifuged at 2900 × g for 25 min. The supernatant (plasma) was transferred into cryovials and stored at −80°C until analyses. For the analysis, 500 µl of plasma or 0.5 g of tissue was homogenized with 2 volumes of Tris-sucrose-EDTA buffer (0.25 M; pH 7.4). Twenty microliters of sodium dodecyl sulfate was added to the mixture and vortexed for 1 min. The homogenate was extracted with a methanol (40 ml): deionized water (10 ml): chloroform (15 ml) solution. Centrifugation was done at 2000 × g for 20 min to facilitate separation of aqueous and organic phases. To remove moisture, the organic phase was passed through anhydrous sodium sulfate. The extraction efficiency was determined by a naphthalene surrogate with recovery at ∼90%. The organic phase was dried under N2 and resuspended in 0.5 ml methanol. Particulates in the extracts were removed by passing the extracts through Acrodisc filters (0.45 μm; 25 mm diameter; Gelman Sciences, Ann Arbor, Michigan). The final filtrates were stored in amber screw-top vials at 4°C until analyses.
Quantitative HPLC Analyses
B(a)P metabolites were resolved by a high-pressure liquid chromatography (HPLC) (model 1200 Agilent Technologies, Wilmington, Delaware) equipped with a fluorescence detector. Using an autosampler, 30 μl of sample was injected onto a C18 reversed-phase column (ODS Hypersil, 5 μm, 4.6 × 250 mm; Agilent). The column was eluted at 33°C at a flow rate of 1.0 ml/min with a ternary gradient of water:methanol:ethanol (%) as follows: 40:40:20 in 20 min, followed by 30:46:24 in 10 min, 100% methanol in 10 min, and lastly, 40:40:20 for 5 min. The excitation and emission wavelengths for the fluorescence detector were 244 and 410 nm, respectively. The B(a)P concentrations in plasma and whole cortex samples were calculated by comparing the retention times and peak areas of samples with those of standards using an HPLC Chemstation software (DOS series, Hewlett Packard). The following B(a)P metabolite standards were used for identification and quantitation of metabolites: B(a)P-trans-4,5-dihydrodiol(+), B(a)P-trans-7,8-dihydrodiol (+), B(a)P-trans-9,10-dihydrodiol (+), B(a)P-3,6-dione, B(a)P-6,12-dione, 3-hydroxy B(a)P, and 9-hydroxy B(a)P.
Analysis of Bioavailability Data
The metabolite concentration data were expressed as mean ± SE. Data from the CNS disposition studies were analyzed for statistical significance by Student’s t test or analysis of variance (ANOVA). Wherever appropriate, following significant interactions, independent group mean comparisons were done by Fisher's least square (LSD).
Spatial Discrimination Reversal Learning Task
Acclimatization
Beginning on PND 40, offspring animals were singularly caged, weighed, and put on a restrictive diet. Animals were fed 2 pellets (Rat Chow 5012: Purina Mills, St. Louis, Missouri) every day until they reached 175 g, which is ∼85% of their age-appropriate free feeding weight. During the experiment, animals were weighed daily to ensure that they were healthy and maintained an appropriate body weight. Animals received sucrose pellets during experimental sessions as food rewards and also received 1 to 1½ food pellets while they were in their home cages in the animal care facility. The amount of food animals received while in their home cage depended on the individual animal’s weight and their performance and weight that day in the experiment. Animals that had higher omissions or made more correct responses received fewer food pellets in their home cage, while animal that made more incorrect response received more food in their home cage. Also, if an animal’s weight for that day was <85% of their free feeding body weight then they received more pellets in their homecage.
Testing apparatus
Behavioral procedures were conducted in 7 commercial Model ENV-008 operant chambers purchased from Med-Associates, St. Albans, Vermont. Each chamber contained 1 rear-mounted lever and 2 front, retractable levers, and a pellet dispenser situated between the 2 front (left and right) levers filled with 48 mg sucrose pellets (Bio-Serve, Inc., New Frenchtown, New Jersey). The apparatus also featured Sonalert tones (2900 and 4500 Hz, nominally; calibrated to 70 dbC), a house lamp (28 V 100 Ma), and a 28 V DC, 100 mA stimulus light above each lever. Each chamber was encased in a sound-attenuating cabinet, with a built-in ventilating fan that circulated air into the experimental environment and masked white noise. Programs for experimental procedures and data collection/statistical analysis were written using MED-PC IV (Med-Associates). Session events were recorded with 0.01-s resolution.
Lever-press training
An autoshaping procedure using a Fixed Time 300-s schedule was implemented in overnight to establish lever-pressing as described in Paletz et al. (2007) and Reed et al. (2006). In brief, after 240″ the left lever extended into the chamber and a light over that lever lit. A press on that lever resulted in the delivery of a 45-mg sucrose pellet, withdrawal of the lever, turning off the light, and resetting the timer to 0. If there was no response then the sucrose pellet was delivered at 300″. After 10 lever-presses, a simple Fixed Ratio 1 (FR 1) schedule was introduced; the lever remained available continuously, every lever-press was reinforced, and no free pellets were delivered. This continued until 100 responses occurred. Then, an FR 1 was in place for the right lever and the left one was unavailable. Finally, the left and right levers were unavailable and an FR1 was implemented on the rear lever.
The final training task was to establish a 2-lever response sequence in which a rear-lever press (trial-initiation response) extended a front lever that, if pressed (choice response), resulted in the delivery of a sucrose pellet. Each trial began with an alternating 2900 Hz tone (0.3 s on, 0.6 s off). When the rear lever was pressed, the tone stopped and either the left or right front levers extended into the chamber, determined pseudo-randomly (the same lever did not extend 3 times in a row), and the light above the extended lever was illuminated until that lever was pressed. Immediately after being pressed, the lever retracted, the light turned off, a pellet was delivered, and the trial ended. To limit position bias during initial training, the lever was extended into the same side of the chamber until it was pressed (correction procedure); only after this response was the side for the subsequent trial re-determined. If the rear lever was not pressed within 5 min after the onset of the tone, the trial ended without a sucrose pellet. If the front lever was not pressed within 5 min after it was extended, the trial ended without a sucrose pellet. There was a 10-s interval between trials. Initially, rear-lever presses during the ITI had no programmed consequences, but after 6 trials ending with sucrose reinforcement, ITI responses reset the incrementing timer to 0 s. The 2-lever response sequence was used to prevent rats from standing in one place throughout a session. The trial-initiation response (the first lever press) also ensured that the rat was on-task. Sessions lasted 100 trials or until 12 cumulative trials ended in reinforcement, with the latter also being the criterion for ending training.
