Abstract
In the context of the great concern about the impact of human activities on the environment, we studied 403 commensal Escherichia coli/Escherichia clade strains isolated from several animal and human populations that have variable contacts to one another. Multilocus sequence typing (MLST) showed a decrease of diversity 1) in strains isolated from animals that had an increasing contact with humans and 2) in all strains that had increased antimicrobial resistance. A specific B1 phylogroup clonal complex (CC87, Institut Pasteur schema nomenclature) of animal origin was identified and characterized as being responsible for the increased antimicrobial resistance prevalence observed in strains from the environments with a high human-mediated antimicrobial pressure. CC87 strains have a high capacity of acquiring and disseminating resistance genes with specific metabolic and genetic determinants as demonstrated by high-throughput sequencing and phenotyping. They are good mouse gut colonizers but are not virulent. Our data confirm the predominant role of human activities in the emergence of antimicrobial resistance in the environmental bacterial strains and unveil a particular E. coli clonal complex of animal origin capable of spreading antimicrobial resistance to other members of microbial communities.
Keywords: Escherichia coli, antimicrobial resistance, phylogroup B1, clonal complex 87, commensal
Introduction
Escherichia coli is a well-known commensal of the gastrointestinal tract of vertebrates, including humans (Tenaillon et al. 2010), but it is also involved in intestinal and extraintestinal pathologies (Croxen and Finlay 2010). Despite the existence of recombination (Tenaillon et al. 2010), E. coli species have a clonal population structure (Desjardins et al. 1995), with the delineation of at least seven main phylogenetic groups (Clermont et al. 2013). The chromosomal plasticity of the strains helps E. coli to adapt to the varying selective pressures that can be found in different environments (Touchon et al. 2009). The recent outbreak of infections and deaths caused by E. coli O104:H4 is a reminder of the risk that can directly affect humans once E. coli has acquired new traits (Karch et al. 2012). The other major health issue associated with E. coli is its role in the emergence and the dissemination of antimicrobial resistance. Most of the resistance properties emerge from commensal bacteria in the gastrointestinal tract (Andremont 2003) where 1) bacteria exist at a high density, allowing horizontal resistance gene transfer between strains from a single species and/or between species or even genera and 2) therapeutic practices in humans and domestic animals that involve use of antimicrobial agents, allow for the selection of resistant strains (Fantin et al. 2009). One of the mechanisms involved in the spread of antimicrobial resistance is the emergence of some specific clones that acquire resistance genes, mostly via mobile genetic elements such as gene cassettes, transposons, integrative genetic elements, and plasmids and that due to an increase in fitness become widespread (Woodford et al. 2011). The most characterized and widespread clones spreading resistance in E. coli are the clonal group A (CGA), belonging to the D phylogroup and harboring cotrimoxazole resistance (Manges et al. 2001) and the sequence type (ST)131 O25b (phylogroup B2) (Clermont, Lavollay, et al. 2008) and ST405 O102 (phylogroup D) (Mihaila et al. 2010) clones that produce extended-spectrum beta-lactamases (ESBLs). Several hypotheses have been proposed to explain such evolutionary success as a fine tuning between the chromosome and the resistance genes (Deschamps et al. 2009) or the arrival of antimicrobial resistance on a clone that already had a high fitness (Brisse et al. 2012; Vimont et al. 2012). However, broad population studies that can capture the evolution in action are lacking, precluding the precise understanding of the phenomenon.
In a previous work (Skurnik et al. 2006), we found an anthropogenic origin of antimicrobial resistance observed in E. coli isolated from the gastrointestinal tract of animals, with a regular increase in the level of antimicrobial resistance associated with an increase of the density of the human population living in the vicinity of these animals. In this work, to decipher the origin of the emergence of antimicrobial resistance, we further characterized this unique collection, gathered in the 2000s, and encompassing commensal wild, farm, and pet animals (Skurnik et al. 2006) as well as humans (Skurnik et al. 2005) strains. By combining molecular phylogeny and epidemiology followed by in vitro and in vivo experiments and finally genomic and metabolic analysis using high-throughput sequencing and microarray screening, we identified and characterized a single clonal complex (CC) of animal origin, CC87, with unique genetic and metabolic specificities. Strains from this CC87 are prone to acquire antimicrobial resistance and possess a high fitness in the gastrointestinal tract, allowing their dissemination to humans. Thus, CC87 might function as a natural reservoir from which other, more pathogenic bacterial species can pick up drug resistance markers and cause outbreaks in which multidrug-resistant isolates are involved.
Results
General Characteristics of the Reservoirs of Antimicrobial Resistance Collection
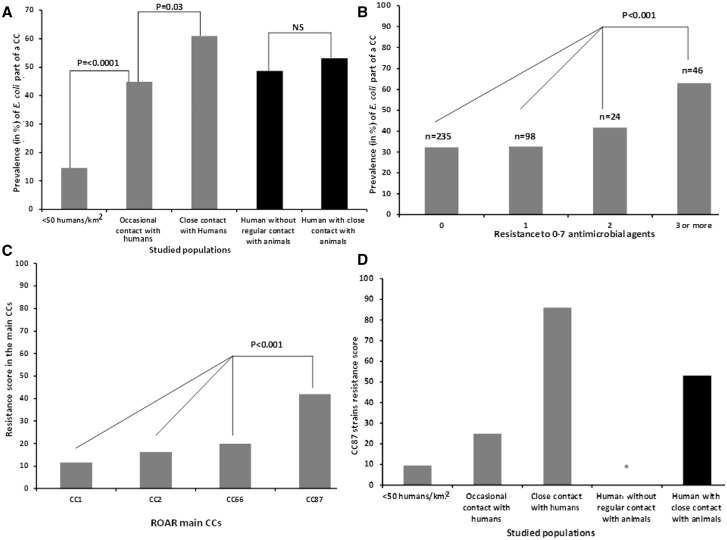
The subject of this study is an unique collection of 403 fecal E. coli/Escherichia clade strains called the “reservoirs of antimicrobial resistance (ROAR)” collection, gathered from animals (Skurnik et al. 2006) and humans (Skurnik et al. 2005) exposed to an increasing level of antimicrobial agents. Animals from the first category are wild animals living in Antarctica, the pristine forest in Gabon, the Pyrenees (France), and Fontainebleau forest (France). This group of animals has very little contact with humans, and thus it is considered to have a low level of antimicrobial exposure. Animals from the second category had occasional contact with humans and are from free-range cattle and poultry in the Pyrenees. The pet dogs constitute the last category: the animals living in close contact with humans, also in the Pyrenees. Thus, this group is considered to be exposed to antimicrobials at the highest level. The human populations originating from Brittany in France were split in two matched categories: 1) pig farmers (PF), considered to be more exposed to antimicrobial agents as they live in a close contact with pigs in intensive farms that are routinely treated with antimicrobial agents (Aubry-Damon et al. 2004), and 2) bank and insurance workers (BIW), considered to be less exposed to antimicrobial agents as they have a limited contact with farm animals and less likely to be carriers of animal bacterial strains. We assessed the phylogenetic relationships between the strains using the MLST data from the Institut Pasteur schema (Jaureguy et al. 2008) by either reconstructing a phylogenetic tree from complete nucleotide sequences (supplementary fig. S1, Supplementary Material online) or generating a minimum spanning tree from the allelic profiles (fig. 1). Both results were congruent, in agreement with the robustness of the clonal structure of the species (Tenaillon et al. 2010). Two hundred sixty-two STs and 27 CCs encompassing 147 strains were identified (fig. 1 and Supplementary table S1, Supplementary Material online). As presented in figure 1, a total of 235 strains (represented in green) were susceptible to all antimicrobial agents tested, 122 strains (in yellow) were resistant to one or two antimicrobial agents, and 46 strains (in red) were considered multiresistant (resistant to at least three antimicrobial agents). A total of four CCs (CC1, CC2, CC66, and CC87) regrouped at least 10 different strains (fig. 1). As shown in figure 2A, 60.9% of the commensal E. coli isolated from animals living in a close contact with humans were part of a CC, versus 44.8% (P = 0.03) of the ones isolated from animals with an occasional contact with humans or 14.5% of those isolated from wild animals (P < 0.0001) (fig. 2A). In contrast, no difference was found between the strains isolated from humans with (PF) or without (BIW) regular contact with farm animals (53.2% vs. 48.7% of strains being part of a CC) (fig. 2A). This result was also found when restricting the analysis only to strains isolated in France (data not shown), thereby excluding a geographical sampling bias.
Fig. 1.
Analysis of the microevolutionary relationships between the 403 E. coli/Escherichia clade strains according to their MLST allelic profile using a minimum spanning tree algorithm. A total of 262 STs and 27 CCs encompassing 147 strains were identified. Among these 27 CCs, the 4 represented by more than 10 strains are indicated. They correspond to CC2 (phylogroup A, STc10 according to Achtman schema) (Wirth et al. 2006) (http://mlst.warwick.ac.uk/mlst/dbs/Ecol last accessed November 12, 2015), CC87 (phylogroup B1, STs 58 and 155 according to Achtman schema), CC66 (complete phylogroup C, STc23 according to Achtman schema), and CC1 (phylogroup B2, STc95 according to Achtman schema). The strains are colored according to their antimicrobial resistance pattern: susceptible to all antimicrobial agents, resistant to 1–2 antimicrobial agents, and resistant to at least three antimicrobial agents.
