Abstract
Patients with cirrhosis have an increased risk of developing liver cancer and a higher rate of mortality. Cirrhosis currently has no known cure, and patients may benefit from new agents aimed at alleviating their complications and slowing down the rate of disease progression. Therefore, the effects of the orally bioavailable synthetic triterpenoid 2-cyano-3,12-dioxooleana- 1,9(11)-dien-28-oate-ethyl amide (CDDO-EA, RTA 405), which has potent antioxidative and antiinflammatory properties, was evaluated in a chronic carbon tetrachloride (CCl4)-induced model of liver cirrhosis and hepatocellular carcinoma (HCC). Mice were injected with CCl4 (to induce fibrosis and cirrhosis) or placebo biweekly for 12 weeks followed by CDDO-EA in the diet for 18 weeks with continued biweekly injections of CCl4. Chronic CCl4 administration resulted in cirrhosis, ascites, and HCC formation, associated with increased serum transforming growth factor-β1, hepatic hydroxyproline content, and increased serum bilirubin. CDDO-EA, whose administration commenced after establishment of liver fibrosis, decreased liver fibrosis progression, serum bilirubin, ascites, and HCC formation and markedly increased overall survival. CDDO-EA also attenuated -TNFα (tumor necrosis factor-α), α-SMA (alpha smooth muscle actin), augmented -IL-10 levels, and improved histologic and serologic markers of fibrosis. Conclusions: CDDO-EA mitigates the progression of liver fibrosis induced by chronic CCl4 administration, which is associated with the induction of antifibrogenic genes and suppression of profibrogenic genes.
Keywords: liver fibrosis, carbon tetrachloride, RTA 405, triterpenoid, antioxidant, antiinflammatory, hepatocellular carcinoma, cirrhosis, CDDO-EA, and end stage liver disease
Cirrhosis, a major clinical issue both in health care cost and direct patient care (annual death rate of 27 000), is an important cause of liver-related deaths in the United States (Minino et al., 2006), and its incidence is on the rise (Lefton et al., 2009; Mehta and Rothstein, 2009). Cirrhosis results from a variety of insults that include infections (Hepatitis C Virus), autoimmune processes, metabolic changes (nonalcoholic steatohepatitis), drugs of abuse, and chronic alcohol consumption, which ultimately results in chronic liver injury (Arundel and Lewis, 2007; Lee and Seremba, 2008). To model human chronic liver injury, the prolonged administration of carbon tetrachloride (CCl4) is a well-established animal model system commonly used for investigating mechanisms involved in the induction of liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) (Kang et al., 2012; Sakata et al., 1987).
Liver fibrosis results from chronic injury and excessive accumulation of extracellular matrix (ECM) proteins. Advanced fibrotic scars, disrupting the hepatic architecture and the subsequent development of regenerating hepatocyte nodules, eventually lead to portal hypertension and liver failure. The pathogenesis of liver fibrosis is believed to depend on the activation of hepatic stellate cells (HSCs) that participate in the process of fibrotic scar formation through a complex interaction of matrix metalloproteinases (MMPs) and their inhibitors, tissue inhibitor of metalloproteinases (TIMPs). More importantly, HSCs participate in the production of collagen type I which is major component of fibrotic tissue formation (Friedman, 2008b). Besides HSC, other nonparenchymal and immune cells also contribute to this process by disrupting normal homeostasis between matrix production and degradation (Jarnagin et al., 1994). The relevance of the immune system to liver fibrosis has been demonstrated in several mouse models. One example shows that toxin induced liver injury activates natural killer T-cells (NK T-cells) leading to inhibition of liver regeneration via a tumor necrosis factor-α (TNFα) dependent mechanism (Wei et al., 2010). In addition, these activated NK T-cells target activated HSCs via retinoic acid early inducible 1/NKG2D-dependent and tumor necrosis factor–related apoptosis-inducing ligand–dependent mechanisms to limit the extent of fibrosis (Radaeva et al., 2006).
The search for novel antifibrotic drugs to combat this important clinical issue is ongoing, and the synthetic triterpenoid 2-cyano-3,12-dioxooleana- 1,9(11)-dien-28-oate-ethyl amide (CDDO-EA) is a potential candidate. CDDO-EA, CDDO-Me, and many related compounds, including CDDO-Im, activate the Keap1-Nrf2 pathway, which facilitates induction of an array of antioxidative enzymes and alleviation of inflammation (Reisman et al., 2009a). In addition to its antifibrotic-properties, its potential applicable efficacy in combating liver cancer may be related to its ability to enhance the expression of TGF-β-dependent genes (Sporn et al., 2011; Suh et al., 2003), induce apoptosis (Zou et al., 2007, 2008), and inhibit interleukins such as IL-6 through direct interaction with both STAT3 and IKK (Ahmad et al., 2008; Duan et al., 2009). Semisynthetic triterpenoids also inhibit constitutive STAT3 phosphorylation and block the degradation of IKB-α when challenged with TNFα (Shishodia et al., 2006). CDDO-Me, an analog of CDDO-EA, has been used in several different cancer models. In human leukemia cell lines, CDDO-Me inhibits both constitutive and inducible NF-κB (Shishodia et al., 2006), whereas in lung cancer models it suppresses IFN-γ action and induces heme oxygenase-1 (Liby et al., 2007).
Despite its multiple mechanisms of action, the potential preventive role of CDDO-EA in liver cirrhosis/fibrosis has not been evaluated. In this study, we sought to determine if the oral administration of CDDO-EA would alter the course of liver fibrosis. Mice were treated with CCl4 for 12 weeks and then administered CDDO-EA for 18 weeks (whereas CCl4 challenge continued), and end stage liver disease outcomes were assessed for reversibility of fibrosis sequelae. Our results indicate that CDDO-EA treatments ameliorate CCl4-induced liver injury and fibrosis. Moreover, early treatment with CDDO-EA in the course of this disease alters ascites and HCC formation and markedly reduces mortality.
