Abstract
Debate continues on how to measure and weight diseases in multimorbidity. We quantified the association of a broad range of chronic diseases with physical health–related qualify of life and used these weights to develop and validate a multimorbidity weighted index (MWI). Community-dwelling adults in 3 national, prospective studies—the Nurses' Health Study (n = 121,701), Nurses' Health Study II (n = 116,686), and Health Professionals Follow-up Study (n = 51,529)—reported physician-diagnosed diseases and completed the Short Form 36 physical functioning (PF) scale over multiple survey cycles between 1992 and 2008. Mixed models were used to obtain regression coefficients for the impact of 98 morbid conditions on PF. The MWI was formed by weighting conditions by these coefficients and was validated through bootstrapping. The final sample included 612,592 observations from 216,890 participants (PF mean score = 46.5 (standard deviation, 11)). The association between diseases and PF varied severalfold (median, −1.4; range, −10.6 to 0.8). End-stage organ diseases were associated with the greatest reduction in PF. The mean MWI score was 4.8 (median, 3.7; range, 0–53), and the mean number of comorbid conditions was 3.3 (median, 2.8; range, 0–34). This validated MWI weights diseases by severity using PF, a patient-centered outcome. These results suggest that simple disease count is unlikely to capture the full impact of multimorbidity on health-related quality of life, and that the MWI is feasible and readily implemented.
Keywords: comorbidity, health-related quality of life, multimorbidity, multiple chronic conditions, physical functioning, Short Form 36
More adults have multimorbidity—multiple, concurrent morbid conditions—than have a single chronic disease (1). Older adults are disproportionately burdened: 80% of adults aged 65 years or older have multimorbidity. However, the prevalence of multimorbidity is high, at 45%, for all US adults (1). Multimorbidity is associated with poor health outcomes and high health-care utilization and costs (2, 3). With therapeutic advances and aging populations, the prevalence of multimorbidity continues to rise, and better tools to measure multimorbidity are needed for patient care, resource allocation, and the prevention of multimorbidity progression and complications.
Debate continues on the definition and measurement of multimorbidity for clinical care and research (4–6). Heterogeneity in the impact of chronic conditions is not characterized optimally, and this hampers consistent, systematic efforts to study multimorbidity. In many prior studies, investigators have counted diseases or weighted them in nonstandardized ways. A few studies have weighted diseases by their impact on health-related quality of life (HRQoL), but these studies have been limited by restricted sample sizes and disease inventories and have focused on specialized populations with an index disease (7–9). Other indices were designed using administrative data to predict mortality among hospitalized patients with index diseases (10, 11). To date, no validated measure has incorporated the effects of disease severity and disease burden on physical HRQoL for a comprehensive set of diseases in the ambulatory setting.
We developed a new measure of multimorbidity using data from 3 prospective cohort studies of community-dwelling adults: the Nurses' Health Study (NHS), NHS II, and the Health Professionals Follow-up Study (HPFS). Unlike static administrative data, these longitudinal data afford a unique opportunity to assess the overall effect of diseases on multimorbidity at multiple time points and for varying disease durations. We examined associations of self-reported physician-diagnosed diseases with physical functioning (PF) to develop and validate a multimorbidity weighted index (MWI). We aimed to address ongoing challenges in measuring multimorbidity by testing the contribution of a broad array of diseases to an individual's overall disease burden using a patient-centered rating of disease impact. At the same time, we sought to develop an index that could be derived from simple disease inventories to maximize its utility.
METHODS
Study population
Participants in 3 prospective cohort studies with national samples—NHS (121,701 female nurses aged 30–55 years in 1976), NHS II (116,686 female nurses aged 25–42 years in 1989), and HPFS (51,530 male health professionals aged 40–75 years in 1986)—comprised the study population. Participants in the 3 studies receive biennial questionnaires regarding newly diagnosed medical conditions, medications, and health behavior, and the studies have a follow-up rate of more than 90% per cycle. We included participants who reported the occurrence (or absence) of chronic disease and who completed the Short Form 36 (SF-36) 10-item PF scale in the same year (12). We excluded participants who were missing data on age, disease status, or PF. We also excluded observations from assessments of women made while they were pregnant, due to a possible independent association between physical HRQoL and pregnancy (13).
Chronic disease assessment
Chronic diseases and conditions refer to those with long duration and generally slow progression that are considered largely irreversible and persistent through adulthood (14, 15). Conditions partially resolved or complicated by surgery (e.g., hip fracture) were evaluated as potential morbid conditions for the index, and acute and subacute conditions fully resolved by definitive surgery (e.g., appendectomy) were not. Self-reported diseases were used in the present analysis for consistency with the approaches used in most nationally representative surveys and patient-centered clinical instruments. Proxy respondents could assist with questionnaire completion, and whether a participant received assistance was assessed in NHS in 2000 and 2004. Among NHS participants who reported dementia, 68% (n = 173) reported receiving assistance with questionnaire completion.