Fig. 2.
CC characteristics in the ROAR collection. Prevalence in percent of CCs in the 403 E. coli/Escherichia clade strains according to (A) animal and human vicinity and (B) antimicrobial resistance (absence = 0, 1, 2 or at least resistance to 3 antimicrobial agents). (C) Antimicrobial resistance expressed as RSs (Murray et al. 1990) in the main CCs (encompassing at least 10 strains) found in the studied population (CC1, CC2, CC66, and CC87). (D) Antimicrobial RSs according to the animal and human vicinity in CC87 strains. The star in the panel (D) indicates that no CC87 strain was found in the population of human without regular contact with animals.
Association between CC and Antimicrobial Resistance
Analysis of the MLST profiles of the ROAR collection revealed that the prevalence of CCs (fig. 2B) was dependent on the antimicrobial resistance phenotype of the commensal strains that constitute the ROAR collection with a prevalence of CCs increasing with the number of resistance markers detected, from a prevalence of 32% in the strains susceptible to the seven antimicrobial agents tested, or in the strains resistant to one antimicrobial agent, versus a prevalence of 41.6% in the strains resistant to two antimicrobial agents and 63% (29/46) in the strains with resistance to at least three antimicrobial agents (P<0.001). Further analysis of the antimicrobial resistance in the ROAR collection (table 1) confirmed the association between CC and antimicrobial resistance and identified the belonging to a CC as a strong risk factor for antimicrobial resistance for the entire ROAR collection, the CCs explaining up to 13% of the overall antimicrobial resistance (defined as the sum of antibiotic resistance, P = 1.7 × 10−12).
Table 1.
Proportion of Antimicrobial Resistance Explained by the Predictor.
| Parameter | Adj. Sqr (%) | Sum of Resistance (P*) | Resistance > = 2 (P*) |
|---|---|---|---|
| Geographic origin | 14.4 | 2.852E-12 | 1.474E-13 |
| Category (Wild/Farm/Pet/BIW/PF) | 14.2 | 2.367E-13 | 6.626E-14 |
| CC | 13.3 | 1.707E-12 | 1.762E-05 |
| Host | 11.5 | 2.154E-05 | 6.555E-07 |
| aer | 8.3 | 2.165E-09 | 8.413E-08 |
| iroN | 5.5 | 1.191E-06 | 3.770E-05 |
| eae | 2.6 | 0.001 | 0.011 |
| Phylogroup | 2.4 | 0.039 | 0.004 |
| afaD | 2.3 | 0.001 | 0.061 |
| VF(Total) | 1.5 | 0.011 | 0.030 |
| fyuA | 0.7 | 0.057 | 0.255 |
| cnf1 | 0.6 | 0.064 | 0.039 |
| sfa | 0.6 | 0.066 | 0.190 |
| kpsE | 0.2 | 0.937 | 0.803 |
| stx2 | 0.2 | 0.796 | 0.503 |
| pap | 0.2 | 0.704 | 0.918 |
| hly | 0.2 | 0.195 | 0.662 |
| stx1 | 0.2 | 0.562 | 0.963 |
| ST | 0.2 | 0.536 | 0.536 |
Note.—The parameters of the analysis of the ROAR collection used to study the antimicrobial resistance are 1) for the host: nature, geographic origin (Antarctica, Africa, or Europe), or category, 2) for the bacteria: phylogroup (A, B1, D, B2, or other), MLST (ST and CC), extraintestinal VFs, analyzed both individually and together. The presented proportion of antibiotic resistance explained by each predictor corresponds to the adjusted squared (Adj. Sqr) correlation obtained from standard linear regression. P*: P values from univariate analyses of each predictor.
To control for potential confounding factors such as geography or categories, we repeated the performed table 1 for CC, while adjusting for these two variables (supplementary table S2, Supplementary Material online) and recovered the association between CC87 and antibiotic resistance, confirming the robustness of the observation.
Association between CC87 and Antimicrobial Resistance
Extension of the analysis of the link between antimicrobial resistance and CCs (fig. 2B and table 1) revealed that among the CCs in our study comprising at least 10 strains, only CC87, which encompassed 17 strains (fig. 1, supplementary table S3, Supplementary Material online), had an increased association with antimicrobial resistance. First, we found that strains of CC87 had an antimicrobial resistance score (RS), an indicator of the antimicrobial resistance of a collection of strains (Murray et al. 1990), of 42%, which is significantly higher than in all the other more common CCs present in our study (fig. 2C). Second, we found that the RS of the CC87 strains isolated from animals was increasing with human vicinity (fig. 2D). Finally, while the RS was significantly higher in 1) the strains isolated from humans living in a close contact with animals than in those living without a close contact (P = 0.01) (fig. 3A) and 2) in the strains isolated from animals living in a close contact to humans than in those isolated from animals living with an occasional contact to humans (P < 0.000001) (fig. 3A), these differences disappeared when CC87 was removed from the study (fig. 3B). This unexpected and major role of CC87 in the previously described significant difference of RS between the studied populations was further confirmed by analyzing separately the resistance to amoxicillin, streptomycin, and sulphonamides and tetracycline (supplementary fig. S2, Supplementary Material online). As for the overall RS, for each antimicrobial agent tested individually, when CC87 was removed, the differences in susceptibility between the different populations were reduced (supplementary fig. S2, Supplementary Material online).
Fig. 3.
Antimicrobial resistance and CC87 in the ROAR collection. Antimicrobial RSs according to the animal and human vicinity in all the 403 E. coli/Escherichia clade strains of the collection (A), in all the strains of the collection excluding the CC87 (B), and in all the 403 strains of the collection according to the phylogenetic groups, the phylogroup B1 being considered with and without the CC87 strains (C). (D) Tetracycline MIC distribution of the E. coli/Escherichia clade strains detected as nonsusceptible by the disk diffusion method with the classification according to the CLSI clinical breakpoints. Note that only strains with a MIC > 64 µg/ml have a tet gene detected by multiplex PCR.
CC87 Phylogenetic Group and Antimicrobial Resistance
Although CC87 belongs to the phylogenetic group B1, the strong association described above between CC87 and antimicrobial resistance was not found in the other B1 strains in our study. As shown in figure 3C, while overall, the phylogenetic group B1 was overrepresented among the strains with at least three antimicrobial resistance properties detected (>40%) versus 20%, 11%, 9%, and 12% of the resistant strains belonging to the groups A, B2, D, or others, respectively, when CC87 was removed from the B1 group, only 10% of the strains with resistance to at least three antimicrobial agents were left in phylogenetic group B1. Of note, even though CC87 strains exhibit a certain level of heterogeneity, as CC87 is composed of five STs with at least five O-types (O9, O11, O40, O88, and O118) and a variable pattern of extraintestinal virulence factors (VFs) not correlated to the STs (supplementary table S3, Supplementary Material online), these strains were present in all the studied animal populations as well as in PF but were absent among the strains isolated from matched (for age, sex, and geographic origin, i.e., the same district in Brittany, France) humans living without a close contact to animals (BIW) (fig. 2D).
CC87 and Tetracycline Resistance
During our analysis of the role of CC87 in the prevalence of resistance to each antimicrobial agent, we found a different trend for tetracycline resistance compared to resistance to other antimicrobial agents. For example, for amoxicillin, streptomycin, and sulphonamides, the prevalence of resistance among the commensal E. coli strains isolated from animals living in an occasional contact with humans or without contact to humans was always less than 15% and 5%, respectively. However, for tetracycline, the prevalence of resistance in these two populations was greater than 40% and 20% (supplementary fig. S2, Supplementary Material online), respectively. In addition, in sharp contrast to the other antimicrobial agents tested, no particular involvement of strains from CC87 was found. To further study this observation, we closely analyzed the minimum inhibitory concentrations (MICs) of the strains defined as nonsusceptible (resistant and intermediate level of resistance) to the tetracycline. This approach revealed a dual population of these nonsusceptible strains, with a group of resistant strains with MICs > 96 µg/ml and a group of resistant and strains with intermediate level of resistance with MICs < 32 µg/ml (fig. 3D). Once this distinction was realized, we were able to explain the discrepancy associated with tetracycline resistance and found that overall strains from the CC87 were significantly associated with antimicrobial resistance, whatever drug was tested, excepted for the low level resistance to tetracycline (fig. 4A).
Fig. 4.
CC87 and statistical characteristics of the ROAR collection. (A) Odds ratio of antimicrobial resistance for strains from CC87. Resistance ≥ 2 corresponds resistance to at least two antimicrobial agents. (B) Factorial analysis of correspondence describing the associations among the various variables of the 403 E. coli/Escherichia clade strains. The variables in black are as follow: the phylogenetic groups (A, B1, B2, D, C, E, F, UG = ungrouped, clades = Escherichia clades), the VFs (kpsE, sfa, iroN, aer, pap, hly, cnf1, fyuA, afaD, stx1, stx2, eae and ST), the virulence categories (absence of VF = VF = 0, 1 to 3 VFs = VF = 1–3, 4 to 7 VFs = VF = 4–7), the resistance to seven antimicrobial agents (amoxicillin = AMX-R, sulphonamide = SUL-R, chloramphenicol = CHL-R, kanamycin = KAN-R, streptomycin = STR-R, nalidixic acid = NAL-R and tetracycline = TET-R), the detection of the tet genes, the MIC to tetracycline (MIC < 2 µg/ml, MIC = 2 to 16 µg/ml; MIC > 16 µg/ml), the effect of the efflux pump inhibitor PAβN (Phe-Arg-beta-naphtylamide) on the MIC of tetracycline, the three resistance categories (resistance to none of the antimicrobial agents tested = R = 0, resistance to 1 to 3 antimicrobial agents = R = 1–3, resistance to 4 to 7 antimicrobial agents = R = 4–7), the origins of the strains (wild animals = WA, domestic animals = DA, pet animals = PA, human with 2 populations: BIW and PF). The CCs are indicated in white. The association of CC87 with the plasmid-borne antimicrobial resistances is indicated by the circle.
