Abstract
STUDY QUESTION
Do high oxygen tension and high glucose concentrations dysregulate p66Shc (Src homologous-collagen homologue adaptor protein) expression during mouse preimplantation embryo culture?
SUMMARY ANSWER
Compared with mouse blastocysts in vivo, P66Shc mRNA and protein levels in blastocysts maintained in vitro increased under high oxygen tension (21%), but not high glucose concentration.
WHAT IS KNOWN ALREADY
Growth in culture adversely impacts preimplantation embryo development and alters the expression levels of the oxidative stress adaptor protein p66Shc, but it is not known if p66Shc expression is linked to metabolic changes observed in cultured embryos.
STUDY DESIGN, SAMPLES/MATERIALS, METHODS
We used a standard wild-type CD1 mouse model of preimplantation embryo development and embryo culture with different atmospheric oxygen tension and glucose media concentrations. Changes to p66Shc expression in mouse blastocysts were measured using quantitative RT-PCR, immunoblotting and immunofluorescence followed by confocal microscopy. Changes to oxidative phosphorylation metabolism were measured by total ATP content and superoxide production. Statistical analyses were performed on a minimum of three experimental replicates using Students’ t-test or one-way ANOVA.
MAIN RESULTS AND THE ROLE OF CHANCE
P66Shc is basally expressed during in vivo mouse preimplantation development. Within in vivo blastocysts, p66Shc is primarily localized to the cell periphery of the trophectoderm. Blastocysts cultured under atmospheric oxygen levels have significantly increased p66Shc mRNA transcript and protein abundances compared to in vivo controls (P < 0.05). However, the ratio of phosphorylated serine 36 (S36) p66Shc to total p66Shc decreased in culture regardless of O2 atmosphere used, supporting a shift in the mitochondrial fraction of p66Shc. Total p66Shc localized to the cell periphery of the blastocyst trophectoderm and phosphorylated S36 p66Shc displayed nuclear and cytoplasmic immunoreactivity, suggesting distinct compartmentalization of phosphorylated S36 p66Shc and the remaining p66Shc fraction. Glucose concentration in the culture medium did not significantly change p66Shc mRNA or protein abundance or its localization. Blastocysts cultured under low or high oxygen conditions exhibited significantly decreased cellular ATP and increased superoxide production compared to in vivo derived embryos (P < 0.05).
LIMITATIONS/REASONS FOR CAUTION
This study associates embryonic p66Shc expression levels with metabolic abnormalities but does not directly implicate p66Shc in metabolic changes. Additionally, we used one formulation of embryo culture medium that differs from that used in other mouse model studies and from clinical media used to support human blastocyst development. Our findings may, therefore, be limited to this media, or may be a species-specific phenomenon.
WIDER IMPLICATIONS OF THE FINDINGS
This is the first study to show distinct immunolocalization of p66Shc to the trophectoderm of mouse blastocysts and that its levels are abnormally increased in embryos exposed to culture conditions. Changes in p66Shc expression and/or localization could possibly serve as a molecular marker of embryo viability for clinical applications. The outcomes provide insight into the potential metabolic role of p66Shc. Metabolic anomalies are induced even under the current optimal culture conditions, which could negatively impact trophectoderm and placental development.
LARGE SCALE DATA
Not applicable.
STUDY FUNDING AND COMPETING INTEREST(S)
Canadian Institutes of Health Research (CIHR) operating funds, Ontario Graduate Scholarship (OGS). There are no competing interests.
Keywords: blastocyst; embryo culture; metabolism; mitochondria; p66Shc; preimplantation embryo; reactive oxygen species, stress adaptor
Introduction
In assisted reproductive technologies, embryo culture routinely follows in vitro fertilization (IVF) to permit growth to the blastocyst stage. Despite improvements in culture medium formulations and the use of physiological oxygen environments, the rate of successful pregnancy after embryo culture remains low. In 2014, the average live birth rate per IVF cycle for women in Canada was 23% (CFAS, 2015). Low success rates may be due to exposure of the preimplantation embryo to stresses introduced by the artificial culture environment (Feuer and Rinaudo, 2012; Wale and Gardner, 2015). The mammalian preimplantation embryo may adapt to these adverse culture conditions, however, these stress-induced responses can result in major changes to gene expression, epigenetic modifications and cellular metabolism (Rinaudo and Schultz, 2004; Wale and Gardner, 2012; de Waal et al., 2014). These changes are currently undetectable according to non-invasive morphological assessment methods, and thus embryos selected by morphology for transfer may still not be the most developmentally competent. This is a particular concern in current efforts to reduce multiple pregnancies by performing single embryo transfer (Grady et al., 2012).
To further advance embryo culture and optimize culture parameters, it is important to understand the biological mechanisms of the preimplantation embryo and its interactions with the reproductive tract environment in vivo and the culture environment in vitro. Metabolism has emerged as an important research avenue in efforts to understand how culture conditions affect the developmental competence of early embryos (Gardner et al., 2001; Seli et al., 2010; Wale and Gardner, 2013). Modulating oxygen tension during embryo culture alters glucose metabolism, demonstrating that the culture atmosphere can dramatically influence embryo metabolism and subsequent viability (Wale and Gardner, 2012). This may affect the later stages of development in particular, as the trophectoderm must generate ATP to power the Na+/K+ ATPases and form the blastocoele cavity (Betts et al., 1998; Houghton et al., 2003). The adaptor protein p66Shc is responsive to oxygen tension and is involved in the bovine embryo's oxidative stress response by promoting permanent embryo arrest and apoptosis under adverse environmental conditions (Favetta et al., 2007a; Betts et al., 2014). P66Shc is a member of the Shc1 family of adaptor proteins with functions in growth factor receptor signaling, reactive oxygen species (ROS) production and oxidative phosphorylation metabolism (Migliaccio et al., 1997, 1999; Nemoto et al., 2006; Acin-Perez et al., 2010). Loss-of-function studies in mouse embryonic fibroblasts (MEFs) and more recently in HeLa cells provide evidence that p66Shc is involved in ATP production by oxidative phosphorylation (Nemoto et al., 2006; Soliman et al., 2014). Dysregulated p66Shc function in the mammalian embryo may therefore not only negatively impact development through high ROS production inducing embryo arrest or apoptosis (Favetta et al., 2004, 2007b; Betts et al., 2014), but also may affect cellular metabolism (Favetta et al., 2007a).
To define a new metabolic route through which preimplantation embryo culture may affect early embryonic development, the objective of our study was to determine if p66Shc expression changes in cultured embryos compared to in vivo derived embryos, and if altered p66Shc expression is a marker of altered embryo metabolism. In the following study, we use a well-defined preimplantation mouse embryo culture model to modulate atmospheric conditions (oxygen) and culture media (glucose concentration) to determine their effects on p66Shc expression and readouts of oxidative phosphorylation metabolism. Our results demonstrate that preimplantation developmental variations in p66Shc expression observed in vivo are further exacerbated by culture and correlate with aberrant mitochondrial ATP and ROS production.
Materials and Methods
Animal source and ethical approval
Experimental protocols were approved by the Canadian Council of Animal Care and the University of Western Ontario Animal Care and Veterinary Services (Watson #2010-021). Female and male CD1 mice were obtained from Charles River Canada (St-Constant, Quebec, Canada). Mice were housed in the conventional manner, with a 12 h light/dark cycle and access to food and water ad libitum. For all experiments, mice were euthanized by CO2 asphyxiation.
Embryo collection and culture
Three-to-four weeks old female mice were injected i.p. with 7.5 IU pregnant mare's serum gonadotrophin (Merck Animal Health, Canada) followed by injection of 7.5 IU hCG (Merck Animal Health, Canada) 48 h later. Female mice were then placed with males for mating. Confirmation of mating was determined by checking for the presence of a vaginal plug the next morning; presence of a vaginal plug indicated embryonic day 0.5 (E0.5). Embryos were flushed with M2 medium (Sigma Aldrich, Canada) from the oviducts and/or uteri of female mice according to the number of hours post-injection (hpi): zygotes (18 hpi), 2-cell embryos (44 hpi), 8-cell embryos (68 hpi) and blastocysts (90 hpi). Zygotes were briefly incubated in M2 medium containing 1% hyaluronidase (Sigma Aldrich, Canada) to remove cumulus cells. Embryos were washed twice in M2, then transferred to Extraction Buffer or radioimmunoprecipitation assay buffer (RIPA buffer, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris) until analysis, or to pre-equilibrated KSOMaa (potassium simplex optimized media with amino acids) Evolve medium supplemented with 1% bovine serum albumin (Zenith Biotech, USA). Embryos were cultured under low (5% O2) or high (in air: ~20%) oxygen tensions in a 5% CO2, 37°C incubator. For glucose experiments, d- or l-glucose (Sigma Aldrich, Canada) was added to KSOMaa Evolve to the desired concentration and embryos were cultured under low oxygen. For transcriptional inhibition experiments, 10 mg/ml α-amanitin (Sigma Aldrich, Canada) in water was diluted to 10 μg/ml in KSOMaa Evolve.