Spatial discrimination and reversal testing
The procedure for the original discrimination (OD) and spatial discrimination reversal task were similar to that of lever press training, except for the following exceptions. First, both front levers were extended (and lights above them turned on) after the rear lever was pressed. Second, if the rear lever was not pressed within 15 s after the onset of the alternating tone, or a front lever was not pressed within 15 s after it was extended, the levers retracted and the trial ended without reinforcement. Third, responding on only one of the front levers was reinforced; responses on the other lever ended the trial without reinforcement. Fourth, there was no correction procedure during testing. Initially, only left-lever presses were followed by reinforcement (OD, or OD-left). The requirement that a trial begin with a rear lever-press remained in place throughout the experiment. After subjects completed at least 3 consecutive sessions with 85% (51 out of 60 trials) or more of the left-lever trials ending in reinforcement, only right-lever presses were reinforced (first reversal, or R1-right). After reaching the same performance criterion, only left lever-presses were reinforced (second reversal, or R2-left). Each session lasted 60 trials.
Statistical analysis of data
For reversal learning paradigm, all statistical analyses were performed using GraphPad Prism 5.0 b (GraphPad Software Inc., La Jolla, California). The Type I error rate (α) was set at 0.05 for all tests. In order to provide a full characterization of behavior, 6 dependent variables were analyzed, with reversal number treated as a within-subject variable for the OD and each reversal. The 6 variables were:
Sessions to criterion: the total number of sessions required to reach criterion for each reversal.
Reinforced trials: the total number of reinforced trials during each reversal.
Omissions: the total number of trials without a rear-lever or front-lever press for each reversal.
Errors: the total number of incorrect presses on a front lever for each reversal.
Rear-lever latency: average time per trial between the onset of the trial (alternating tone) and the rear-lever press for the first session of each reversal. Trials without a rear lever-press were scored as 15 s.
Choice latency: average time per trial between a rear-lever press and a front-lever press for the first session of each reversal. Trials without a choice response were scored as 15 s.
Exposure effects were examined using two-way ANOVAs with exposure as a between group factor and session as a repeated measure. This was conducted on OD, Reversal 1, and Reversal 2. Significant ANOVAs were followed by pairwise Bonferroni post testing among the control and B(a)P experimental groups to determine differences, if any, in cohort groups; F-ratios, degrees of freedom, and P-values are reported for all two-way ANOVAs, and P-values are reported for Bonferroni post test. Only significant effects are reported for each variable. If not reported, the P-value was >.1.
Expression Profiling Via Western Blot Analysis
Quantifying experience-dependent upregulation of protein expression in response to acquisition of novelty
A comparison of the protein content of Arc in neocortical tissue derived from offspring was conducted and compared with a housekeeping gene GAPDH. Medial prefrontal cortices (75–100 mg) from caged control and exposed offspring versus spatial discrimination trained control and exposed offspring (<PND70) were used for the experiments as shown in Figure 1.
FIG. 1.
Timeline of in utero B(a)P dosing, metabolite determinations, behavioral testing procedures, and Arc protein expression (see text for details).
Statistical analysis of protein expression
Arc protein expression was quantitated from Western blots using Image J software (National Institutes of Health, Bethesda, Maryland; open source Image J software available at http://rsb.info.nih.gov/ij/. The resulting densitometric signal band intensities ± SE from at least 3 experiments were normalized to the corresponding GAPDH loading levels and plotted as bar graphs. Percentage band intensities ± SE relative to GAPDH was plotted on the Y-axis versus experimental group on the X-axis for caged control, caged 600 and 1200 μg/kg B(a)P offspring versus spatial discrimination trained control and 600 and 1200 μg/kg B(a)P offspring.
RESULTS
Toxicological Observations
There were no significant differences in the number of pups born per litter between control and any B(a)P-exposed dams (Table 1). This is consistent with a previous report from our group (Brown et al., 2007). During the in utero and subsequent pre-weaning exposure periods, there were no identifiable B(a)P-related effects on conventional/reproductive indices of toxicity. No convulsions, tremors, or abnormal movements were noted in any of the dams or offspring from control or B(a)P-exposed litters. There were no significant differences in body weight gain between control and B(a)P-exposed offspring over the course of this study (Figure 2). We previously reported treatment-related differences in brain to body weight ratios only on PND15 and PND30 between control and B(a)P-exposed (McCallister et al., 2008). In that study, the brain-to-body weight ratios from B(a)P-exposed offspring were greater than controls (McCallister et al., 2008) on PND15 and PND30. It is interesting to note that postnatal modulatory effects on PND15 and PND30 quantified as robust reductions in temporal developmental expression of glutamatergic receptor subunits or glutamatergic signaling proteins subsequent to in utero exposure to environmental aryl hydrocarbon receptor agonists have been previously reported (Brown et al., 2007; Hood et al., 2006; Li et al., 2012). The body weight growth curves for control and in utero B(a)P-exposed offspring reveal no significant differences in the pre-weaning growth curves.
TABLE 1.
B(a)P Exposure Has No Effect on the Number of Pups Born Per Litter
| Group | Dams | Pups/litter (range) | Pups/litter* (mean) |
|---|---|---|---|
| Control | 17 | 8–12 | 10.4 ± 0.3 |
| 150 µg/kg BW | 7 | 7–12 | 11.6 ± 0.6 |
| 300 µg/kg BW | 6 | 8–14 | 10.8 ± 0.8 |
| 600 µg/kg BW | 15 | 9–15 | 11.7 ± 0.5 |
| 1200 µg/kg BW | 9 | 8–13 | 11.1 ± 0.6 |
In utero B(a)P exposure results in the absence of a reproductive toxicological effect. Shown are the average numbers of pups born/litter. Values represent the mean ± SE.