MATERIALS AND METHODS
Animals, reagents used, and experimental design
CCl4 and all other chemicals used in these studies were from Sigma-Aldrich (St. Louis, Missouri), unless specified otherwise. Male mice between ages of 8 and 12 weeks were obtained from The Jackson Laboratory [(C57BL/6J mice) Bar Harbor, Maine]. All animal studies were carried out in compliance with accepted standards of humane animal care as described in the Guide for the Care and Use of Laboratory Animals (Council, 1996) and were approved by the UT Southwestern Institutional Animal Care and Use Committee. Mice were euthanized by CO2 inhalation consistent with the AVMA Guidelines on Euthanasia. Mice were injected intraperitoneally with CCl4 dissolved in corn oil in a 3:1 ratio (vol/vol) at a dose of 1–2 mg/kg body weight, or control animals received an equal volume of corn oil starting at week 0 and continued as defined in the different experimental protocols (Supplementary Figure 1). Briefly, CCl4 was administered biweekly for 30 weeks. CDDO-EA (also referred to as RTA 405, Reata Pharmaceuticals, Inc., Irving, TX) was then introduced in the ad libitum diet of the mice (200 mg/kg) at week 12 for a period of 18 weeks with continued biweekly injections of CCl4. The diet composition was as follows: 95% Purina diet 5002, 1.25% Ethanol, 3.75% Neobee Oil, 0.02% RTA 405, and 0.03% FD&C Red Dye#40. The placebo diet used in this study includes a vehicle without the dye (or RTA 405) with the difference made up with the Purina diet 5002. At the end of the experiment, animals were sacrificed at the indicated times after a 1-week washout to eliminate acute effects of CCl4 and assessed for fibrosis, HCC, and ascites formation. For the acute CCl4 toxicity experiments, animals were placed on CDDO-EA diet for 2 weeks prior to the administration of CCl4 as described above. Animals were sacrificed 8 h post CCl4 injection to assess the acute effects on CYP2E1 and alanine transaminase (ALT) levels.
Serum liver function assay
Following euthanasia, blood samples were collected for isolation of plasma for assessment of liver injury. Serum levels of ALT, aspartate transaminase, albumin, total bilirubin, creatinine, sodium, gamma-glutamyl transpeptidase (GGT), total protein, alkaline phosphatase, and blood urea nitrogen (BUN) were measured using Vitros-250 Chemistry Analyzer at the University of Texas-Southwestern (Dallas, Texas) core facility.
Immunoblotting
Western blotting was performed as previously described (Getachew et al., 2010). Immunodetection was performed using the Pierce ECL Western Blotting Substrate (Thermo Scientific, Rockford, Illinois). Anti-α-SMA (alpha smooth muscle actin) mouse monoclonal and anti-CYP 2E1 polyclonal rabbit primary antibodies were obtained from Abcam, Inc. (Cambridge, Massachusetts). Beta-actin and GAPDH purified antibody, HRP-conjugated anti-rabbit and HRP-conjugated anti-rabbit Ig secondary antibodies were obtained from Pierce Biotechnology (Thermo Scientific, Rockford, Illinois). Survivin and Nrf2 antibodies were purchased from Fisher Scientific (Chicago, Illinois).
Quantitative RT-PCR of liver mRNA
RNA isolation and real time PCR analysis has been previously described (Getachew, et al., 2010). Primer sequences used are listed in Supplementary Table 1, and unless otherwise indicated, all primers are of mouse origin.
Evaluation of ascites and HCC
The volume of ascites was estimated at the time the mice were euthanized. After laparotomy, the abdominal fluid was drained by paper strips that were then weighed before and after placement in the abdominal cavity for absorbing the ascites fluid. Livers were removed and grossly visible lesions on the surface of the liver were counted and sized. Numbers of tumors and their size and histopathology were evaluated in a nonblinded fashion. The livers were then sectioned beginning at the medial hilar surface, and stained with hematoxylin & eosin (H&E) per standard histologic techniques.
Histopathological examination
H&E, Masson's trichrome and Sirius red staining protocols have been previously described (Dooley et al., 2003). To evaluate the relative fibrosis area, the measured collagen area was divided by the net field area and then multiplied by 100. From each animal analyzed, the amount of fibrosis as a percentage was measured and the average value presented using the Sirius red method.
Hepatic hydroxyproline content
Hydroxyproline content was assessed as previously described (Kliment et al., 2011). Following homogenization in 6 N hydrochloric acid (HCl), liver tissues underwent hydrolysis at 110°C for a period of 18 h. After cooling of the hydrolysate, it was treated with activated charcoal, filtered, evaporated, and resuspended in distilled water at which point chloramine T was added. Five minutes later, Ehrlich’s reagent was added to the mixture, followed by incubation for a period of 30 min at 60°C. Using a reagent blank containing the complete system without added tissue, samples were read on a spectrophotometer at 560 nm. The hepatic hydroxyproline content was expressed as micrograms per gram of tissue (dry weight).
Cell line experimental culture conditions
Primary human HSCs (HHSCs, Cat# 5300) (ScienCell Research Laboratories) from a frozen stock were purchased and cultured in stellate cell medium (SteCM, Cat# 5301) at density of 5 × 105 per 1 ml volume according to manufacturer’s instruction. CDDO-EA was dissolved in dimethyl sulfoxide at a concentration of 10 mM, and aliquots were stored at −80°C. The stock solution was diluted to the desired final concentrations with growth medium just prior to use. Escherichia coli LPS (100 ng/ml; Sigma-Aldrich Co., St. Louis, Missouri) was used 6 h daily to activate HHSC cells for 3 days followed by media change. RNA and protein extraction were performed at day 3 post induction. Plating cells under normal conditions for 5 days with daily medium change achieved culture activation without the addition of LPS. A rat hepatoma cell line (FAO) was cultured in Ham’s F12 Media with 15%–18% fetal bovine serum, and supplemented with 1% penicillin/streptomycin (10 000 units/ml/10 000 µg/ml), 5% L-glutamate (29 mg/ml), and 5% sodium pyruvate (11 mg/ml) [Sigma-Aldrich] was used in the cell based experiments.