Real time quantitative RT-PCR
Pools of 20 embryos collected from 1–3 mice were stored in Extraction Buffer (Life Technologies, USA) at −80°C until use. Total RNA was extracted using the PicoPure RNA isolation kit (Life Technologies, USA) according to the manufacturer's guidelines. For glucose treatment experiments, 0.5 pg of exogenous luciferase mRNA (Promega, USA) was added to the extract prior to ethanol precipitation. Eluted RNA was reverse transcribed to cDNA using SuperScript III (Life Technologies, USA) according to manufacturer's instructions, with final concentrations of 150 ng random hexamers (Life Technologies, USA) and 2 pmol p66Shc-specific reverse primer (Table I). Real time quantitative RT-PCR (qRT-PCR) was performed in a CFX384 thermocycler (BioRad, Canada) with each reaction containing 7 μl PerfeCTa SYBR Green 2X SuperMix (Quanta BioSciences, USA), 200 nM each of forward and reverse primers (see Table I for all primer sequences) and 4 μl cDNA (equivalent to 0.25 embryo per reaction). PCR conditions are as follows: 95°C for 3 min, followed by 45 cycles of 95°C for 15 s, 59°C for 15 s, and 72°C for 30 s. Relative transcript abundance was determined using the delta-delta CT method using expression of Ppia (peptidylprolyl isomerase A) and H2afz (H2A Histone Family, Member Z), or luciferase, for normalization (Mamo et al., 2007). To determine amplification specificity after PCR amplification of p66Shc in blastocyst cDNA, PCR products were purified using the PureLink Quick Gel Extraction and PCR Purification Kit (Life Technologies, USA) according to manufacturer's instructions. PCR products were sequenced by the Robarts Research Institute DNA Sequencing Facility (London, Ontario, Canada). Amplified p66Shc PCR products displayed 96% sequence identity to Mus musculus src homology 2 domain-containing transforming protein C1 (Shc1), transcript variant 1 (NM_001113331.2) after BLAST analysis (NCBI database), indicating specific amplification of the p66Shc isoform.
Table I.
Oligonucleotide primer sequences used in quantitative RT-PCR.
| Expected product size | ||
|---|---|---|
| Src homology 2 domain containing (p66Shc) (for reverse transcription) | 5′-GGTGGATTCCTGAGATACTGTTT-3′ | N/A |
| p66Shc (qRT-PCR) | F: 5′-CCGACTACCCTGTGTTCCTTCTT-3′ | 111 bp |
| R: 5′-CCCATCTTCAGCAGCCTTTCC-3′ | ||
| Peptidylprolyl isomerase 1A (Ppia) | F: 5′-GTCCTGGCATCTTGTCCATG-3′ | 126 bp |
| R: 5′-TGCCTTCTTTCACCTTCCCA-3′ | ||
| H2A histone family member Z (H2afz) | F: 5′-CGCAGAGGTACTTGAGTTGG-3′ | 176 bp |
| R: 5′-TCTTCCCGATCAGCGATTTG-3′ | ||
| Luciferase | F: 5′-TTGACAAGGATGGATGGCTAC-3′ | 336 bp |
| R: 5′-TTCGGTACTTCGTCCACCAAAC-3′ | ||
Western blot analysis
Pools of 30–50 embryos collected from two to four mice were stored in RIPA buffer containing protease and phosphatase inhibitor cocktails (Millipore, USA) at -80°C until use. Total protein lysates were resolved on a 4–12% Bis-Tris gel (Life Technologies) and transferred to a polyvinylidene diflouride membrane (PVDF: Millipore, USA). Membranes were blocked in 5% skim milk or 5% bovine serum albumin in PBS with 0.1% Tween-20 (PBST, Sigma Aldrich) for 1 h at room temperature, followed by overnight incubation in primary antibody at the indicated concentration at 4°C. Primary antibodies used were: anti NT-Shc (Acris Antibodies, USA, 1:100), anti-(phospho S36) p66Shc (Abcam, USA, 1:100), anti-(phospho Y239/Y240) p66Shc (Cell Signaling Technologies, USA, 1:500), and horse-radish peroxidase (HRP)-conjugated anti β-actin (Sigma Aldrich, Canada, 1:20,000). Membranes were then incubated in HRP-conjugated secondary antibody (Jackson Laboratories, USA). Membranes were visualized by detection of Forte enhanced chemiluminescence (Millipore, USA). Densitometry analysis was performed in Image Lab 4.0 (BioRad, USA).
HT-22 culture and transfection
The HT-22 cell line (immortalized mouse hippocampal cells) and human p66Shc-HA expression plasmid were obtained from Dr Robert Cumming (University of Western Ontario, London, Canada). Cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Life Technologies, USA), at 37°C and 5% CO2 in air. Cells were transfected with the p66Shc-HA expression plasmid using Lipofectamine 3000 according to the manufacturer's protocol (Life Technologies, USA), fixed in 4% paraformaldehyde in PBS and processed for immunofluorescence and confocal microscopy.
Immunofluorescence and confocal microscopy
Embryos were fixed in 2% paraformaldehyde in PBS and permeabilized in 0.1% Triton X-100 in PBS (Sigma Aldrich, Canada) for 30 min. Fixed cells were blocked in 5% normal goat serum (Sigma Aldrich, Canada) for 1 h at room temperature, followed by overnight incubation in primary antibody at the indicated concentration at 4°C. Primary antibodies used were: anti NT-Shc (Acris Antibodies, 1:100), anti phospho-S36-p66Shc (Abcam, 1:100), anti CDX2 (Abcam, 1:100), anti HA-Alexa 647 (Santa Cruz, USA, 1:50). Embryos were incubated in rabbit-anti-mouse Alexa 488 (Life Technologies) for 30 min, followed by incubation in goat-anti-rabbit Alexa 488 (Life Technologies) for signal amplification. For caudal type homeobox 2 (CDX2) immunoreactivity, embryos were incubated in goat-anti-rabbit Alexa 547 (Life Technologies). Cells were counterstained with 0.5 µg/ml DAPI (Sigma Aldrich, Canada) and mounted on a glass microscope slide in VectaShield antifade medium (Vector Laboratories, USA). Cells were imaged with a laser scanning confocal microscope (Zeiss LSM510). Laser settings were unchanged when detecting the same primary antibody.
ATP content assay
Pools of five blastocysts collected from individual mice after treatment under each oxygen tension group were transferred to 96-well plates containing KSOMaa Evolve. ATP content was measured using the Luminescent ATP Detection Assay Kit (Abcam, USA) according to manufacturer's guidelines. Luminescence was quantified using an eight-point ATP standard curve (0.78–100 pmol) and normalized to blastocyst cell number.
MitoSOX superoxide staining
Blastocysts from each oxygen tension group were transferred to KSOMaa Evolve containing 5 μM MitoSOX red mitochondrial superoxide indicator (Life Technologies, USA) and incubated for 1 h at 37°C, 5% CO2, 5% O2 (in vivo and low oxygen groups) or in air (high oxygen groups). Blastocysts were transferred to a drop of PBS covered by embryo culture grade mineral oil (Zenith Biotech, USA) for imaging. Blastocysts were imaged using laser scanning confocal microscopy (Zeiss LSM510). Relative fluorescence was quantified by measuring the mean gray value in Image J (National Institutes of Health, MD, USA). Only blastocyst images with visible inner cell mass were quantified for fluorescence and compared between groups.
Blastocyst cell counts
Blastocysts were fixed in 4% paraformaldehyde in PBS, permeabilized in 0.2% Triton X-100 in PBS, and stained with DAPI for 1 h at room temperature. Stained blastocysts were imaged using laser scanning confocal microscopy, with three z-stacks taken per embryo. DAPI-positive nuclei from three stacks were counted using ImageJ.
Statistical analyses
Experiments were performed a minimum of three times using independent replicates with the indicated sample sizes. Statistical analyses were performed in Graph Pad Prism (6.0) for Student's t-test (unpaired, two-tailed and equal variance) or one-way ANOVA followed by Tukey's honestly significant difference test to correct for multiple comparisons. Values presented in figures are the mean ± SEM. Probability values less than 0.05 (P < 0.05) were considered statistically significant.