Further studying the particularity of the tetracycline resistance pattern in the ROAR collection, we found that a tet gene encoding tetracycline resistance was present in all the strains with a MIC > 64 µg/ml, with tet(A) and tet(B) being the most common tet genes identified (supplementary table S1, Supplementary Material online). Except for three tet(E) genes identified in Escherichia clade V strains of different STs (supplementary table S1, Supplementary Material online), no tet gene was detected among the resistant strains with a MIC < 32 µg/ml (supplementary table S1, Supplementary Material online) suggesting a different mechanism of resistance in these strains. To identify the mechanism of the low level of resistance to tetracycline, we tested the MICs in the presence or the absence of an efflux pump inhibitor, Phe-Arg-β-naphthylamide (supplementary fig. S3A, Supplementary Material online). Resistance to tetracycline was inhibited suggesting that efflux pumps might be involved. A single strain (ROAR154) had a MIC of 6 µg/ml in the presence and absence of Phe-Arg-β-naphtylamide (supplementary fig. S3A, Supplementary Material online). The ROAR strains with a low level of resistance to the tetracycline were further screened for alternative tet genes (tet G, J, L, M, W, Y, X2, and tet(39)) without any success.
At the end, we were able to conclude that for the tetracycline-resistant strains with a tet gene, the CC87 had the same role on the prevalence of the RS among the studied population than the other major antimicrobial agents analyzed separately (supplementary fig. S3B, Supplementary Material online). In contrast, no role of the CC87 strains was found for the tetracycline-resistant strains with no detected tet gene (supplementary fig. S3C, Supplementary Material online). In fact, we found that tetracycline-resistant strains without a tet gene were mainly present in the populations with no general effect of CC87, that is, isolated from the wild animals and the animals without close contact with humans.
CC87 and Efficiency of Conjugation
To provide a better overview of the data, we conducted a factorial analysis of correspondence (fig. 4B). The variables on the plane F1/F2, which accounted for 28.41% of the total variance, distinguished the characters of resistance to antimicrobial agents and the high RSs, which were projected on the positive values of F1 from the absence of resistance, which was projected on the negative values of F1. Similarly, the origins of the strains were also distinguished on this axis with the wild and domestic animal origins on the negative values and the pet animal, BIW, and PF origins on the positive values. The presence of the tet genes was linked to high of MICs to tetracycline on the positive values of F1 and the presence of an inhibition of resistance to tetracycline by the efflux pump inhibitor Phe-Arg-β-naphthylamide to a low level of tetracycline resistance (MIC ≤ 16 µg/ml) on the positive values of F1 and F2. The factor 2 appeared to characterize the intrinsic virulence of the strains. Thus, the absence of VF was projected on the positive values of F2, whereas the VF score of 4–7 and the most part of VFs were projected on its negative values. The phylogenetic groups were projected along this axis, the groups B1 and A on its positive values, whereas the groups D, F, and B2 on its negative values. This analysis confirmed the strong association between CC87, the presence of a tet gene, the high tetracycline MIC, and resistance to amoxicillin, streptomycin, and sulphonamide and at a lesser extend chloramphenicol (fig. 4B).
Dissemination of tetracycline, amoxicillin, streptomycin, and sulphonamide resistance is often due to gene transmission by conjugation, and for each of these antimicrobial agents, CC87 was associated with antimicrobial resistance. In parallel, the only time we found a lack of association between antimicrobial resistance and CC87 was with the specific portion of tetracycline resistance that was mediated by another mechanism. Thus, we hypothesized that the link between CC87 and antimicrobial resistance could be linked to the transmission of antimicrobial resistance genes via conjugation. To test this hypothesis, we studied the conjugation abilities of antimicrobial resistance of strains from our collection from CC87 or other CCs. For this experiment, we used two different donor strains, E. coli 13 and 38, both of which are clinical isolates carrying a plasmid containing the gene blaTEM-1 encoding amoxicillin resistance.
We measured the frequency of conjugation of amoxicillin resistance genes between these donors and five CC87 strains (three ST21 strains: ROAR61, ROAR72, and ROAR82 and two ST87 strains: ROAR105 and ROAR205) on the one hand or five non-CC87 control strains on the other. Control strains included two strains (ROAR28 and ROAR171) from the same phylogenetic group than the CC87 strains (B1) and three strains from two different phylogenetic groups commonly found in feces (supplementary table S1, Supplementary Material online), ROAR79 (phylogroup B2), ROAR155, and ROAR394 (phylogroup A). All recipients were susceptible to amoxicillin.
For the selection of transconjugants, a kanamycin resistance gene was inserted in all the ROAR strains. As presented in figure 5A, in all circumstances, the CC87 strains were more efficient in acquisition of amoxicillin resistance compared to the non-CC87 strains. Furthermore, we found that CC87 strains were more efficient than non-CC87 strains to acquire the plasmid carrying the gene blaCTX-M-15 (fig. 5B) encoding the ESBL CTX-M-15, a source of an ongoing pandemic resistance to third generation of cephalosporin in the hospital as well as in the community (Woerther et al. 2013). Notably, once these commensal CC87 strains have acquired this ESBL, they were able to efficiently transfer the resistance to clinically relevant pathogenic E. coli strains (fig. 5C) suggesting that they might play a major role in spread of drug resistance to human pathogenic bacteria.
Fig. 5.
CC87 and bacterial conjugation abilities. Efficiency of conjugation of (A) the β-lactamase gene blaTEM between two different donors (E. coli 14 and 38) and CC87 or non-CC87 strains, (B) the ESBL gene blaCTX-M-15 in direction of CC87 or non-CC87 strains, and (C) the same ESBL gene blaCTX-M-15 from CC87 strains to clinical and pathogenic strains. CC87 strains tested: ROAR61, 72, 82, 104 and 205. Non-CC87 strains tested: ROAR28, 79, 155, 171, 394.
To avoid any bias in the comparison of CC87 and non-CC87 strains, efficiency of conjugation and plasmid incompatibility groups were also determined. Indeed the presence of an incompatible plasmid may limit the efficiency of plasmid retention and therefore bias the analysis. Incompatibility groups of plasmids carried by strains used for conjugation assays are shown in supplementary table S4, Supplementary Material online. The two TEM-1-producing E. coli isolates (13 and 38) carried a multireplicon IncF (FIB-FII) plasmid, whereas the CTX-M-15-producing E. coli strain harbored a FIA-FII plasmid. E. coli ROAR61 and 155 carried an IncFIB plasmid. E. coli ROAR72 harbored an IncI plasmid. Each of the E. coli strains ROAR394, 28 and 205 harbored a multireplicon IncF plasmid (FIB-FII, FIB-FIC-FII1, and FIA-FIB-FII2, respectively). The remaining ROAR strains used for the conjugation assays did not carry any plasmid as confirmed by plasmid DNA extraction by the Kieser method (supplementary fig. S4, Supplementary Material online) (Kieser 1984). E. coli strains often carry IncF plasmids and these plasmids have been reported to be commonly found in fecal microbiota of humans and animals, regardless resistance genes (Johnson et al. 2007). IncF plasmids are usually multireplicon plasmids carrying FIA, FIB, and FII replicons in different combinations (Carattoli 2009). This multireplicon allows not only replication in a broad range of host bacteria but also the acquisition of plasmids carrying incompatible replicons (Villa et al. 2010). Regarding incompatibility groups of the studied plasmids, we were able to rule out that the difference in rates of conjugative transfer found between CC87 and non-CC87 strains was due to plasmid incompatibility.
