Abstract
Background
This study aimed to investigate the role of miR-184 in the proliferation and apoptosis of keratinocyte (HaCaT cells).
Material/Methods
HaCaT cells were cultured in a growth medium. The miR-184 was transfected with siRNA, then cell viability and apoptosis were assayed by MTT and flow cytometry, respectively. The colony-forming efficacy of HaCaT cells were detected as well. mRNA expressions of basic fibroblast growth factor (bFGF) and transforming growth factor (TGF)-β1 were measured with RT-PCR. The expressions of apoptosis-related proteins caspase-3 and Bcl-x in HaCaT cells were determined by Western blot.
Results
After miR-184 was transfected with siRNA, cell viability and colony forming ability decreased significantly, and apoptosis was significantly increased. The expressions of growth factors TGF-β1 and bFGF mRNAs, as well as apoptosis-related proteins Bcl-x, in HaCaT cells declined significantly after miR-184 was transfected with siRNA. In addition, the expression of pro-apoptotic protein caspase-3 increased significantly.
Conclusions
Our results suggest distinct roles of miR-184 during the growth, proliferation, and apoptosis of keratinocytes.
MeSH Keywords: Apoptosis, Cell Survival, Keratinocytes, MicroRNAs, Transfection
Background
Impaired wound healing and its medical complications result in multiple adverse health outcomes worldwide [1]. Epithelial keratinocyte proliferation and differentiation are essential elements of wound repair [2]. Keratinocytes are the predominant epithelial cell in skin and are involved in complex processes to maintain epidermal homeostasis [3]. In some inflammatory skin disorders, such as psoriasis, an imbalance between proliferation and differentiation of keratinocytes leads to epidermal hyperproliferation in excess of the extent of skin injury. However, the pathological mechanism behind these disorders is complex and poorly understood [4].
Growing evidence points toward the roles of small non-coding RNAs, called microRNAs (miRNAs), which usually regulate gene expression by targeting the 3′-untranslated region (UTR) of mRNA for degradation of the mRNA or suppression of translation [5]. It is estimated that about 60% of genes are regulated by miRNAs [6], and changes in miRNA expression affect physiological and pathological processes [7]. miR-184 is a single-copy gene that is evolutionarily conserved at the nucleotide level, from flies to humans [8]. It has been implicated in mediating neurological development and apoptosis, as well as modulating stem cell proliferation and growth [9,10]. However, the functions of miR-184 in keratinocyte proliferation and apoptosis are poorly defined.
HaCaT cells are immortalized human keratinocytes, which have growth characteristics similar to that of normal human skin keratinocytes. Therefore, in the present study, the role of miR-184 in the proliferation and apoptosis of HaCaT cells was investigated. In order to further study the molecular mechanisms, the mRNA expressions of growth factors, including basic fibroblast growth factor (bFGF) and transforming growth factor (TGF)-β1, and the expressions of apoptosis-related proteins caspase-3 and Bcl-xin HaCaT cells, were respectively determined by RT-PCR and Western blot analysis. Our study aimed to provide evidence to demonstrate that miR-184 regulates the proliferation and apoptosis of keratinocytes.
Material and Methods
Cell culture
HaCaT cells were used in this study. HaCaT cells were cultured in a growth medium with Dulbecco’s modified Eagle medium (DMEM), 10% fetal bovine serum (FBS), 100 unit/mL penicillin, and 100 mg/mL streptomycin (Life Technologies, UK) at 37°C in a tissue culture incubator (5% CO2).
Transfection
siRNA miR-184 and negative control scrambled siRNAs were transfected with Lipofectamine 2000 according to the manufacturer’s protocol. Liposome and DNA were incubated in DMEM. The liposome-DNA mixture was added to standard 6-well tissue culture plates containing HaCaT cells in 500 μL of DMEM. Fresh medium was added on the next day. At 48 h post-transfection, the cells were visualized by phase contrast and fluorescence microscopy at a magnification of 400× using an Olympus IX70 microscope (Olympus Optical Co., Ltd., Japan).
MTT assay
Cell viability was assessed by MTT assay. Briefly, HaCaT cells were cultured in a 96-well plate. At the indicated time points, 0.5 mg/ml MTT was added to each well and incubated at 37°C for 4 h. Then, 200 μL dimethyl sulfoxide (DMSO) was added to each well at room temperature. The optical density (OD) was measured at 490 nm using a Molecular Devices VERSAmax microplate reader (Molecular devices, Sunnyvale, CA, USA).