Results
P66Shc expression increases in blastocysts during mouse preimplantation development
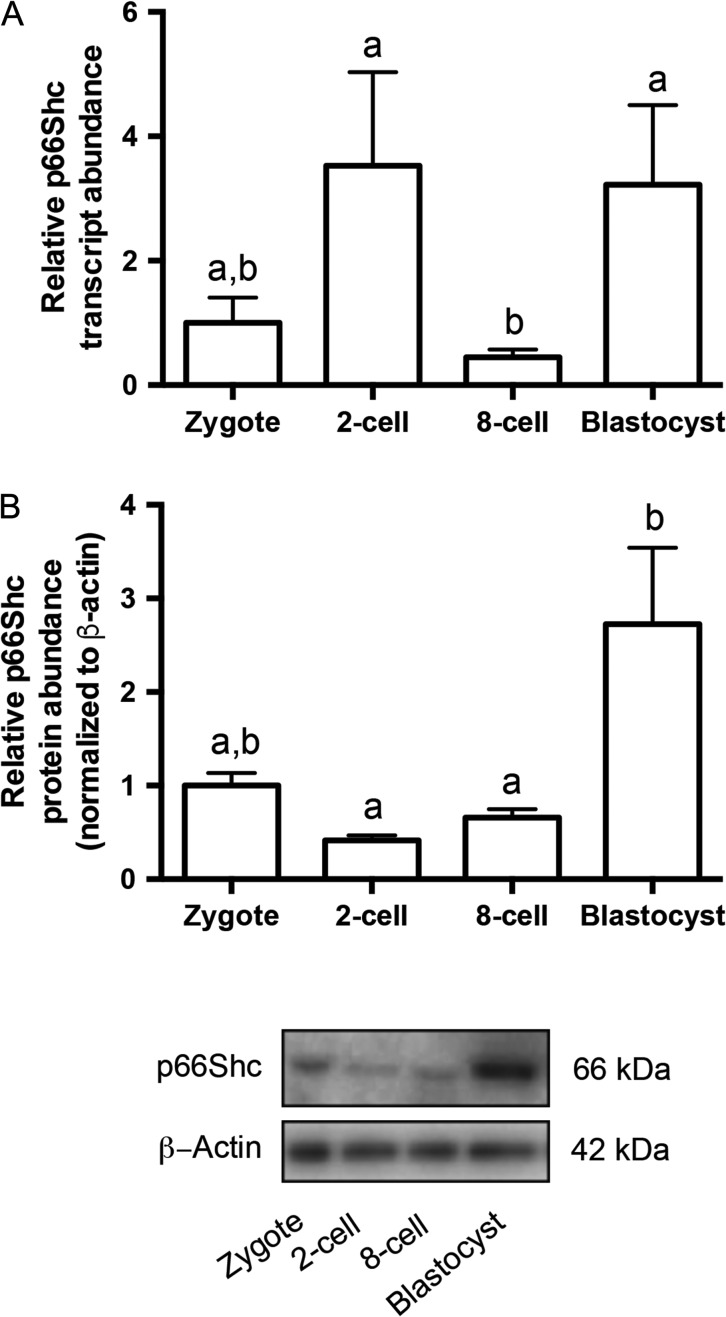
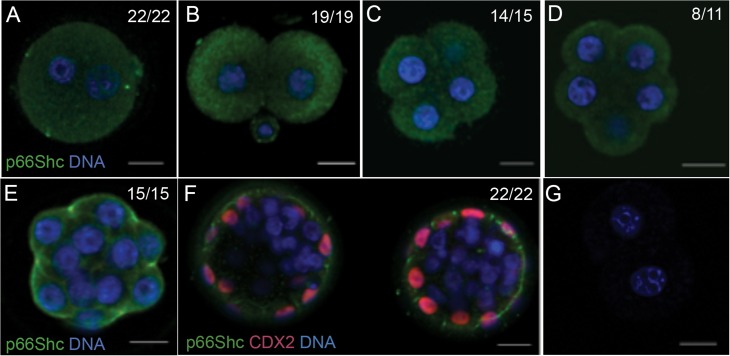
P66Shc mRNA and protein have been previously detected in bovine (Favetta et al., 2004) and murine embryos (Ren et al., 2014), but an analysis of expression during the progression of mouse preimplantation development in vivo has not been carried out. To determine the expression profile of p66Shc during preimplantation development, we performed real time qRT-PCR and immunoblotting on pools of embryos from four developmental stages. P66Shc mRNA transcript and protein were detectable in all stages observed. We observed a significant increase in both transcript and protein abundance from the 8-cell to blastocyst stages. P66Shc mRNA or protein did not significantly change between the zygote and 8-cell stages (Figs. 1A and B; for uncropped immunoblot, see Supplementary Fig. 1A). To determine the cellular localization of p66Shc during preimplantation development, we performed whole mount immunofluorescence followed by confocal microscopy using a p66Shc-specific antibody on embryos from six developmental stages. We observed p66Shc immunoreactivity throughout the cytoplasm of pre-compaction stage embryos (Fig. 2A–D), with restriction to the apical cell periphery of compacted 16 cell morulae (Fig. 2E). To determine if p66Shc localization is restricted to the trophectoderm lineage, we co-stained blastocysts with CDX2. Of all blastocysts observed, p66Shc showed detectable cell periphery localization in only CDX2 positive cells (Fig. 2F). P66Shc immunoreactivity was undetectable in CDX2 negative cells (Fig. 2F). These results indicate that p66Shc expression is normally upregulated in the blastocyst and may be restricted primarily to the trophectoderm of in vivo produced blastocysts.
Figure 1.
p66Shc expression increases during mouse preimplantation development in vivo. (A) Quantitative real time RT-PCR (qRT-PCR) for p66Shc relative transcript abundance was performed on three replicates of pools of 20 embryos per stage. P66Shc relative transcript abundance significantly increases from eight cell to blastocyst-stage embryos (n = 3, mean ± SEM, P = 0.0476 1W-ANOVA). (B) Immunoblotting for total p66Shc protein abundance was performed on three replicates of pools of 30–50 embryos per stage. P66Shc relative protein abundance increases from eight cell to blastocyst-stage embryos (n = 3, mean ± SEM, P = 0.0331 one way-ANOVA). A representative blot is shown (for uncropped immunoblot, see Supplementary Fig. 1A).
Figure 2.
p66Shc progressively localizes to the cell periphery during mouse preimplantation development. Immunofluorescence and confocal microscopy for p66Shc was performed on 10–20 embryos per stage. Representative confocal images are shown: (A) Zygote (B) 2-cell embryo (C) 4-cell embryo (D) 8-cell non-compacted embryo (E) 8–16 cell compacted morula (F) Blastocyst, counterstained for caudal type homeobox 2 (CDX2) (G) Primary antibody omitted. Green = p66Shc, Red = CDX2, Blue = DAPI. Scale bar = 20 μm.
Validation of NT-Shc antibody specificity
To verify that the antibodies used to detect p66Shc and phosphorylated (S36) p66Shc only recognized the 66-kDa Shc isoform by immunofluorescence confocal microscopy, we cultured mature neurons known to have undetectable basal p66Shc expression (Ventura et al., 2002). We performed immunofluorescence using both antibodies on the mouse HT-22 hippocampal cell line. HT-22 cells transfected with a HA-tagged p66Shc DNA construct showed p66Shc and HA immunoreactivity, while non-transfected cells showed no detectable p66Shc or HA immunoreactivity (Supplementary Fig. 2A). Transfected HT-22 cells also displayed phosphorylated S36 p66Shc and HA immunoreactivity compared to undetectable levels in non-transfected cells (Supplementary Fig. 2B). These results validate the use of these antibodies for immunofluorescent detection of p66Shc and S36-phosphorylated p66Shc cell localization in mouse preimplantation embryos.
P66Shc expression is sensitive to oxygen tension, but not glucose concentration, during embryo culture
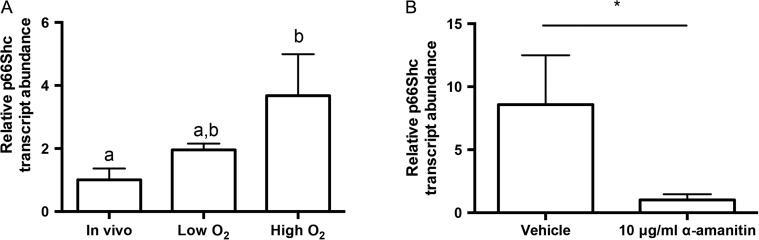
Under in vivo conditions, p66Shc expression levels may be fine-tuned to prevent adverse developmental events. Given our observations within in vivo derived mouse embryos, we then aimed to determine whether certain embryo culture conditions induce aberrant changes in embryonic p66Shc expression levels. Mouse zygotes were cultured to three developmental stages under low oxygen tension (5% O2) or high oxygen tension (21% O2). Embryos cultured in low or high oxygen tensions in KSOMaa Evolve show comparable rates of blastocyst formation (Supplementary Fig. 3A). However, blastocysts cultured in low oxygen have significantly increased cell numbers compared to flushed in vivo-derived blastocysts (Supplementary Fig. 3B). Real time qRT-PCR was performed on pools of embryos to determine changes in p66Shc transcript abundance. Blastocysts examined after 96 h of culture showed increasing p66Shc transcript abundance with increasing oxygen tension (Fig. 3A). This increase was dependent on de novo transcription of p66Shc, as the increase in p66Shc abundance was abolished in blastocysts cultured at high oxygen tension in the presence of the transcriptional inhibitor α-amanitin (Fig. 3B). It is interesting to note that some p66Shc transcripts were still detectable in treated blastocysts, suggesting that maternally stored p66Shc may still be present at the blastocyst stage (Fig. 3B). Overall, these observations suggest that p66Shc is actively transcribed by the embryo under atmospheric oxygen conditions.
