Abstract
STUDY QUESTION
Do the organ culture conditions, previously defined for in vitro murine male germ cell differentiation, also result in differentiation of rat spermatogonia into post-meiotic germ cells exhibiting specific markers for haploid germ cells?
SUMMARY ANSWER
We demonstrated the differentiation of rat spermatogonia into post-meiotic cells in vitro, with emphasis on exhibiting, protein markers described for round spermatids.
WHAT IS KNOWN ALREADY
Full spermatogenesis in vitro from immature germ cells using an organ culture technique in mice was first reported 5 years ago. However, no studies reporting the differentiation of rat spermatogonia into post-meiotic germ cells exhibiting the characteristic protein expression profile or into functional sperm have been reported.
STUDY DESIGN, SAMPLES/MATERIALS, METHODS
Organ culture of testicular fragments of 5 days postpartum (dpp) neonatal rats was performed for up to 52 days. Evaluation of microscopic morphology, testosterone levels, mRNA and protein expression as measured by RT-qPCR and immunostaining were conducted to monitor germ cell differentiation in vitro. Potential effects of melatonin, Glutamax® medium, retinoic acid and the presence of epidydimal fat tissue on the spermatogenic process were evaluated. A minimum of three biological replicates were performed for all experiments presented in this study. One-way ANOVA, ANOVA on ranks and student's t-test were applied to perform the statistical analysis.
MAIN RESULTS AND THE ROLE OF CHANCE
Male germ cells, present in testicular tissue pieces grown from 5 dpp rats, exhibited positive protein expression for Acrosin and Crem (cAMP (cyclic adenosine mono phosphate) response element modulator) after 52 days of culture in vitro. Intra-testicular testosterone production could be observed after 3 days of culture, while when epididymal fat tissue was added, spontaneous contractility of cultured seminiferous tubules could be observed after 21 days. However, no supportive effect of the supplementation with any factor or the co-culturing with epididymal fat tissue on germ cell differentiation in vitro or testosterone production was observed.
LIMITATIONS, REASONS FOR CAUTION
The human testis is very different in physiology from the rat testis, further investigations are still needed to optimize the organ culture system for future use in humans.
WIDER IMPLICATIONS OF THE FINDINGS
The successful differentiation of undifferentiated spermatogonia using the testis explant culture system might be employed in future to produce sperm from human spermatogonia as a clinical tool for fertility preservation in boys and men suffering infertility.
LARGE SCALE DATA
None.
STUDY FUNDING AND COMPETING INTEREST(S)
This work was supported financially by the Frimurare Barnhuset in Stockholm, the Paediatric Research Foundation, Jeanssons Foundation, Sällskåpet Barnåvard in Stockholm, Swedish Research Council/Academy of Finland, Emil and Wera Cornells Foundation, Samariten Foundation, the Swedish Childhood Cancer Foundation as well as through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet. All authors declare no conflicts of interests.
Keywords: testis, acrosin, crem, spermatogonial stem cells (SSCs), fertility preservation, organ culture, meiosis
Introduction
Fertility preservation is becoming a growing research field. It involves methods and procedures to retain fertility for those who are at high risk to lose it (Anderson et al., 2015; Jahnukainen et al., 2015). Indications for fertility preservation in males include any of the conditions that require gonadotoxic treatment such as cancer, systemic lupus erythematosus, sickle cell anemia, Fanconi anemia, multiple sclerosis and renal diseases (Silva and Brunner, 2007; Mersereau and Dooley, 2010; Miller et al., 2012; Estes, 2015; Gajjar et al., 2015; Jahnukainen et al., 2015). Furthermore, spontaneous conditions that can cause failure in spermatogenesis are also considered an indication for fertility preservation or restoration, such as cryptorchidism or Klinefelter's syndrome (Jahnukainen and Stukenborg, 2012; Goossens et al., 2013; Rives et al., 2013; Picton et al., 2015). Childhood cancer patients represent an important patient group who might benefit from the fertility preservation techniques. Due to advancement in anti-cancer treatments for children, childhood cancer survivors are increasing in number dramatically in the past few decades. Nevertheless, these improvements in management and survival did not coincide with a reduction in the treatment-related gonadotoxicity, which has created a rising demand for fertility preservation (Jahnukainen et al., 2015).
In males, spermatogenesis entails three phases; mitosis, which involves proliferation of the spermatogonial stem cells (SCCs); meiosis, which involves the formation of haploid round spermatids from the diploid spermatogonia through the pre-meiotic spermatocytes; and spermiogenesis, which involves the differentiation of round spermatids into mature spermatozoa with no further cell division (Griswold, 2016). Most often, gonadotoxic treatments target the proliferating cells in the body. In the pre-pubertal human testis, the only germ cells present are the undifferentiated Adark and Apale spermatogonia. Together with the proliferating Sertoli cells, mainly undifferentiated spermatogonia are affected by the gonadotoxic treatment (Jahnukainen et al., 2011). Hence, it is not surprising that the pre-pubertal human testis is greatly affected by gonadotoxic treatment, which can lead to ablation of germ cells and infertility later in life (Relander et al., 2000; Chemes, 2001; Jahnukainen et al., 2011; Vassilakopoulou et al., 2016).
In order to model the clinical situation for such pre-pubertal patients, where the only available germ cells are SSCs, an in vitro maturation (IVM) procedure should achieve the maturation of male germ cells from the immature stage (pre-meiotic spermatogonia) to the mature stage (post-meiotic spermatids). To date, there are in principle two techniques that have been used for IVM; ex vivo organ culture, and three-dimensional (3D) cell culture (Jahnukainen and Stukenborg, 2012). These two techniques ensure that the testicular cells will have a spatial arrangement similar to the one they have in vivo (Staub, 2001; Stukenborg et al., 2009). Indeed, it has been reported that 3D culture was successful in differentiating male germ cells in vitro from both rats and humans (Lee et al., 2006, 2007). However, in these studies, the starting material was not obtained from pre-pubertal subjects, which would be important for modeling the situation of childhood cancer patients, who cannot deliver a semen sample for cryopreservation. Meanwhile, much effort has been directed to testicular organ culture since the 1960s (Steinberger et al., 1964; Stukenborg et al., 2009). Nevertheless, it was never possible to show full spermatogenesis in vitro in any species, until Gohbara and colleagues published their paper in 2010 (Gohbara et al., 2010). One year later, the same group showed for the first time that it is possible to differentiate spermatogonia in murine pre-pubertal testes into functional sperm in vitro (Sato et al., 2011). Since then, others have also reported similar data using murine pre-pubertal testis pieces (Dumont et al., 2015; Arkoun et al., 2015, 2016).
In order to approach the clinical application of this method, the success of IVM in mice should be repeated in other species, in order to confirm the reproducibility and culture conditions before transferring to the human situation. In an attempt to do so, our aim in this study was to investigate the possibility of producing mature rat male germ cells in vitro from immature pre-pubertal pieces of testis, exploiting the optimal organ culture conditions that were published for mouse previously.
Materials and methods
Animals
Newborn male Sprague-Dawley rats were purchased from Charles River (Sulzfeld, Germany), transported with their mothers to Karolinska Institutet (Stockholm, Sweden) and sacrificed by decapitation at 5 dpp of age.
Ethical approval
The use and handling of animals was approved by the ethics committee for experimental laboratory animals at Karolinska Institutet (N489/11 and N280/14).