CC87 and Commensalism
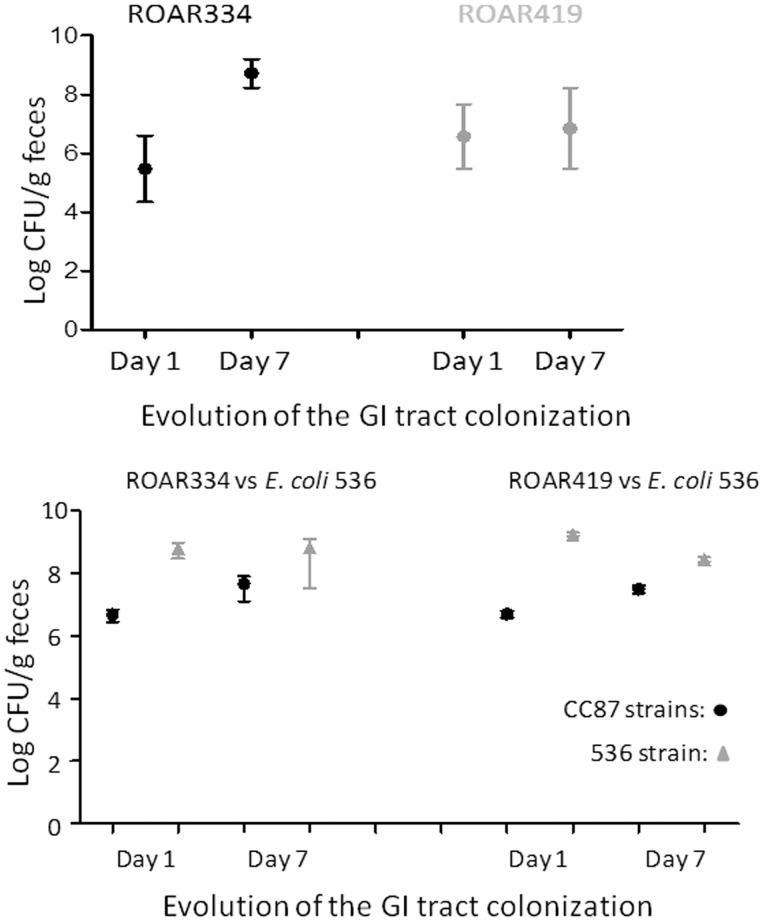
As acquisition of antimicrobial resistance has previously been described to be associated with an in vivo fitness cost (Andersson and Hughes 2010), we next tested the ability of CC87 strains ROAR334 (ST87) and 419 (ST24) to colonize the mouse gastrointestinal tract. Each strain carried six antimicrobial resistance markers (the maximum found in our study), with ROAR334 being isolated from a dog and ROAR419 being isolated from a PF, respectively (supplementary table S3, Supplementary Material online). To assess the gut colonization ability of these CC87 strains, we used a modified streptomycin treated mouse model that allows testing of strains not resistant to high levels of streptomycin (Vimont et al. 2012). We first tested the two strains in monocolonization. Both strains easily colonized the gut at a high level in 1 day and persisted for up to day 7 when the experiment was stopped as no further changes in colonization levels were observed (median: >108 CFU/g of feces and >106 CFU/g of feces for ROAR334 and ROAR419, respectively) (fig. 6A). Next, we tested these strains in competition with E. coli 536, a strain from the B2 phylogroup, that is known to be an excellent competitor in the mouse gut (Diard et al. 2010). The E. coli 536 strain is susceptible to amoxicillin, whereas the ROAR strains were amoxicillin resistant (supplementary table S3, Supplementary Material online). This allowed us to plate the feces on media containing amoxicillin to distinguish the strains. Furthermore, the strain identity was confirmed by performing specific polymerase chain reaction (PCRs) on isolated clones on plates (B2 phylogroup and O6-type determination for the 536 strain and B1 phylogroup determination and fyuA detection for the ROAR strains) (data not shown). The tested ROAR strains were able to colonize and persist in the mouse gut, even in the presence of the 536 strain (median: >107 CFU/g of feces for both strains) (fig. 6B). These results suggest that antimicrobial resistance of CC87 strains does not reduce their in vivo fitness and colonization abilities.
Fig. 6.
Commensal abilities of CC87 strains. Mouse digestive tract colonization assays. The day of inoculation, 104–105 E. coli ROAR bacteria were administered in 200 µl of PBS by oral route to mice free of coliform flora, either alone (A) or mixed at a ratio of 1:1 with the E. coli 536 strain, a well-known good colonizer of the mouse gut (Diard et al. 2010). On days 1 and 7 after bacterial administration, the sizes of bacterial populations in the intestine of mice were evaluated by plating dilutions of weighed fresh feces on LB agar with or without appropriate antimicrobial agents. Four mice were used per experiment. GI, gastrointestinal.
Finally, to confirm that CC87 strains were true commensal strains, without any intrinsic extraintestinal virulence causing damage to the host, we tested three CC87 strains with different profiles in a murine model of extraintestinal virulence: ROAR72 (ST21) without any antimicrobial resistance but with four extraintestinal VFs, ROAR47 (ST21) with resistance to one antimicrobial agent and no VF, and ROAR419 (ST24) with resistance to several antimicrobial agents and three VFs (supplementary table S3, Supplementary Material online). In all instances, and despite the presence of VFs, no mouse was killed after challenge by a CC87 strain, as well as after challenge with the known commensal strain K-12 MG1655. Contrary to that, all mice infected with the known uropathogenic strain CFT073 belonging to the B2 phylogroup were killed (table 2). This result confirmed that drug-resistant CC87 strains are excellent colonizers of the gastrointestinal tract but are avirulent under the tested conditions.
Table 2.
CC87 Strains and Mouse Model of Sepsis.
| Escherichia coli Strain Used for the Challenge | Number of Survivors/Number of Mice Infected | |
|---|---|---|
| Controls | ||
| Commensal strain | K-12 MG1655 | 10/10 |
| Uro-virulent strain | CFT073 | 0/10 |
| CC87 strains | ||
| Susceptibility to all antimicrobial agents tested | ROAR72 | 10/10 |
| Resistance to one antimicrobial agent | ROAR47 | 10/10 |
| Resistance to multiple antimicrobial agents | ROAR419 | 10/10 |
Genomic Analysis of the CC87 Strains
Using high-throughput sequencing (Illumina technology), we sequenced the full genomes of seven distinct CC87 E. coli strains: four fully antimicrobial susceptible strains, all belonging to ST21 (ROAR47, 61, 72, and 82) and three strains belonging to ST24 and 87 with one or more antimicrobial resistance genes: ROAR205, 334, and 419 (supplementary table S3, Supplementary Material online). We then performed comparative genomic analysis using 128 complete E. coli genomes (Lescat et al. 2014), including three previously sequenced CC87 strains, TA271 (mammal commensal, Dasyurus viverrinus), B574 (bird commensal, Lichenostomus penicillatus), and H591 (human urinary tract infection). We first reconstructed the phylogenetic history of the strains by using single-nucleotide polymorphisms of 500 genes of the E. coli core genome. The CC87 strains clustered as a single clade within the 22 non-CC87 B1 strains (data not shown). When analyzing only the B1 phylogroup strains (both CC87 and non-CC87) rooted on the closest E. coli plylogroup, that is, the phylogroup C, to avoid long branch attraction effect, it appears that the CC87 has emerged relatively recently in the B1 phylogeny as the strains of the complex are on terminal branches with small branch lengths (fig. 7). However, a diversification of the CC87 strain can be observed, confirming the heterogeneity of the CC87 suggested by the MLST and O-typing assays. To detect genes with a specific presence/absence pattern in CC87, we then searched for genes that were either almost exclusively present in CC87 among B1 or exclusively absent from CC87 and mapped them on the strain phylogeny (fig. 7). As annotation or assembly errors are possible, we allowed for one or two strains within each group to have a different pattern. By this way, we identified 33 genes, 20 of which were parts of three blocks of synteny. Two of these blocks of synteny were present in the CC87 and absent from the other B1 strains, the other one presented the opposite pattern and was exclusively deleted in CC87 (fig. 7). The two main characteristics of the CC87 as determined in this study are their high abilities to: 1) acquire and disseminate antimicrobial resistance genes through horizontal transfer and 2) colonize the gastrointestinal tract. Analysis of the genes specifically missing and specifically present in CC87 strains (supplementary table S5 and fig. 7, Supplementary Material online) revealed genetic determinants that could partially explain these two characteristics. First, we identified a gene annotated as Eta271v1_1520040 in ROAR271 (supplementary table S5, Supplementary Material online) that was specifically present in all CC87 strains. This gene is predicted to encode an analog to the accessory colonization factor heat-resistant agglutinin 1 (Hra1) (Bhargava et al. 2009; Mancini et al. 2011) (supplementary table S5, Supplementary Material online), thus its presence might explain the good in vivo fitness of the CC87 strains. We also found that all the CC87 strains were specifically missing the so called “death-gene” ygcR (Amitai et al. 2009). YgcR has been previously described to be involved in bacterial cell death when triggered by DNA damage cause by antimicrobial agents (Amitai et al. 2009). The specific presence of Hra1 associated with the specific absence of ygcR in CC87 strains could therefore confer a double advantage to these strains: higher abilities to colonize the gastrointestinal tract that could persist even when their hosts are exposed to an increased antimicrobial pressure. Lastly, we found that all CC87 strains contained numerous additional metabolic genes predicted to be involved in the transport and the utilization of sugars (supplementary table S5, Supplementary Material online), and it has been demonstrated that sugar catabolic pathways are essential for E. coli intestine colonization (Chang et al. 2004).
Fig. 7.
Specific genic presence/absence pattern of CC87 among B1 strains. The phylogeny of the B1 strains rooted on the phylogroup C was computed with 500 core genes. Gene presence/absence is illustrated for the set of genes that presented a contrasted signature between CC87 strains and non-CC87-B1. Among this set, four groups of linked genes are represented: metabolic genes; transporters; genes with alternative functions; and genes of unknown functions.