Figure 3.
Culture and high oxygen tension increases the relative p66Shc mRNA abundance in mouse blastocysts. (A) RT-qPCR for p66Shc was performed on four replicates of pools of 20 blastocysts. There is a significant increase in p66Shc mRNA abundance in blastocysts cultured at high oxygen tension compared to in vivo controls (n = 4, mean ± SEM, P = 0.0305 one way-ANOVA). (B) Blastocysts cultured for 24 h in 10 μg/ml α-amanitin showed significantly decreased p66Shc transcript abundance compared to controls (n = 3, mean ± SEM, P = 0.0477 Student's t-test).
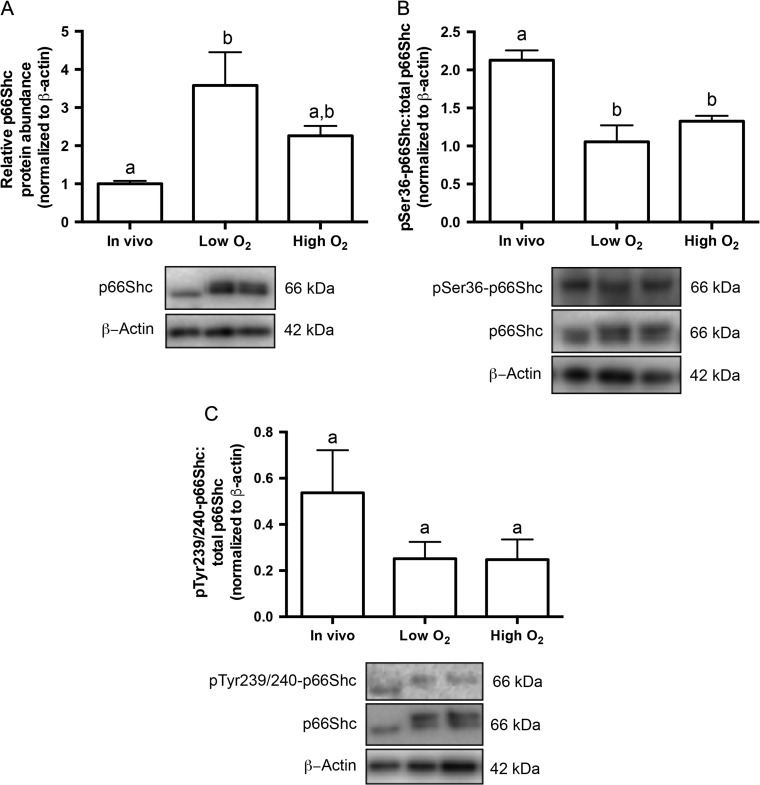
We next aimed to determine if p66Shc protein abundance also increased with increasing oxygen tension. Immunoblotting for total p66Shc on pools of embryos showed a significant increase in p66Shc protein abundance in cultured blastocysts compared to in vivo derived blastocysts (Fig. 4A). This induction of p66Shc expression was unique to the blastocyst stage, as p66Shc transcript abundance decreased and protein abundance was unchanged in cultured 2-cell and 8-cell embryos (Supplementary Fig. 4A and B). We then saw that culture in both low and high oxygen tensions significantly decreased the ratio of phosphorylated S36 p66Shc to total p66Shc in blastocysts, suggesting a possible change in the mitochondrial fraction of p66Shc in cultured blastocysts (Fig. 4B) as serine-36 phosphorylation of p66Shc promotes Pin-1, isomerization, and ultimately p66Shc translocation to the mitochondria (Pinton et al., 2007). Oxygen tension did not alter the ratio of phosphorylated Y239/Y240 p66Shc to total p66Shc (Fig. 4C). These are two residues on Shc1 proteins that are known to be phosphorylated after interaction with receptor tyrosine kinases (Gotoh et al., 1997). This result suggests that the shift in the 66-kDa band seen in cultured blastocysts may be due to an alternative (e.g. Ser138, Y317) or novel post-translational modification induced by culture.
Figure 4.
Culture and high oxygen tension increases the relative p66Shc protein abundance in mouse blastocysts. (A) Immunoblotting for p66Shc was performed on four replicates of pools of 50 blastocysts. P66Shc protein abundance significantly increases in blastocysts cultured at low oxygen tension compared to in vivo controls (n = 4, mean ± SEM, P = 0.0306 one way-ANOVA). A representative blot is shown (for uncropped immunoblot, see Supplementary Fig. 1B). (B) Immunoblotting for phosphorylated p66Shc on serine 36 (S36) and total p66Shc was performed on three replicates of pools of 40–50 blastocysts. The ratio of phosphorylated serine 36 (S36)-p66Shc to total p66Shc significantly decreases in blastocysts cultured in low and high oxygen tensions compared to controls (n = 3, mean ± SEM, P = 0.0057 for low O2; P = 0.0219 for high O2 one way-ANOVA). A representative blot is shown (for uncropped immunoblot, see Supplementary Fig. 1C). (C) Immunoblotting for phosphorylated tyrosine 239/240 (Y239/Y240)-p66Shc and total p66Shc was performed on three replicates of pools of 20–30 blastocysts. The ratio of phospho Y239/Y240-p66Shc to total p66Shc does not significantly in cultured blastocysts compared to controls (n = 3, mean ± SEM, P = 0.5043, one way-ANOVA). A representative blot is shown (for uncropped immunoblot, see Supplementary Fig. 1D).
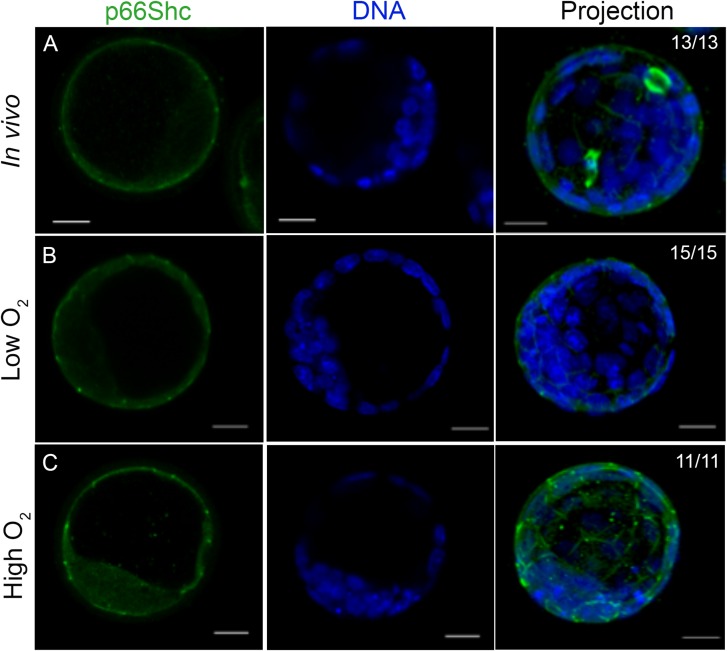
To determine if p66Shc cellular localization changed with embryo culture, cultured blastocysts were stained for p66Shc immunoreactivity and were compared to freshly flushed, in vivo derived blastocysts. Blastocysts cultured in high oxygen conditions showed an increase in p66Shc fluorescence intensity and detectable diffuse p66Shc staining in inner cells, compared to that of in vivo and low oxygen cultured blastocysts (Fig. 5A–C). As we did not co-stain for lineage markers, we cannot definitively identify these inner cells as the inner cell mass. However, p66Shc fluorescence is undetectable in inner cells of in vivo derived and low oxygen blastocysts in culture, suggesting that high oxygen may induce abnormal p66Shc expression in the inner cell mass. To determine the localization of phosphorylated S36 p66Shc, cultured blastocysts were stained for phosphorylated S36 p66Shc immunoreactivity and compared to in vivo controls. Consistent with the immunoblotting results, neither the fluorescence levels of phosphorylated S36 p66Shc nor its localization appeared to change between treatment groups. However, phosphorylated S36 p66Shc did show a distinct cellular localization pattern compared to total p66Shc, showing cytoplasmic and nuclear immunoreactivity in the outer and inner cells of the blastocyst (Fig. 6A–C). In addition, phosphorylated S36 p66Shc was also detectable in inner cells of the in vivo produced blastocyst while total p66Shc was not. We observed similar cytoplasmic staining of both total p66Shc and pSer36-p66Shc in HT-22 cells, suggesting that the NT-Shc (total p66Shc) antibody can detect phosphorylated p66Shc. However, we overexpressed p66Shc in these cells, whereas we assessed basal p66Shc expression in mouse blastocysts. Overexpression of the protein and differences in antibody sensitivities may explain why total p66Shc fluorescence appears to differ from pSer36-p66Shc fluorescence in blastocysts. (Figs. 5A and 6A). The localization pattern may also suggest that the phosphorylated S36 p66Shc fraction in blastocysts produced in vivo or in culture may be localized to a distinct compartment in the cytoplasm or nucleus compared to non-phosphorylated, or p66Shc phosphorylated at a different residue.