All chronic diseases and conditions listed on the biennial questionnaires were considered for analysis (see Web Tables 1 and 2, available at http://aje.oxfordjournals.org/). We also considered diseases voluntarily listed by participants, based on prevalence or inclusion in previous indices, so this index could be compared with others (Figure 1). When a disease was not sufficiently prevalent (such that a stable estimate could not be obtained) and could not be combined with similar diseases, the disease was not included in the index.
Figure 1.
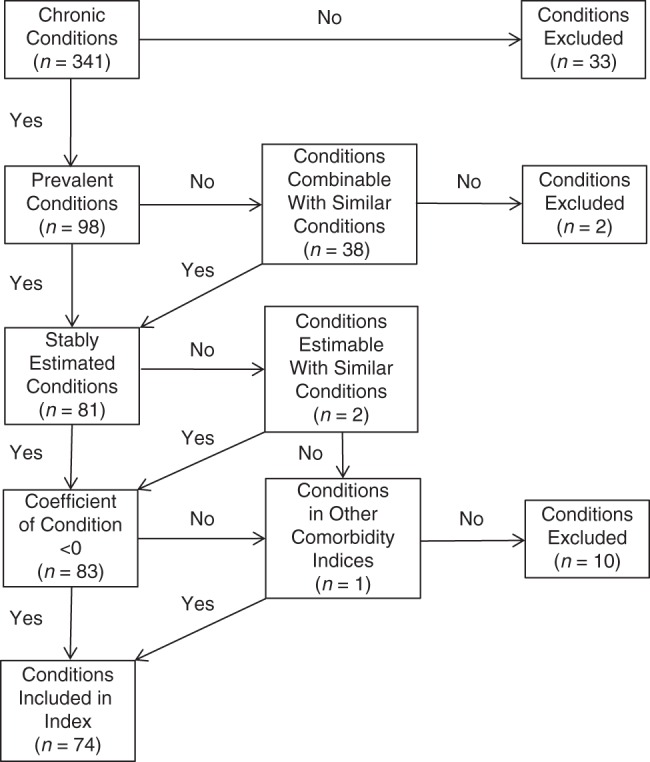
Inclusion and exclusion criteria used for disease conditions assessed in 3 nationally sampled cohorts (Nurses' Health Study, Nurses' Health Study II, and Health Professionals Follow-up Study) to be considered for inclusion in a multimorbidity weighted index. Data were gathered between 1992 and 2008. After grouping of combined conditions, there were 81 conditions with stably estimated coefficients.
HRQoL assessment
The SF-36 PF scale was used to weigh the burden of individual diseases. SF-36 is an instrument for assessing HRQoL that is used widely in health services research (16). As opposed to instruments specific to age, sex, or disease, the SF-36 is a generic HRQoL measure applied to diverse diseased and healthy individuals (12) and is ideal for understanding the impact and scope of disease burden.
We used the PF scale to assess functional status and morbidity. Lower PF is associated with several negative health outcomes and has been used to measure the late-stage impact of diseases on patients (17). The mean PF score and its standard deviation are standardized to the US general population, and scores are transformed to a mean of 50 (standard deviation, 10) with a range of 0–100 for lowest to highest functioning. PF was assessed every 4 years starting in 1992 for NHS (1992, 1996, 2000, 2004) and in 1993 for NHS II (1993, 1997, 2001). For HPFS, PF was assessed in 1996 and 2008. In addition, the full physical and mental component summaries were available in NHS and NHS II. The physical component summary incorporates weighted combinations of the 8 SF-36 scales—including physical deficits, bodily pain, vitality, general health perceptions, emotional deficits, social functioning, and mental health—and was highly correlated with PF in women (Pearson's r = 0.802, 95% confidence interval: 0.800, 0.803; P < 0.0001).
Statistical analysis
With continuous PF as the outcome, we tested candidate morbid conditions as predictors. We used mixed models to account for repeated assessments of PF within individuals over time and to allow for between- and within-individual variation (18). We used an unstructured covariance structure, which yielded the minimum Akaike information criterion. We used regression coefficients to develop a weighted combination of all morbid conditions to be used for the MWI. We adjusted for age (linear and quadratic), calendar time, and other conditions as potential confounders. We did not adjust for medications because treatment, including potential side effects, was considered part of the burden of an individual condition.
Regression coefficients were obtained separately for each cohort to maximally adjust for conditions available in each cohort (e.g., sex-specific diseases). Coefficients from all 3 cohorts were then pooled using fixed-effect meta-analysis to obtain the DerSimonian-Laird estimator (19).