Isolation of mouse hepatic stellate cells
Mouse HSCs (MHSCs) were isolated using the collagenase-pronase perfusion protocol that has been previously described (Bataller et al., 2003). Briefly, mice were anesthetized and the portal vein was accessed with an 18G catheter. Perfusion was performed as described by Bataller et al. (2003) with perfusate containing pronase E, collagenase D, and DNase in sequential manner for total of 45 min. The liver was then removed and incubated for 15 min in a solution containing DNase and collagenase D and afterwards filtered through a sterile nylon 120 µm mesh. Subsequent cell washes and suspension was performed in a solution containing Accudenz solution to achieve a gradient white layer isolate that contains HSCs. Cell sorting and purification were performed using a CyAnTM LX flow cytometer (Dako Cytomation, Carpintaria, California) and FlowJo software (TreeStar, Ashland, Oregon). Cell gating was performed on singlets, excluding doublets, after depletion of Kupffer cells using anti-F4/80 (BD Biosciences) and anti-CD11b (BD Biosciences). MHSC activation was achieved as described above using chronic CCl4 administration over 30 weeks. Cells that were isolated were stained for collagen-1α1 and DAPI.
Statistical evaluation
Data are presented as mean ± standard error (SE). To detect differences among groups, statistical significance was evaluated by t test (for comparison of 2 groups) or 1-way ANOVA (followed by a posttest using Bonferroni for comparison of multiple groups; Graph Pad Prism Version 5 [Graph Pad Software, San Diego, California]); differences at the level of *P < .05, **P < .01, and ***P < .001 are noted separately.
RESULTS
CDDO-EA Reduces Liver Fibrosis Progression
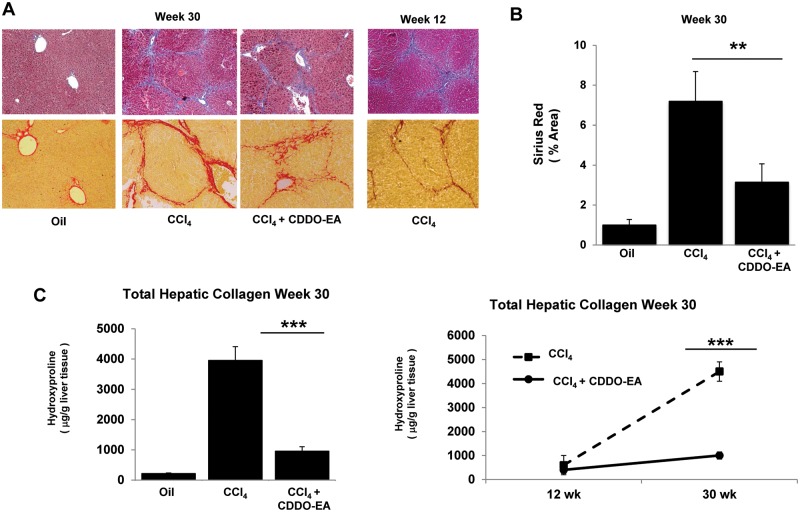
To characterize the importance of CDDO-EA in reducing liver fibrosis in the course of CCl4-induced liver injury, wild type (C57BL/6J) mice were treated with 2 mg/kg of CCl4 over a course of 30 weeks. Administration of CCl4 resulted in marked fibrosis as determined by Masson's trichrome and Sirius red stains as depicted in Figure 1A. All control (oil treated) mice revealed a normal distribution of collagen, while extensive collagen deposition and nodule formation was evident in liver tissue from CCl4 only treated groups. Sirius red staining demonstrated that CDDO-EA administration was associated with a marked decrease in the amount of fibrotic tissue compared with control groups with CCl4-induced liver injury (Figure 1A), which was significant upon quantification of the area of the injury (Figure 1B). Further, analysis of hydroxyproline content was consistent with the histological findings and demonstrated that CDDO-EA almost completely inhibited accumulation of hepatic collagen induced by chronic CCl4 administration (Figure 1C).
FIG. 1.
CCl4-treated mice exhibit decreased level of fibrosis after CDDO-EA rescue treatment. A, Liver histology was assessed with trichrome and Sirius red stain. Data show a representative slide in all groups (magnification at 20×). Slides depict time from week 30 (left 3 panels) as well as week 12 (right panel). The amount of Sirius red stain collagen is shown in (B) as a histogram. C, Total hepatic collagen content is shown as hydroxyproline content. Data represent the mean ± SE of all mice (N = 10 per group). *P < .05, **P < .01, ***P < .001.
CDDO-EA Reduces End Stage Liver Disease Complications and Improves Overall Survival
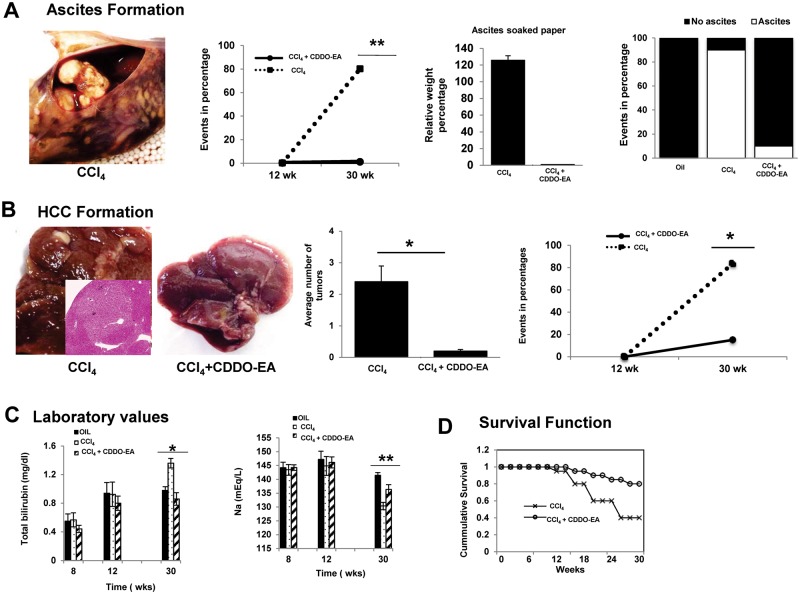
CDDO-EA treatment resulted in an almost complete reduction to naïve control levels in complications of end stage liver disease including overall mortality. Ascites, spleen size, and HCC formation were evaluated. The amount of ascites formation was noted to be higher in CCl4-treated mice compared to CCl4 plus CDDO-EA-treated groups (Figure 2A). Spleen sizes were similar in both groups of mice (data not shown). As shown in the Supplementary Table 2, other liver tests did not have statistically different values. To investigate whether CDDO-EA reduces HCC formation, livers of mice were visually inspected at experimental termination for the formation of tumor nodules. As depicted in Figure 2B, higher numbers of HCCs were noted in CCl4-treated mice, and CDDO-EA treatment resulted in a marked reduction in the absolute numbers of HCC formed. CCl4 administration produced tumors that were 3 mm to 1.5 cm in size and were either single or multiple nodules with well-differentiated HCC histological characteristics but remarkably, only a single 5 mm tumor was noted in the CCl4 + CDDO-EA treated cohort. Assessment of liver function tests showed CDDO-EA treated groups had near normal levels of total bilirubin and serum sodium, whereas the CCl4 treated only group had decreased serum sodium and elevated total bilirubin (Figure 2C). Further, assessment of overall mortality demonstrated CDDO-EA-treated mice had greater than 80% survival, while the CCl4 only treated group had only a 30% survival rate after 30 weeks (Figure 2D).