Figure 5.
Total p66Shc becomes detectable in the inner cells of mouse blastocysts cultured under atmospheric oxygen tension. Representative immunofluorescence and confocal microscopy images for total p66Shc in pools of 10–15 blastocysts per treatment group. (A) In vivo flushed blastocysts. (B) Blastocysts after 96 h culture under low oxygen tension. (C) Blastocysts after 96 h culture under high oxygen tension. Green = p66Shc, Blue = DAPI. Scale bar = 20 μm.
Figure 6.
Phosphorylated S36 p66Shc localization does not change in cultured mouse blastocysts. Representative immunofluorescence and confocal microscopy images for phosphorylated (S36) p66Shc in pools of 15–20 blastocysts per treatment group. (A) In vivo flushed blastocysts. (B) Blastocysts after 96 h culture under low oxygen tension. (C) Blastocysts after 96 h culture under high oxygen tension. Green = p66Shc, Blue = DAPI. Scale bar = 20 μm.
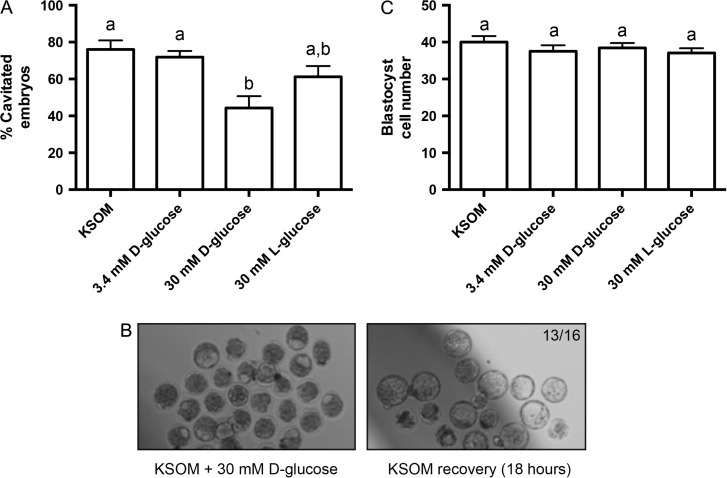
In addition to its role in mediating the oxidative stress response, several studies have implicated p66Shc in regulating cellular glucose uptake through growth factor receptor signaling, actin cytoskeleton regulation, or modulation of anaerobic respiration (Natalicchio et al., 2009; Soliman et al., 2014). Thus, we next aimed to determine if p66Shc expression is sensitive to culture medium glucose concentration, a component often modified in embryo culture to simulate in vivo microenvironmental conditions. We cultured flushed 8-cell stage embryos for 24 h in KSOM varying in glucose concentrations under low oxygen tension: 0.2 mM (standard KSOM), 3.4 mM (equivalent to normal mouse oviductal glucose levels, (Gardner and Leese, 1990)), 30 mM d-glucose (hyperglycemia, (Moley et al., 1998)) and 30 mM l-glucose to control for increased osmolarity. We observed that embryos cultured in 30 mM d-glucose have decreased rates of blastocyst cavitation (Fig. 7A). The embryos did not fail to cavitate due to glucose toxicity, as 18 h culture in 0.2 mM d-glucose rescued cavitation (Fig. 7B). Furthermore, cell number in non-cavitated embryos did not significantly change with high glucose culture compared to control, suggesting that these embryos were not developing slower than the controls (Fig. 7C).
Figure 7.
High glucose media concentrations reversibly inhibit mouse embryo cavitation. (A) Percent cavitation of blastocysts after 24 h culture in each treatment group, indicated by the formation of any cavity in the embryo (n = 4, mean ± SEM, P = 0.0052 1W-ANOVA). (B) Bright field microscopy images of embryos after 24 h treatment in 30 mM d-glucose, followed by recovery in low glucose potassium simplex optimized media (KSOM) for 18 h. Arrows in the left panel indicate examples of embryos classified as non-cavitated. Thirteen of sixteen non-cavitated embryos after high glucose treatment cavitated after 18 h of recovery. (C) Blastocyst cell number after 24 h culture in each treatment group (n = 19–21 per group, mean ± SEM, P = 0.5099 one way-ANOVA).
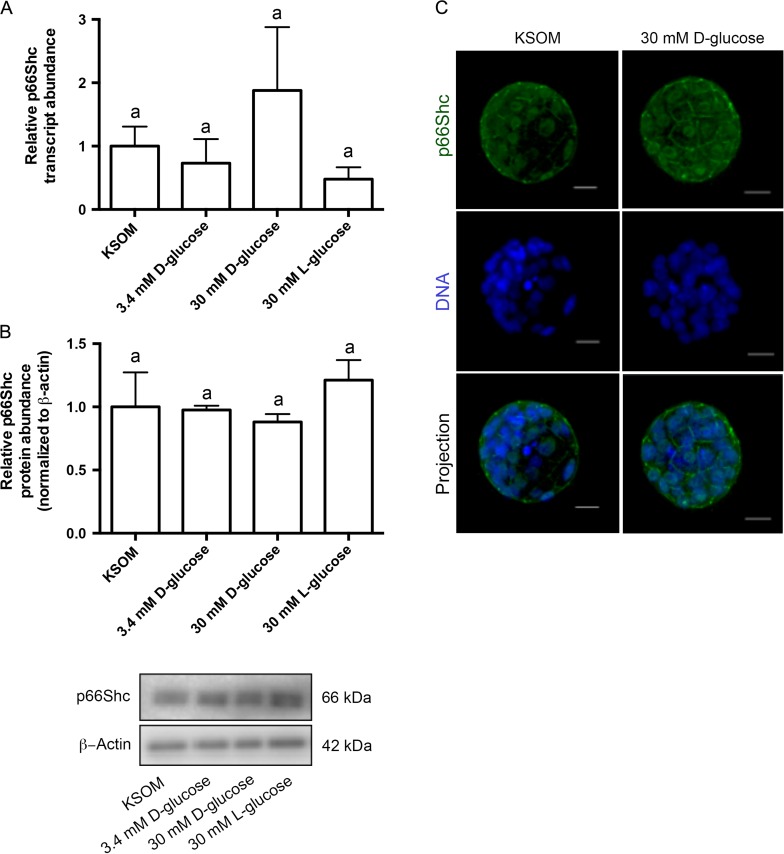
To determine if p66Shc expression changed during culture in high glucose, we performed qRT-PCR and immunoblotting for p66Shc in pools of blastocysts cultured in the four glucose concentrations. Neither transcript levels nor protein abundance significantly changed in embryos cultured in varying glucose conditions (Fig. 8A and B), suggesting that p66Shc expression levels are not sensitive to increased glucose in embryo culture media. To determine if p66Shc cellular localization changed with glucose concentration, embryos cultured in 30 mM d-glucose were stained for p66Shc immunoreactivity and compared to embryos cultured in KSOM. We saw comparable peripheral and cytoplasmic p66Shc immunoreactivity in non-cavitated embryos after high glucose culture compared to controls, suggesting that p66Shc cellular localization is not impacted by media glucose concentrations (Fig. 8C).
Figure 8.
High glucose media concentrations do not significantly change relative p66Shc mRNA and protein abundance in mouse blastocysts. (A) qRT-PCR was performed on pools of 10 blastocysts for relative p66Shc transcript abundance, normalized to levels of exogenously added luciferase (n = 3, mean ± SEM, P = 0.3783 one way-ANOVA). (B) Immunoblotting was performed on pools of 30 blastocysts per treatment group for total p66Shc protein abundance, normalized to levels of β-actin. A representative blot is shown (n = 3, mean ± SEM, P = 0.5549 one way-ANOVA). (C) Representative immunofluorescence and confocal microscopy images of blastocysts cultured in 30 mM d-glucose (right panel) and KSOM only (left panel) for total p66Shc reactivity. Green = p66Shc, Blue = DNA. Scale bar = 20 μm.