As an initial screen for potential effect modification by age in the disease-PF association, we compared regression coefficients for participants above and below the cohort-specific median age (64 years, 42 years, and 67 years for NHS, NHS II, and HPFS, respectively). The impact of conditions on HRQoL in the 2 NHS cohorts was also compared; women in NHS were on average 20 years older than women in NHS II. There was no consistent, significant effect modification by age with specific diseases, so coefficients from younger and older participants within each cohort were combined. With no qualitative differences or a priori hypotheses on specific age-disease associations, multiple testing with formal interaction terms was not pursued.
All diseases, particularly rare diseases (prevalence <0.25%), were evaluated for stability of coefficients to preserve precision. A stable estimate could not be directly obtained for cirrhosis, but stable estimates for other end-stage organ diseases—including congestive heart failure, chronic kidney disease, and chronic obstructive pulmonary disease—were made and had a consistent magnitude of severity (change in PF score of −4.0 to −4.8). Thus, the median of these estimates (−4.3) was used to weight cirrhosis. For bladder cancer, the median of scores for 5 genitourinary, pelvic organ, and lower digestive tract cancers (changes in PF score: cervical cancer, −0.72; colon cancer, −1.2; ovarian cancer, −1.9; prostate cancer, −0.40; uterine cancer, −0.75) was used to obtain an estimate (−0.99). Diseases for which a reliable estimate could not be determined (e.g., hyperthyroidism) or that were associated with improved PF were excluded from the MWI. Hepatitis was included for purposes of comparison with other comorbidity measures. All other conditions not specifically assessed in the questionnaire were adjusted for in the model through a stable estimate obtained for the category “other diagnosed diseases.”
The sum of chronic diseases weighted by severity was used to create the MWI score in PF units. For MWI scores to be easily interpretable and comparable with simple counts of disease, we recalibrated the index result to disease units by dividing the MWI score in PF units by the mean coefficient weighted by inverse variance (i.e., the average impact of disease on PF).
The MWI was internally validated through bootstrapping (20) and cross-validation. For core conditions routinely available and present throughout follow-up in at least 1 cohort, which spanned a range of prevalences and disease severity, we estimated the bias and confidence intervals for our estimates through bootstrapping. With unrestricted sampling, we created 100 random independent samples with replacement that were each the same size as our original cohort. For each sample, we again computed estimates of the associations between diseases and observed PF, and we compared them with the original regression coefficients through relative and absolute differences. As further cross-validation, we compared estimates obtained from single and combined years of data using all possible combinations. All analyses were conducted using SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Participant characteristics
Complete data on the presence of chronic disease, HRQoL, and demographic factors were available from the period 1992–2008 for 216,890 participants (173,657 women, 43,233 men). NHS contributed 390,711 observations made at 4 assessment points (1992, 1996, 2000, and 2004), NHS II contributed 312,332 observations made at 3 assessment points (1993, 1997, and 2001), and HPFS contributed 74,447 observations made at 2 assessment points (1996 and 2008) for a total of 777,490 observations. Women who were pregnant at the time of assessment (n = 5,347) represented 2.5% of the cohort. Among participants with complete data on age and chronic disease, we excluded observations that did not have completed SF-36 PF scales; this left a final sample of 612,592 observations. Participants had a mean age of 55 (standard deviation, 13) years, and 26% of participants were aged 65 years or older at baseline (Table 1).
Table 1.
Characteristics of Participants at Year of Study Entry for 3 Cohorts of Health-Care Professionals,a United States, 1989–1996
| Characteristic | Nurses' Health Study (Women; n = 72,589) |
Nurses' Health Study II (Women; n = 86,221) |
Health Professionals Follow-up Study (Men; n = 36,488) |
|||
|---|---|---|---|---|---|---|
| % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | |
| Age, years | 64.1 (8.2) | 38.1 (4.6) | 63.3 (9.4) | |||
| White race | 93.7 | 94.2 | 96.9 | |||
| US region | ||||||
| Northeast | 58.5 | 33.7 | 28.3 | |||
| Midwest | 19.8 | 32.2 | 27.5 | |||
| South | 7.4 | 15.4 | 20.7 | |||
| West | 12.2 | 14.8 | 23.2 | |||
| Body mass indexb | 26.1 (5.0) | 26.1 (6.1) | 23.4 (8.5) | |||
| Smoking status | ||||||
| Never smoker | 43.4 | 65.3 | 42.7 | |||
| Past smoker | 42.0 | 27.0 | 45.0 | |||
| Current smoker | 13.9 | 7.6 | 5.5 | |||
| Physical examination in past year | ||||||
| Screening, no symptoms | 69.0 | 70.7c | 65.6 | |||
| Symptoms present | 19.0 | 18.8c | 18.1 | |||
| No examination | 11.2 | 10.2c | 15.5 | |||
| Parityd | ||||||
| 0 (nulliparous) | 5.7 | 23.4 | ||||
| 1 | 7.1 | 16.6 | ||||
| 2 | 27.9 | 37.2 | ||||
| ≥3 | 57.8 | 22.9 | ||||
| Score from the physical functioning scale | 50.1 (8.2) | 52.8 (6.5) | 51.0 (8.6) | |||
Abbreviation: SD, standard deviation.
a These analyses were based on data gathered from the Nurses’ Health Study in 1992, the Nurses’ Health Study II in 1989 or 1993, and the Health Professionals Follow-up Study in 1996.
b Body mass index was calculated as weight (kg)/height (m)2.
c In the Nurses’ Health Study II, physical examination was assessed in 1989 because the data were unavailable for 1993.
d Data available for women only.