FIG. 2.
in utero B(a)P exposure has no effect on birth indices. Shown are the gross body weights for experimental groups over the period from PND 0 through PND 15. White bars represent control offspring, grey bars represent in utero B(a)P exposed offspring. (Values are mean + SE with P < .05.) The data represent litter means of body weight from a representative subset [n = 8 of control litters; n = 3 for B(a)P litters] of total litters [n = 17 for control litters; n = 7 for 150 µg/kg BW B(a)P; n = 6 for 300 µg/kg BW B(a)P; n = 15 for 600 µg/kg BW B(a)P and n = 9 for 1200 µg/kg BW B(a)P litters].
Bioavailability and Metabolite Distribution
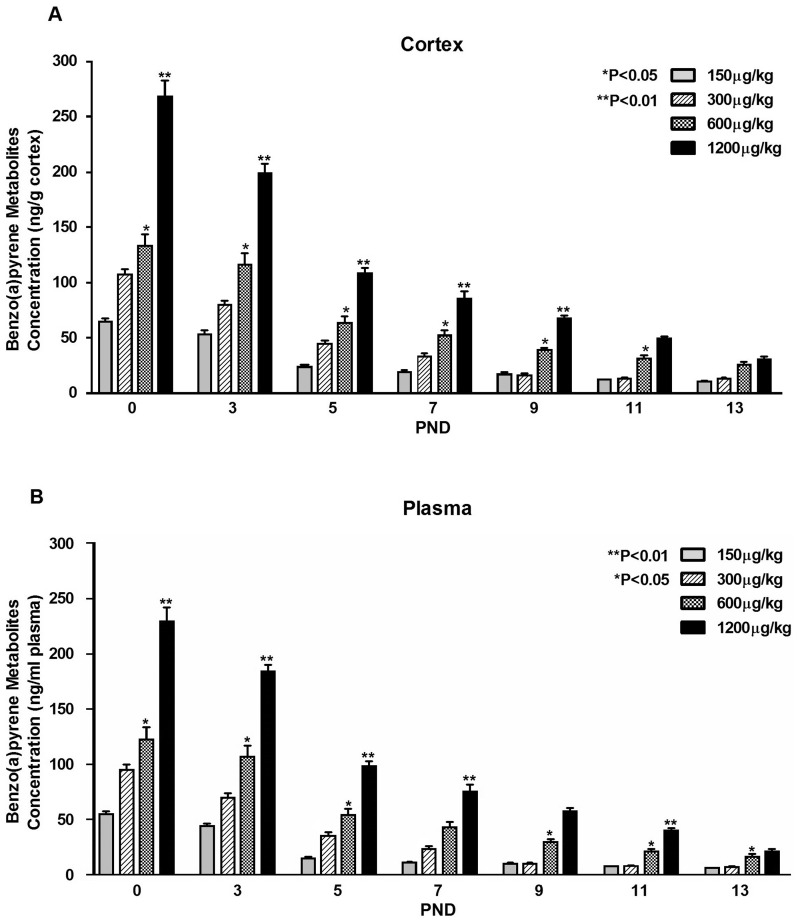
HPLC was used to determine the profile of B(a)P metabolites as a result of in utero exposure. To rule out the possibility or background contribution of similarly structured B(a)P-like endogenous compounds, we examined control offspring plasma and whole brain tissue for total B(a)P metabolites. No metabolites were detected in the control offspring. However, a dose-dependent increase and a post B(a)P exposure time-dependent decrease in total B(a)P metabolite concentrations were observed in plasma and whole brain neocortical tissue and is shown in Figures 3A and B.
FIG. 3.
Time-course distribution of bioavailable B(a)P total metabolite concentration measured in brain and plasma tissues. Timed-pregnant dams received 150, 300, 600, and 1200 µg/kg BW B(a)P via gavage daily on days E14, E15, E16, and E17. Offspring pups were killed on PND0, 3, 5, 7, 9, 11, and 13 and metabolite concentrations were determined. Values represent mean ± SE with *P < .05 for n = 3 litters for control and n = 3 litters for B(a)P-exposed. Owing to the limited volume of blood that could be obtained at PND0 and PND5, whole blood was used in lieu of plasma, whereas plasma was used for the remaining time points for metabolite analysis. Cerebrocortical brain tissue was used for metabolite concentrations analysis. The asterisks denote statistical significance. A, Brain or B, Plasma.
The total B(a)P metabolite tissue burden in the brain was shown to increase as evidenced by, the concentrations of B(a)P metabolites, such as B(a)P toxification and 9,10-epoxide and 3(OH)B(a)P. These increases indicate the activity B(a)P toxification and detoxification pathways, respectively, and are shown in Figure 4. What is interesting is that the concentration of B(a)P 4,5-diol, 7,8-diol, 9,10-epoxide metabolites remain over 50% of the total tissue burden during the pre-weaning period. The 3-hydroxy metabolite is elevated on PND9 through PND13.
FIG. 4.
Qualitative distribution of metabolites. Percentage distribution of B(a)P metabolites from cerebral cortex in offspring pups [600 µg/kg BW B(a)P]. Pups were killed on PND0, 3, 5, 7, 9, 11, and 13 and metabolite concentrations were determined and authenticated using standards obtained from the National Cancer Institute. In as much as the volume of sample on PND0 and PND3 were small and whole brain tissue was used for analysis.
Spatial Discrimination Reversals
There was no significant difference in the total number of correct or incorrect lever-presses or omissions throughout the course of all phases (OD, R1, and R2) (data not shown). The principle interest, however, was in behavior in the first session of the OD and, especially, of the reversals. Overall performance was assessed by examining the time required to complete the session. Rear-lever latency reflects task disengagement or the presence of other behavior (eg, exploring or grooming) during the ITI, which can affect the latency to start the self-initiated trial. Choice (front-lever) latency is sometimes associated with either impulsive responding (very short latencies) or an ambiguous situation in which the correct response is not clearly indicated (very long latencies). Figure 5 shows that there are no significant differences in the session time (panel A) but offspring in both exposure groups pressed the rear lever and completed the trials more quickly than the controls on the first reversal (panels B and C).