Changes to p66Shc expression in culture correlate with altered embryo metabolism
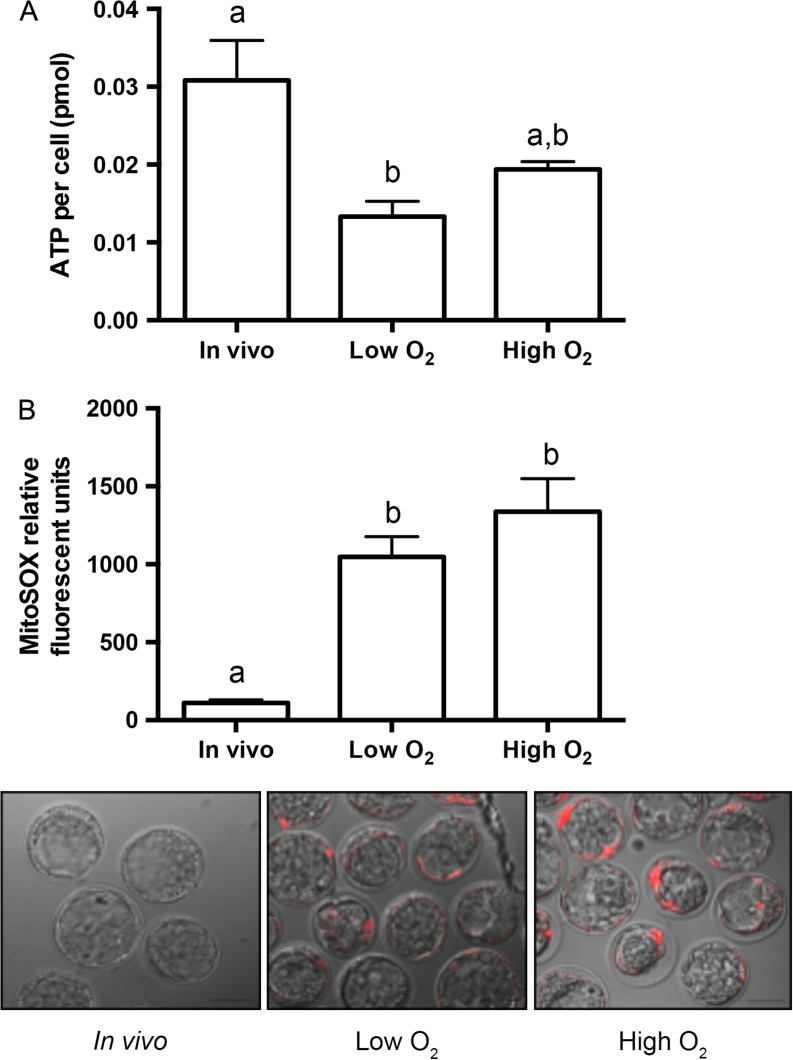
To determine if increased p66Shc expression levels in cultured embryos could be a marker of altered embryo metabolism, we performed two metabolic assays on blastocysts derived in vivo and after culture under low and high oxygen. We first assessed total ATP content of blastocysts from each group, and observed that ATP levels per cell significantly decreased in blastocysts cultured under low oxygen compared to in vivo blastocysts (Fig. 9A). As oxidative phosphorylation in the trophectoderm is the major source of cellular ATP in the blastocyst (Houghton, 2006), we then assayed for production of superoxide in the same treatment groups. Superoxide is a free radical produced as a by-product of oxidative phosphorylation that is normally present at low levels and is readily scavenged by superoxide dismutase. Blastocysts were incubated in MitoSOX red superoxide indicator and imaged using confocal microscopy. We observed that blastocysts cultured in low and high oxygen tensions showed significantly higher MitoSOX fluorescence compared to in vivo controls, suggesting increased superoxide production or decreased antioxidant scavenging in these culture conditions (Fig. 9B). Our results suggest that even under low oxygen conditions, cultured blastocysts contain less ATP and increased superoxide levels compared to in vivo derived blastocysts, correlating with increased mRNA and protein abundance of p66Shc.
Figure 9.
Increased p66Shc expression correlates with decreased ATP and increased superoxide in cultured mouse blastocysts. (A) Total ATP content was quantified from pools of five blastocysts in each treatment group and normalized to blastocyst cell number. ATP content per cell significantly decreases in blastocysts cultured in low oxygen for 96 h compared to in vivo controls (n = 3, mean ± SEM, P = 0.0.0199 one way-ANOVA). Mean cell numbers for each treatment group are: in vivo = 27.43 ± 10.31 (n = 46), low oxygen = 35.03 ± 7.36 (n = 31), high oxygen = 31.41 ± 9.49 (n = 30). (B) MitoSOX relative fluorescence was quantified in blastocysts in each treatment group. MitoSOX fluorescence significantly increases in blastocysts cultured under low or high oxygen compared to in vivo controls (in vivo n = 28, low oxygen n = 26, high oxygen n = 23, mean ± SEM, P < 0.0001 one way-ANOVA). Representative images of MitoSOX staining are shown in the three panels.
Discussion
Here we demonstrate that p66Shc is basally expressed in mouse preimplantation embryos and its expression is altered by embryo culture. We also show that dysregulated p66Shc expression coincides with metabolic changes in culture that may negatively affect embryo developmental viability. Our results suggest that p66Shc mRNA is stored in the oocyte and degraded during the maternal-to-embryonic transition. P66Shc mRNA is later upregulated by the blastocyst stage, and predominately located at the cell periphery of trophectoderm cells. Blastocysts grown in vitro show increasing p66Shc mRNA and protein abundance with increasing oxygen tension, coupled with alterations to phosphorylated residues that have implications for the proteins cellular compartmentalization and function. These changes appear to be oxygen-sensitive, while changing media glucose concentrations did not significantly affect p66Shc expression levels in the blastocyst. Lastly, we are the first to correlate these changes in culture and high oxygen tension to dysregulated ATP and superoxide production within in vitro produced blastocysts.
Our expression analysis of p66Shc during in vivo blastocyst development suggests that p66Shc is normally upregulated during the eight-cell embryo to blastocyst transition. This basal level of expression during in vivo development implies that despite promoting apoptosis, p66Shc expression may be necessary for survival and prevents blastocysts from being selected against during development. One possible biological function of p66Shc during preimplantation development may be the promotion of oxidative phosphorylation. Basal oxygen consumption in 66Shc-null MEFs decreases by 30–50% with no change in mitochondrial or cytochrome c content, with a compensatory increase in ATP production by anaerobic respiration (Nemoto et al., 2006). There is also a evidence suggesting that in MEFs, p66Shc forms a complex with cytochrome c in the inner mitochondrial membrane to regulate pyruvate dehydrogenase kinase, ultimately regulating the activity of pyruvate dehydrogenase) depending on the redox state of cytochrome c (Acin-Perez et al., 2010). In the mouse blastocyst, the trophectoderm produces ATP through oxidative phosphorylation to support development, but the inner cell mass is relatively metabolically quiescent (Houghton, 2006). Metabolic differences between the two embryonic lineages could account for our immunolocalization results, as p66Shc appears to localize predominately to the trophectoderm in vivo and under low oxygen conditions, suggesting that p66Shc could be involved in trophectoderm metabolism. Although our study did not directly test the role of p66Shc in oxidative phosphorylation, we have correlated increasing p66Shc transcript and protein abundances after embryo culture with alterations to ATP and superoxide production, suggesting that dysregulated p66Shc levels in the embryo may have a negative impact on embryo metabolism.
Studies of p66Shc in mammalian embryos have thus far focused primarily on the apoptosis- and senescence-promoting functions of p66Shc, basally or in stress-inducing culture conditions. In bovine pre-attachment embryos, siRNA-mediated knockdown of p66Shc reduces levels of intracellular ROS, DNA damage and apoptosis in untreated and oxidant-treated culture conditions (Betts et al., 2014). Early bovine embryos exhibit high levels of developmental arrest (>50%) in culture (Leidenfrost et al., 2011), likely due to suboptimal culture conditions, which could result in increased p66Shc transcript levels, leading to senescence (permanent embryo arrest) and apoptosis. Because of species-specific differences in early development, or better optimized conditions, mouse preimplantation embryos from inbred strains exhibit high developmental rates with >75% of zygotes reaching the blastocyst stage in optimized media and low oxygen conditions (Karagenc et al., 2004). It is possible that p66Shc expression is carefully regulated during preimplantation development, such that both abnormally high and low p66Shc expression levels are detrimental to the embryo.