HRQoL characteristics
At baseline, the mean PF score was 51.5 (standard deviation, 7.4) units, close to the normed mean of 50 units and the age- and sex-adjusted means for the general population; it did not differ by sex (Table 1). During follow-up, PF decreased by an average of 0.25 units per year in men in HPFS, less than 0.25 units per year in women in NHS II, and 0.5 units per year in women in NHS.
Comparison of chronic disease and PF association by sex
Some diseases were associated with nominally worse PF for one sex than for the other, but most diseases did not have consistent clinically significant differences by sex (Web Table 2). Because most diseases were associated with a similar decrement in PF units in men and women, we focused on sex-independent models.
Prevalent chronic diseases and HRQoL
Participants reported 374 diseases and conditions through the questionnaire or listed them as other diagnoses, and 106 were sufficiently prevalent for consideration for the index (Figure 1, Web Table 1). After combining groups of rare diseases, such as connective tissue diseases, we stably obtained estimates for 81 diseases and conditions. Conditions were excluded if they had unstable estimates or were associated with improvements in PF. After the incorporation of estimates obtained for cirrhosis, bladder cancer, and hepatitis, 74 diseases and conditions were included in the final index. Of these, 95% were assessed through questionnaire and 5% were listed by participants.
Among the 74 conditions, the median impact on PF score by a disease was −1.4 (range, −10.6 to 0.8). Diseases with the highest prevalence and greatest impact on PF are shown in Table 2. Even among the most common diseases, the impact on PF varied greatly (median, −0.6; range, −3.5 to 0.1) (Table 2). Hypercholesterolemia was most prevalent but had virtually no impact on PF. In contrast, osteoarthritis, the fourth most prevalent condition, had more than 10-fold greater impact on PF than hypercholesterolemia and twice the mean impact of all diseases. Other highly prevalent diseases, including benign breast disease and colon polyps, were not significantly associated with PF. Skin conditions, including squamous cell carcinoma, were associated with improved PF, likely due to confounding from outdoor leisure-time and physical activity among high-functioning individuals.
Table 2.
Adjusted Impact of Chronic Diseases and Conditions as Measured on the Short Form 36 Physical Functioning Scale for the Most Prevalent and Most Severe Diseases Recorded Among 3 Cohorts of Health-Care Professionals,a United States, 1992–2008
| Medical Condition | No. of Participants | %b | Coefficientc | Standard Error | P Value |
|---|---|---|---|---|---|
| Most prevalent conditions | |||||
| Elevated cholesterol | 233,871 | 38.5 | −0.34 | 0.025 | <0.0001 |
| High blood pressure | 176,855 | 29.1 | −1.53 | 0.027 | <0.0001 |
| Osteoarthritis | 149,180 | 24.5 | −3.52 | 0.029 | <0.0001 |
| Benign breast disease | 133,271 | 21.9 | 0.11 | 0.022 | <0.0001 |
| Depression | 53,886 | 20.8 | −1.29 | 0.042 | <0.0001 |
| Asthma | 54,022 | 8.9 | −1.62 | 0.040 | <0.0001 |
| Cataract | 59,460 | 8.2 | −0.29 | 0.037 | <0.0001 |
| Prostate cancer | 5,061 | 8.1 | −0.40 | 0.126 | <0.0001 |
| Herniated disc | 42,734 | 7.1 | −3.27 | 0.042 | <0.0001 |
| Osteoporosis | 47,879 | 6.6 | −1.00 | 0.049 | <0.0001 |
| Basal cell carcinoma | 37,003 | 6.1 | 0.25 | 0.043 | <0.0001 |
| Periodontal disease | 32,171 | 5.6 | −0.16 | 0.063 | 0.01 |
| Diabetes | 30,610 | 5.0 | −2.67 | 0.055 | <0.0001 |
| Uterine fibroid | 30,340 | 5.0 | −0.03 | 0.039 | 0.46 |
| Premenstrual syndrome | 30,237 | 5.0 | −0.41 | 0.038 | <0.0001 |
| Most severe conditions | |||||
| Multiple sclerosis | 3,210 | 0.5 | −10.60 | 0.155 | <0.0001 |
| Knee replacement surgery | 1,943 | 0.3 | −9.11 | 0.317 | <0.0001 |
| Parkinson disease | 1,355 | 0.2 | −8.82 | 0.264 | <0.0001 |
| Amyotrophic lateral sclerosis | 152 | 0.02 | −7.45 | 0.759 | <0.0001 |
| Lung cancer | 1,147 | 0.2 | −6.25 | 0.258 | <0.0001 |
| Dementia, including Alzheimer disease | 305 | 0.1 | −6.10 | 0.440 | <0.0001 |
| Congestive heart failure | 3,442 | 0.6 | −4.76 | 0.165 | <0.0001 |
| Chronic obstructive pulmonary disease | 15,771 | 3.5 | −4.32 | 0.078 | <0.0001 |
| Chronic kidney disease | 722 | 0.1 | −3.98 | 0.330 | <0.0001 |