Testicular tissue culture
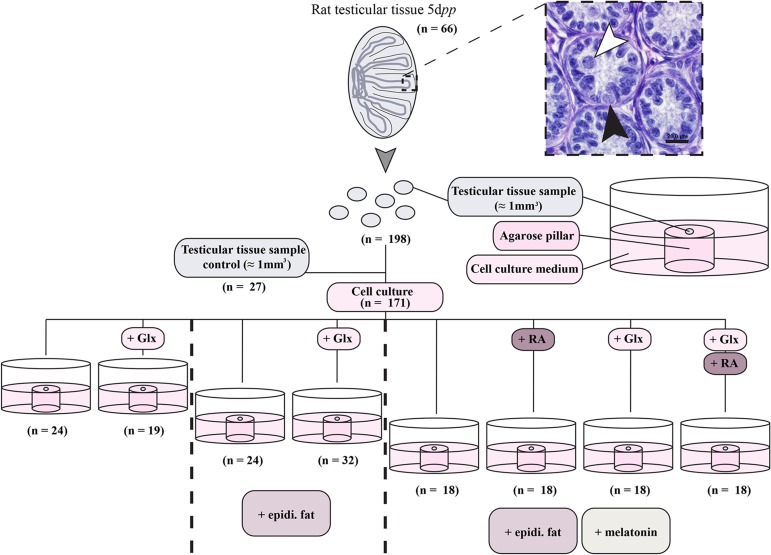
The testicular tissue culture was designed as previously described by Sato et al. (2011). Briefly, agarose pillars were prepared by mixing pre-autoclaved 0.7% (w/v) SeaKem® LE agarose (50004, Lonza, Basel, Switzerland) with the relevant culture medium 1:1 to give a final concentration of 0.35% (w/v) agarose. The agarose-medium mixture was left to solidify in culture dishes at room temperature (RT) and then cut into small pillars (approximately 7 mm diameter and 5 mm height), which were transferred into the six well plates (three pillars per well). After decapitation of the animals by scissors without anesthesia, following the ethics approved by Karolinska Institutet, testes from the 5 dpp rats were removed and placed in in Minimum Essential Medium alpha (MEMα, 22561-021, Gibco, Thermo Fisher Scientific, MA, USA) culture medium supplemented with 1% (v/v) penicillin/streptomycin (Pen/Strep, 15140-122, Gibco) on ice. Testes were decapsulated and cut with sterile forceps and scissors into small pieces (≈1 mm3 in size for each). The small testicular pieces were placed on top of the agarose pillars, one piece per pillar, and the relevant culture medium was placed in the wells of 6 well plates so that it reached the edge of the pillar without covering the testicular tissue pieces (Fig. 1). The culture medium consisted of either MEMα without Glutamax (22561-021, Gibco) or MEMα with Glutamax (32561-029, Gibco) both supplemented with 10% (v/v) Knock-out Serum Replacement (KSR, 10828-028, Gibco) and 1% (v/v) penicillin/streptomycin, Melatonin (M5250, Sigma Aldrich, Munich, Germany, final concentration 10−7 M) or retinoic acid (RA, R2625, Sigma Aldrich, final concentration 10−6 M) or a combination of both was added to the culture medium, depending on the composition intended. The stock solutions for melatonin and RA were prepared in Dimethyl Sulfoxide (DMSO; D2650, Sigma Aldrich) with the concentrations 10−2 and 10−1 M respectively. Stock solutions were kept light-protected at −20°C and later upon usage they were diluted in the assigned culture medium for each culture condition to the concentrations 10−4 and 10−3 M respectively to form the working concentrations. From these working concentrations, 1:1000 dilution with the assigned culture medium were made to give their final concentrations in the culture medium, 10−7 and 10−6 M respectively. Small pieces (≈1 mm3 in size for each) of epididymal fat were extracted from the pre-pubertal 5 dpp rats and placed in direct contact with the testicular tissue pieces on the agarose pillars in some wells, depending on the experiment. The culture conditions used were as follow: condition (1) MEMα without Glutamax + 1% (v/v) pen/strep + 10% (v/v) KSR; condition (2) MEMα with Glutamax + 1% (v/v) pen/strep + 10% (v/v) KSR; condition (3) MEMα without Glutamax + 1% (v/v) pen/strep + 10% (v/v) KSR + epididymal fat; condition (4) MEMα with Glutamax + 1% (v/v) pen/strep + 10% (v/v) KSR + epididymal fat; condition (5) MEMα without Glutamax + 1% (v/v) pen/strep + 10% (v/v) KSR + epididymal fat + melatonin; condition (6) MEMα without Glutamax + 1% (v/v) pen/strep + 10% (v/v) KSR + epididymal fat + melatonin + RA; condition (7) MEMα with Glutamax + 1% (v/v) pen/strep + 10% (v/v) KSR + epididymal fat + melatonin; and condition (8) MEMα with Glutamax + 1% (v/v) pen/strep + 10% (v/v) KSR + epididymal fat + melatonin + RA. An overview of the different culture conditions is provided in Fig. 1. The rat testicular tissue was cultured at 34.5°C and 5% CO2 and 95% air for up to 52 days, and the culture medium was replaced once per week.
Figure 1.
Schematic overview of different culture conditions used for culturing 5 days post parum (dpp) rat testicular tissue. In total, 66 testes of 5dpp old rats were used to obtain 198 testicular tissue samples sized about 1 mm3. Twenty-seven tissue samples were used as controls. The remaining samples were cultured on agarose pillars in organ culture conditions containing cell culture medium (minimal essential medium α + 1% penicillin/streptomycin + 10% (v/v) knock-out serum replacement) with the additions as stated in the figure. A representative picture of a 5dpp old rat testis is shown demonstrating the presence of gonocytes (white arrowheads) and spermatogonia (black arrowheads) in the tissue at the beginning of the culture. The scale bar is 20 µm. Abbreviations: Glutamax (Glx), epididymal fat (epidi. fat), and retinoic acid (RA).
Embedding and sectioning
Samples were fixed either in formaldehyde 4% in PBS (02176, Histolab, Gothenburg, Sweden) or in Bouin's solution (HT10132, Sigma-Aldrich) at 4°C overnight, and then dehydrated in ascending ethanol concentrations (30%, 50% and 70% (v/v) ethanol), each for 24 h at room temperature. Afterwards, samples were dehydrated in ascending ethanol concentrations (80%, 96% and 99.6% (v/v) ethanol), each for at least 6h at room temperature. Samples were then transferred into butyl acetate (45860, Sigma-Aldrich) stood overnight at room temperature followed by embedding in melted paraffin (ParaplastX-TRA®, P3808, Sigma-Aldrich) at 61°C overnight. After cooling down, paraffin-embedded samples were cut into 5 µm sections using a Biocut sectioning machine (Reichert-Jung, NY, USA) and sections were placed on microscope slides (10143352, Superfrost Plus, Thermo Scientific, MA, USA) and dried at 37°C overnight.
Immunostaining
Sections of formaldehyde 4% fixed samples were de-paraffinized in xylene and rehydrated in descending ethanol concentrations from 99.6% (v/v) to 70% (v/v) (99.6%, 96% and 70%) each for 10 min at room temperature. The sections were then washed in Tris-buffered saline (TBS) for 5 min and antigen retrieval was performed using citrate buffer (pH 6.0) at 95°C for 30 min, followed by cooling down at room temperature for another 30 min. Subsequently, sections were used in Ddx4/Ki67 and Ddx4/Crem double staining, in Acrosin immunofluorescent (IF) staining, or in Acrosin and Crem Immunohistochemical staining.
For Ddx4/Ki67 and Ddx4/Crem immunofluorescence (IF) double staining, the same method previously described in Reda et al., (2014) was followed. Briefly, sections were washed after antigen retrieval for 5 min in TBS and endogenous peroxidase was blocked using H2O2 3% (v/v) in methanol for 30 min at room temperature. Afterwards, sections were washed in TBS for 5 min and blocked with a blocking buffer formed of normal chicken serum 20% (v/v) (NChS, C5405, Sigma-Aldrich) and bovine serum albumin 5% (w/v) (BSA, 001-000-162, Jackson Immuno Research, PA, USA) in 1× TBS (TBS/NChS/BSA). Sections were then incubated overnight at 4°C either with rabbit polyclonal anti-Ddx4 primary antibody (ab13840, Abcam, Cambridge, UK 1:500, final concentration 2 µg/ml) or rabbit IgGs (ab27478, Abcam, 1:100, final concentration 2 µg/ml) as a negative control, both diluted in the blocking buffer (TBS/NChS/BSA). After washing in TBS three times for 5 min each, sections were incubated for 30 min at room temperature with Horseradish peroxidase (HRP)-conjugated chicken anti-rabbit secondary antibody (SC-2963, Santa Cruz, CA, USA, 1:200, final concentration 2 µg/ml) diluted in blocking buffer (TBS/NChS/BSA). After washing three times for 5 min each in TBS, the TSA™ Plus Fluorescein System (NEL741001KT, Perkin Elmer Life Sciences, Boston, USA) was applied according to the manufacturer's protocol. Subsequently, antigen retrieval was repeated again on the sections as above and sections were cooled for 30 min. Sections were then incubated in H2O2 3% (v/v) in TBS-Tween 0.1% (v/v) for 30 min at room temperature. After washing for 5 min in TBS, sections were blocked in the same blocking buffer (TBS/NChS/BSA) for 30 min at room temperature, followed by incubation overnight at 4°C with either rabbit monoclonal anti-Ki67 primary antibody (ab16667, Abcam, 1:200, final concentration 5 µg/ml), rabbit polyclonal anti-Crem primary antibody (SC-440, Santa cruz, 1:400, final concentration 0.5 µg/ml) or rabbit IgGs as a negative control (1:400–1:40, final concentration 0.5–5 µg/ml). All antibodies and IgGs were diluted in the same blocking buffer (TBS/NChS/BS). Sections were then washed in TBS three times for 5 min each and incubated again for 30 min at room temperature with HRP-conjugated chicken anti-rabbit secondary antibody (SC-2963, Santa Cruz, 1:200, final concentration 2 µg/ml) diluted in the blocking buffer (TBS/NChS/BSA). After washing three times in TBS for 5 min each, TSA™ Plus Cyanine 3 System (NEL744001KT, Perkin Elmer Life Sciences) was applied according to the manufacturer's protocol and the sections were mounted in VECTASHIELD mounting medium with DAPI (4’,6-Diamidino-2-Phenylindole; H-1500, VECTOR, CA, USA).