Consistent with our findings in our mouse embryo culture model, there is strong evidence associating p66Shc induction with negative developmental outcomes under adverse bovine and murine embryo culture conditions. Bovine embryos grown in oviductal epithelial cell co-culture, considered a suboptimal culture environment, show a significantly increased p66Shc transcript abundance compared to culture under chemically defined synthetic oviductal fluid media at lower oxygen tension. This increase was associated with increased markers of oxidative stress (intracellular ROS, DNA damage) and embryo arrest (Favetta et al., 2007b). Mouse embryos cultured in media containing arsenic show significantly decreased blastocyst development and increased p66Shc immunofluorescence intensity, suggesting that p66Shc may mediate a stress response to arsenic. Treatment with the antioxidant N-acetyl cysteine rescued apoptosis and p66Shc expression levels observed in arsenic-treated embryos, suggesting that arsenic-induced ROS increases p66Shc expression, which may in turn further increase ROS and lead to apoptosis (Zhang et al., 2010). Preimplantation development under both cases improved when p66Shc was knocked down by RNA interference (Favetta et al., 2007a; Betts et al., 2014; Ren et al., 2014). Previous RNA-interference experiments may have normalized an adverse environment-induced ‘spike’ in p66Shc expression, but not completely deplete the embryo of maternal- or zygotic-derived p66Shc, thus masking any loss-of-function phenotype. Maternally derived p66Shc function may be important to preimplantation development, as embryo cleavage and blastocyst development is impaired when p66Shc is knocked down in immature bovine oocytes (Favetta et al., 2007a). We are the first to show that p66Shc transcript and protein expression is upregulated at the blastocyst stage during mouse in vivo development, indicating that p66Shc may also have an important physiological function other than promoting apoptosis and embryo arrest.
We found that the induction of p66Shc transcription in cultured blastocysts appears to be specific to oxygen, as increasing media glucose concentrations did not significantly change p66Shc transcript abundance compared to controls. Oxygen-sensitive induction in our results is consistent with findings that p66Shc transcription can be regulated by the Nrf2-antioxidant response element (ARE) pathway under stress-inducing conditions. Chromatin immunoprecipitation assays performed in hemin-treated human erythroleukemic cells demonstrated that Nrf2 binds to an ARE enhancer upstream of the transcriptional start site of p66Shc and that Nrf2 induction of expression is isoform-specific (Miyazawa and Tsuji, 2014). This could be the upstream mechanism in our model of p66Shc transcriptional upregulation in blastocysts cultured under high oxygen. High glucose concentrations in the culture media did not significantly change p66Shc expression in blastocysts, but did affect cavitation. This is consistent with previous reports of hyperglycemic conditions negatively affecting blastocyst development (Fraser et al., 2007). Thus it is unlikely that the cell's response to high glucose regulates the transcription of p66Shc, but instead may affect other genes known to be involved in cavitation (e.g. ATPase Na+/K+ transporting subunit beta 1, aquaporin 3, aquaporin 9 and cadherin 1). Furthermore, it is not known whether p66Shc is important for the regulation of glucose uptake in preimplantation embryos or if this function is dependent on mammalian target of rapamycin or growth factor receptor signaling pathways (Natalicchio et al., 2009; Soliman et al., 2014). It is possible that p66Shc could mediate a response to high glucose levels in embryos independent of an increase in its transcript or protein abundance, through phosphorylation of certain residues.
It is possible that culture conditions increase p66Shc expression to promote its apoptotic functions, removing it from its metabolic function in the mitochondria. Our results suggest that culture-mediated changes to phosphorylated residues on p66Shc may impact its cellular compartmentalization and may ultimately be a key factor in its cellular function. Subcellular fractionation of untreated MEF lysates showed that p66Shc is detectable in the soluble, mitochondrial and endoplasmic reticulum fractions (Orsini et al., 2004). Phosphorylation of the serine-36 residue, which is unique to the 66 kDa isoform of the Shc1 family, has been implicated in its cellular localization. Serine-36 phosphorylation of p66Shc under oxidizing conditions increases its association with the prolyl isomerase Pin-1, ultimately resulting in p66Shc translocation to the mitochondria. Fibroblasts lacking Pin-1 have a decreased mitochondrial fraction of p66Shc after H2O2 treatment compared to wild type fibroblasts, linking the modification of this residue to the protein's mitochondrial localization (Pinton et al., 2007). In our study, blastocysts cultured under low or high oxygen conditions show decreased ratios of phosphorylated 36 to total p66Shc, suggesting that these conditions may decrease the mitochondrial fraction of p66Shc despite an increase in total p66Shc protein abundance. This alteration in cellular localization may affect the functions of p66Shc in the mitochondria, which our results of altered embryo metabolism may reflect.
Despite using optimal culture conditions, both p66Shc expression and the metabolic parameters measured were significantly altered in blastocysts grown under low oxygen tension. No significant difference between increased superoxide production in blastocysts after culture in low or high oxygen tension suggests that oxidative phosphorylation metabolism may be adversely affected regardless of oxygen tension, or that there is another parameter in the microenvironment that must be further optimized to limit metabolic alterations in cultured embryos. Levels of p66Shc may therefore be an indicator of altered blastocyst metabolism, particularly of the trophectoderm, which is responsible for generating nearly all of the blastocyst's ATP content (Houghton, 2006). Altered expression levels and/or p66Shc function in culture may lead to adverse trophectoderm development through increases in ROS-mediated apoptosis or decreases in ATP production, which may impact implantation and placentation.
Our study did not follow up on peri- and post-implantation stage embryos and p66Shc expression levels, but we suspect that p66Shc expression is likely altered in the trophoblast or post-implantation trophoblast-derived tissues after embryo culture. Supporting this is evidence that p66Shc CpG promoter methylation is decreased in human placental tissue of intrauterine growth restricted neonates compared to neonates appropriate and small for gestational age (Tzschoppe et al., 2013). This is also consistent with the finding that most culture-induced embryo abnormalities affect the trophoblast and placenta, and to a lesser extent the fetal tissues (Fauque et al., 2010; de Waal et al., 2014). Post-implantation development is affected by oxygen tension, as transferred blastocysts cultured at 20% oxygen show a significant increase in the number of uterine resorption sites and a decrease in living fetuses compared to blastocysts cultured at 5% oxygen (Karagenc et al., 2004). Additionally, transferred blastocysts cultured in both oxygen tensions resulted in decreased fetal weights compared with freshly flushed blastocysts, suggesting that some component of culture aside from oxygen tension impacts fetal development (Harlow and Quinn, 1979). These studies used different culture medium conditions from ours that may be critical to the developmental outcomes observed. Culture effects observed in our study may impact fetal development through abnormal increases in p66Shc expression and/or altered oxidative phosphorylation metabolism.
Our study correlated increased p66Shc expression levels with altered oxidative phosphorylation metabolism, however, we did not directly implicate a mechanism for p66Shc involvement in dysregulated metabolism. Further work must be performed to determine the mechanism of p66Shc function during preimplantation development and its implications for post-implantation development. Additionally, we used one formulation of embryo culture medium that differs from media used in other mouse model studies and from clinical media used to support human blastocyst development. Our findings may be limited to this media, or may be a species-specific phenomenon. However, as increases in p66Shc expression due to culture are observed in another large animal model (bovine) (Favetta et al., 2007b), it would be interesting to explore whether cultured human embryos exhibit varying p66Shc levels and if this correlates with developmental outcome. For clinical applications, using increased p66Shc expression as a molecular marker of altered metabolism may impact on which blastocyst may be the most developmentally competent for embryo transfer.
Supplementary Material
Acknowledgements
The authors thank Dr Robert Cumming (The University of Western Ontario) for donation of the HT-22 cell line and HA-p66Shc expression plasmid. Confocal microscopy was performed at the Integrated Microscopy Laboratory at the Biotron Experimental Climate Change Research Centre (The University of Western Ontario, London, Ontario, Canada).
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/
Authors’ roles
Study conception and design: N.A.E., A.J.W., D.H.B. Performed the experiments: N.A.E. Data analysis: N.A.E., A.J.W., D.H.B. Drafted and proofread the manuscript: N.A.E., A.J.W., D.H.B.
Funding
Funding for this study was provided by Canadian Institutes of Health Research operating funds to A..J.W. and D.H.B. (MOP 130396). N.A.E. is supported by an Ontario Graduate Scholarship.
Conflict of interest
None declared.