| Rheumatoid arthritis | 25,811 | 4.2 | −3.79 | 0.062 | <0.0001 |
| Stroke | 6,863 | 1.1 | −3.79 | 0.108 | <0.0001 |
| Hip fracture | 5,571 | 0.8 | −3.56 | 0.122 | <0.0001 |
| Hip replacement surgery | 8,191 | 1.1 | −3.55 | 0.104 | <0.0001 |
| Peripheral artery disease | 6,841 | 0.9 | −3.25 | 0.111 | <0.0001 |
| Connective tissue disease | 4,373 | 0.7 | −3.02 | 0.128 | <0.0001 |
a These analyses were based on a total of 612,592 observations gathered from the Nurses’ Health Study, the Nurses’ Health Study II, and the Health Professionals Follow-up Study.
b Percentage prevalence of each condition based on the sum of observations from all years and cohorts in which that particular condition was assessed; the prevalence is not necessarily the overall sum of observations for all years and all cohorts, as in the case of core non–sex-specific conditions assessed in all years.
c Regression coefficients of PF scores were adjusted for age, sex, cohort, and all other chronic diseases and conditions.
Cancers varied in their impact on PF score (range, −0.40 to −6.25). Lung cancer had the worst impact on PF, followed by ovarian cancer, leukemia and lymphoma, colon cancer, and breast cancer.
Chronic diseases with the lowest HRQoL
Diseases with the greatest adverse impact on PF are shown in Table 2. Neurological diseases, including multiple sclerosis and Parkinson disease, were associated with the greatest decrement in PF. Neurological diseases had 4 times’ greater impact on PF than did the average disease.
After neurological diseases, late- or end-stage organ diseases—including congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease, and cerebrovascular disease—had the next-greatest effect on diminution of PF, generally 2–3 times worse than the average of all diseases.
Musculoskeletal conditions were far more prevalent than neurological and end-stage organ diseases and were associated with large impacts on PF (Table 2). Participants who underwent knee replacement surgery, which likely reflects the severity of knee conditions such as osteoarthritis, had the second-worst PF scores for all diseases and conditions (−9.11, 95% confidence interval: −9.73, −8.49; P < 0.0001). Participants who underwent hip replacement surgery had PF scores similar to those with osteoarthritis.
Multimorbidity weighted index
The pooled estimates of PF obtained from participants with and without specific diseases formed the basis for weighting diseases in the MWI. The absolute values of the declines in PF units for a participant's conditions were summed to compute the individual's MWI score. Conditions with a positive impact on PF score were excluded. MWI score was computed for each participant in PF units and recalibrated (by a factor of 1.1) units of disease count.
The average participant had an average MWI score of 4.8 (median, 3.7; range, 0–53) at the last measurement of PF in these cohorts (Table 3). Women in NHS had MWI scores twice as high as those of women in NHS II, on average, consistent with their older age. Over half the women in NHS II had MWI scores of less than 2. On average, men had higher MWI scores than women. The MWI spanned a wider distribution of values than simple disease count in both men and women. MWI scores for the most common conditions weighted by their impacts on PF were only moderately correlated with simple disease count (Pearson's r = 0.522, 95% confidence interval: 0.521, 0.523; P < 0.0001).
Table 3.
Mean Values (With Standard Deviations) for Multimorbidity Weighted Index Score and Simple Disease Count for Participants in 3 Cohorts of Health-Care Professionals,a United States, 2008
| Characteristic | Combined Cohorts (n = 165,138) | Nurses’ Health Study (n = 64,557) | Nurses’ Health Study II (n = 76,876) | Health Professionals Follow-up Study (n = 23,705) |
|---|---|---|---|---|
| No. of chronic diseases | 3.3 (2.4) | 3.9 (2.6) | 2.2 (2.0) | 5.2 (3.1) |
| Multimorbidity weighted index, average weighted disease units | 4.8 (4.5) | 6.1 (4.9) | 3.0 (3.2) | 6.9 (5.0) |
| Multimorbidity weighted index, physical functioning scale units | 5.0 (4.8) | 6.4 (5.2) | 3.2 (3.4) | 7.2 (5.3) |
a These analyses were based on data gathered by the Nurses’ Health Study, the Nurses’ Health Study II, and the Health Professionals Follow-up Study between 1992 and 2008.
