Abstract
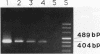
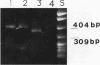
AIMS--To develop a sensitive and specific polymerase chain reaction (PCR) based system for detecting genomic variation in JC virus. To apply this system to formalin fixed, paraffin wax embedded brain tissue from patients with and without progressive multifocal leucoencephalopathy (PML). METHODS--A pair of primers (JC1 and JC2) were designed to be complementary to the early and late regions of JC and BK polyomaviruses, respectively. A third primer (JC3), internal to JC1 and JC2, was designed to be specific for JC virus. The specificity of JC3 was investigated by amplifying plasmids with BK or JC virus genomes. Sensitivity was estimated by titration of a plasmid containing JC virus genome. Seven brains from patients with PML (PMLB) and 30 from patients without PML (non-PMLB) were amplified using JC1 and JC2, followed by JC1 and JC3. Amplification of the beta globin gene was used as an amplification control. RESULTS--Amplification with JC1 and JC2 was common for JC and BK viruses, but with JC1 and JC3 it was specific for JC virus. The sensitivity of the system was 25 copies of JC plasmid per 10 microliters of digested tissue. Five out of seven PMLB and 28 of the 30 non-PMLB amplified for beta globin, but only the PMLB gave a signal with polyoma primers. Hypervariation of the length of the regulatory region of the JC isolates in the PML tissues was consistent with the presence of multiple strains of JC. CONCLUSIONS--Variation in the regulatory region of JC virus can be specifically and sensitively detected from routinely processed, paraffin wax embedded brain tissue. Variation in the regulatory region is common in PML derived JC strains, but JC virus was not detectable in non-PMLB tissue.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chesters P. M., Heritage J., McCance D. J. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J Infect Dis. 1983 Apr;147(4):676–684. doi: 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- Esiri M. M., Morris C. S., Millard P. R. Fate of oligodendrocytes in HIV-1 infection. AIDS. 1991 Sep;5(9):1081–1088. doi: 10.1097/00002030-199109000-00003. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Bream G. L., Cannella M. T. Human polyomavirus JC virus genome. J Virol. 1984 Aug;51(2):458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Y. M., Mehal W. Z., Fleming K. A. In vitro amplification of hepatitis B virus sequences from liver tumour DNA and from paraffin wax embedded tissues using the polymerase chain reaction. J Clin Pathol. 1989 Aug;42(8):840–846. doi: 10.1136/jcp.42.8.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Y. M., Patel P., Wainscoat J. S., Sampietro M., Gillmer M. D., Fleming K. A. Prenatal sex determination by DNA amplification from maternal peripheral blood. Lancet. 1989 Dec 9;2(8676):1363–1365. doi: 10.1016/s0140-6736(89)91969-7. [DOI] [PubMed] [Google Scholar]
- Loeber G., Dörries K. DNA rearrangements in organ-specific variants of polyomavirus JC strain GS. J Virol. 1988 May;62(5):1730–1735. doi: 10.1128/jvi.62.5.1730-1735.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. D., King D. M., Slauch J. M., Frisque R. J. Differences in regulatory sequences of naturally occurring JC virus variants. J Virol. 1985 Jan;53(1):306–311. doi: 10.1128/jvi.53.1.306-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke W., Smith R. A., Lancaster W. D., Goldstein D. A., Tourtellotte W. W., Spizizen J. In vitro-labeled DNA for detecting viral genomes in multiple sclerosis: I. Papovaviruses. Neurology. 1977 Aug;27(8):736–740. doi: 10.1212/wnl.27.8.736. [DOI] [PubMed] [Google Scholar]
- Mori M., Aoki N., Shimada H., Tajima M., Kato K. Detection of JC virus in the brains of aged patients without progressive multifocal leukoencephalopathy by the polymerase chain reaction and Southern hybridization analysis. Neurosci Lett. 1992 Jul 20;141(2):151–155. doi: 10.1016/0304-3940(92)90883-9. [DOI] [PubMed] [Google Scholar]
- Mori M., Kurata H., Tajima M., Shimada H. JC virus detection by in situ hybridization in brain tissue from elderly patients. Ann Neurol. 1991 Apr;29(4):428–432. doi: 10.1002/ana.410290414. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Eckroade R. J., Dessel B. H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971 Jun 19;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Telenti A., Aksamit A. J., Jr, Proper J., Smith T. F. Detection of JC virus DNA by polymerase chain reaction in patients with progressive multifocal leukoencephalopathy. J Infect Dis. 1990 Oct;162(4):858–861. doi: 10.1093/infdis/162.4.858. [DOI] [PubMed] [Google Scholar]
- Terry R. D., DeTeresa R., Hansen L. A. Neocortical cell counts in normal human adult aging. Ann Neurol. 1987 Jun;21(6):530–539. doi: 10.1002/ana.410210603. [DOI] [PubMed] [Google Scholar]
- Yogo Y., Kitamura T., Sugimoto C., Ueki T., Aso Y., Hara K., Taguchi F. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J Virol. 1990 Jun;64(6):3139–3143. doi: 10.1128/jvi.64.6.3139-3143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]