For the Acrosin IF staining, sections after the antigen retrieval were blocked with a blocking buffer formed of normal donkey serum 10% (v/v) (NDS, 017-00-121, Jackson Immuno Research) and BSA 1% (w/v) in 1× TBS (TBS/NDS/BSA) for 30 min at room temperature. Sections were then incubated overnight at 4°C with either rabbit polyclonal anti-Acrosin primary antibody (NBP2-14260, Novus Biologicals, CO, USA,1:100, final concentration 4 µg/ml) or rabbit IgGs (1:50, final concentration 4 µg/ml) as a negative control, both were diluted in the same blocking buffer (TBS/NDS/BSA). After washing in TBS three times for 5 min each, sections were incubated at room temperature for 30 min with Cy3-conjugated donkey anti-rabbit secondary antibody (711-166-152, Jackson Immuno Research, 1:300, final concentration 4.6 µg/ml) diluted in the same blocking buffer (TBS/NDS/BSA). Sections were then washed in TBS three times for 5min each and mounted in VECTASHIELD mounting medium with DAPI.
For the Acrosin and Crem immunohistochemistry staining, sections were blocked before antigen retrieval for endogenous peroxidase enzyme with H2O2 0.3% (v/v) in ethanol 96% for 10 min at room temperature. After antigen retrieval, sections were washed in 1× TBS and then blocked for non-specific protein binding with a blocking buffer formed of 10% (v/v) goat serum (S-1000, Vector) and 1% (w/v) BSA in 1×TBS for 30 min at room temperature. Afterwards, sections were incubated overnight at 4°C with either rabbit polyclonal anti-Acrosin primary antibody (NBP2-14260, Novus Biologicals, CO, USA,1:100, final concentration 4 µg/ml) or rabbit polyclonal anti-Crem primary antibody (SC-440, Santa Cruz, 1:200, final concentration 1 µg/ml) or rabbit IgGs (1:50–1:200, final concentration 1–4 µg/ml) as a negative control. All primary antibodies and rabbit IgGs negative control were diluted in the same blocking buffer (10% (v/v) goat serum in 1% (w/v) BSA in 1×TBS). After washing in 1×TBS for three times, sections were incubated at room temperature for 1 h with biotinylated goat anti-rabbit IgG secondary antibody ready to use (ab64256, Abcam, no dilution, final concentration 5 µg/ml). Sections were then washed with 1×TBS for 3 times and the Vectastain ABC elite Standard kit (PK-6100, Vector) was applied on the sections according to the manufacturer's protocol (sections were incubated with the ABC buffer at 37°C for 30 min). After washing again in 1×TBS for 3 times, ImPACT™ DAB (3, 3’-Diaminobenzidine) kit (SK-4105, Vector) was used for detection according to the manufacturer's protocol. Sections were later counterstained with hematoxylin (Mayer's Hemalaun solution, 1092491000, Merck) for 5 s and washed in running tap water for 2 min. Subsequently, sections were dehydrated using ascending ethanol concentrations (70%, 96% and 99.6% (v/v)) followed by xylene, and mounted finally in Entellan® new (1079610100, Merck).
For the evaluation of Ddx4/Ki67 double staining, the number of tubules containing Ddx4 positive/Ki67 positive cells per the total number of tubules in a section (germ cell proliferation index) was compared in two culture conditions (MEMα + 10% (v/v) KSR ± Glutamax). A total of 60–80 tubules were counted for each culture condition.
For the evaluation of Ddx4/Crem staining, the number of tubules containing Ddx4 positive/Crem positive cells per the total number of tubules in a section was counted and compared across the different culture conditions. A total of 100–450 tubules were counted for each condition.
For the evaluation of Acrosin staining, the number of tubules containing Acrosin positive cells per the total number of tubules in a section was counted and compared within the different culture conditions. A total of 100–450 tubules were counted for each setting.
Periodic acid-Schiff staining (PAS staining) and morphological evaluation
Sections of Bouin's solution fixed samples were de-paraffinized in xylene and rehydrated in descending ethanol concentrations from 99.6% (v/v) to 70% (v/v) (99.6%, 96% and 70%) each for 10 min at room temperature. Afterwards, PAS kit (101646, Merck, Darmstadt, Germany) was used to stain the sections according to the manufacturer's protocol. Briefly, after re-hydration, sections were washed in distilled water twice for 5 min each and incubated in periodic acid for 5 min. This was followed by thorough washing under running tap water and then twice in distilled water for 5 min each. Later on, sections were incubated in Schiff's reagent for 15 min. Sections were then washed thoroughly again under running tap water and twice in distilled water for 5 min each. Then, samples were either counter-stained with hematoxylin (Mayer's Hemalaun solution, 1092491000, Merck) for 2 min or dehydrated directly without counter-staining. Directly afterwards, counter-stained sections were washed in running tap water for 2 min, dehydrated using ascending ethanol concentrations (70%, 96% and 99.6% (v/v)) followed by xylene, and mounted finally in Entellan® new (1079610100, Merck). The different types of male germ cells that were identified depended on the morphological aspects described earlier (Russel et al., 1990). Primary spermatocytes were recognized by the larger size of cell and nucleus compared to the other germ cell types, and by the chromatin threads of the condensed chromosomes. Round spermatids were recognized by the smaller size of cell and nucleus compared to the other germ cell types, and by the presence of the acrosomal cap. The number of tubules containing primary spermatocytes or round spermatids per the total number of tubules in a section was used as an index for the germ cell differentiation progress. The total number of tubules counted per culture condition varied between 80 and 180 tubules.
For the gross morphologic evaluation, 4× pictures of the PAS-stained sections were taken and the necrotic region in a section was identified and quantified relative to the total surface area of the same section (as a percentage) using the ImageJ software (National Institutes of Health, USA). For statistical analysis, 3–9 biological replicates were recruited from each culture condition and the percentage of necrotic area was compared between the different conditions.
Microscopy and time lapse
Examination of PAS and IF stained sections was performed using an Eclipse E800 microscope (Nikon, Tokyo, Japan). Pictures of these evaluations were taken with a 12.5 million-pixel cooled digital color camera system (Olympus DP70, Tokyo, Japan). Time lapse was examined with an Eclipse TE200 (Nikon) microscope, and pictures were taken with an Infinity1 camera (Lumenera, Ontario, Canada). Frames for the time lapse were taken every one second and represented in the videos with the real-time pace. The surface area data was obtained and analyzed using the ImageJ software (National Institutes of Health, USA).
Testosterone assay
To evaluate the testosterone production from the immature rat testicular tissue cultured in different conditions, samples after 3, 10, 20, and 52 days of culture from the different culture conditions were snap frozen and kept in −80°C for later use in testosterone assay. For testosterone extraction, ethyl acetate was used as previously described in Reda et al. (2014). Briefly, each sample was sonicated in 200 µl phosphate buffered saline (PBS) using Vibra-cell™ (Sonics & Materials Inc, Newtown, USA) and 500 µl ethyl acetate was added to the homogenate. The mixture was vigorously shaken for 15 min on an automatic shaker at 900 rpm followed by centrifugation at 3000g for 2 min. The upper layer was then separated and placed in a new Eppendorf tube, and ethyl acetate extraction was repeated once again on the residue. The two extraction fractions were pooled and left to evaporate overnight at room temperature, and the testosterone left in the tube was dissolved in 200 µl PBS. Subsequently, testosterone was measured by an ELISA kit (EIA-1559, DRG instruments, Marburg, Germany) with an intra-assay CV < 5% and inter-assay CV < 10% according to the manufacturer's protocol. The microplate reader Fluostar Omega (BMG LabTech, Ortenberg, Germany) was used for reading the absorbance at 450 nm. The standards provided with the kit were used for generating a standard curve while each sample and standard was run in duplicates. Weights of the testicular tissue pieces were measured prior to the extraction process and the amount of testosterone produced per each sample was adjusted to the sample weight.