FIG. 2.
CDDO-EA treatment reduces complication of end stage liver disease and improves mortality. A, Representative ascites photograph at week 30 in CCl4 treated group. Longitudinal graph shows percentage of ascites formation. Histogram shows relative weight of soaked ascites paper (middle panel) whereas right panel depicts event noted per group for the formation of ascites. B. Representative photograph of gross livers from mice at week 30 post treatment of CCl4 plus CDDO-EA. Histogram depicts average number of tumors identified per group. Data are presented as mean ± SE (n = 10 per group). Longitudinal graph shows percentage of HCC formation after CCl4 treatment for 12 weeks followed by 18 more weeks of CDDO-EA. C. Liver function test over time in CDDO-EA treated versus nontreated. Data represent mean ± SE (n = 20 per time point). D, CCl4 treated mice had > 70% mortality after 30 weeks while addition of CDDO-EA at week 12 had < 20% mortality during the same period of time. (N = 40 per group). *P < .05, **P < .01, ***P < .001.
CDDO-EA Reduces CCl4-Induced Fibrosis Without Altering CYP2E1 Levels
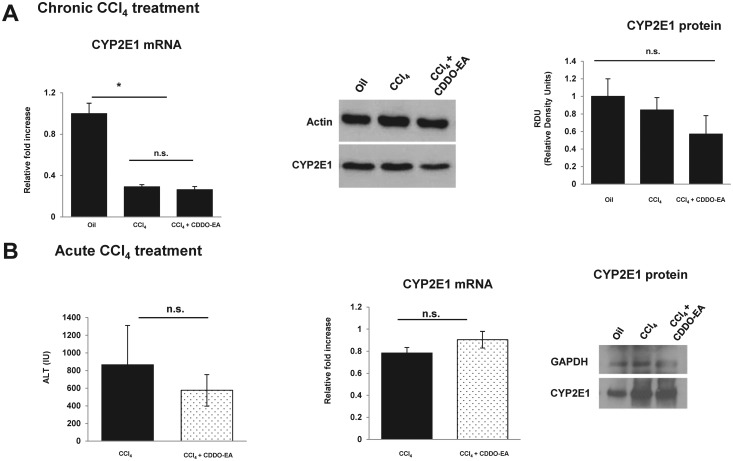
CYP2E1 mRNA and protein expression were evaluated by real time PCR and Western blot analysis, respectively. As depicted in Figure 3A, mRNA expression of CYP2E1 was decreased in the CCl4 group and CDDO-EA + CCl4 groups. The addition of CDDO-EA did not alter either mRNA or protein expression of CYP2E1. FAO cell lines were used to test the ability of CDDO-EA to induce CYP2E1 but no statistically significant elevation of either mRNA and protein expression was observed (Data provided in Supplementary section and Supplementary Figure 2). In addition, to assess the impact of CCl4 on expression of CYP2E1, CCl4 was administered in acute setting and mRNA and Western blot was assessed. As depicted in Figure 3B no difference was noted but ALT measurements showed a trend toward reduction in CDDO-EA group.
FIG. 3.
CDDO-EA reduces CCl4-induced fibrosis without altering CYP2E1 levels compared with CCl4 treatment. A, mRNA and protein expression expression of CYP2E1 was assessed at 30 weeks post CCl4 treatment versus CCl4 + CDDO-EA. Histogram depicts relative density units of protein expression. B, Acute effect of CCl4 was assessed with measurement of ALT, mRNA and protein expression expression of CYP2E1. Protein expression was assessed via Western blot analysis. Data are presented as mean ± SE (n = 10 per group). Each experiment was performed in duplicate. *P < .05, **P < .01, ***P < .001.
CDDO-EA Attenuates Fibrosis Correlating with Altered Levels of Proinflammatory and Antiinflammatory Cytokines
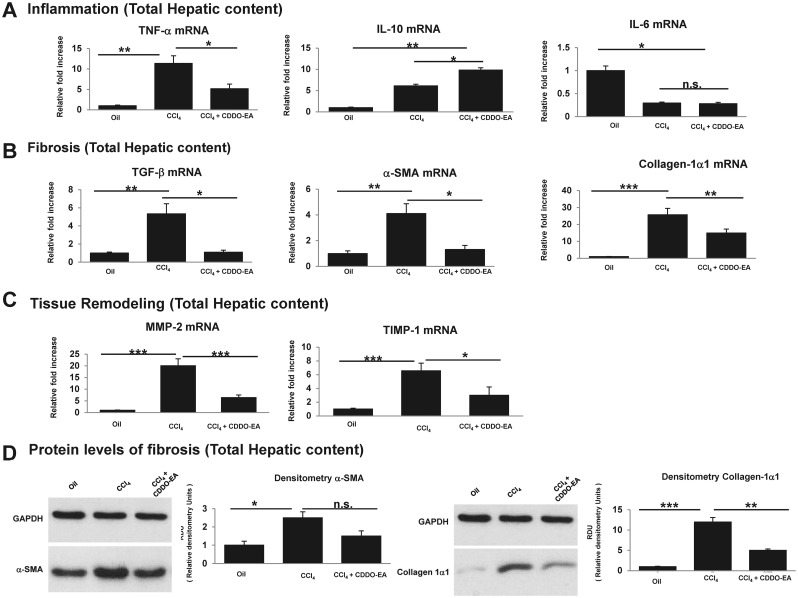
Synthetic triterpenoids amplify expression of antiinflammatory cytokines and attenuate expression of proinflammatory cytokines (Deeb et al., 2010; Duan et al. 2009; Sporn et al. 2011; Suh et al. 2003). The livers of CCl4-treated and CDDO-EA-treated mice were assessed for levels of various proinflammatory and antiinflammatory cytokines. Proinflammatory cytokine mRNA encoding for TNFα were up regulated in CCl4-treated groups, and decreased with CDDO-EA treatment. IL-6 mRNA levels were reduced in all groups of CCl4-treated mice. IL-10, in contrast, was markedly elevated in all groups of CCl4-treated mice and was further upregulated by the administration of CDDO-EA (Figure 4A). No differences were noted in other cytokines, including IL-1β, IL-18 and IL-4 (data not shown). As a main positive regulator of fibrosis, TGF-β was significantly reduced in CDDO-EA-treated mice. In parallel, production of collagen-1α1 and α-SMA was noted to be reduced in CDDO-EA treated group (Figs. 4B and D). Also depicted in Figure 4C, analysis of MMP2 and TIMP1, which are implicated in tissue remodeling, showed down-regulation associated with CDDO-EA treatment.