The second main feature of the CC87 strains is their significantly higher abilities to acquire antimicrobial resistance genes via conjugation. In favor of a genetic component of this feature, we found that among the genes present in CC87 strains but absent from other B1 phylogroup strains were genes encoding GntR and AraC regulators, two members of the superfamilies of helix-turn-helix (HTH) DNA-binding domain proteins (Tong et al. 1996). Interestingly, it has recently been reported that mutations within helix-turn-helix DNA-binding motifs may reduce binding of regulator to its target DNA and therefore decrease mating efficiency (Rodriguez-Maillard et al. 2010).
High-Throughput Phenotypic Analysis of CC87 and Non-CC87 Strains' Metabolism
Having found several additional genetic determinants associated with the bacterial transport and the utilization of sugars in CC87 strains, we decided to complete our genetic analysis with a high-throughput phenotypic approach to compare CC87 and non-CC87 strains metabolism. Our first step was to comprehensively compare all the metabolic characteristics of the multisusceptible strains ROAR61, ST21 (CC87), and ROAR394 (phylogroup A) found to have significantly different abilities to acquire antimicrobial resistance (fig. 5). For this initial screening, we have selected the CC87 strain called ROAR61 from the ST21 because it is the most common ST among the CC87 strains isolated from the ROAR wild animals, while the non-CC87 strain called ROAR394 was selected as it is part of the phylogenetic group A, very commonly isolated from the human commensal microbiota (Tenaillon et al. 2010). Our second step was to confirm any signal found during the initial screening and to determine if these results could explain differences found in conjugation abilities of strains that also result in changes in antimicrobial resistance profile. For the screening part of our approach, we used the commercially available Phenotype MicroArray (Biolog). We obtained data from 25 96-well plates in which nearly every well had different metabolites, substrates, or conditions. This allowed us to quickly generate phenotypic data differentiating the selected CC87 (ROAR61) and non-CC87 (ROAR394) strains. Out of the 1,152 compounds and conditions tested (supplementary table S6, Supplementary Material online), 55 were associated with an increased fitness of the non-CC87 strain and 108 with a positive fitness of the CC87 strains (fig. 8A). Interestingly, the CC87 strain was more resistant to high concentration of 1-10 phenanthroline (fig. 8B), a known inhibitor of bacterial conjugation (Ou and Reim 1976). We therefore focused the follow-up of our screening step to determine if all CC87 strains are more resistant to this compound. The MICs to 1-10 phenanthroline of 14 CC87 and non-87 strains were further tested. We found that the MICs of the 7 CC87 strains we sequenced (ROAR47, 61, 72, 82, 205, 334, and 419) were all at 64 µg/ml, while all seven non-CC87 strains (ROAR55, 355, 365, 377, 394, 420, and 431) had a MIC at 16 µg/ml, suggesting that higher conjugation abilities of CC87 might be explained in part by higher resistance to 1-10 phenanthroline (and possibly other inhibitors of conjugation) (fig. 8, supplementary table S6, Supplementary Material online).
Fig. 8.
High-throughput Biolog phenotypic analysis of strains ROAR61 (CC87) and ROAR394 (non-CC87). (A) For each metabolite, the OD590nm after 24 h of growth is indicated. Metabolites influencing the bacterial growth of CC87 and non-CC87 are grouped according to the normalized ratios (ROAR61/ROAR394). Are represented: example of metabolites associated with an increased fitness of the non-CC87 strain (ratio < 0.5); example of metabolites with an increased fitness of the CC87 strain (ratio > 2); and no effect. (B) Individual growth curves of strains ROAR61 (square) and 394 (triangle) with (solid line) or without (dotted line) 1,10-phenanthroline (32 mg/l).
Development of a Rapid and Easy PCR Assay for the Detection of CC87 Strains
To perform large-scale epidemiological studies of CC87 strains without requiring time and resource consuming MLST analyses, a PCR approach based on two CC87 allele-specific primers targeting icd was developed to quickly identify these strains (supplementary fig. S5, Supplementary Material online). This test was validated on 185 B1 phylogroup strains from the ROAR collection for which MLST data were available (n = 129) and from two other previously reported collections, one including the ECOR strains (Clermont et al. 2011) (n = 49) and the other the completely sequenced Broad Institute strains (n = 7). In addition to the 17 CC87 strains from the ROAR collection, we found seven additional CC87 strains, including the ECOR34 strain and four strains sequenced at the Broad Institute (B574, B799, H591, and TA271), in agreement with the data of the complete genome sequencing (fig. 7). These strains originated from wild animals (birds and mammal), a dog, and humans and were all except two commensals. Altogether, our test showed a specificity and a sensitivity of 100%. Furthermore, this analysis also suggests a widespread of CC87 in nature, and major impact it can play in a spread of antimicrobial resistance to other, pathogenic bacteria.
Discussion
The ROAR collection constitutes a unique source of information on the consequences of the interaction between animals and humans on commensal strains of E. coli. The strains from this collection were isolated from the feces of five different populations, from wild animals to humans living in a close contact with animals in an intensive farm (PF). For each isolate of the collection, a comprehensive analysis was done that included MLST, determination of the O-types, the presence of the main VFs, and antimicrobial resistance characterization.
The first signal that emerged from this analysis was a decrease of the diversity of strains, which was dependent on the proximity of the animals to humans (fig. 2A). This result is in agreement with the previous finding on almost 2,000 commensal isolates (Escobar-Paramo et al. 2006) that human microbiota is characterized by a lower intrahost diversity than the nonhuman mammal ones and that domestication decreases the intrahost diversity. Furthermore, certain CCs of the ROAR collection were also associated with an increased prevalence of antimicrobial resistance (fig. 2B). Altogether, these results are consistent with antimicrobial pressure in the environment of the hosts selecting for a resistant population of low diversity within the gut microbiota.
The further study of the association between CCs, human vicinity, and antimicrobial resistance led to the identification of a CC particularly linked to the antimicrobial resistance: the CC87. This single CC by itself can explain part of the differences in the antimicrobial resistance patterns between the different studied populations, that is, the animals with occasional or close contact to humans and the humans with or without contact to animals (fig. 3A and B and supplementary fig. S2, Supplementary Material online). CC87 has been isolated in all animal categories, but was only found in humans with contact to farm animals. Furthermore, CC87 belongs to the B1 phylogroup, a group that was previously very rarely found in humans but highly prevalent in animal microbiota (Tenaillon et al. 2010). These data strongly support the hypothesis of an animal reservoir of CC87. We also confirmed that the strains of this CC are true commensals as they can effectively colonize the gut of the mice despite the cost of antimicrobial resistance (Andersson and Hughes 2010), even in the presence of other E. coli competitors (fig. 6). They do not possess intestinal VFs, and despite the presence of up to four extraintestinal VFs, they do not have any intrinsic virulence assessed in a mouse sepsis model (Picard et al. 1999) (table 2). Our data are in line with epidemiologic data from the literature showing that B1 phylogroup strains very rarely cause human extraintestinal infections (Lefort et al. 2011). Strains of CC87 are not of recent emergence as they exhibit a certain level of diversity, with several STs, O-types and VF patterns (supplementary table S3, Supplementary Material online).
A global analysis of the data (fig. 4) points to the association between CC87 and plasmid-mediated antimicrobial resistance properties, that is, resistance to amoxicillin, streptomycin, and sulphonamides (supplementary fig. S2, Supplementary Material online) and high level tetracycline resistance (MIC ≥ 32 µg/ml) based on the presence of tet genes (fig. 3). Interestingly, we found high abilities of the CC87 strains to acquire antimicrobial resistance by conjugation (fig. 5). This increased capacity of resistance acquisition explains the parallel acquisition of distinct tet genes [tet(A) and tet(B)] in several distinct genetic backgrounds of CC87 (supplementary table S3, Supplementary Material online). These multiple acquisitions of antimicrobial resistance genes within members of a single CC is indicative of a convergence, a hallmark of selection, with a fine tuning between the genetic background of the strains and the determinants of resistance, as it has been reported in ESBL-producing E. coli (Branger et al. 2005; Deschamps et al. 2009). High efficiency of these strains to acquire and disseminate antimicrobial resistance determinants could be partially explained by the metabolic and/or genetic characteristics specific to the strains from the CC87. In favor of the metabolic hypothesis, here we report several CC87-specific genes associated with sugar metabolism as well as an increased level of resistance to the 1-10 phenanthroline for all tested strains from the CC87. Indeed, this compound has been previously described as being able to inhibit bacterial conjugation (Ou and Reim 1976), thus it is tempting to speculate that a similar compound can be present in the environment or be produced by other microbes of the microbiota, thus conferring an advantage to CC87 strains. Another hypothesis that could explain the high level of commensalism of the CC87 strains and their abilities to acquire and then transfer antimicrobial resistance determinants is the presence in all sequenced strains of additional helix-turn-helix DNA-binding motif proteins that have been described to be associated with mating efficiency (Rodriguez-Maillard et al. 2010). In parallel, CC87 strains possess genetic determinants such as a gene encoding for an analog of the adhesin Hra1 and numerous genes involved in sugar metabolism that could explain the high capacities to be commensal found in this study. CC87 strains could also display a higher level of resistance to the antimicrobial-induced stresses by missing the so called “death gene” ygcR responsible of the cell death after the DNA damage induced by antimicrobial agents (Amitai et al. 2009).