Gene expression analysis
Samples of testicular tissue after 52 days of culture under different conditions were snap frozen and kept at −80°C until RNA extraction was performed. The RNA was isolated from control and cultured testicular tissue using TRIzol® Reagent (15596-018, Invitrogen, Thermo Fisher Scientific MA, USA) and RNeasy Mini Kit (74104, Qiagen, Venlo, Netherlands) according to the manufacturer's protocol. 0.2 µg RNA per sample was utilized for synthesizing 20 µl of cDNA (10 ng/µl) using the IScript cDNA synthesis kit (170-8891, Bio-Rad, CA, USA) according to the manufacturer's protocol.
The iCycler iQ™ multicolor RT-PCR detection system (Bio-Rad) was applied to perform the qPCR, while the iCycler iQ® ver.3.1 software (Bio-Rad) was used for data analysis. TaqMan® probes for the genes assessed, along with the TaqMan® Gene Expression Master Mix (4369510, Applied Biosystems, Thermo Fisher Scientific), were used to perform the qPCR. A list of all the genes assessed as well as the assay numbers of all the TaqMan® probes used are included in the Supplementary Table I. The thermal cycle consisted of initial 10 min at 95°C followed by 45 cycles of 2 steps; denaturation at 95°C for 15 s and one-step elongation at 60°C for 1 min. All samples were run in duplicates and the ddCt method was used for expressing the fold change in gene expression (2-ddCt). The Ct value of any gene assessed was normalized to the Actb (beta Actin) Ct value, whereas the fold change in gene expression was represented relative to the expression of the same gene in pre-pubertal 5 dpp rat testis (pre-culture) control.
Statistical analysis
Student's t-test, One-way ANOVA, and ANOVA on ranks were applied to perform the statistical analysis using the Sigma Plot software ver.12.0 (Systat Software Inc., IL, USA) as stated in the figure legends. Each experimental condition was repeated at least three times, while the means and standard deviations were used in statistical analysis and in the figures. The P value was considered significant if it was ≤ 0.05.
Results
Morphologic assessment
When the 5 dpp rat testicular pieces, cultured in organ culture for 52 days, were sectioned and stained with PAS, two distinct regions in the sections could be identified; a peripheral intact region and a central necrotic area. Using the ImageJ software, analysis of the PAS-stained sections revealed that the necrotic area represented between 17 ± 7.0% and 49 ± 10.4% of the section area in the different culture conditions. The plain medium with no supplementation (MEMα + 10% (v/v) KSR) had significantly less necrotic area per section surface area compared to the other culture conditions (Supplementary Figure 1).
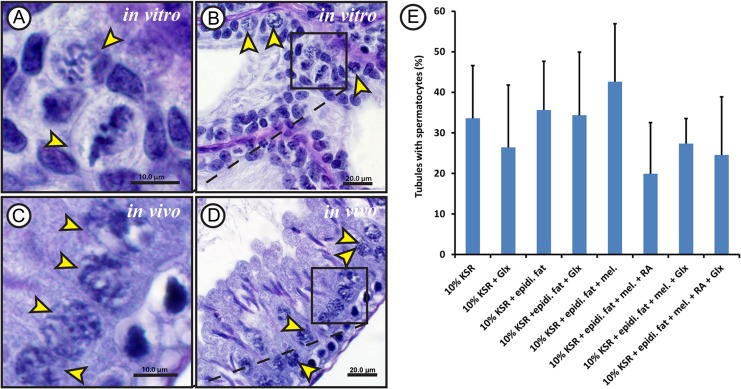
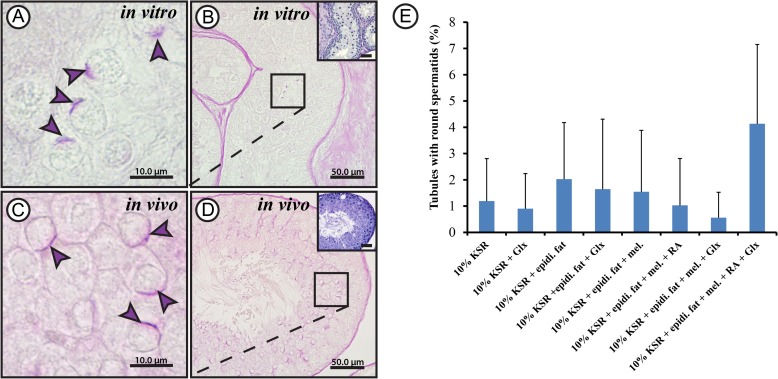
Gonocytes and spermatogonia were the only germ cell types present in the 5 dpp rat testes, used as starting material in the beginning of the experiments (Fig. 1). After 52 days in culture, cells exhibiting morphological features similar to spermatocytes (the condensed chromatin threads; Fig. 2A and B) and round spermatids (the acrosomal cap; Fig. 3A and B), which are seen in 60 dpp rat (Figs. 2C and D; 3C and D), could be observed. The percentage of tubules containing spermatocytes or round spermatids per sample section differed slightly between the various treatments. However, these differences were not statistically significant due to the high variance of each treatment group from experiment to experiment. Cells exhibiting morphological characteristics of spermatocytes could be observed in 20 ± 12.6% to 43 ± 14.3% of tubules (Fig. 2E), while those showing characteristics of round spermatids where present in 0.5 ± 0.9% to 4 ± 3.0% of the evaluated tubules (Fig. 3E).
Figure 2.
Morphological evaluation results for spermatocytes obtained from rat testicular tissue culture. Periodic acid Schiff's staining (PAS) with haematoxylin counter staining for Bouin's solution-fixed paraffin-embedded testicular tissue cultured for 52 days in minimum essential medium alpha (MEMα) + 10% (v/v) knock-out serum replacement (KSR) (A and B) and 60 dpp adult rat testis control (C and D) showing primary spermatocytes. Higher magnifications of B and D are shown in A and C, respectively. Hematoxylin was used for counter-staining. The yellow arrow heads show primary spermatocytes. The scale bars are 10 µm (A and C) and 20 µm (B and D). Percentage of tubules containing spermatocytes (E) compared between the different culture conditions used. MEMα, minimal essential medium α;KSR, knock-out serum replacement; Glx, Glutamax; epidi. fat, epididymal fat; mel, melatonin; RA, retinoic acid. Values are represented in mean ± SD (n = 6–12). For statistical analysis, one-way ANOVA test was applied to compare between the percentages of tubules from the different culture conditions.
Figure 3.
Morphological evaluation results for round spermatids obtained from rat testicular tissue culture. Periodic acid Schiff's staining (PAS) of Bouin's solution-fixed paraffin-embedded testicular tissue cultured for 52 days in minimum essential medium alpha (MEMα) + 10% (v/v) knock-out serum replacement (KSR) (A and B) and 60 days postpartum adult rat testis control (C and D) showing round spermatids. Higher magnifications of B and D are shown in A and C, respectively. Hematoxylin was used for counter-staining in the same samples to show overall organization in the tissue (small inserts; B and D). The violet arrow heads show acrosomal caps. The scale bars are 10 µm (A and C) and 50 µm (B and D). Percentage of tubules containing spermatids exhibiting acrosomal caps (E) compared between the different culture conditions used; MEMα, minimal essential medium α;KSR, knock-out serum replacement; Glx, Glutamax; epidi. fat, epididymal fat; mel, melatonin; RA, retinoic acid. Values are mean ± SD (n = 6–12). For statistical analysis, one-way ANOVA test was applied to compare between the percentages of tubules from the different culture conditions.
Germ cell proliferation
In order to evaluate the effect of Glutamax supplementation with a final concentration of 1.87 mM (as pre-supplemented by the culture medium provider; Gibco, Thermo Fisher Scientific) on the proliferation pattern of germ cells after 52 days in vitro, samples from two culture conditions (MEMα + 10% (v/v) KSR ± Glutamax) were double stained for the germ cell marker Ddx4 and the cell proliferation marker Ki67 (data not shown). The percentage of tubules containing cells expressing Ddx4 and Ki67 was used as germ cell proliferation index. The comparison between both conditions using student's t-test, however, revealed no significant difference regarding the germ cell proliferation index (99 ± 1.3% and 80 ± 13.9% for (+) Glutamax and (−) Glutamax respectively).