FIG. 4.
CDDO-EA attenuates fibrosis by altering expressions of proinflammatory and antiinflammatory mediators. A, mRNA expression of proinflammatory and antiinflammatory cytokines. B, mRNA expression of profibrogenesis cytokines was assessed which includes TGF-β, collagen-1α1, and α-SMA. C, mRNA expression of tissue remodeling cytokines was assessed at baseline and week 30 post-CCl4 treatment in the presence of CDDO-EA versus control. Data are presented as mean ± SE (n = 10 per group). D, A representative Western blot showing α-SMA and collagen-1α1 with relative amount of protein expression determined by densitometry were measured at week 30 after CCl4 versus CCl4 for 12 week followed by 18 weeks of CDDO-EA + CCl4. Data are presented as mean ± SE of all mice (N = 10 per group). Each figure legend depicts an independently performed 2 experiments. *P < .05, **P < .01, ***P < .001.
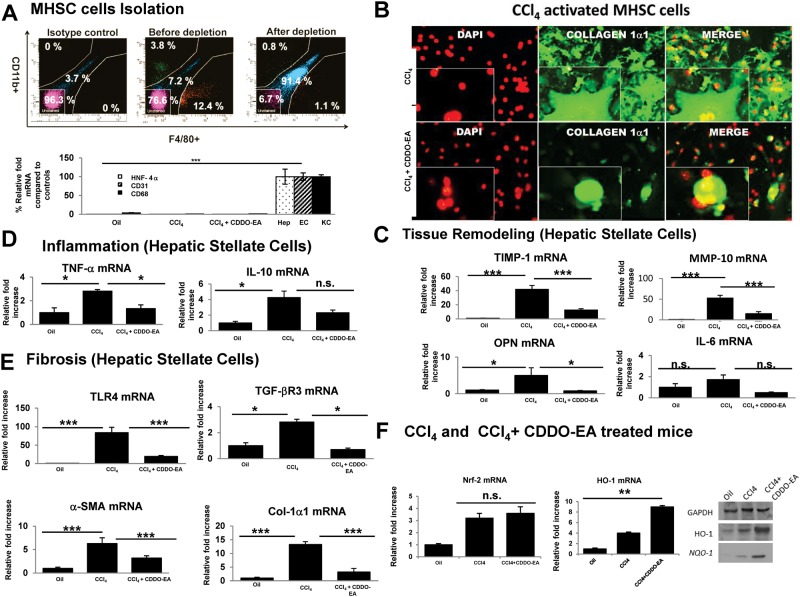
CDDO-EA Attenuates In Vivo MHSC Profibrogenic Cytokines
To assess the role of CDDO-EA on MHSC, MHSC cells were isolated and analyzed by quantitative real-time PCR, Western blot, flow cytometry, and cell staining. The purity of MHSC was achieved by depleting macrophages and B-cells as shown in Figure 5A. CDDO-EA treatment resulted in attenuation of MHSC activation as evidenced by immunohistochemistry stain in Figure 5B and mRNA cytokine expression of inflammatory and fibrotic mediators (Figs. 5C–E). Further analysis of these groups showed that downstream targets of Nrf2 gene were up regulated in CDDO-EA treated mice (Figure 5F).
FIG. 5.
Mouse hepatic stellate cells (MHSC) expresses profibrogenic genes with CCl4 administration that is attenuated by CDDO-EA. A, MHSC cells were isolated and purified. Flow as well as mRNA data show isolation purity of MHSC cells after depletion. B, MHSC cells isolated were stained with collagen-1a1 to assess degree of activation. Pictogram shows a representative figure. C, mRNA expression in MHSCs of tissue remodeling cytokines. D, mRNA expression in MHSCs of inflammatory cytokines. E, mRNA expression of fibrogenetic cytokines were measured at week 30 after CCl4 versus CCl4 for 12 week followed by 18 weeks of CDDO-EA+ CCl4. (F) mRNA expression in MHSCs for Nrf-2 and HO-1 as well as protein levels of HO-1 and Nqo-1 were measured. Data are presented as mean ± SE of all mice (N = 10 per group). Each figure legend depicts an independently performed 2 experiments. *P < .05, **P < .01, *** P < .001.