FIG. 5.
in utero exposed offspring require less time to perform the spatial discrimination reversal task. On P40–60, spatial discrimination and reversal experiments were conducted on control offspring and offspring exposed in utero to 600 and 1200 µg/kg BW B(a)P. This graph displays the average session time (top), rear press latency (middle), and choice latency (bottom) during the first session of each condition. P < .05 for the 600 and 1200 µg /kg BW B(a)P groups compared with controls animals. Values are means ± SE.
On the first day of the first reversal, there was a dose-related decrease in correct responses and corresponding increase in incorrect responses (P < .05, Figure 6). However, there were no significant differences in the number of omissions on the first day of conditioning.
FIG. 6.
In utero B(a)P exposure decreased choice accuracy in the spatial discrimination reversal task. On P40–60, spatial discrimination and reversal behavioral experiments were conducted on control offspring and offspring prenatally exposed in utero to 600 and 1200 µg/kg BW B(a)P. This graph displays the average number of correct (top), incorrect (middle), and omissions (bottom) during the first session of each condition. P < .05 for the 600 and 1200 µg/kg BW B(a)P groups compared with controls animals. Values are means ± SE.
Figure 7 shows representative subjects to illustrate the acquisition of OD and the 2 reversals. Panels A, B, and C, represent 0, 600, and 1200 μg/kg exposure groups, respectively. Within each panel, correct responses, errors, and omissions are in the top row and trial-initiation and choice response latencies are in the second row. The OD, reversal 1, and reversal 2 are shown from left to right.
FIG. 7.
Representative graphs of the dependent measures for each of the conditions for control (A), 600 µg/kg BW (B), and 1200 µg/kg BW (C) B(a)P-exposed offspring. The abscissa shows sessions. The ordinate denotes trials (top graph in each panel) or sessions (bottom graph in each panel). The gaps in data represent reversals. Dotted line in all 3 graphs represents the criterion for each task which is 85% accuracy. The non-filled circles show correct trials (reinforced trials); the filled circles show errors, and the filled squares show omission trials for the top graph in each panel. The open squares show latency (in seconds) to make a rear lever choice to initiate the trial. The filled squares show the latency to make a choice between the front 2 levers.
Overall, responding was well maintained in all groups; there were very few omissions. The OD was acquired rapidly for all 3 exposure groups, with 85% accuracy occurring after 2 sessions for the subjects shown. The first reversal was characterized by a large number of incorrect responses and a small number of correct responses that persisted for several sessions, with more incorrect responses for the B(a)P-exposed offspring groups. Response acquisition for the third reversal proceeded more quickly, resembling the OD.
Trial-initiation (rear-lever) latencies began long and grew shorter across trials, reflecting increased control over behavior by the requirements of the SDR task. Choice latencies remained low throughout. Although difficult to see because of the scale, the choice latencies were higher for the first session of a reversal.
Expression of Arc-Associated Protein
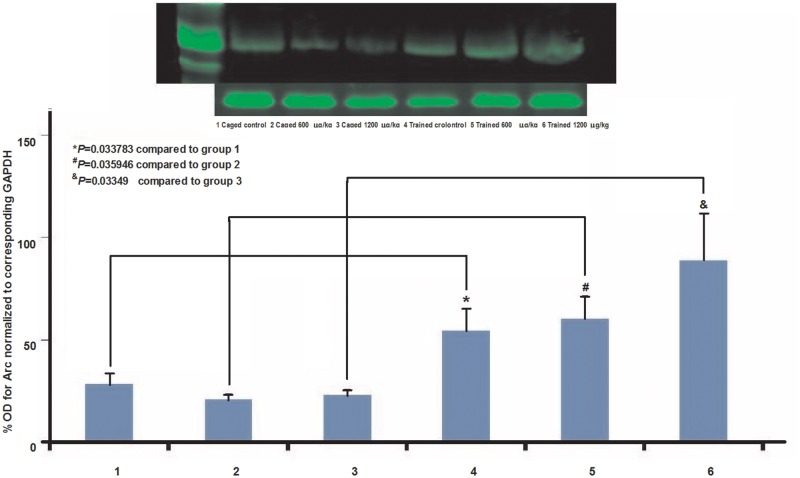
To determine how in utero exposure to B(a)P affects behavioral adverse outcome pathways on a molecular level we looked at the expression of the Arc protein in the cortex of rat offspring after the behavioral experiments were concluded. The learning deficit phenotype correlated well with the downregulation of cortical Arc expression in a somewhat dose-dependent manner. The Western blot experiments (Figure 8) showed that offspring control animals that were magazine trained and shaped for lever pressing exhibited higher levels of Arc mRNA expression than caged controls. This upregulation of Arc expression was downregulated if not inhibited as a consequence of in utero exposure to B(a)P in caged animals. However, there is a dose-dependent increase in the offspring trained for the behavioral task which may be because of the time it takes for the high-dosed animals to complete the task compared with the control trained offspring (Figure 8).
FIG. 8.
Arc protein expression in cortices from offspring exposed in utero to B(a)P. Expression of Arc protein is shown to be virtually unchanged in home cage control and home cage B(a)P-exposed offspring (lanes 1–3). Conversely, Arc protein expression is shown to be significantly upregulated in B(a)P-exposed offspring that underwent spatial discrimination testing. The result suggests that the increase in protein expression was in response to acquisition of novelty in these offspring as compared with a lack thereof, in their home cage controls.