Gene expression analysis
Gene expression analysis was performed to confirm the morphological observations in terms of male germ cell differentiation in vitro. mRNA samples of testicular tissue cultured for 52 days were assessed for genes important for different steps during spermatogenesis (post-meiotic makers: Acr (Acrosin), Crem, Prm1 (Protamine 1); meiotic markers: Sycp3 (Synaptonemal complex protein 3), Boll (Boule-like RNA-binding protein); pre-meiotic markers: Kit (V-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog), Zbtb16 (Zinc finger-and BTB-domain containing 16), Dazl (Deleted in azoospermia-like), as well as the general germ cell marker Ddx4). Testicular tissue cultured for 52 days showed in general a slight elevation in gene expression profile, when compared to the control pre-pubertal 5 dpp rat testis. However, this elevation did not reach statistical significance in any of the genes investigated, except for the Sycp3, where the plain culture setting (only MEMα + KSR 10% (v/v)) had a significantly upregulated Sycp3 expression compared to the control pre-pubertal 5 dpp rat testis (Supplementary Figure 2). Meanwhile, comparing the adult 60 dpp rat testis control to the different culture conditions and the pre-pubertal 5 dpp rat testis control revealed that the adult testis control had significantly higher gene expression compared to the different culture conditions and the pre-pubertal control, regarding the genes Crem, Prm1, Sycp3, Boll, Acr, Dazl, and Ddx4, except for the plain culture setting (only MEMα + KSR 10% (v/v)), where Sycp3 was not significantly different from the adult testis control (Supplementary Figure 2). Moreover, no significant difference in gene expression between the different culture conditions could be observed, except in the expression of Sycp3, where the plain culture setting with no supplements (MEMα + 10% (v/v) KSR) had significantly higher Sycp3 expression compared to two of the culture conditions (Supplementary Figure 3).
Male germ cell differentiation
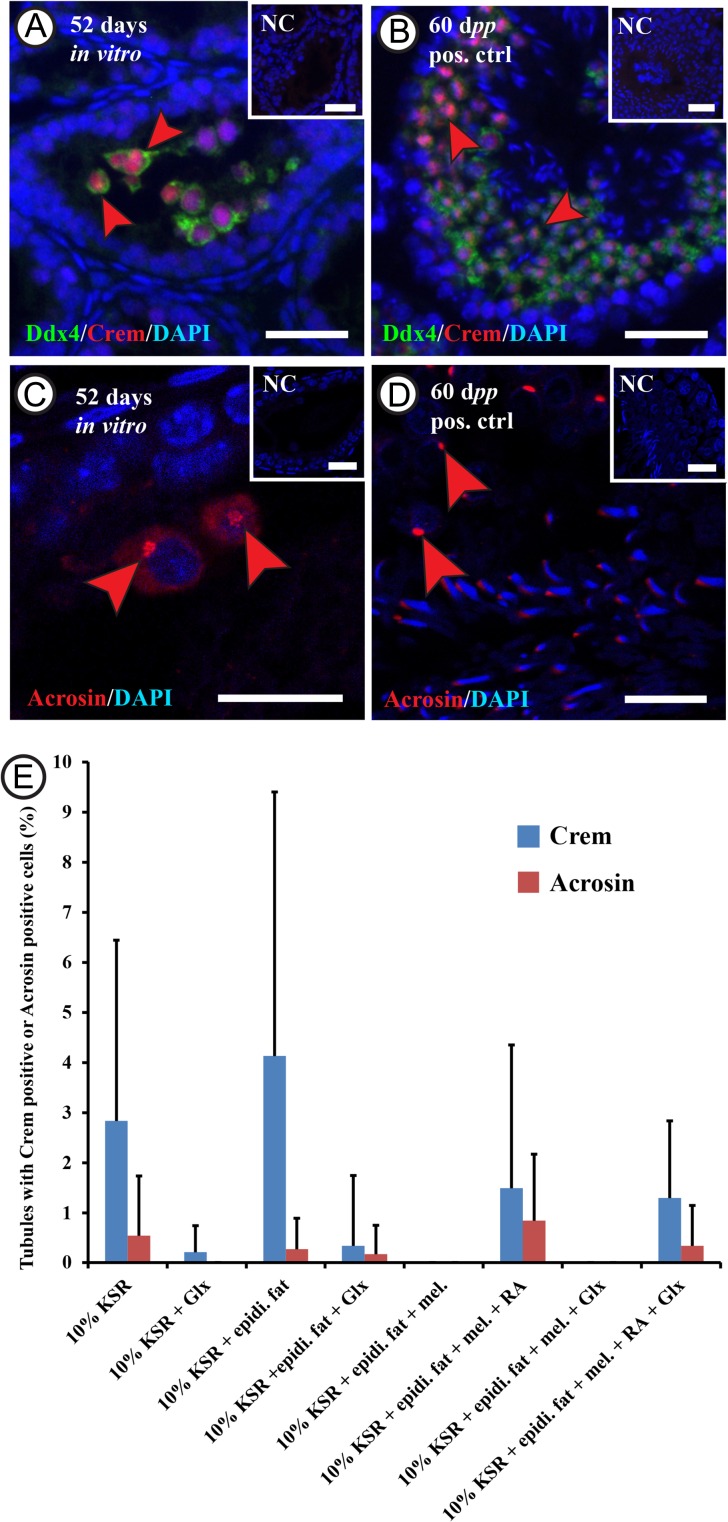
While the morphologic evaluation suggested the presence of cells exhibiting the characteristics known for spermatocytes and round spermatids, we wished to confirm using additional methods. Therefore, double IF staining for Ddx4 and Crem (the Crem protein is expressed in late spermatocytes and round spermatids) as well as IF staining for Acrosin (the Acrosin protein is expressed in spermatids) and immunohistochemical staining for Crem and Acrosin were performed (Fig. 4, Supplementary Figure 3). The percentage of tubules containing Ddx4 positive germ cells, and also expressing Crem was up to 4 ± 5.2%, while the percentage of tubules containing Acrosin positive cells was up to 0.8 ± 1.3% (Fig. 4). No significant differences concerning the expression of Crem or Acrosin could be detected when comparing the different culture conditions. Moreover, some cells showed a dim cytoplasmic signal of Acrosin in tubules from the different culture conditions. The percentage of tubules containing dim Acrosin positive cells in a sample section reached up to 6 ± 6.2% from the different culture conditions (Supplementary Figure 4). This number did not differ significantly between the different culture conditions. However, when combining the numbers of seminiferous tubules containing dim or bright Acrosin positive cells, the percentage of these tubules increased to 6 ± 6.9% and the plain culture setting with no supplements (MEMα + 10% (v/v) KSR) had significantly higher percentage of tubules with Acrosin positive cells than the other conditions (Supplementary Figure 4).
Figure 4.
Assessment of the rat male germ cell differentiation in vitro.Double immunofluorescent staining for 5 days postpartum (dpp) rat testicular tissue after culturing in minimum essential medium alpha (MEMα) + 10% (v/v) knock-out serum replacement (KSR) for 52 days, showing cells double positive for Ddx4 (DEAD box polypeptide 4; in green) and Crem (cAMp (cyclic adenosine mono phosphate) response element modulator; in red) labeled with red arrowheads (A and B), and Acrosin positive cells (in red) labeled with the red arrowheads (C and D). Rabbit IgGs were used instead of the primary antibodies in the negative control and DAPI (4’,6-Diamidino-2-Phenylindole) was utilized in counterstaining (in blue). The scale bars are 20 µm A and B, and negative controls), and 10 µm (C and D). The graph (E) shows the percentage of tubules containing Crem positive cells (in blue) or Acrosin positive cells (in red) after culturing the 5 dpp rat testicular tissue for 52 days in the different culture conditions. Values are mean ± SD (n = 3–12 (Crem), n = 3–9 (Acrosin)). For statistical analysis, ANOVA on ranks test was applied to compare between the percentages of tubules from the different culture conditions. Abbreviations: minimal essential medium α (MEMα), melatonin (mel.), knock-out serum replacement (KSR), Glutamax (Glx), epididymal fat (epidi. fat), and retinoic acid (RA).
To explore more about the Crem positive cells and the different Acrosin intensity profiles, immunohistochemical staining for Acrosin and Crem was performed. The results, in both the cultured tissues and the control adult tissue, revealed that Crem positive cells had a uniform intensity profile and localization (nuclear), while Acrosin expression could be observed as two intensity profiles; a weak cytoplasmic signal as well as a strong acrosomal signal (Supplementary Figure 3).
Functionality of Leydig and peritubular cells in vitro
To evaluate the effect of culture time on testosterone production, and thereby the functionality of Leydig cells in the culture, rat immature 5 dpp testicular tissue was cultured in a plain setting MEMα + 10% (v/v) KSR. Samples were subjected to testosterone extraction followed by measurement after 3, 10 or 52 days in vitro and the results showed that testosterone production was significantly higher at 3 days of culture (261 ± 27.9 pmol/g testis weight) than at 10 days (110 ± 15.5 pmol/g testis weight) or at 52 days of culture (152 ± 18.7 pmol/g testis weight) as shown in Supplementary Figure 5. To evaluate the effect of epididymal fat combined with different culture conditions on testosterone production, testicular tissue of 5 dpp rats was cultured for 52 days in MEMα + 10% (v/v) KSR + epididymal fat under different culture conditions (with or without Glutamax, with or without melatonin, and with or without RA) (Supplementary Figure 6). Testosterone levels were found to fluctuate between 46 ± 39.9 and 98 ± 19.7 pmol/g testis weight. However, there was no statistically significant difference between these values.