References
- Acin-Perez R, Hoyos B, Gong J, Vinogradov V, Fischman DA, Leitges M, Borhan B, Starkov A, Manfredi G, Hammerling U. Regulation of intermediary metabolism by the PKCdelta signalosome in mitochondria. FASEB J 2010;24:5033–5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts DH, Bain NT, Madan P. The p66(Shc) adaptor protein controls oxidative stress response in early bovine embryos. PLoS One 2014;9:e86978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts DH, Barcroft LC, Watson AJ. Na/K-ATPase-mediated 86Rb+ uptake and asymmetrical trophectoderm localization of alpha1 and alpha3 Na/K-ATPase isoforms during bovine preattachment development. Dev Biol 1998;197:77–92. [DOI] [PubMed] [Google Scholar]
- CFAS Human Assisted Reproduction 2015 Live Birth Rates for Canada. 2015http://www.cfas.ca/index.php?option=com_content&view=article&id=1415%3Ahuman-assisted-reproduction-2015-live-birth-rates-for-canada&catid=929%3Apress-releases&Itemid=130.
- de Waal E, Mak W, Calhoun S, Stein P, Ord T, Krapp C, Coutifaris C, Schultz RM, Bartolomei MS. In vitro culture increases the frequency of stochastic epigenetic errors at imprinted genes in placental tissues from mouse concepti produced through assisted reproductive technologies. Biol Reprod 2014;90:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauque P, Mondon F, Letourneur F, Ripoche M-A, Journot L, Barbaux S, Dandolo L, Patrat C, Wolf J-P, Jouannet P et al. In vitro fertilization and embryo culture strongly impact the placental transcriptome in the mouse model. PLoS One 2010;5:e9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favetta LA, Madan P, Mastromonaco GF, St John EJ, King WA, Betts DH. The oxidative stress adaptor p66Shc is required for permanent embryo arrest in vitro. BMC Dev Biol 2007. a.7:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favetta LA, Robert C, St John EJ, Betts DH, King WA. p66shc, but not p53, is involved in early arrest of in vitro-produced bovine embryos. Mol Hum Reprod 2004;10:383–392. [DOI] [PubMed] [Google Scholar]
- Favetta LA, St John EJ, King WA, Betts DH. High levels of p66shc and intracellular ROS in permanently arrested early embryos. Free Radic Biol Med 2007. b.42:1201–1210. [DOI] [PubMed] [Google Scholar]
- Feuer S, Rinaudo P. Preimplantation stress and development. Birth Defects Res C Embryo Today 2012;96:299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser RB, Waite SL, Wood KA, Martin KL. Impact of hyperglycemia on early embryo development and embryopathy: in vitro experiments using a mouse model. Hum Reprod 2007;22:3059–3068. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M, Stevens J, Schoolcraft WB. Noninvasive assessment of human embryo nutrient consumption as a measure of developmental potential. Fertil Steril 2001;76:1175–1180. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Leese HJ. Concentrations of nutrients in mouse oviduct fluid and their effects on embryo development and metabolism in vitro. J Reprod Fertil 1990;88:361–368. [DOI] [PubMed] [Google Scholar]
- Gotoh N, Toyoda M, Shibuya M. Tyrosine phosphorylation sites at amino acids 239 and 240 of Shc are involved in epidermal growth factor-induced mitogenic signaling that is distinct from Ras/mitogen-activated protein kinase activation. Mol Cell Biol 1997;17:1824–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady R, Alavi N, Vale R, Khandwala M, McDonald SD. Elective single embryo transfer and perinatal outcomes: a systematic review and meta-analysis. Fertil Steril 2012;97:324–331. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- Harlow GM, Quinn P. Foetal and placental growth in the mouse after pre-implantation development in vitro under oxygen concentrations of 5 and 20 %. 1979363–370. [DOI] [PubMed] [Google Scholar]
- Houghton FD, Humpherson PG, Hawkhead J A, Hall CJ, Leese HJ. Na+, K+, ATPase activity in the human and bovine preimplantation embryo. Dev Biol 2003;263:360–366. [DOI] [PubMed] [Google Scholar]
- Houghton FD. Energy metabolism of the inner cell mass and trophectoderm of the mouse blastocyst. Differentiation 2006;74:11–18. [DOI] [PubMed] [Google Scholar]
- Karagenc L, Sertkaya Z, Ciray N, Ulug U, Bahçeci M. Impact of oxygen concentration on embryonic development of mouse zygotes. Reprod Biomed Online 2004;9:409–417. [DOI] [PubMed] [Google Scholar]
- Leidenfrost S, Boelhauve M, Reichenbach M, Güngör T, Reichenbach HD, Sinowatz F, Wolf E, Habermann FA. Cell arrest and cell death in mammalian preimplantation development: lessons from the bovine model. PLoS One 2011;6:e22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo S, Gal AB, Bodo S, Dinnyes A. Quantitative evaluation and selection of reference genes in mouse oocytes and embryos cultured in vivo and in vitro. BMC Dev Biol 2007;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 1999;402:309–313. [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Mele S, Salcini AE, Pelicci G, Lai KM, Superti-Furga G, Pawson T, Di Fiore PP, Lanfrancone L, Pelicci PG. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J 1997;16:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa M, Tsuji Y. Evidence for a novel antioxidant function and isoform-specific regulation of the human p66Shc gene. Mol Biol Cell 2014;25:2116–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moley KH, Chi MM, Knudson CM, Korsmeyer SJ, Mueckler MM. Hyperglycemia induces apoptosis in pre-implantation embryos through cell death effector pathways. Nat Med 1998;4:1421–1424. [DOI] [PubMed] [Google Scholar]
- Natalicchio A, De Stefano F, Perrini S, Laviola L, Cignarelli A, Caccioppoli C, Quagliara A, Melchiorre M, Leonardini A, Conserva A et al. Involvement of the p66Shc protein in glucose transport regulation in skeletal muscle myoblasts. Am J Physiol Endocrinol Metab 2009;296:E228–E237. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Combs CA, French S, Ahn BH, Fergusson MM, Balaban RS, Finkel T. The mammalian longevity-associated gene product p66shc regulates mitochondrial metabolism. J Biol Chem 2006;281:10555–10560. [DOI] [PubMed] [Google Scholar]
- Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, Martin-Padura I, Pelliccia G, Trinei M, Bono M et al. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem 2004;279:25689–25695. [DOI] [PubMed] [Google Scholar]
- Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR et al. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science 2007;315:659–663. [DOI] [PubMed] [Google Scholar]
- Ren K, Li X, Yan J, Huang G, Zhou S, Yang B, Ma X, Lu C. Knockdown of p66Shc by siRNA injection rescues arsenite-induced developmental retardation in mouse preimplantation embryos. Reprod Toxicol 2014;43:8–18. [DOI] [PubMed] [Google Scholar]
- Rinaudo P, Schultz RM. Effects of embryo culture on global pattern of gene expression in preimplantation mouse embryos. Reproduction 2004;128:301–311. [DOI] [PubMed] [Google Scholar]
- Seli E, Vergouw CG, Morita H, Botros L, Roos P, Lambalk CB, Yamashita N, Kato O, Sakkas D. Noninvasive metabolomic profiling as an adjunct to morphology for noninvasive embryo assessment in women undergoing single embryo transfer. Fertil Steril 2010;94:535–542. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- Soliman MA, Abdel Rahman AM, Lamming DW, Birsoy K, Pawling J, Frigolet ME, Lu H, Fantus IG, Pasculescu A, Zheng Y et al. The adaptor protein p66Shc inhibits mTOR-dependent anabolic metabolism. Sci Signal 2014;7:ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschoppe A, Doerr H, Rascher W, Goecke T, Beckmann M, Schild R, Struwe E, Geisel J, Jung H, Dötsch J. DNA methylation of the p66Shc promoter is decreased in placental tissue from women delivering intrauterine growth restricted neonates. Prenat Diagn 2013;33:484–491. [DOI] [PubMed] [Google Scholar]
- Ventura A, Luzi L, Pacini S, Baldari CT, Pelicci PG. The p66Shc longevity gene is silenced through epigenetic modifications of an alternative promoter. J Biol Chem 2002;277:22370–22376. [DOI] [PubMed] [Google Scholar]
- Wale PL, Gardner DK. Oxygen regulates amino acid turnover and carbohydrate uptake during the preimplantation period of mouse embryo development. Biol Reprod 2012;87:24. [DOI] [PubMed] [Google Scholar]
- Wale PL, Gardner DK. Oxygen affects the ability of mouse blastocysts to regulate ammonium. Biol Reprod 2013;89:75. [DOI] [PubMed] [Google Scholar]
- Wale PL, Gardner DK. The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Hum Reprod Update 2015;22:dmv034 . [DOI] [PubMed] [Google Scholar]
- Zhang C, Liu C, Li D, Yao N, Yuan X, Yu A, Lu C, Ma X. Intracellular redox imbalance and extracellular amino acid metabolic abnormality contribute to arsenic-induced developmental retardation in mouse preimplantation embryos. J Cell Physiol 2010;222:444–455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.