DISCUSSION
Our results show that in utero B(a)P exposure produces deficits in spatial discrimination reversals during adolescence (PND 40–60) in the offspring. The rodent adolescent period is considered to span ∼PND 25–56 based on cortical development as well as behavioral markers (Spear, 2000, 2007). Therefore, the age period examined here represents the late adolescence/early adulthood. The spatial discrimination reversal procedure was selected because it has proven to be an efficient test that is sensitive to environmental toxicants that negatively impact the CNS (Gilbert and Rice, 1987; Kulig et al., 1996; Reed et al., 2006; Schantz and Bowman, 1989; Widholm et al., 2003).
During the first reversal (R1-right), the rats exposed in utero to 600 and 1200 μg/kg BW of B(a)P perseverated on the left lever; the lever on which the OD had been acquired. This likely reflects enhanced resistance to extinction in the B(a)P-exposed offspring and perhaps excessive reinforcer efficacy, as has been shown with developmental methylmercury exposure (Newland et al., 2015). The enhanced perseveration was accompanied by shorter choice latencies in the B(a)P-exposed offspring. Long latency choice responses occur when a new discrimination is being acquired and then they shorten as the discrimination stabilizes. While subtle because of the scale of the ordinate, this is present in Figure 7 where choice latencies tended to be longer early in OD and at the first reversal (see also Paletz et al., 2007). It is also evident in the trend toward shorter latencies across reversals in control (Figure 5B, also Paletz et al., 2007).
If short latencies reflect improved discrimination then why are perseverative errors associated with short latencies in the B(a)P-exposed rats? This could occur if the B(a)P-exposed offspring are responding too quickly, a pattern that might be termed impulsive. A similar pattern, perseveration accompanied by short choice latencies, was also reported in rats exposed prenatally to methylmercury (Paletz et al., 2007). Since such exposures were also associated with impaired response inhibition (Newland et al., 2013), it is possible that the persistent responding on the previously reinforced lever reflects a failure to inhibit that response. The response pattern seen with gestational methylmercury exposure led to a hypothesis of impaired balance between the direct impact of reinforcement and the inhibitory control imposed by the prefrontal cortex (Newland et al., 2015). In a sense, the reinforcers appeared to be too efficacious. Interestingly, this imbalance is also part of what characterizes adolescence (Spear, 2000, 2007) so the prenatal exposure may have exacerbated an adolescent response pattern. While this can only be hypothesized at present, it is consistent with the behavioral pattern seen here, the disruption of glutamatergic activity as discussed below, and the important changes in glutamatergic systems that occur during adolescence. The reason for the decreased trial-onset latencies in B(a)P-exposed offspring is unclear at present, but such a pattern is not inconsistent with enhanced reinforcement efficacy since that would lead to B(a)P-exposed offspring starting a reinforced trial more quickly.
These effects on behavior are likely explained by previous data from our lab which suggested that in utero exposure to B(a)P produces reproducible negative modulation of cerebrocortical neural activity in response to sensory input in the offspring (McCallister et al., 2008). Specifically, that normal functioning of cerebrocortical areas are impaired for at least 4 months after parturition as a result of gestational exposure to B(a)P. Our demonstration of gestational exposure-induced repression of evoked cortical activity correlated well with down regulation of NMDA-NR2B receptor subunit expression. In the S1 cortex, this glutamatergic deficit phenotype is characterized by a robust diminution in sensory stimulus evoked cortical neural activity. These observations of a persistent reduction in the initial shorter latency epoch (3–20 ms) from in utero B(a)P-exposed offspring were nearly identical to that which was observed for in utero dioxin-exposed offspring (Hood et al., 2006). In the latter instance for control offspring, this shorter latency response has been demonstrated to be completely dependent on glutamatergic receptors (Armstrong-James et al., 1993) localized in thalamocortical synapses of cortical layer IV. Alternatively, responses with latencies between 11 and 20 ms have been found to be dependent on NMDA type glutamate receptor subunit expression (Armstrong-James et al., 1993; Rema et al., 1998). Therefore, it can be concluded that the repression of these early latency responses in B(a)P-exposed offspring is a result of modulation of NMDA receptor subunit expression and function, and possibly of other types of glutamate receptors. Collectively, our results have established that in utero exposure to low doses of B(a)P during critical developmental windows in cortical structures results in a robust negative modulation of glutamate receptor expression (Li et al., 2012).
The fact that in utero exposure to B(a)P results in a robust diminution of cortical neuronal activity in adult offspring poses an interesting question as to whether the bioavailability of maternal B(a)P contributes to the causal mechanism for the observed neurotoxicity. Our bioavailability data and the empirical determinations of quantitative distribution of B(a)P metabolites from whole brain offspring suggest that metabolites accumulate and persist in offspring brain tissues up to PND20. These findings are very consistent with and corroborate our earlier results (Brown et al., 2007; Wu et al., 2003) to demonstrate translocation of metabolites from dam to fetus during the in utero period and persistence in tissues throughout the pre-weaning period (PND20). Such perinatal exposure pathways via lactational transfer of B(a)P from mother to the newborn has been reported for rats (Tozuka et al., 2004), ruminants (Lapole et al., 2007), and humans (Zanieri et al., 2007). In such exposure scenarios suckling offspring pups get a second exposure during the postnatal period subsequent to in utero exposure (via placental transfer; Sanyal and Li, 2007). As was discussed in McCallister et al. (2008), in addition to transplacental transfer of maternal metabolites, fetal metabolism of B(a)P transferred through the placenta (Kihlstrom, 1986) may also contribute to the global B(a)P metabolite pool in pre-weaning offspring. This bioavailable pool of reactive B(a)P metabolites such as the B(a)P 7,8 diol 9,10-epoxide in developing cerebrocortical structures may also potentiate the reported special discrimination deficits in this report.
In utero B(a)P exposure likely produces many of its long-lasting effects in offspring by potentiating cortical-based sensory deprivation caused by B(a)P repression of glutamate receptor subunit expression during the perinatal period. When these results from low input activity are compared with similar effects of in utero B(a)P exposure, this raises the possibility that exposure during gestation produces a ‘central’ deprivation to effectively reduce cortical activity below the levels needed for normal experience-dependent maturation of synaptic function. It remains a possibility that the delay in response onset could be due to slow conduction and ‘sluggish’ synapses in the somatosensory circuit pathway. In addition, both the magnitude of response and latency might be affected via trigeminal or thalamic relay neurons in the S1 pathway. We continue to work on resolving these complex issues.