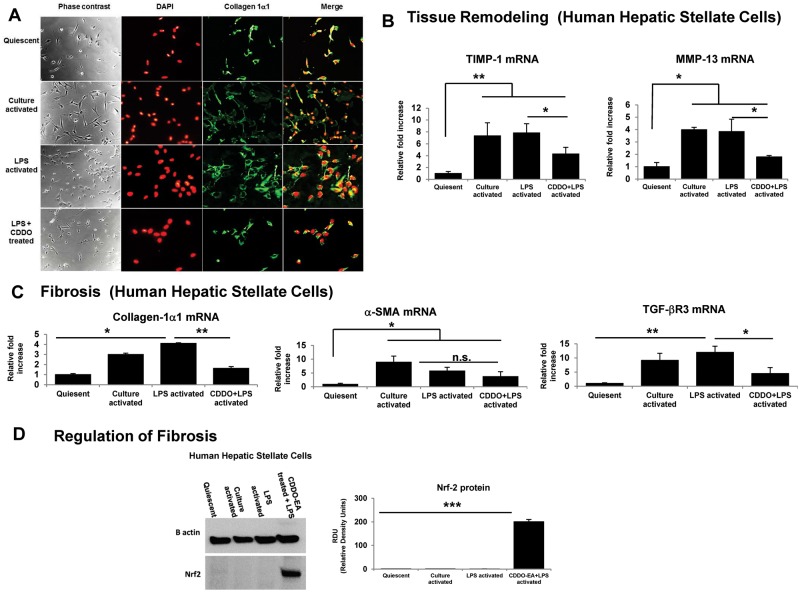
CDDO-EA Treatment Attenuates LPS Activation of HHSC Cells In Vitro
To assess the role of CDDO-EA on HHSC activation, HHSC were treated in vitro as detailed in the methods section. Cells that were treated with CDDO-EA showed lower level of markers of stellate cell activation (Figure 6A) and a reduction in markers of proinflammatory cytokines (Figs. 6B and C). In addition, HHSCs that were treated with CDDO-EA showed a significant elevation of Nrf2 protein levels (Figure 6D) when compared with the LPS treated group and controls. However, CDDO-EA treatment did not result in an alteration of levels of survivin, another known regulator of antiapoptotic pathway (Data shown in Supplementary Figure 3).
FIG. 6.
CDDO-EA treatment attenuates LPS induced HHSC cells activation in vitro. A, A representative immunohistochemistry pictogram showing culture activation, LPS activation and CDDO-EA + LPS activation. B, mRNA expression of HHSC tissue remodeling cytokine (C) mRNA expression of HHSC fibrogenic cytokine (D) protein expression of potential effectors of CDDO-EA mechanism of action. Data are presented as mean ± SE of the 4 different groups tested. (N = 3 per group). Each figure legend depicts 2 independently performed experiments. *P < .05, **P < .01, ***P < .001.
DISCUSSION
The role of ECM in advancing liver fibrosis in response to chronic injury is already documented (Friedman, 2008b). During liver injury, quiescent HSCs become activated and acquire a myofibroblast-like phenotype (Friedman, 2008a) with the excess production of collagen-1α1 and α-SMA and reduced ECM degradation. The perpetuation of this proinflammatory and profibrogenic background, mediated by the interplay of paracrine and autocrine molecules, ultimately replaces the normal liver architecture with fibrotic tissue and regenerative parenchymal nodules (Friedman, 2008b).
This study demonstrates that CDDO-EA, an orally available antioxidant/antiinflammatory synthetic small molecule, treatment leads to decreased liver fibrosis. CDDO-EA attenuates the chronic liver fibrosis and continual remodeling and correlates with decreased levels of expression of α-SMA protein, suggesting CDDO-EA suppresses the activation of HSCs. This is further supported by the significant amount of fibrosis reduction depicted with Sirius red staining and the halting of fibrosis progression when CDDO-EA was given to the mice starting after week 12 of CCl4 treatment (Figure 1C). We also demonstrated that the administration of CDDO-EA reduces the incidence and accumulation of ascites (Figure 2A). In mice that received CDDO-EA at an early fibrosis stage, ascites formation was abrogated.
We also evaluated the potential effects of CDDO-EA on HCC incidence and formation. In agreement with previous observations (Fujii et al., 2010), administration of CCl4 resulted in increased HCC formation. In this study, we were able to demonstrate that treatment of CDDO-EA at the early stage of advanced fibrosis resulted in a marked reduction in the formation of HCC (Figure 3C). The antitumorigenic properties of CDDO-EA and its analogs have been evaluated and validated in a variety of cancer models including prostate, pancreatic cancer, and colorectal cancer cells. In these cancer models, synthetic triterpenoid compounds attenuate ROS generation and inhibit Akt, NF-κB and mTOR signaling proteins (Deeb et al., 2010; Gao et al., 2010), as well as directly interact with STAT3 and IKK to decrease constitutive IL-6 secretions (Liby et al., 2010). Suppression of IL-6 (Figure 5A) in MHSCs, STAT3, and NF-κB levels (data not shown) following CDDO-EA administration was also observed and consistent with a previous finding.(Shishodia et al., 2006) Also Inami et al reported that HCC formation is enhanced via Nrf2 pathway through p62 activation (Inami et al., 2011). CDDO-EA has multiple mechanisms that include activation of Nrf2, which in a controlled manner plays an important antiinflammatory role. Our findings suggest that the smaller number of HCCs observed in CDDO-EA-treated mice may be due to mechanisms that either inhibits de novo tumor formation and/or regression of tumors that were formed. In contrast, in a constitutive activation state Nrf2 can participate in the development of HCC. Our current findings suggest that other factors such as the degree of fibrosis and MMP-2 interaction with the ECM may also play a pivotal role in altering the fate of HCC formation in the liver.
This study also demonstrates that administration of CDDO-EA to mice with CCl4-induce liver fibrosis is associated with the attenuation of several profibrogenic and proinflammatory mediators, as well as increases in some antiinflammatory mediators in chronic liver injury. CDDO-EA decreased the expression of proinflammatory cytokines including TNF-α and Osteopontin (OPN). IL-10, a major antiinflammatory cytokine, which reduces expression of TGF-β, TNFα, and TIMP-1 (Zhang et al., 2006), was upregulated by CDDO-EA (Figure 4A), which is consistent with previous finding (Kang et al., 2012).
The hepatoprotection related to CDDO-EA is also associated with changes in the levels of MMP-2 and TIMP-1 mRNA expression (Figure 4C), suggesting enhanced fibrolytic activity. Additionally, MMP-2 enhanced activity has been associated with cancer progression and metastatic invasion via type IV collagen degradation (Mook et al., 2004) and by proteolytically activating TGF-β (Gialeli et al., 2011). The levels of TGF-β, a known potent mitogen that activates quiescent HSC to myofibroblast-like cells (Friedman, 2008a), is suppressed in the CDDO-EA-treated groups (Figure 4B). Potential mechanisms of action for the effect of CDDO-EA on TGF-β expression include downregulation of SOCS-3 (data not shown), a known activator of TGF-β transcription (Niwa et al., 2005; Ogata et al., 2006), and also attenuation of TLR-4 levels (Figure 5E). TLR-4 has been shown to be a major regulator of TGF-β expression (Seki et al., 2007).