Regarding the peritubular cells, when testicular tissue of these rats was cultured with epididymal fat from the same animals for 52 days, spontaneous contractility after 3 weeks of culture until the end of the culture after 52 days could be observed (Supplementary Video 1 and 2). The contractions, suggesting the activation of peritubular cells, could be observed in up to 66% of the testicular tissue pieces cultured in direct contact with epididymal fat. Although the spontaneous activation of peritubular cells could be observed by the addition of epididymal fat, a positive effect on the differentiation of male germ cells was not observed.
Discussion
To our knowledge, the first full spermatogenesis in vitro from immature germ cells using an organ culture technique in mice was reported 5 years ago (Sato et al., 2011). However, studies reporting the differentiation of spermatogonia into post-meiotic germ cells exhibiting the characteristic protein expression profile or the functional sperm from other mammalian species are missing. In order to transfer the technique to clinics, it has to be repeated successfully in other species, including in humans. Rats, although they are rodents as mice are, exhibit features of spermatogenesis which are somewhere between mice and humans. For example, rats take longer time for full spermatogenesis than mice, but shorter than humans (Adler, 1996). In addition, the rat testis shows delayed recovery after irradiation compared to mice, but it has a shorter recovery period than humans (Abuelhija et al., 2013). Hence, the rat model is considered a step forward from the mouse model towards the clinical application. Recently, a study reported the differentiation of rat male germ cells in vitro (Liu et al., 2016). Unfortunately, the authors based their findings only upon morphologic analysis of the cultured testicular tissue and did not include data on protein expression levels of post-meiotic markers expressed by haploid cells.
In 2009, we demonstrated the differentiation of murine male pre-meiotic germ cells into post-meiotic germ cells in vitro, using the 3D culture system (Stukenborg et al., 2009). However, when we applied the same methodology to the rat model, we encountered some difficulties regarding germ cell differentiation, most probably due to the dis-organized orientation of the germ cells in relation to the Sertoli cells, as we discussed in an earlier publication (Reda et al., 2014). Moreover, the efficiency with the 3D system was extremely low using the mouse model (Stukenborg et al., 2009). Thus, along with the successful report from Sato and colleagues using the organ culture condition in mice (Sato et al., 2011), we exploited the organ culture approach in order to achieve the germ cell maturation in vitro using the rat model. Therefore, in this study, we applied the successful IVM described in mice to rat testis pieces, with the focus on morphologic characteristics but also on the expression of post-meiotic markers Crem and Acrosin on protein expression by immunostaining.
Acrosin and Crem were used as markers for post-meiotic male germ cells. Crem protein is expressed in mammalian male germ cells from the late pachytene spermatocyte stage until the mature spermatids are released (Foulkes et al., 1992). Hence, we used Crem in this study as a marker for the germ cell differentiation process. In addition to Crem, Acrosin, which is a serine protease found in the acrosome of the post-meiotic haploid male germ cells, is used as marker for round and elongated spermatids (Sato et al., 2011). It is expressed as an inactive zymogen, Proacrosin, and stored in the acrosome until fertilization (Adham et al., 1989; Nayernia et al., 1994). In rats, the Acr gene should be transcribed in the diploid stage, and is translationally suppressed, and finally, it is expressed in the post-meiotic haploid round spermatid stage (Florke et al., 1983; Nayernia et al., 1994). We saw two intensity profiles for Acrosin in our tissues: a small population of strongly-expressing cells, and a relatively larger population of faintly-expressing cells (cytoplasmic expression). We believe that this faint intensity profile could be due to Acrosin expression in an earlier maturation step, prior to concentration in the acrosomal vesicle during the round spermatid steps. Meanwhile, the Crem immunohistochemical staining confirmed the expression of Crem with a uniform intensity profile and nuclear localization in round spermatids. Studies in mice demonstrated a uniform faint Acrosin expression in the cytoplasm of pachytene spermatocytes (Ventela et al., 2000). After these germ cells completed meiosis, the cytoplasmic Acrosin accumulated and resulted in a strong expression in round spermatids (Ventela et al., 2000). Although similar expression profiles were observed in cells cultured for 52 days in the current study, this issue is intriguing and needs to be addressed in future studies.
The organ culture of rat testicular tissue was performed for up to 52 days, to ensure that the whole process of spermatogenesis, which in vivo takes 52–53 days in rats, starting from type A spermatogonia, present at the age of 5 dpp in rats (Clermont and Harvey, 1965; Legendre et al., 2010), could be completed within the culture period. The culture medium, MEMα with 10% (v/v) KSR, was used as it has been shown earlier to support male germ cell differentiation in vitro in mice (Sato et al., 2011). Indeed, it was possible to reproduce in rat the success in IVM of immature male germ cells in mouse, using the same culture conditions. This was evaluated morphologically and confirmed by the presence of Acrosin and Crem on protein level by immunostaining. However, the efficiency of the organ culture system was low, as only 0.5 ± 1.0% to 4.1 ± 3.0% of the tubules contained round spermatids exhibiting the formation of an acrosomal cap (as identified via PAS). Other recently published studies reported that murine testicular tissue cultured in organ culture resulted in 22 ± 2.8% of the tubules containing round spermatids (Dumont et al., 2015). If repeated by others, the efficiency of murine germ cell differentiation in vitro into round spermatids would be far higher than the rats as described in this study.
Nevertheless, we believe our protein expression levels do represent true post-meiotic cells because gene expression analysis data revealed that there was an upregulation in most of the genes involved in male germ cell differentiation including Crem, Prm1, Sycp3, Boll, Dazl, and Ddx4. Despite that, this upregulation did not always reach significance in all of the culture conditions, as evidenced by the scarcity of differentiated cells. There was a slight upregulation in Acr expression in the cultured tissue compared to the control pre-pubertal 5 dpp rat testis as well, but it did not reach significance for the same reason. These genes are known to be significantly upregulated in the adult mature male germ cells (Supplementary Figure 2). In general, germ cell differentiation in vitro was not consistent in the organ culture system, as it appeared in some sporadic tubules and not in others under the same culture conditions. Using different culture conditions did not change this fact.
We had hypothesized that adding selected key nutrients to the medium might improve the yield and consistency of the cultures. Glutamax, melatonin, and RA were used in attempts to improve in vitro germ cell maturation. We had shown previously that Glutamax has some positive effects on germ cell viability and differentiation in vitro in rats using a re-aggregated 3D culture system (Reda et al., 2014). Melatonin, N-acetyl-5-methoxytryptamine, is produced mainly by the pineal gland, but is also produced directly by the testis (Tijmes et al., 1996). It is a multifunctional molecule that has several roles in the reproductive system (Kinson, 1976; Carlomagno et al., 2011). We hypothesized its utility in these cultures because it has been reported to have anti-cytotoxic effects against chemotherapy and radiation, based on its anti-oxidant properties. Melatonin can scavenge the reactive oxygen species (ROS) produced in tissues due to chemotherapy or radiation (Leon et al., 2004; Take et al., 2009; Madhu et al., 2015). Hence, we assumed that melatonin would have a positive effect on the survival of the cultured testicular tissue in vitro. We tested a concentration of 10−7 M melatonin, as it was the concentration that is shown previously to have a protective effect on cells in vitro (Wang et al., 2014, 2015; Song et al., 2015).
Regarding RA, it is essential for the differentiation of rat male germ cells in vivo (Van Pelt and De Rooij, 1990; Gely-Pernot et al., 2015), the release of the mature spermatids into the seminiferous tubule lumen (Vernet et al., 2008), and initiating and maintaining the seminiferous cycle (Hogarth and Griswold, 2013). In addition, RA is known to support the differentiation of pluripotent cells into male germ cell lineage in vitro (Miryounesi et al., 2014; Zhang et al., 2015), and also the differentiation of male germ cells into meiotic cells in vitro (Riboldi et al., 2012; Travers et al., 2013). The concentration of retinoic acid used here in this study was 10−6 M, which was found in the previous studies to support germ cell differentiation in vitro (Travers et al., 2013; Zhang et al., 2015).
Finally, we investigated the effect of co-culturing epididymal fat with immature testis pieces in vitro, as it was shown previously that epididymal white adipose tissue (EWAT) is important for normal spermatogenesis (Srinivasan et al., 1986; Chu et al., 2010). In order to mimic the situation in vivo, where both tissues (testicular tissue and epididymal fat) have the same age and grow together simultaneously, epididymal fat from immature animals was used in the current study.