Many research groups have demonstrated differential effects, depending upon the neurotoxicant investigated on subunits of glutamate receptors. (Chen et al., 2004; Grova et al., 2007; Guilarte and McGlothan, 1998; Hood et al., 2006; Nihei and Guilarte, 1999; Wormley et al., 2004a, b). We continue to establish that in utero exposure to B(a)P degrades the function of cortical areas in a distinctive and quantifiable manner. The physiological deficits reported herein are supported by previous data demonstrating reductions in NMDA NR2B mRNA and protein levels. (Li et al., 2012; McCallister et al., 2008), These new data also corroborate earlier observations (Brown et al., 2007; Hood et al., 2006; Wu et al., 2003) and demonstrate that in utero B(a)P-exposure-induced effects result in diminished expression of NMDA receptor subunits and occur at a time when excitatory synapses are being formed for the first time in cortical areas. Down-regulation of this receptor leads to a sustained decrease in synaptic functioning when excitatory synapses are formed for the first time. If in utero exposure to B(a)P modulates temporal glutamate receptor subunit mRNA developmental expression, then this may contribute to the reduced capacity for the homeostatic maintenance of synaptic plasticity mechanisms during development. In McCallister et al. (2008), the mRNA expression of the NMDA receptor subunit NR2A was quantified in parallel with behavioral and electrophysiology studies. The present studies suggest that in later-life, NR2B expression may remain suppressed and may contribute to the observed behavioral learning deficits.
Another gene that is important for long-term potentiation, synaptic plasticity, and spatial learning is Arc-associated protein (Guzowski et al., 2000; Rosi et al., 2005). A prior study conducted in our laboratory by Wormley et al. (2004b) found that Arc expression to be in significantly up-regulated in animals that were magazine trained and shaped on an FR1 schedule as compared with untrained caged control animals (Wormley et al., 2004b). Our data indicate that early life exposure to environmental contaminants poses a significant threat to normal growth and differentiation processes in the developing brain (Jacobson and Jacobson, 1997). Our finding that in utero B(a)P exposure suppressed cortical Arc expression in caged control offspring while stimulating an apparent up-regulation of Arc in exposed animals that underwent discrimination training is consistent this overall hypothesis and suggests therapeutic strategies going forward to potentially overcome or mitigate the effects from in utero exposure.
The consequence of a developmental neocortical tissue metabolite burden can then be appreciated within the context of the data reported in Perera et al. (2009). The study reported on of 5-year-old children who had been exposed in utero to 0.49–34.48 ng/m3 PAHs. More than half of these children ended up in the high-PAH exposure group. When they were tested at 12, 24, and 36 months, children in the high-exposure group had significantly lower full-scale/verbal IQ scores than the low-PAH exposure group. Another study on the same cohort (Perera et al., 2011) found that the children exposed in utero to PAHs had higher cord PAH adducts. These adducts are associated with higher symptom scores of Anxious and Depressed at 4.8 years and Attention Problems at 4.8 and 7 years, and according to Diagnostic and Statistical Manual of Mental Disorders (DSM)-oriented Anxiety Problems at the 4.8 years. These results suggest that PAH exposure, measured by DNA adducts, may adversely affect child behavior, potentially affecting school performance (Perera et al., 2011).
ACKNOWLEDGMENTS
We would also like to thank Russell E. Savage, Ph.D. for critical review of the manuscript.
FUNDING
This study was supported in part by R56 ES017448-01 (D.B.H.) from the National Institute of Environmental Health Sciences, Grant 5RO1CA142845-04 from the National Cancer Institute (A.R.), Grant RRO3032 to Meharry Medical College, Meharry Medical College-Vanderbilt University Diversity Neuroscience Training Grant T32MH065782 (D.B.H. and M.M.M.) and start-up funds (D.B.H.) from the Division of Environmental Health Sciences, College of Public Health, The Ohio State University.
REFERENCES
- Armstrong-James M., Welker E., Callahan C. A. (1993). The contribution of NMDA and non-NMDA receptors to fast and slow transmission of sensory information in the rat SI barrel cortex. J. Neurosci. 13, 2149–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J. (1995). Fetal origins of coronary heart disease. BMJ 311, 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J. (2003). The midwife, the coincidence, and the hypothesis. BMJ 327, 1428–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A., Derenne A. (2000). Progressive-ratio schedules: effects of later schedule requirements on earlier performances. J. Exp. Anal. Behav. 73, 291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. A., Khousbouei H., Goodwin J. S., Irvin-Wilson C. V., Ramesh A., Sheng L., McCallister M. M., Jiang G. C., Aschner M., Hood D. B. (2007). Down-regulation of early ionotrophic glutamate receptor subunit developmental expression as a mechanism for observed plasticity deficits following gestational exposure to benzo(a)pyrene. Neurotoxicology 28, 965–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. H., Lee Y. F., Chan M. H., Lo P. S. (2004). The role of N-methyl-d-aspartate receptors in neurobehavioral changes induced by toluene exposure during synaptogenesis. Ann. N. Y. Acad. Sci. 1025, 552–555. [DOI] [PubMed] [Google Scholar]
- Gilbert S. G., Rice D. C. (1987). Low-level lifetime lead exposure produces behavioral toxicity (spatial discrimination reversal) in adult monkeys. Toxicol. Appl. Pharmacol. 91, 484–490. [DOI] [PubMed] [Google Scholar]