As only a total of 17 CC87 strains are part of the ROAR collection, this low number could constitute a limitation to our study. However, we have developed a quick, easy, and robust PCR assay to detect these strains among E. coli of phylogroup B1 (supplementary fig. S5, Supplementary Material online) and found additional CC87 strains in our collections originating worldwide. We also established the correspondence between our MLST scheme and the Achtman MLST scheme (Wirth et al. 2006) and determined that our CC87 corresponds to the ST58 and 155 (Clermont et al. 2015). In the Achtman MLST database (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli last accessed November 12, 2015), we found 52 strains from various continents (Europe, Africa, North and South America, and Asia) belonging to ST58 and 155 and originating from humans, wild, and domestic animals, and the environment, confirming that these strains are widespread. Of particular interest, six of these strains produce an ESBL CTX-M enzyme, usually encoded by a plasmid-borne gene. Similarly, two ST155 strains with plasmid-borne antibiotic resistance genes have been reported from small rodents living in close contact to humans in French Guiana (Grall et al. 2015). Nevertheless, our findings need to be confirmed on additional strain collections.
Overall, in a context of an increasing concern about the impact of human activities on the environment, careful analysis of a unique collection of E. coli strains from different animal and human populations led to the discovery of a CC of E. coli strains, CC87, that by itself explains the increased antimicrobial resistance of animal commensal strains caused by exposure to humans. On the basis of our data, we propose the following scenario, summarized in the schematic presented in figure 9: first, the CC87 encompasses commensal animal strains with unique metabolic and genetic characteristics that can easily acquire and transfer plasmid-borne antimicrobial resistance by conjugation. Then, from use of antimicrobials by farmers/ranchers, to pet owners, and veterinarians, there is major antimicrobial pressure of anthropogenic origin. Therefore, CC87 strains and other bacterial strains similarly efficient at colonization and conjugation can become resistant to antimicrobial agents and these antimicrobial-resistant strains can spread easily between hosts, including humans, as their capability to colonize the gut of a new host is not hampered by the cost of resistance. This raises the concern of a dissemination of commensal multiresistant CC87 strains in human populations as well as the concern of the diffusion of the antimicrobial resistance carried by these strains to other noncommensal, pathogenic strains. We recently reported (Roux et al. 2015) that in contrast to a general belief in the field, antimicrobial resistance was often associated with increased in vivo fitness and virulence during bacterial infection. The findings of this study that commensal strains of animal origin such as the CC87 strains are also able to associate fitness and antimicrobial resistance, and are then able to disseminate in humans, stress the urgent need for a better control of the use of antimicrobial agents not only among humans but also among animals.
Fig. 9.
Schematic presentation of the acquisition and dissemination of antimicrobial resistance by CC87 strains.
Materials and Methods
Ethics Statements
All in vivo experiments were performed in accordance with the ARRIVE guidelines. The mouse septicemia model was conducted following the European and National regulations for housing and care of laboratory animals after pertinent review and approval by the Bioethics Committee at Santiago de Compostela University and by the French Veterinary Services (certificate number A 75-18-05). The mouse gut colonization model was conducted after approval by the Debré-Bichat Ethics Committee for Animal Experimentation (Protocol Number 2012-17/722-0076) in accordance to the European Decret and French law on the protection of animals. All possible measures were taken to minimize animal suffering and to ensure animal welfare. When necessary, animals were sacrificed by lethal intraperitoneal injection of phenobarbital after volatile anesthesia with sevoflurane.
Bacterial Strains
Four hundred three E. coli/Escherichia clade I to V strains originating from humans (Skurnik et al. 2005) and animals (Skurnik et al. 2006) were studied. The complete characteristics of the strains are given in supplementary table S1, Supplementary Material online. The kanamycin-resistant strains used as recipient strains in conjugation experiments were constructed by inserting a kanamycin resistance cassette in the noncoding region between lacA and cynX using the Dastsenko and Wanner protocol (Datsenko and Wanner 2000). Two clinical E. coli strains (Nos. 13 and 38) isolated from urinary tract infections in the 2000s in France, harboring a blaTEM-1 gene on a plasmid and belonging to the phylogroup B2, were used as donor strains for the transfer of amoxicillin resistance. A clinical strain (No. 209) carrying the ESBL gene blaCTX-M-15 (B2 phylogroup; blood origin) was used for the experiments on acquisition and transfer of ESBL. The pathogenic clinical strains IAI32 (Escherichia clade I; blood origin), IAI59 (B2 phylogroup; urine origin), IAI69 (B2 phylogroup; miscellaneous origin), and IAI73 (B2 phylogroup; blood origin) (Picard et al. 1999) were used as recipients of antimicrobial resistance transferred by CC87 strains. Strains from the B1 phylogenetic group used to validate the CC87 allele-specific PCR were coming from Clermont et al. (2011) and from the strains sequenced at the Broad Institute of Harvard and MIT in the frame of the “Escherichia coli Antibiotic Resistance Sequencing Project” (http://www.broadinstitute.org/ last accessed November 12, 2015).
Phylotyping, Virulence Gene Content, and O-Typing of the Strains
MLST was performed as in Jaureguy et al. (2008) using the partial sequences of eight housekeeping genes (dinB, icdA, pabB, polB, putP, trpA, trpB, and uidA) (http://www.pasteur.fr/mlst last accessed November 12, 2015). BioNumerics v6.5 (Applied-Maths, Belgium) was used to perform minimum spanning tree analysis. Phylogenetic reconstruction was performed using PHYML (Guindon et al. 2005). Eighteen genes encoding VFs were detected by PCR as in Clermont et al. (2011) and encompassed both extraintestinal (kpsE, sfa/foc, iroN, aer, papC, hly, cnf1, and fyuA) and intestinal (afaD, ipaH, stx1, stx2, eae, bfpA, aatA, aaiC, LT, and ST) VFs. O-types were determined in a subset of strains by PCR as in Clermont et al. (2007, 2011).
Antimicrobial Drug Susceptibility Testing and Detection of Tet Genes
Antimicrobial susceptibilities to seven main antimicrobial agents (amoxicillin, sulphonamides, chloramphenicol, kanamycin, streptomycin, nalidixic acid, tetracycline) were determined by disc diffusion, according to the recommendations of the Clinical Laboratory Standards Institute (CLSI, http://clsi.org/ last accessed November 12, 2015). The RS was defined as the ratio of the total number of observed resistances to the total number of possible resistance × 100 (Murray et al. 1990). For the tetracycline resistance testing, all non-susceptible strains, according to the CLSI classification, were considered as resistant and determination of the tetracycline MICs was performed using E-test (bioMérieux). A multiplex PCR was used to detect tetracycline resistance genes tet(A) to tet(E) (Nawaz et al. 2006). The genes tet(G), (J), (L), (M), (W), (Y), (X2), and tet(39) were also screened by PCR (Aminov et al. 2002; Agersø and Guardabassi 2005). Inhibition of efflux mechanisms was performed using the efflux pump inhibitor Phe-Arg-β-naphtylamide (Kadlec et al. 2007).
Conjugation Experiments
Conjugation was performed on solid media as previously described (Phornphisutthimas et al. 2007). The efficiency of conjugation was measured as the number of transconjugants per donor cell by plating on agar media containing the appropriate antimicrobial agents. For each individual strains, biological replicates were performed during independent experiments at different times.
Plasmid Extraction and Determination of Incompatibility Groups
Plasmid DNA extraction from the strains used for conjugation assays was performed by the Kieser method (Kieser 1984). Plasmid incompatibility groups were determined using an adapted PCR-based replicon typing (Carattoli 2009; Villa et al. 2010). Five multiplex PCRs were used for the detection of HI1, HI2, I1, X, L/M, N, FIA, FIB, W, Y, P, FIC, A/C, T, and FIIs replicons. Replicons F, K, B/O, R, and U were detected by simplex PCR. All the repF replicons were subsequently sequenced. Replicons FII1, FII2, NewXXX (also named ZK), LVPK, and Met were detected using the PCR method developed by D. Decré and G. Arlet (G. Arlet, personal communication). The repA, traU, and parA genes were detected by PCR as described elsewhere (Poirel et al. 2012).
Allele-Specific PCR Amplification of CC87 Strains
PCR reaction was carried out in a 20-µl volume containing 4 µl of 5X buffer (supplied with Taq polymerase) giving a final MgCl2 concentration at 1.5 mM, 20 pmol of each primer, 200 µM each dNTP, 2 U of Taq polymerase (Promega, Charbonnières-les-Bains, France), and 3 µl of bacterial lysate or 2 µl of DNA (∼200 ng). PCR was performed with an Eppendorf Mastercycler with MicroAm tubes in the following conditions: denaturation 4 min at 94°C, 30 cycles of 5 s at 94°C, and 20 s at 63°C, and a final extension step of 5 min at 72°C. The primers used for allele-specific amplification of CC87 strains, located in icd, were icdCC87.f (5’-CCGAAGGCGCGTTTAAAGAT-3’) and icdCC87.r (5’-CGTCAGAAATGTAGTCACCA-3’); they generate a 226 bp product. This gene was chosen as it contained CC87-specific single-nucleotide polymorphisms allowing the design of the allele-specific primers. In addition, primers trpBA.f (5’-CGGCGATAAAGACATCTTCAC-3’) and trpA2.r (5’-GCAACGCGGCCTGGCGGAAG-3’) located in trpA were added in the same reaction to obtain a PCR control amplicon of 489 bp in all tested strains as in (Clermont, Lescat, et al. 2008). PCR products were loaded on 2% agarose gel with SYBR Safe DNA gel stain (Invitrogen, Cergy Pontoise, France). After electrophoresis, gels were photographed under UV light.