Internal validation
We obtained bootstrapped estimates for 62 core conditions assessed in all years in at least 1 cohort, which included a range of prevalences and disease severity. Estimates for PF in our index consistently approached the original parameter estimates (Web Figure 1). Further, these estimates had excellent reliability and precision, as demonstrated through tight confidence intervals from 100 bootstrapped resamplings (Web Table 3). Conditions with the greatest relative difference (e.g., colon polyps) were also those with a positive impact on PF and that were thus excluded. Further adjustment or replacement of original coefficient estimates was unnecessary given the reliability of the resamplings.
DISCUSSION
To better characterize the growing population of persons with multiple chronic conditions, we propose a patient-centered, quantitative measure of multimorbidity that may be applied readily in research and ambulatory practice. We have demonstrated that individual conditions vary widely, up to severalfold, in their impacts on PF. This suggests that simple disease counts are unlikely to capture the full impact of multimorbidity on health, and methods weighted to physical HRQoL or other validated measures are feasible, informative, and easily implemented. The interpretability of our index, derived from 98 chronic diseases, is 2-fold: Because the average disease conferred a decrement in PF of approximately 1 unit, it provides estimates of both an individual's burden of “average” diseases and expected decline in PF. Thus, a 1-point increase in MWI score represents a 1-point decrement in PF.
Strengths and limitations
Given the large number of individuals who have provided information on HRQoL repeatedly over time, this is among the largest quantitative studies of morbidity in community-dwelling adults to have been conducted to date. The cohort studies we analyzed contributed rigorous, reliable data on physician-diagnosed chronic diseases (which participants were experienced in reporting), diagnosis dates, HRQoL (as measured through the SF-36), and numerous predictors and health outcomes. Our sample of 216,890 adults and 612,592 observations enabled us to develop a precise and comprehensive index. Moreover, because the index relies on self-reported data, it approximates approaches used in most nationally representative surveys and patient-centered clinical instruments. The index can be applied to clinical and research settings without further refinement, because past medical history is the only information necessary to calculate the MWI. Using self-reported diseases also avoids potential recording errors that occur in administrative data, such as incomplete documentation, incomplete or mistaken diagnosis, miscoding, and bias due to hospital reimbursement (21). Nevertheless, we acknowledge that not all potential diseases were included, particularly rare conditions that, by definition, affect a minority of individuals. We also collected information on diseases by means of both explicit questionnaire and spontaneous open reporting, and these methods may capture diseases of different severity.
The MWI is weighted to physical HRQoL and adjusted for depression, but it does not capture the impact of disease conditions on mental HRQoL. We examined the impact of various conditions on the SF-36 mental composite summary, but the effect size and range were smaller than for PF. The MWI assumes an additive effect of diseases but does not account for potential multiplicative effects, which would require examining first- and higher-order interactions for several potential disease combinations.
Our study sample included adults from all regions of the United States but was not explicitly nationally representative. However, the prevalence of diseases in this sample reflected those previously reported in US and other populations (22). Further, participant characteristics, such as obesity and tobacco abuse, were similar to those found generally in the United States (23) and many other industrialized nations (24, 25). Finally, although disease prevalences may differ by race/ethnicity or other socioeconomic factors, the conditional associations of diseases and conditions with PF are likely to be robust to these factors. While other measures of HRQoL may have been considered, a primary strength of the SF-36 is that its content and scoring are standardized and thus may be meaningfully used to monitor and compare clinical practice outcomes.
Comparison with prior studies
The escalating prevalence of multimorbidity has been accompanied by investigative efforts to better characterize adults with multimorbidity and develop frameworks to optimize their care. More than 100 definitions have been proposed for multimorbidity (5, 26). One of the most commonly used measures of multimorbidity is a simple disease count (≥2 chronic diseases) (27). While simple disease count is easily computed, ambiguity persists regarding which diseases should contribute toward the disease count. Prior studies have included a heterogeneous group of diseases, often based on the availability of diseases assessed or those hypothesized to influence outcomes such as HRQoL and mortality. As a consequence, multimorbidity is likely underestimated due to a ceiling effect, whereby the multimorbidity scale has not included a sufficiently comprehensive range of morbid conditions to capture and distinguish participants with more severe multimorbidity. Second, a simple count does not account for the diversity and severity of diseases. We have demonstrated that both common and rare diseases have wide-ranging impacts on PF, from no deficit to severalfold greater deficits. This suggests that treating all diseases equally, as with a simple count, may oversimplify their full impact on health. Third, a simple count underrates morbidity in individuals with single but devastating diseases. For example, an individual with multiple sclerosis alone would not be considered to have multimorbidity despite having severalfold worse HRQoL (plus the associated burden of medication use and health-care utilization) than an individual with multiple conditions of lesser severity. Some multimorbidity indices weight diseases through nonstandard, semiquantitative measures, such as subjective ratings by patients or providers (3, 28). However, these indices are less translatable, and findings obtained using them are less comparable with those of other studies. Similarly, indices developed in specialized clinical populations may not be generalizable (7, 9). Finally, our study included young and middle-aged adults, who may also carry high levels of multimorbidity. In a prior study, Lawson et al. (29) reported worse impacts on preference-weighted HRQoL in younger adults compared with older adults, with no differences by sex.