- Grova N., Valley A., Turner J. D., Morel A., Muller C. P., Schroeder H. (2007). Modulation of behavior and NMDA-R1 gene mRNA expression in adult female mice after sub-acute administration of benzo(a)pyrene. Neurotoxicology 28, 630–636. [DOI] [PubMed] [Google Scholar]
- Guilarte T. R., McGlothan J. L. (1998). Hippocampal NMDA receptor mRNA undergoes subunit specific changes during developmental lead exposure. Brain Res. 790, 98–107. [DOI] [PubMed] [Google Scholar]
- Guzowski J. F., Lyford G. L., Stevenson G. D., Houston F. P., McGaugh J. L., Worley P. F., Barnes C. A. (2000). Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J. Neurosci. 20, 3993–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattemer-Frey H. A., Travis C. C. (1991). Benzo-a-pyrene: environmental partitioning and human exposure. Toxicol. Ind. Health 7, 141–157. [DOI] [PubMed] [Google Scholar]
- Hodos W. (1961). Progressive ratio as a measure of reward strength. Science 134, 943–944. [DOI] [PubMed] [Google Scholar]
- Hood D. B., Nayyar T., Ramesh A., Greenwood M., Inyang F. (2000). Modulation in the developmental expression profile of Sp1 subsequent to transplacental exposure of fetal rats to desorbed benzo[a]pyrene following maternal inhalation. Inhal. Toxicol. 12, 511–535. [DOI] [PubMed] [Google Scholar]
- Hood D. B., Woods L., Brown L., Johnson S., Ebner F. F. (2006). Gestational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure effects on sensory cortex function. Neurotoxicology 27, 1032–1042. [DOI] [PubMed] [Google Scholar]
- Jacobson J. L., Jacobson S. W. (1997). Evidence for PCBs as neurodevelopmental toxicants in humans. Neurotoxicology 18, 415–424. [PubMed] [Google Scholar]
- Kheramin S., Body S., Herrera F. M., Bradshaw C. M., Szabadi E., Deakin J. F., Anderson I. M. (2005). The effect of orbital prefrontal cortex lesions on performance on a progressive ratio schedule: implications for models of inter-temporal choice. Behav. Brain Res. 156, 145–152. [DOI] [PubMed] [Google Scholar]
- Kihlstrom I. (1986). Placental transfer of benzo(a)pyrene and its hydrophilic metabolites in the guinea pig. Acta Pharmacologica et Toxicologica 58, 272–276. [DOI] [PubMed] [Google Scholar]
- Koger S. M., Schettler T., Weiss B. (2005). Environmental toxicants and developmental disabilities: a challenge for psychologists. Am. Psychol. 60, 243–255. [DOI] [PubMed] [Google Scholar]
- Koya E., Spijker S., Voorn P., Binnekade R., Schmidt E. D., Schoffelmeer A. N., De Vries T. J., Smit A. B. (2006). Enhanced cortical and accumbal molecular reactivity associated with conditioned heroin, but not sucrose-seeking behaviour. J. Neurochem. 98, 905–915. [DOI] [PubMed] [Google Scholar]
- Kulig B., Alleva E., Bignami G., Cohn J., Cory-Slechta D., Landa V., O'Donoghue J., Peakall D. (1996). Animal behavioral methods in neurotoxicity assessment: SGOMSEC joint report. Environ. Health Perspect. 104(Suppl 2), 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapole D., Rychen G., Grova N., Monteau F., Le Bizec B., Feidt C. (2007). Milk and urine excretion of polycyclic aromatic hydrocarbons and their hydroxylated metabolites after a single oral administration in ruminants. J. Dairy Sci. 90, 2624–2629. [DOI] [PubMed] [Google Scholar]
- Li Z., Chadalapaka G., Ramesh A., Khoshbouei H., Maguire M., Safe S., Rhoades R. E., Clark R., Jules G., McCallister M., et al. (2012). PAH particles perturb prenatal processes and phenotypes: protection from deficits in object discrimination afforded by dampening of brain oxidoreductase following in utero exposure to inhaled benzo(a)pyrene. Toxicol. Sci. 125, 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox S. A., Schafe G. E. (2011). The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for reconsolidation of a Pavlovian fear memory. J. Neurosci. 31, 7073–7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallister M. M., Maguire M., Ramesh A., Aimin Q., Liu S., Khoshbouei H., Aschner M., Ebner F. F., Hood D. B. (2008). Prenatal exposure to benzo(a)pyrene impairs later-life cortical neuronal function. Neurotoxicology 29, 846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobini S., Chiang T. J., Ho M. Y., Bradshaw C. M., Szabadi E. (2000). Comparison of the effects of clozapine, haloperidol, chlorpromazine and d-amphetamine on performance on a time-constrained progressive ratio schedule and on locomotor behaviour in the rat. Psychopharmacology 152, 47–54. [DOI] [PubMed] [Google Scholar]
- Newland M. C. (1995). Motor function and the physical properties of the operant: applications to screening and advanced techniques. In Neurotoxicology (Slikker W. J., Chang L. W., Eds.), pp. 265–299. Academic Press, San Diego. [Google Scholar]
- Newland M. C., Hoffman D. J., Heath J. C., Donlin W. D. (2013). Response inhibition is impaired by developmental methylmercury exposure: acquisition of low-rate lever-pressing. Behav. Brain Res. 253, 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland M. C., Reed M. N., Rasmussen E. (2015). A hypothesis about how early developmental methylmercury exposure disrupts behavior in adulthood. Behav. Process. 114, 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihei M. K., Guilarte T. R. (1999). NMDAR-2A subunit protein expression is reduced in the hippocampus of rats exposed to Pb2+ during development. Brain Res. Mol. Brain Res. 66, 42–49. [DOI] [PubMed] [Google Scholar]
- Paletz E. M., Day J. J., Craig-Schmidt M. C., Newland M. C. (2007). Spatial and visual discrimination reversals in adult and geriatric rats exposed during gestation to methylmercury and n − 3 polyunsaturated fatty acids. Neurotoxicology 28, 707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P., Bellone C., Zhou Q. (2013). NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 14, 383–400. [DOI] [PubMed] [Google Scholar]