High-Throughput Biolog Phenotyping
Two representative E. coli strains (CC87 strain ROAR61 and non-CC87 strain ROAR394) were analyzed by Biolog (Hayward, CA) Phenotype Microarrays (PM1 to PM20). Prior to inoculation, E. coli isolates were grown on BUGB agar plates (Hayward, CA) at 30°C. Cells suspensions were prepared and inoculated in accordance with the manufacturer’s instructions. Plates were incubated at 37°C, and optical density at 590 nm (OD590) was monitored using a Synergy 2 microplate reader (Bio-Tek). The 24-h OD590 reading of each well was subtracted by the OD590 value obtained at T 0 h. For each well, we calculated the ratio of the normalized 24-h OD590 ROAR61/ROAR394 and ratios >2 or < 0.5 were considered biologically relevant besides their statistical significance (P < 0.05).
Illumina Sequencing and Mapping and Phylogenetic Analysis
Chromosomal DNA was extracted from seven ROAR strains of the CC87: 4 strains belonging to ST21 (ROAR47, 61, 72, and 82) and three strains belonging to ST24 and ST87 (ROAR205, 334 and 419) using DNeasy Blood and Tissue Kit (Qiagen) from 1 ml of overnight culture grown in LB. DNA quality and amount was measured using Nanodrop 1000 spectrophotometer (Thermo Scientific). One microgram of genomic DNA was sheared using Q500 sonicator (QSonica), and the fragments were used to prepare libraries using a NEBNext Ultra DNA library kit (New England Biolabs). Libraries were barcoded and sequenced as 50-nucleotide single-end reads on the Illumina HiSeq2000 flow cell. Reads were aligned using CLC-Genomics Workbench software. At least 17 million reads were obtained for each sample.
The seven ROAR sequencing data sets were assembled from seven fastq files containing 50 base pair length single reads using SOAPdenovo version 2.04 (Luo et al. 2012). The contigs were then mapped, using BWA 0.7.10 (Li and Durbin 2010) to a data set of 128 complete E. coli genomes that are listed in Lescat et al. (2014). For each contig, we identified the reference E. coli genome with the highest similarity. We extracted from that reference the gene annotations within the contig mapping region. We could therefore identify the gene repertoire of each sequenced genome and extract their sequence. Gene presence and absence were recorded in the 128 reference genomes using homology and synteny to define the homologous gene categories and extended to include the strains we sequenced. We also extracted and aligned the sequences of 500 genes from the core E. coli genome using MUSCLE v3.8.31 (Edgar 2004). We concatenated these 500 genes into one large sequence for each strain that was then used to create a phylogeny using PhyML v3.0 (Guindon et al. 2010) under the HKY85 substitution model.
Animal Models
Intrinsic extraintestinal virulence was tested in a mouse model of sepsis following neck subcutaneous inoculation of 2 × 108 bacteria as in Picard et al. (1999). Gut colonization of the strains was studied in 6-week-old female mice (Charles River, CD-1) pretreated with streptomycin (Vimont et al. 2012).
Statistical Analysis
A factorial analysis of correspondence (Greenacre 1992) was used to describe associations among the various variables with SPAD.N software (Cisia, Saint Mandé, France).
We evaluated the marginal association between antimicrobial resistance, defined either as the total number of resistance or as the resistance to two or more antimicrobial agents, and parameters 1) specific to the host: nature, geographic origin (Antarctica, Africa or Europe), or category and 2) specific to the bacteria: phylogroup (A, B1, D, B2, or other), MLST (ST and CC), extraintestinal VFs, using standard linear regression. For nonordinal categorical variables (e.g., geographic origin), we coded each category as a dummy variable, using arbitrarily a reference category. We also derived for each of these predictors the adjusted r-squared (to account for the number of categories included) for association with the total number of resistance. Finally, because these predictors were correlated, it was not possible to evaluate objectively the relative importance of each of them in a multivariate model. Then, we derived the odds ratios between the CC87 strains and resistance for six specific antimicrobial agents and between CC87 strains and resistance to two or more antimicrobial agents. Odds ratios were estimated through logistic regression. The CC87 strains were coded as a binary variable, whereas all non-CC87 strains were merged into a single reference group. However, strains from CC1, CC2, and CC66 were excluded from the analysis as they displayed significant marginal association with antimicrobial resistance.
Significant differences between groups were determined by the χ2 test using the R statistical system software (http://www.r-project.org, last accessed November 12, 2015). P < 0.05 was considered significant.
Supplementary Material
Supplementary figures S1–S5 and tables S1–S6 are available at Molecular Biology and Evolution online (http://www.mbe.oxfordjournals.org/ last accessed November 12, 2015).
Acknowledgments
This work was originally supported by the Alliance for the Prudent Use of Antibiotics (APUA) in the frame of the Reservoirs of Antibiotic Resistance (ROAR) project 2006–2007. We are grateful to Guillaume Arlet for providing us the clinical E. coli strains exhibiting the blaTEM-1 gene. We thank Jennifer O'Malley for her technical support.
References
- Agersø Y, Guardabassi L. 2005. Identification of Tet 39, a novel class of tetracycline resistance determinant in Acinetobacter spp. of environmental and clinical origin. J Antimicrob Chemother. 55:566–569. [DOI] [PubMed] [Google Scholar]
- Aminov RI, Chee-Sanford JC, Garrigues N, Teferedegne B, Krapac IJ, White BA, Mackie RI. 2002. Development, validation, and application of PCR primers for detection of tetracycline efflux genes of gram-negative bacteria. Appl Environ Microbiol. 68:1786–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai S, Kolodkin-Gal I, Hananya-Meltabashi M, Sacher A, Engelberg-Kulka H. 2009. Escherichia coli MazF leads to the simultaneous selective synthesis of both “death proteins” and “survival proteins”. PLoS Genet. 5:e1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DI, Hughes D. 2010. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat Rev Microbiol. 8:260–271. [DOI] [PubMed] [Google Scholar]
- Andremont A. 2003. Commensal flora may play key role in spreading antibiotic resistance. ASM News 63:601–607. [Google Scholar]
- Aubry-Damon H, Grenet K, Sall-Ndiaye P, Che D, Cordeiro E, Bougnoux ME, Rigaud E, Le Strat Y, Lemanissier V, Armand-Lefevre L, et al. 2004. Antimicrobial resistance in commensal flora of pig farmers. Emerg Infect Dis. 10:873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava S, Johnson BB, Hwang J, Harris TA, George AS, Muir A, Dorff J, Okeke IN. 2009. Heat-resistant agglutinin 1 is an accessory enteroaggregative Escherichia coli colonization factor. J Bacteriol. 191:4934–4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branger C, Zamfir O, Geoffroy S, Laurans G, Arlet G, Thien HV, Gouriou S, Picard B, Denamur E. 2005. Genetic background of Escherichia coli and extended-spectrum beta-lactamase type. Emerg Infect Dis. 11:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisse S, Diancourt L, Laouenan C, Vigan M, Caro V, Arlet G, Drieux L, Leflon-Guibout V, Mentre F, Jarlier V, et al. 2012. Phylogenetic distribution of CTX-M- and non-extended-spectrum-beta-lactamase-producing Escherichia coli isolates: group B2 isolates, except clone ST131, rarely produce CTX-M enzymes. J Clin Microbiol. 50:2974–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob Agents Chemother. 53:2227–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DE, Smalley DJ, Tucker DL, Leatham MP, Norris WE, Stevenson SJ, Anderson AB, Grissom JE, Laux DC, Cohen PS, et al. 2004. Carbon nutrition of Escherichia coli in the mouse intestine. Proc Natl Acad Sci U S A. 101:7427–7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont O, Christenson JK, Denamur E, Gordon DM. 2013. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep. 8:58–65. [DOI] [PubMed] [Google Scholar]
- Clermont O, Gordon D, Denamur E. 2015. Guide to the various phylogenetic classification schemes for Escherichia coli and the correspondence among schemes. Microbiology 161:980–988. [DOI] [PubMed] [Google Scholar]
- Clermont O, Johnson JR, Menard M, Denamur E. 2007. Determination of Escherichia coli O types by allele-specific polymerase chain reaction: application to the O types involved in human septicemia. Diagn Microbiol Infect Dis. 57:129–136. [DOI] [PubMed] [Google Scholar]
- Clermont O, Lavollay M, Vimont S, Deschamps C, Forestier C, Branger C, Denamur E, Arlet G. 2008. The CTX-M-15-producing Escherichia coli diffusing clone belongs to a highly virulent B2 phylogenetic subgroup. J Antimicrob Chemother. 61:1024–1028. [DOI] [PubMed] [Google Scholar]
- Clermont O, Lescat M, O'Brien CL, Gordon DM, Tenaillon O, Denamur E. 2008. Evidence for a human-specific Escherichia coli clone. Environ Microbiol. 10:1000–1006. [DOI] [PubMed] [Google Scholar]
- Clermont O, Olier M, Hoede C, Diancourt L, Brisse S, Keroudean M, Glodt J, Picard B, Oswald E, Denamur E. 2011. Animal and human pathogenic Escherichia coli strains share common genetic backgrounds. Infect Genet Evol. 11:654–662. [DOI] [PubMed] [Google Scholar]