Surprisingly, the supplementation with these levels of Glutamax, melatonin, or RA did not improve the rat male germ cell differentiation in vitro, which suggests that they are not the only players in the differentiation process. This is consistent with the report by Sato, who reported success in producing post-meiotic spermatids using only MEM and KSR (Sato et al., 2011). Nor did these supplements improve testosterone production. Notably, we have shown that the testosterone production after 52 days is significantly less than the production in the first 3 days. It is worth noting that our cultures lacked hCG or LH, known stimulators of Leydig cell steroidogenesis, and this remains fertile ground for future experiments. Meanwhile, the proliferation endpoint (Ki67) revealed that the germ cells were still proliferating after 52 days in culture, although Glutamax supplementation had no significant effect on this germ cell proliferation.
It has been reported that epididymal fat is important for spermatogenesis in vivo, in rats and mice (Srinivasan et al., 1986; Chu et al., 2010). The authors have suggested the presence of an unidentified growth factor, secreted by the epididymal fat, which stimulates spermatogenesis in the seminiferous tubules. However, we found no effect of the epididymal fat co-cultured with the testicular tissue in vitro on spermatogenesis or germ cell differentiation. In addition, we could detect no effect of epididymal fat on testosterone production, which matches with the results from previous reports (Chu et al., 2010). Strikingly, we did observe improved spontaneous contractility in the rat seminiferous tubules after 3 weeks of co-culture with epididymal fat, which lasted until the end of the experiment at 52 days. This spontaneous contractility was never seen when the tubules were cultured without epididymal fat. Rat seminiferous tubule contractility has been investigated previously (Hovatta, 1972; Maekawa et al., 1996). It is driven mainly by the peritubular myoid cells, which become functional at 15 days of age in vivo, after they start exhibiting actin filaments.
In conclusion, we report here the successful in vitro differentiation of undifferentiated rat spermatogonia into cells exhibiting morphologic features and protein markers of round spermatids using organ culture conditions previously described for mice. Although the complete rat spermatogenesis in vitro, resulting in functional sperm, could not be demonstrated in the current study, we consider this a step forward towards the clinical application after the success achieved in mouse. However, as the human testis is still much different in physiology from the rat testis, further investigations are still needed to optimize the organ culture system for future use in humans.
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/.
Acknowledgment
The authors thank E. Villow for technical assistance.
Authors’ roles
Reda A.: Study design, data acquisition, analysis and interpretation, drafting the article and final approval of the submitted version.
Hou M.: Data acquisition and analysis, drafting the article and final approval of the submitted version.
Winton T.R.: Data analysis and interpretation, drafting the article and final approval of the submitted version.
Chapin R.E.: Data analysis and interpretation, drafting the article and final approval of the submitted version.
Söder O.: Data analysis and interpretation, drafting the article and final approval of the submitted version.
Stukenborg J.B.: Study design, data acquisition, analysis and interpretation, drafting the article and final approval of the submitted version.
Funding
Frimurare Barnhuset in Stockholm, the Paediatric Research Foundation, Jeanssons Foundation, Sällskåpet Barnåvard in Stockholm, Swedish Research Council/ Academy of Finland, Emil and Wera Cornells Foundation, Samariten Foundation, the Swedish Childhood Cancer Foundation as well as through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet.
Conflict of interest
None of the authors has received benefits or grants that are relevant for this study from other sources than the research foundations listed above.
References
- Abuelhija M, Weng CC, Shetty G, Meistrich ML. Rat models of post-irradiation recovery of spermatogenesis: interstrain differences. Andrology 2013;1:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adham IM, Klemm U, Maier WM, Hoyer-Fender S, Tsaousidou S, Engel W. Molecular cloning of preproacrosin and analysis of its expression pattern in spermatogenesis. Eur J Biochem 1989;182:563–568. [DOI] [PubMed] [Google Scholar]
- Adler ID. Comparison of the duration of spermatogenesis between male rodents and humans. Mutat Res 1996;352:169–172. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Mitchell RT, Kelsey TW, Spears N, Telfer EE, Wallace WH. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol 2015;3:556–567. [DOI] [PubMed] [Google Scholar]
- Arkoun B, Dumont L, Milazzo JP, Rondanino C, Bironneau A, Wils J, Rives N. Does soaking temperature during controlled slow freezing of pre-pubertal mouse testes influence course of in vitro spermatogenesis. Cell Tissue Res 2016;364(3):661–674. doi: 10.1007/s00441-015-2341-2. [DOI] [PubMed] [Google Scholar]
- Arkoun B, Dumont L, Milazzo JP, Way A, Bironneau A, Wils J, Mace B, Rives N. Retinol improves in vitro differentiation of pre-pubertal mouse spermatogonial stem cells into sperm during the first wave of spermatogenesis. PloS One 2015;10:e0116660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlomagno G, Nordio M, Chiu TT, Unfer V. Contribution of myo-inositol and melatonin to human reproduction. Eur J Obstet Gynecol Reprod Biol 2011;159:267–272. [DOI] [PubMed] [Google Scholar]
- Chemes HE. Infancy is not a quiescent period of testicular development. Int J Androl 2001;24:2–7. [DOI] [PubMed] [Google Scholar]
- Chu Y, Huddleston GG, Clancy AN, Harris RB, Bartness TJ. Epididymal fat is necessary for spermatogenesis, but not testosterone production or copulatory behavior. Endocrinology 2010;151:5669–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont Y, Harvey SC. Duration of the cycle of the seminiferous epithelium of normal, hypophysectomized and hypophysectomized-hormone treated albino rats. Endocrinology 1965;76:80–89. [DOI] [PubMed] [Google Scholar]
- Dumont L, Arkoun B, Jumeau F, Milazzo JP, Bironneau A, Liot D, Wils J, Rondanino C, Rives N. Assessment of the optimal vitrification protocol for pre-pubertal mice testes leading to successful in vitro production of flagellated spermatozoa. Andrology 2015;3:611–625. [DOI] [PubMed] [Google Scholar]
- Estes SJ. Fertility preservation in children and adolescents. Endocrinol Metab Clin North Am 2015;44:799–820. [DOI] [PubMed] [Google Scholar]
- Florke S, Phi-van L, Muller-Esterl W, Scheuber HP, Engel W. Acrosin in the spermiohistogenesis of mammals. Differentiation 1983;24:250–256. [DOI] [PubMed] [Google Scholar]
- Foulkes NS, Mellstrom B, Benusiglio E, Sassone-Corsi P. Developmental switch of CREM function during spermatogenesis: from antagonist to activator. Nature 1992;355:80–84. [DOI] [PubMed] [Google Scholar]
- Gajjar R, Miller SD, Meyers KE, Ginsberg JP. Fertility preservation in patients receiving cyclophosphamide therapy for renal disease. Pediatr Nephrol 2015;30:1099–1106. [DOI] [PubMed] [Google Scholar]
- Gely-Pernot A, Raverdeau M, Teletin M, Vernet N, Feret B, Klopfenstein M, Dennefeld C, Davidson I, Benoit G, Mark M et al. . Retinoic acid receptors control spermatogonia cell-fate and induce expression of the SALL4A transcription factor. PLoS Genet 2015;11:e1005501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohbara A, Katagiri K, Sato T, Kubota Y, Kagechika H, Araki Y, Ogawa T. In vitro murine spermatogenesis in an organ culture system. Biol Reprod 2010;83:261–267. [DOI] [PubMed] [Google Scholar]
- Goossens E, Van Saen D, Tournaye H. Spermatogonial stem cell preservation and transplantation: from research to clinic. Hum Reprod 2013;28:897–907. [DOI] [PubMed] [Google Scholar]
- Griswold MD. Spermatogenesis: the commitment to meiosis. Physiol Rev 2016;96:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth CA, Griswold MD. Retinoic acid regulation of male meiosis. Curr Opin Endocrinol Diabetes Obes 2013;20:217–223. [DOI] [PubMed] [Google Scholar]