- Perera F. P., Li Z., Whyatt R., Hoepner L., Wang S., Camann D., Rauh V. (2009). Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics 124, e195–e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F. P., Wang S., Vishnevetsky J., Zhang B., Cole K. J., Tang D., Rauh V., Phillips D. H. (2011). Polycyclic aromatic hydrocarbons-aromatic DNA adducts in cord blood and behavior scores in New York city children. Environ. Health Perspect. 119, 1176–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. H. (1999). Polycyclic aromatic hydrocarbons in the diet. Mut. Res. 443, 139–147. [DOI] [PubMed] [Google Scholar]
- Ploski J. E., Pierre V. J., Smucny J., Park K., Monsey M. S., Overeem K. A., Schafe G. E. (2008). The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J. Neurosci. 28, 12383–12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh A., Inyang F., Hood D. B., Archibong A. E., Knuckles M. E., Nyanda A. M. (2001). Metabolism, bioavailability, and toxicokinetics of benzo(alpha)pyrene in F-344 rats following oral administration. Exp. Toxicol. Pathol.53, 275–290. [DOI] [PubMed] [Google Scholar]
- Ramesh A., Walker S. A., Hood D. B., Guillen M. D., Schneider K., Weyand E. H. (2004). Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int. J. Toxicol. 23, 301–333. [DOI] [PubMed] [Google Scholar]
- Reed G. A., Jones B. C. (1996). Enhancement of benzo[a]pyrene diol epoxide mutagenicity by sulfite in a mammalian test system. Carcinogenesis 17, 1063–1068. [DOI] [PubMed] [Google Scholar]
- Reed M. N., Paletz E. M., Newland M. C. (2006). Gestational exposure to methylmercury and selenium: effects on a spatial discrimination reversal in adulthood. Neurotoxicology 27, 721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rema V., Armstrong-James M., Ebner F. F. (1998). Experience-dependent plasticity of adult rat S1 cortex requires local NMDA receptor activation. J. Neurosci.18, 10196–10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T. W. (1996). Dissociating executive functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 351, 1463–1470; discussion 1470–1471. [DOI] [PubMed] [Google Scholar]
- Rosi S., Ramirez-Amaya V., Vazdarjanova A., Worley P. F., Barnes C. A., Wenk G. L. (2005). Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression. J. Neurosci. 25, 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal M. K., Li Y. L. (2007). Deleterious effects of polynuclear aromatic hydrocarbon on blood vascular system of the rat fetus. Birth Defects Res. B Dev. Reprod. Toxicol. 80, 367–373. [DOI] [PubMed] [Google Scholar]
- Schantz S. L., Bowman R. E. (1989). Learning in monkeys exposed perinatally to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Neurotoxicol. Teratol. 11, 13–19. [DOI] [PubMed] [Google Scholar]
- Schiltz C. A., Bremer Q. Z., Landry C. F., Kelley A. E. (2007). Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression. BMC Biology 5, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz C. A., Kelley A. E., Landry C. F. (2005). Contextual cues associated with nicotine administration increase arc mRNA expression in corticolimbic areas of the rat brain. Eur. J. Neurosci. 21, 1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W., Dayan P., Montague P. R. (1997). A neural substrate of prediction and reward. Science 275, 1593–1599. [DOI] [PubMed] [Google Scholar]
- Schultz W., Tremblay L., Hollerman J. R. (1998). Reward prediction in primate basal ganglia and frontal cortex. Neuropharmacology 37, 421–429. [DOI] [PubMed] [Google Scholar]
- Sheng L., Ding X., Ferguson M., McCallister M., Rhoades R., Maguire M., Ramesh A., Aschner M., Campbell D., Levitt P., et al. (2010). Prenatal polycyclic aromatic hydrocarbon exposure leads to behavioral deficits and downregulation of receptor tyrosine kinase, MET. Toxicolog. Sci.118, 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L. P. (2000). The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 24, 417–463. [DOI] [PubMed] [Google Scholar]
- Spear L. P. (2007). Assessment of adolescent neurotoxicity: rationale and methodological considerations. Neurotoxicol. Teratol. 29, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D., LeSage M. G., Glowa J. R. (1998). Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology 139, 169–184. [DOI] [PubMed] [Google Scholar]
- Tozuka Y., Watanabe N., Osawa M., Toriba A., Kizu R., Hayakawa K. (2004). Transfer of polycyclic aromatic hydrocarbons to fetuses and breast milk of rats exposed to diesel exhaust. J. Health Sci. 50, 497–502. [Google Scholar]
- Weiss B., Landrigan P. J. (2000). The developing brain and the environment: an introduction. Environ. Health Perspect. 108(Suppl 3), 373–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm J. J., Seo B. W., Strupp B. J., Seegal R. F., Schantz S. L. (2003). Effects of perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on spatial and visual reversal learning in rats. Neurotoxicol. Teratol. 25, 459–471. [DOI] [PubMed] [Google Scholar]
- Wormley D. D., Chirwa S., Nayyar T., Wu J., Johnson S., Brown L. A., Harris E., Hood D. B. (2004a). Inhaled benzo(a)pyrene impairs long-term potentiation in the F1 generation rat dentate gyrus. Cell Mol. Biol. (Noisy-le-grand) 50, 715–721. [PubMed] [Google Scholar]
- Wormley D. D., Ramesh A., Hood D. B. (2004b). Environmental contaminant-mixture effects on CNS development, plasticity, and behavior. Toxicol. Appl. Pharmacol. 197, 49–65. [DOI] [PubMed] [Google Scholar]
- Wu J., Ramesh A., Nayyar T., Hood D. B. (2003). Assessment of metabolites and AhR and CYP1A1 mRNA expression subsequent to prenatal exposure to inhaled benzo(a)pyrene. Int. J. Dev. Neurosci.21, 333–346. [DOI] [PubMed] [Google Scholar]
- Zanieri L., Galvan P., Checchini L., Cincinelli A., Lepri L., Donzelli G. P., Del Bubba M. (2007). Polycyclic aromatic hydrocarbons (PAHs) in human milk from Italian women: influence of cigarette smoking and residential area. Chemosphere 67, 1265–1274. [DOI] [PubMed] [Google Scholar]
- Zavala A. R., Osredkar T., Joyce J. N., Neisewander J. L. (2008). Upregulation of Arc mRNA expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking behavior. Synapse 62, 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]