Chronic CCl4 treatment resulted in reduction of CYP2E1 mRNA levels but further reduction by the administration of CDDO-EA post 12 weeks of CCl4 treatment was not observed in these experiments (Figure 3A). In addition, treatment of FAO cell line treatment with CDDO-EA did not result in the reduction of CYP2E1 mRNA expression (Supplementary Figure 2). Thus, the protection that is observed by the treatment of CDDO-EA is unlikely to be related to differences in the formation of trichloromethyl free radicals (•CCl3), the electrophilic metabolite of CCl4 that is implicated in the mechanism of CCl4-induced liver injury. This was further confirmed by the treatment of mice that were on CDDO-EA diet 2 week prior to treatment of CCl4 administration. Results as shown in Figure 3B show no alteration of CYP2E1 mRNA and protein level. ALT level analysis show a trend toward a reduced level in CDDO-EA group but that could also be related to the antiinflammatory activity of CDDO-EA as seen in other agents such as acetaminophen (APAP) toxicity (Reisman et al., 2009).
In addition, CDDO-EA resulted in the decreased level of activation of HSC as noted in Figures 5B and D. This was further supported by in vitro model of HHSC cells where CDDO-EA treatment resulted in decreased activation of these cells (Figure 6A). The potential mechanism for the decreased activation may have been related to the up regulation of Nrf2 (Figure 6B). The ability of CDDO-EA and related analogs to activate Nrf2 and induce its antioxidant target genes is a well-established mode of action for synthetic triterpenoids (Reisman et al., 2009a). Nrf2 plays a major role in dampening inflammatory pathways likely contributing to the down regulation of proinflammatory cytokines. Others have shown that survivin, a known antiapoptotic gene plays a major role in protection against CCl4 injury (Li et al., 2010). However, analysis in our study showed no difference in the level of this mediator (Supplementary Figure 3), suggesting its minimal role in the protection that CDDO-EA affords in this model.
In summary, this study demonstrates an important role for CDDO-EA in the early stages of advanced fibrosis in a chronic CCl4-induced liver injury mouse model. Moreover, CDDO-EA improved other sequelae of end stage liver cirrhosis and mortality. The multiple modes of action and diverse pharmacology of CDDO-EA may achieve the results in these studies by attenuation of proinflammatory mechanisms, as well as augmenting antioxidative, antiinflammatory, and anticarcinogenic pathways.
Supplementary Material
ACKNOWLEDGMNTS
The authors thank Dr. Donald L. Rockey (MUSC) for sharing his CCl4 animal protocol. Scott Reisman is an employee of Reata Pharmaceuticals, and Jerry Shay is a scientific advisor to Reata Pharmaceuticals.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
NIH 1K08DK085432 and the Jan and Henri Bromberg Chair in Internal Medicine (Y.G.). The Southland Financial Corporation Distinguished Chair in Geriatric Research (J.W.S.). Part of this work was performed in laboratories constructed with support from National Institute of Health grant C06 RR30414. Reata Pharmaceutical Inc., Irving TX provided CDDO-EA.
REFERENCES
- Ahmad R., Raina D., Meyer C., Kufe D. (2008). Triterpenoid CDDO-methyl ester inhibits the Janus-activated kinase-1 (JAK1)–>signal transducer and activator of transcription-3 (STAT3) pathway by direct inhibition of JAK1 and STAT3. Cancer Res. 68, 2920–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arundel C., Lewis J. H. (2007). Drug-induced liver disease in 2006. Curr. Opin. Gastroenterol. 23, 244–254. [DOI] [PubMed] [Google Scholar]
- Bataller R., Schwabe R. F., Choi Y. H., Yang L., Paik Y. H., Lindquist J., Qian T., Schoonhoven R., Hagedorn C. H., Lemasters J. J., et al. (2003). NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J. Clin. Invest. 112, 272–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council N. R. (National Research Council of the National Academics) (1996). Guide for the Care and Use of Laboratory Animals. National Academy Press, Washington. [Google Scholar]
- Deeb D., Gao X., Jiang H., Janic B., Arbab A. S., Rojanasakul Y., Dulchavsky S. A., Gautam S. C. (2010). Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells through a ROS-dependent mechanism. Biochem. Pharmacol. 79, 350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley S., Hamzavi J., Breitkopf K., Wiercinska E., Said H. M., Lorenzen J., Ten Dijke P., Gressner A. M. (2003). Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology 125, 178–191. [DOI] [PubMed] [Google Scholar]
- Duan Z., Ames R. Y., Ryan M., Hornicek F. J., Mankin H., Seiden M. V. (2009). CDDO-Me, a synthetic triterpenoid, inhibits expression of IL-6 and Stat3 phosphorylation in multi-drug resistant ovarian cancer cells. Cancer Chemother. Pharmacol. 63, 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. L. (2008a). Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 88, 125–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. L. (2008b). Mechanisms of hepatic fibrogenesis. Gastroenterology 134, 1655–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Fuchs B. C., Yamada S., Lauwers G. Y., Kulu Y., Goodwin J. M., Lanuti M., Tanabe K. K. (2010). Mouse model of carbon tetrachloride induced liver fibrosis: Histopathological changes and expression of CD133 and epidermal growth factor. BMC Gastroenterol. 10, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Deeb D., Hao J., Liu Y., Arbab A. S., Dulchavsky S. A., Gautam S. C. (2010). Synthetic triterpenoids inhibit growth, induce apoptosis and suppress pro-survival Akt, mTOR and NF-(Ahmad et al.)B signaling proteins in colorectal cancer cells. Anticancer Res. 30, 785–792. [PMC free article] [PubMed] [Google Scholar]
- Getachew Y., James L., Lee W. M., Thiele D. L., Miller B. C. (2010). Susceptibility to acetaminophen (APAP) toxicity unexpectedly is decreased during acute viral hepatitis in mice. Biochem. Pharmacol. 79, 1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gialeli C., Theocharis A. D., Karamanos N. K. (2011). Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 278, 16–27. [DOI] [PubMed] [Google Scholar]