Implications
Our MWI contributes to the field of multimorbidity in several ways. First, our MWI includes a comprehensive set of 98 distinct morbid conditions for use by other investigators. Second, we demonstrated that diseases vary widely in their impact on physical HRQoL, and thus current methods, such as simple disease counts, may not be the most informative for capturing the burden of multiple chronic conditions. Third, we developed and internally validated our MWI, which uses these weights to assign severity values to individual diseases, providing an easy estimate of physical HRQoL burden from a simple disease inventory. This index allows diseases associated with greater decrements in PF to contribute greater weight to an individual's multimorbidity burden. For example, weighting diseases by their impact on PF rather than their mortality recognizes chronic, debilitating yet not directly fatal diseases, such as osteoarthritis, as having a high impact on multimorbidity. As a consequence, researchers conducting clinical- and population-level studies of community-dwelling adults may now reliably and validly adjust for a comprehensive set of diseases. Clinical applications include identifying risk factors to delay multimorbidity onset and progression as well as refining decision-making and care coordination for patients with multimorbidity—using an index that, ultimately, could be completed by patients themselves and does not rely on administrative data. Such an index can be used to tailor visit frequency or duration and target support services to those at greatest risk.
Future studies
HRQoL is a single patient-centered outcome with which to quantify the severity of individual diseases and their contributions to multimorbidity. Future studies are needed to measure other outcomes—such as patient experiences, barriers to health care, quality of care, receipt of preventive services, health-care utilization and cost, and quality-adjusted life years—and assess the impact of potential interventions in adults with multiple chronic conditions. While the utilization and cost of health services increase with multimorbidity, further studies are needed to assess the association of the MWI with these and other health outcomes. The impact of diseases on mental HRQoL may also be further investigated using other instruments. While our index is based on large prospective cohort studies of community-dwelling adults throughout the United States, further studies in diverse populations are nonetheless necessary to establish the external validity of this index.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of General Medicine and Primary Care, Beth Israel Deaconess Medical Center and Harvard Medical School, Brookline, Massachusetts (Melissa Y. Wei, Kenneth J. Mukamal); Division of General Medicine, University of Michigan, Ann Arbor, Michigan (Melissa Y. Wei); Institute for Healthcare Innovation and Policy, University of Michigan, Ann Arbor, Michigan (Melissa Y. Wei); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Ichiro Kawachi, Olivia I. Okereke); Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Ichiro Kawachi, Olivia I. Okereke); Department of Social and Behavioral Sciences, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Ichiro Kawachi); Department of Psychiatry, Brigham and Women's Hospital, Boston, Massachusetts (Olivia I. Okereke); and Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Kenneth J. Mukamal).
This study was supported by the National Institutes of Health (grants UM1 CA186107 for the Nurses’ Health Study, UM1 CA176726 for Nurses’ Health Study II, and UM1 CA167552 for the Health Professionals Follow-up Study). M.Y.W. was supported through a National Research Service Award from the National Center for Research Resources (grant T32 HP12706-06).
We thank Ellen Hertzmark (Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Boston, Massachusetts) for her statistical advice.
This work was presented at the Society of General Internal Medicine's 37th Annual Meeting, April 23–26, 2014, San Diego, California, and published in abstract form (J Gen Intern Med. 2014;29(suppl 1):S235).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: none declared.
REFERENCES
- 1.Ornstein SM, Nietert PJ, Jenkins RG et al. The prevalence of chronic diseases and multimorbidity in primary care practice: a PPRNet report. J Am Board Fam Med. 2013;265:518–524. [DOI] [PubMed] [Google Scholar]
- 2.Anderson G. Chronic care: making the case for ongoing care. http://www.rwjf.org/en/library/research/2010/01/chronic-care.html Published 2010. Accessed October 10, 2013.