- Croxen MA, Finlay BB. 2010. Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol. 8:26–38. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 97:6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps C, Clermont O, Hipeaux MC, Arlet G, Denamur E, Branger C. 2009. Multiple acquisitions of CTX-M plasmids in the rare D2 genotype of Escherichia coli provide evidence for convergent evolution. Microbiology 155:1656–1668. [DOI] [PubMed] [Google Scholar]
- Desjardins P, Picard B, Kaltenbock B, Elion J, Denamur E. 1995. Sex in Escherichia coli does not disrupt the clonal structure of the population: evidence from random amplified polymorphic DNA and restriction-fragment-length polymorphism. J Mol Evol. 41:440–448. [DOI] [PubMed] [Google Scholar]
- Diard M, Garry L, Selva M, Mosser T, Denamur E, Matic I. 2010. Pathogenicity-associated islands in extraintestinal pathogenic Escherichia coli are fitness elements involved in intestinal colonization. J Bacteriol. 192:4885–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Paramo P, Le Menac'h A, Le Gall T, Amorin C, Gouriou S, Picard B, Skurnik D, Denamur E. 2006. Identification of forces shaping the commensal Escherichia coli genetic structure by comparing animal and human isolates. Environ Microbiol. 8:1975–1984. [DOI] [PubMed] [Google Scholar]
- Fantin B, Duval X, Massias L, Alavoine L, Chau F, Retout S, Andremont A, Mentre F. 2009. Ciprofloxacin dosage and emergence of resistance in human commensal bacteria. J Infect Dis. 200:390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grall N, Barraud O, Wieder I, Hua A, Perrier M, Babosan A, Gaschet M, Clermont O, Denamur E, Catzeflis F, et al. 2015. Lack of dissemination of acquired resistance to beta-lactams in small wild mammals around an isolated village in the Amazonian forest. Environ Microbiol Rep. 7:698–708. [DOI] [PubMed] [Google Scholar]
- Greenacre M. 1992. Correspondence analysis in medical research. Stat Methods Med Res. 1:97–117. [DOI] [PubMed] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 59:307–321. [DOI] [PubMed] [Google Scholar]
- Guindon S, Lethiec F, Duroux P, Gascuel O. 2005. PHYML Online–a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res. 33:W557–W559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaureguy F, Landreau L, Passet V, Diancourt L, Frapy E, Guigon G, Carbonnelle E, Lortholary O, Clermont O, Denamur E, et al. 2008. Phylogenetic and genomic diversity of human bacteremic Escherichia coli strains. BMC Genomics 9:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TJ, Wannemuehler YM, Johnson SJ, Logue CM, White DG, Doetkott C, Nolan LK. 2007. Plasmid replicon typing of commensal and pathogenic Escherichia coli isolates. Appl Environ Microbiol. 73:1976–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadlec K, Kehrenberg C, Schwarz S. 2007. Efflux-mediated resistance to florfenicol and/or chloramphenicol in Bordetella bronchiseptica: identification of a novel chloramphenicol exporter. J Antimicrob Chemother. 59:191–196. [DOI] [PubMed] [Google Scholar]
- Karch H, Denamur E, Dobrindt U, Finlay BB, Hengge R, Johannes L, Ron EZ, Tonjum T, Sansonetti PJ, Vicente M. 2012. The enemy within us: lessons from the 2011 European Escherichia coli O104:H4 outbreak. EMBO Mol Med. 4:841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser T. 1984. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19–36. [DOI] [PubMed] [Google Scholar]
- Lefort A, Panhard X, Clermont O, Woerther PL, Branger C, Mentre F, Fantin B, Wolff M, Denamur E. 2011. Host factors and portal of entry outweigh bacterial determinants to predict the severity of Escherichia coli bacteremia. J Clin Microbiol. 49:777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescat M, Reibel F, Pintard C, Dion S, Glodt J, Gateau C, Launay A, Ledda A, Cruvellier S, Tourret J, et al. 2014. The conserved nhaAR operon is drastically divergent between B2 and non-B2 Escherichia coli and is involved in extra-intestinal virulence. PLoS One 9:e108738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R. 2010. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, He G, Chen Y, Pan Q, Liu Y, et al. 2012. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience 1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini J, Weckselblatt B, Chung YK, Durante JC, Andelman S, Glaubman J, Dorff JD, Bhargava S, Lijek RS, Unger KP, et al. 2011. The heat-resistant agglutinin family includes a novel adhesin from enteroaggregative Escherichia coli strain 60A. J Bacteriol. 193:4813–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manges AR, Johnson JR, Foxman B, O'Bryan TT, Fullerton KE, Riley LW. 2001. Widespread distribution of urinary tract infections caused by a multidrug-resistant Escherichia coli clonal group. N Engl J Med. 345:1007–1013. [DOI] [PubMed] [Google Scholar]
- Mihaila L, Wyplosz B, Clermont O, Garry L, Hipeaux MC, Vittecoq D, Dussaix E, Denamur E, Branger C. 2010. Probable intrafamily transmission of a highly virulent CTX-M-3-producing Escherichia coli belonging to the emerging phylogenetic subgroup D2 O102-ST405 clone. J Antimicrob Chemother. 65:1537–1539. [DOI] [PubMed] [Google Scholar]
- Murray BE, Mathewson JJ, DuPont HL, Ericsson CD, Reves RR. 1990. Emergence of resistant fecal Escherichia coli in travelers not taking prophylactic antimicrobial agents. Antimicrob Agents Chemother. 34:515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz M, Sung K, Khan SA, Khan AA, Steele R. 2006. Biochemical and molecular characterization of tetracycline-resistant Aeromonas veronii isolates from catfish. Appl Environ Microbiol. 72:6461–6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou JT, Reim R. 1976. Effect of 1,10-phenanthroline on bacterial conjugation in Escherichia coli K-12: inhibition of maturation from preliminary mates into effective mates. J Bacteriol. 128:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phornphisutthimas S, Thamchaipenet A, Panijpan B. 2007. Conjugation in Escherichia coli: A laboratory exercise. Biochem Mol Biol Educ. 35:440–445. [DOI] [PubMed] [Google Scholar]
- Picard B, Garcia JS, Gouriou S, Duriez P, Brahimi N, Bingen E, Elion J, Denamur E. 1999. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect Immun. 67:546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirel L, Bonnin RA, Nordmann P. 2012. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob Agents Chemother. 56:559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Maillard JM, Arutyunov D, Frost LS. 2010. The F plasmid transfer activator TraJ is a dimeric helix-turn-helix DNA-binding protein. FEMS Microbiol Lett. 310:112–119. [DOI] [PubMed] [Google Scholar]
- Roux D, Danilchanka O, Guillard T, Cattoir V, Aschard H, Fu Y, Angoulvant F, Messika J, Ricard JD, Mekalanos JJ, et al. 2015. Fitness cost of antibiotic susceptibility during bacterial infection. Sci Transl Med. 7:297ra114. [DOI] [PubMed] [Google Scholar]
- Skurnik D, Le Menac'h A, Zurakowski D, Mazel D, Courvalin P, Denamur E, Andremont A, Ruimy R. 2005. Integron-associated antibiotic resistance and phylogenetic grouping of Escherichia coli isolates from healthy subjects free of recent antibiotic exposure. Antimicrob Agents Chemother. 49:3062–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik D, Ruimy R, Andremont A, Amorin C, Rouquet P, Picard B, Denamur E. 2006. Effect of human vicinity on antimicrobial resistance and integrons in animal faecal Escherichia coli. J Antimicrob Chemother. 57:1215–1219. [DOI] [PubMed] [Google Scholar]
- Tenaillon O, Skurnik D, Picard B, Denamur E. 2010. The population genetics of commensal Escherichia coli. Nat Rev Microbiol. 8:207–217. [DOI] [PubMed] [Google Scholar]
- Tong S, Porco A, Isturiz T, Conway T. 1996. Cloning and molecular genetic characterization of the Escherichia coli gntR, gntK, and gntU genes of GntI, the main system for gluconate metabolism. J Bacteriol. 178:3260–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchon M, Hoede C, Tenaillon O, Barbe V, Baeriswyl S, Bidet P, Bingen E, Bonacorsi S, Bouchier C, Bouvet O, et al. 2009. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet. 5:e1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa L, Garcia-Fernandez A, Fortini D, Carattoli A. 2010. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J Antimicrob Chemother. 65:2518–2529. [DOI] [PubMed] [Google Scholar]
- Vimont S, Boyd A, Bleibtreu A, Bens M, Goujon JM, Garry L, Clermont O, Denamur E, Arlet G, Vandewalle A. 2012. The CTX-M-15-producing Escherichia coli clone O25b:H4-ST131 has high intestine colonization and urinary tract infection abilities. PLoS One 7:e46547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, et al. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol. 60:1136–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerther PL, Burdet C, Chachaty E, Andremont A. 2013. Trends in human fecal carriage of extended-spectrum beta-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev. 26:744–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford N, Turton JF, Livermore DM. 2011. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev. 35:736–755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.