- Inami Y., Waguri S., Sakamoto A., Kouno T., Nakada K., Hino O., Watanabe S., Ando J., Iwadate M., Yamamoto M., et al. (2011). Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J. Cell Biol. 193, 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnagin W. R., Rockey D. C., Koteliansky V. E., Wang S. S., Bissell D. M. (1994). Expression of variant fibronectins in wound healing: Cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J. Cell Biol. 127(6 Pt 2), 2037–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. W., Yoon S. J., Sung Y. K., Lee S. M. (2012). Magnesium chenoursodeoxycholic acid ameliorates carbon tetrachloride-induced liver fibrosis in rats. Exp. Biol. Med. 237, 83–92. [DOI] [PubMed] [Google Scholar]
- Kliment C. R., Englert J. M., Crum L. P., Oury T. D. (2011). A novel method for accurate collagen and biochemical assessment of pulmonary tissue utilizing one animal. Int. J. Clin. Exp. Pathol. 4, 349–355. [PMC free article] [PubMed] [Google Scholar]
- Lee W. M., Seremba E. (2008). Etiologies of acute liver failure. Curr. Opin. Crit. Care 14, 198–201. [DOI] [PubMed] [Google Scholar]
- Lefton H. B., Rosa A., Cohen M. (2009). Diagnosis and epidemiology of cirrhosis. Med. Clin. North Am. 93, 787–799. [DOI] [PubMed] [Google Scholar]
- Li F., Cheng Q., Ling X., Stablewski A., Tang L., Foster B. A., Johnson C. S., Rustum Y. M., Porter C. W. (2010). Generation of a novel transgenic mouse model for bioluminescent monitoring of survivin gene activity in vivo at various pathophysiological processes: Survivin expression overlaps with stem cell markers. Am. J. Pathol. 176, 1629–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liby K., Royce D. B., Williams C. R., Risingsong R., Yore M. M., Honda T., Gribble G. W., Dmitrovsky E., Sporn T. A., Sporn M. B. (2007). The synthetic triterpenoids CDDO-methyl ester and CDDO-ethyl amide prevent lung cancer induced by vinyl carbamate in A/J mice. Cancer Res. 67, 2414–2419. [DOI] [PubMed] [Google Scholar]
- Liby K. T., Royce D. B., Risingsong R., Williams C. R., Maitra A., Hruban R. H., Sporn M. B. (2010). Synthetic triterpenoids prolong survival in a transgenic mouse model of pancreatic cancer. Cancer Prev. Res. 3, 1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta G., Rothstein K. D. (2009). Health maintenance issues in cirrhosis. Med. Clin. North Am. 93, 901–915, viii-ix. [DOI] [PubMed] [Google Scholar]
- Minino A. M., Heron M. P., Smith B. L. (2006). Deaths: Preliminary data for 2004. Nat. Vital Stat. Rep. 54, 1–49. [PubMed] [Google Scholar]
- Mook O. R., Frederiks W. M., Van Noorden C. J. (2004). The role of gelatinases in colorectal cancer progression and metastasis. Biochim. Biophys. Acta 1705, 69–89. [DOI] [PubMed] [Google Scholar]
- Niwa Y., Kanda H., Shikauchi Y., Saiura A., Matsubara K., Kitagawa T., Yamamoto J., Kubo T., Yoshikawa H. (2005). Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene 24, 6406–6417. [DOI] [PubMed] [Google Scholar]
- Ogata H., Chinen T., Yoshida T., Kinjyo I., Takaesu G., Shiraishi H., Iida M., Kobayashi T., Yoshimura A. (2006). Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-beta1 production. Oncogene 25, 2520–2530. [DOI] [PubMed] [Google Scholar]
- Radaeva S., Sun R., Jaruga B., Nguyen V. T., Tian Z., Gao B. (2006). Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology 130, 435–452. [DOI] [PubMed] [Google Scholar]
- Reisman S. A., Buckley D. B., Tanaka Y., Klaassen C. D. (2009). CDDO-Im protects from acetaminophen hepatotoxicity through induction of Nrf2-dependent genes. Toxicol. Appl. Pharmacol. 236, 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata T., Watanabe A., Hobara N., Nagashima H. (1987). Chronic liver injury in rats by carbon tetrachloride inhalation. Bull. Environ. Contam. Toxicol. 38, 959–961. [DOI] [PubMed] [Google Scholar]
- Seki E., De Minicis S., Osterreicher C. H., Kluwe J., Osawa Y., Brenner D. A., Schwabe R. F. (2007). TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat. Med. 13, 1324–1332. [DOI] [PubMed] [Google Scholar]
- Shishodia S., Sethi G., Konopleva M., Andreeff M., Aggarwal B. B. (2006). A synthetic triterpenoid, CDDO-Me, inhibits IkappaBalpha kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of nuclear factor kappaB-regulated gene products in human leukemic cells. Clin. Cancer Res. 12, 1828–1838. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Liby K. T., Yore M. M., Fu L., Lopchuk J. M., Gribble G. W. (2011). New synthetic triterpenoids: Potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J. Nat. Prod. 74, 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh N., Roberts A. B., Birkey Reffey S., Miyazono K., Itoh S., ten Dijke P., Heiss E. H., Place A. E., Risingsong R., Williams C. R., et al. (2003). Synthetic triterpenoids enhance transforming growth factor beta/Smad signaling. Cancer Res. 63, 1371–1376. [PubMed] [Google Scholar]
- Wei H., Wei H., Wang H., Tian Z., Sun R. (2010). Activation of natural killer cells inhibits liver regeneration in toxin-induced liver injury model in mice via a tumor necrosis factor-alpha-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G275–G282. [DOI] [PubMed] [Google Scholar]
- Zhang L. J., Zheng W. D., Shi M. N., Wang X. Z. (2006). Effects of interleukin-10 on activation and apoptosis of hepatic stellate cells in fibrotic rat liver. World J. Gastroenterol. 12, 1918–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W., Chen S., Liu X., Yue P., Sporn M. B., Khuri F. R., Sun S. Y. (2007). c-FLIP downregulation contributes to apoptosis induction by the novel synthetic triterpenoid methyl-2-cyano-3, 12-dioxooleana-1, 9-dien-28-oate (CDDO-Me) in human lung cancer cells. Cancer Biol. Ther. 6, 1614–1620. [DOI] [PubMed] [Google Scholar]
- Zou W., Yue P., Khuri F. R., Sun S. Y. (2008). Coupling of endoplasmic reticulum stress to CDDO-Me-induced up-regulation of death receptor 5 via a CHOP-dependent mechanism involving JNK activation. Cancer Res. 68, 7484–7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.