- 3.Brettschneider C, Leicht H, Bickel H et al. Relative impact of multimorbid chronic conditions on health-related quality of life—results from the MultiCare Cohort Study. PLoS One. 2013;86:e66742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison C, Britt H, Miller G et al. Examining different measures of multimorbidity, using a large prospective cross-sectional study in Australian general practice. BMJ Open. 2014;47:e004694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Reste JY, Nabbe P, Manceau B et al. The European General Practice Research Network presents a comprehensive definition of multimorbidity in family medicine and long term care, following a systematic review of relevant literature. J Am Med Dir Assoc. 2013;145:319–325. [DOI] [PubMed] [Google Scholar]
- 6.Fortin M, Stewart M, Poitras ME et al. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med. 2012;102:142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hays RD, Reeve BB, Smith AW et al. Associations of cancer and other chronic medical conditions with SF-6D preference-based scores in Medicare beneficiaries. Qual Life Res. 2014;232:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothrock NE, Hays RD, Spritzer K et al. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS). J Clin Epidemiol. 2010;6311:1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radner H, Yoshida K, Mjaavatten MD et al. Development of a multimorbidity index: impact on quality of life using a rheumatoid arthritis cohort. Semin Arthritis Rheum. 2015;452:167–173. [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;405:373–383. [DOI] [PubMed] [Google Scholar]
- 11.Elixhauser A, Steiner C, Harris DR et al. Comorbidity measures for use with administrative data. Med Care. 1998;361:8–27. [DOI] [PubMed] [Google Scholar]
- 12.Ware JE Jr, Snow KK, Kosinski M et al. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: The Health Institute; 1993. [Google Scholar]
- 13.Tendais I, Figueiredo B, Mota J et al. Physical activity, health-related quality of life and depression during pregnancy. Cad Saude Publica. 2011;272:219–228. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. The World Health Report 2008—Primary Health Care (Now More Than Ever). Geneva, Switzerland: World Health Organization; 2008:148. [Google Scholar]
- 15.National Center for Health Statistics, Centers for Disease Control and Prevention. Health, United States, 2010: With Special Feature on Death and Dying. (Appendix) Hyattsville, MD: National Center for Health Statistics; 2011: 486–487. [PubMed] [Google Scholar]
- 16.Ware JE Jr, Bayliss MS, Rogers WH et al. Differences in 4-year health outcomes for elderly and poor, chronically ill patients treated in HMO and fee-for-service systems. Results from the Medical Outcomes Study. JAMA. 1996;27613:1039–1047. [PubMed] [Google Scholar]
- 17.Carey EC, Walter LC, Lindquist K et al. Development and validation of a functional morbidity index to predict mortality in community-dwelling elders. J Gen Intern Med. 2004;1910:1027–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. 2nd ed Boston, MA: John Wiley & Sons, Inc.; 2011. [Google Scholar]
- 19.Hertzmark E, Spiegelman D. The SAS METAANAL Macro. http://www.hsph.harvard.edu/wp-content/uploads/sites/271/2012/09/metaanal_user_manual_-_5-24-2012.pdf Published May 24, 2012. Accessed February 24, 2014.
- 20.Efron B, Tibshirani R. An Introduction to the Bootstrap. (Monographs on statistics and applied probability) New York, NY: Chapman & Hall, Inc.; 1993. [Google Scholar]
- 21.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;584:323–337. [DOI] [PubMed] [Google Scholar]
- 22.Lochner KA, Cox CS. Prevalence of multiple chronic conditions among Medicare beneficiaries, United States, 2010. Prev Chronic Dis. 2013;10:E61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnell K, Weiss CO, Lee T et al. The prevalence of clinically-relevant comorbid conditions in patients with physician-diagnosed COPD: a cross-sectional study using data from NHANES 1999–2008. BMC Pulm Med. 2012;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schäfer I, von Leitner EC, Schön G et al. Multimorbidity patterns in the elderly: a new approach of disease clustering identifies complex interrelations between chronic conditions. PLoS One. 2010;512:e15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Baal PH, Engelfriet PM, Boshuizen HC et al. Co-occurrence of diabetes, myocardial infarction, stroke, and cancer: quantifying age patterns in the Dutch population using health survey data. Popul Health Metr. 2011;91:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodman RA, Posner SF, Huang ES et al. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013;10:E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Akker M, Buntinx F, Metsemakers JF et al. Multimorbidity in general practice: prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. J Clin Epidemiol. 1998;515:367–375. [DOI] [PubMed] [Google Scholar]
- 28.Diederichs CP, Wellmann J, Bartels DB et al. How to weight chronic diseases in multimorbidity indices? Development of a new method on the basis of individual data from five population-based studies. J Clin Epidemiol. 2012;656:679–685. [DOI] [PubMed] [Google Scholar]
- 29.Lawson KD, Mercer SW, Wyke S et al. Double trouble: the impact of multimorbidity and deprivation on preference-weighted health related quality of life a cross sectional analysis of the Scottish Health Survey. Int J Equity Health. 2013;12:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