- Hovatta O. Contractility and structure of adult rat seminiferous tubules in organ culture. Z Zellforsch Mikrosk Anat 1972;130:171–179. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Hou M, Schlatt S. Testicular function and fertility preservation in male cancer patients. Best Pract Res Clin Endocrinol Metab 2011;25:287–302. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Mitchell RT, Stukenborg JB. Testicular function and fertility preservation after treatment for haematological cancer. Curr Opin Endocrinol Diabetes Obes 2015;22:217–223. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Stukenborg JB. Clinical review: present and future prospects of male fertility preservation for children and adolescents. J Clin Endocrinol Metab 2012;97:4341–4351. [DOI] [PubMed] [Google Scholar]
- Kinson GA. Pineal factors in the control of testicular function. Adv Sex Horm Res 1976;2:87–139. [PubMed] [Google Scholar]
- Lee JH, Gye MC, Choi KW, Hong JY, Lee YB, Park DW, Lee SJ, Min CK. In vitro differentiation of germ cells from nonobstructive azoospermic patients using three-dimensional culture in a collagen gel matrix. Fertil Steril 2007;10 1016/jfertnstert. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim HJ, Kim H, Lee SJ, Gye MC. In vitro spermatogenesis by three-dimensional culture of rat testicular cells in collagen gel matrix. Biomaterials 2006;27:2845–2853. [DOI] [PubMed] [Google Scholar]
- Legendre A, Froment P, Desmots S, Lecomte A, Habert R, Lemazurier E. An engineered 3D blood-testis barrier model for the assessment of reproductive toxicity potential. Biomaterials 2010;31:4492–4505. [DOI] [PubMed] [Google Scholar]
- Leon J, Acuna-Castroviejo D, Sainz RM, Mayo JC, Tan DX, Reiter RJ. Melatonin and mitochondrial function. Life Sci 2004;75:765–790. [DOI] [PubMed] [Google Scholar]
- Liu F, Cai C, Wu X, Cheng Y, Lin T, Wei G, He D. Effect of knockOut serum replacement on germ cell development of immature testis tissue culture. Theriogenology 2016;85:193–199. [DOI] [PubMed] [Google Scholar]
- Madhu P, Reddy KP, Reddy PS. Role of melatonin in mitigating chemotherapy-induced testicular dysfunction in Wistar rats. Drug Chem Toxicol 2015:1–10. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Kamimura K, Nagano T. Peritubular myoid cells in the testis: their structure and function. Arch Histol Cytol 1996;59:1–13. [DOI] [PubMed] [Google Scholar]
- Mersereau J, Dooley MA. Gonadal failure with cyclophosphamide therapy for lupus nephritis: advances in fertility preservation. Rheum Dis Clin North Am 2010;36:99–108viii. [DOI] [PubMed] [Google Scholar]
- Miller SD, Ginsberg JP, Caplan A, Meyers KE. Sperm banking in adolescent males with nephrotic syndrome: defining the limits of access to fertility preservation. Arch Dis Child 2012;97:765–766. [DOI] [PubMed] [Google Scholar]
- Miryounesi M, Nayernia K, Mobasheri MB, Dianatpour M, Oko R, Savad S, Modarressi MH. Evaluation of in vitro spermatogenesis system effectiveness to study genes behavior: monitoring the expression of the testis specific 10 (Tsga10) gene as a model. Arch Iran Med 2014;17:692–697. [PubMed] [Google Scholar]
- Nayernia K, Reim K, Oberwinkler H, Engel W. Diploid expression and translational regulation of rat acrosin gene. Biochem Biophys Res Commun 1994;202:88–93. [DOI] [PubMed] [Google Scholar]
- Picton HM, Wyns C, Anderson RA, Goossens E, Jahnukainen K, Kliesch S, Mitchell RT, Pennings G, Rives N, Tournaye H et al. . A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod 2015;30:2463–2475. [DOI] [PubMed] [Google Scholar]
- Reda A, Hou M, Landreh L, Kjartansdottir KR, Svechnikov K, Soder O, Stukenborg JB. In vitro spermatogenesis – optimal culture conditions for testicular cell survival, germ cell differentiation, and steroidogenesis in rats. Front Endocrinol 2014;5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relander T, Cavallin-Stahl E, Garwicz S, Olsson AM, Willen M. Gonadal and sexual function in men treated for childhood cancer. Med Pediatr Oncol 2000;35:52–63. [DOI] [PubMed] [Google Scholar]
- Riboldi M, Rubio C, Pellicer A, Gil-Salom M, Simon C. In vitro production of haploid cells after coculture of CD49f+ with sertoli cells from testicular sperm extraction in nonobstructive azoospermic patients. Fertil Steril 2012;98(3):580–590, e4. doi: 10.1016/j.fertnstert.2012.05.039. [DOI] [PubMed] [Google Scholar]
- Rives N, Milazzo JP, Perdrix A, Castanet M, Joly-Helas G, Sibert L, Bironneau A, Way A, Mace B. The feasibility of fertility preservation in adolescents with Klinefelter syndrome. Hum Reprod 2013;28:1468–1479. [DOI] [PubMed] [Google Scholar]
- Russel L, Shinha Hitaim A, Clegg E. Histological and Histopathological Evaluation of the Testis. St. Louis, USA: Cache River Press, 1990. [Google Scholar]
- Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, Kubota Y, Ogawa T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011;471:504–507. [DOI] [PubMed] [Google Scholar]
- Silva CA, Brunner HI. Gonadal functioning and preservation of reproductive fitness with juvenile systemic lupus erythematosus. Lupus 2007;16:593–599. [DOI] [PubMed] [Google Scholar]
- Song J, Kang SM, Lee KM, Lee JE. The protective effect of melatonin on neural stem cell against LPS-induced inflammation. Biomed Res Int 2015;2015:854359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan V, Thombre DP, Lakshmanan S, Chakrabarty AS. Effect of removal of epididymal fat on spermatogenesis in albino rats. Indian J Exp Biol 1986;24:487–488. [PubMed] [Google Scholar]
- Staub C. A century of research on mammalian male germ cell meiotic differentiation in vitro. J Androl 2001;22:911–926. [DOI] [PubMed] [Google Scholar]
- Steinberger E, Steinberger A, Perloff WH. Initiation of spermatogenesis in vitro. Endocrinology 1964;74:788–792. [DOI] [PubMed] [Google Scholar]
- Stukenborg JB, Schlatt S, Simoni M, Yeung CH, Elhija MA, Luetjens CM, Huleihel M, Wistuba J. New horizons for in vitro spermatogenesis? An update on novel three-dimensional culture systems as tools for meiotic and post-meiotic differentiation of testicular germ cells. Mol Hum Reprod 2009;15:521–529. [DOI] [PubMed] [Google Scholar]
- Take G, Erdogan D, Helvacioglu F, Goktas G, Ozbey G, Uluoglu C, Yucel B, Guney Y, Hicsonmez A, Ozkan S. Effect of melatonin and time of administration on irradiation-induced damage to rat testes. Braz J Med Biol Res 2009;42:621–628. [DOI] [PubMed] [Google Scholar]
- Tijmes M, Pedraza R, Valladares L. Melatonin in the rat testis: evidence for local synthesis. Steroids 1996;61:65–68. [DOI] [PubMed] [Google Scholar]
- Travers A, Arkoun B, Safsaf A, Milazzo JP, Absyte A, Bironneau A, Perdrix A, Sibert L, Mace B, Cauliez B et al. . Effects of vitamin A on in vitro maturation of pre-pubertal mouse spermatogonial stem cells. PLoS ONE 2013;8:e82819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pelt AM, De Rooij DG. The origin of the synchronization of the seminiferous epithelium in vitamin A-deficient rats after vitamin A replacement. Biol Reprod 1990;42:677–682. [DOI] [PubMed] [Google Scholar]
- Wang F, Tian X, Zhou Y, Tan D, Zhu S, Dai Y, Liu G. Melatonin improves the quality of in vitro produced (IVP) bovine embryos: implications for blastocyst development, cryotolerance, and modifications of relevant gene expression. PLoS ONE 2014;9:e93641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhou H, Du Z, Chen X, Zhu F, Wang Z, Zhang Y, Lin L, Qian M, Zhang X et al. . Cytoprotective effect of melatonin against hypoxia/serum deprivation-induced cell death of bone marrow mesenchymal stem cells in vitro. Eur J Pharmacol 2015;748:157–165. [DOI] [PubMed] [Google Scholar]
- Vassilakopoulou M, Boostandoost E, Papaxoinis G, de La Motte Rouge T, Khayat D, Psyrri A. Anticancer treatment and fertility: effect of therapeutic modalities on reproductive system and functions. Crit Rev Oncol Hematol 2016;97:328–334. doi: 10.1016/j.critrevonc.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Ventela S, Mulari M, Okabe M, Tanaka H, Nishimune Y, Toppari J, Parvinen M. Regulation of acrosome formation in mice expressing green fluorescent protein as a marker. Tissue Cell 2000;32:501–507. doi: 10.1016/j.critrevonc.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Vernet N, Dennefeld C, Klopfenstein M, Ruiz A, Bok D, Ghyselinck NB, Mark M. Retinoid X receptor beta (RXRB) expression in Sertoli cells controls cholesterol homeostasis and spermiation. Reproduction 2008;136:619–626. [DOI] [PubMed] [Google Scholar]
- Zhang L, Tang J, Haines CJ, Feng H, Teng X, Han Y. RA induces differentiation of multipotent P19 cells towards male germ cell. In Vitro Cell Dev Biol Anim 2015;51:85–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.