Colony formation assay
Colony formation assay was carried out as described earlier [11]. In detail, 500 viable cells (normal HaCaT cells, HaCaT cells transfected with scrambled siRNAs or siRNA miR-184) were seeded into 6-well plates. Plates were incubated in a CO2 incubator for 8–10 days and then fixed with methanol, followed by staining with 0.1% crystal violet solution. Colonies of 50 or more cells were counted using a Zeiss inverted light microscope.
Flow cytometry
The normal HaCaT cells, cells transfected with scrambled siRNAs, and cells transfected with siRNA miR-184 were seeded at a density of 2×105 per 35-mm culture dish. Cell apoptosis was determined by flow cytometry analysis (FACSCanto; BD Biosciences, San Jose, CA) of annexin V and propidium iodide (PI) stained cells (Vybrant apoptosis assay kit; Invitrogen), which characterized early and late apoptosis.
RT-PCR
Total RNA was isolated in Trizol (Sigma, St. Louis, MO, USA). Then, 2 μg total RNA was reverse transcribed into complementary DNA (cDNA) using reverse transcriptase (iScript™ cDNA Synthesis Kit; Bio-Rad Laboratories). For amplification of TGF-β1, bFGF, and β-actin, the primers were employed as follows: TGF-β1, sense 5′-AGCACAGAGCCTCGCCTTT-3′; anti-sense 5′-AGGGTGAGGATGCCTCTCTT-3′; bFGF, sense 5′-AGACACCCATCCGTGAACC-3′; anti-sense 5′-CTTGATGTGAGGGTCGCTCT-3′; β-actin, sense 5′-GTACCTGAACCCGTGTTGCT-3′; anti-sense 5′-GTCCTTGCGGAAGTCAATGT-3′. All the primers were designed using Primer 3 (ABI, USA) and synthesized by Shanghai Sangon Biological Engineering Technology and Services Co., Ltd.
Western blot
HaCaT cells lysate was prepared using RIPA buffer (Pierce Co., Shanghai, China) and the total protein concentration was determined with the BCA method. Then, 50 μg protein was loaded into SDS-PAGE and the gel was transferred onto a nitrocellulose membrane (BioRad). After transfer, the membrane was incubated for 1 h at room temperature with polyclonal rabbit antibodies to human caspase-3 (Santa Cruz), monoclonal antibodies to human Bcl-x (Santa Cruz), and β-actin (Santa Cruz) at 1:500 dilution. Bound antibodies were detected with corresponding secondary antibodies conjugated with horseradish peroxidase: mouse anti-rabbit immunoglobulin (Promega) and goat anti-mouse (Santa Cruz) (1:2000). Bands were developed with ECL developing solution (Pierce Co., Shanghai, China).
Statistical analysis
All variable data were analyzed using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). The results of multiple experiments are presented as mean ±SD. Measurement data were tested by the t test (for 2 groups) or one-way analysis of variance (ANOVA) for more than 3 groups. P<0.05 was considered statistically significant.
Results
Cell viability
MTT assay was performed to investigate the effect of miR-184 on HaCaT cell viability. The results showed that, compared to the control and scramble siRNA groups, the cell viability decreased significantly in the siRNA-miR-184 group (P<0.05) (Figure 1).
Figure 1.
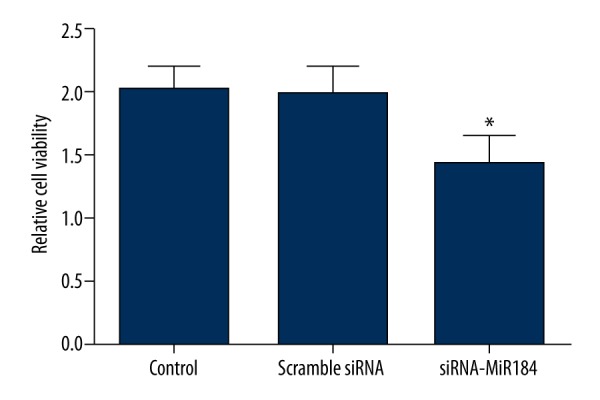
Cell viability assayed by MTT. Cell viability of HaCaT cells decreased significantly upon miR-184 siRNA treatment. Data expressed as mean ±SD (* P<0.05).
Colony formation efficacy
Normal HaCaT cells and HaCaT cells transfected with scrambled siRNAs or siRNA-miR-184 were allowed to form colonies for 8–10 days, after which they were stained with 0.1% crystal violet and counted. siRNA-miR-184 significantly reduced the colony forming ability of HaCaT cells compared with control cells and HaCaT cells transfected with scrambled siRNAs (P<0.05) (Figure 2).
Figure 2.
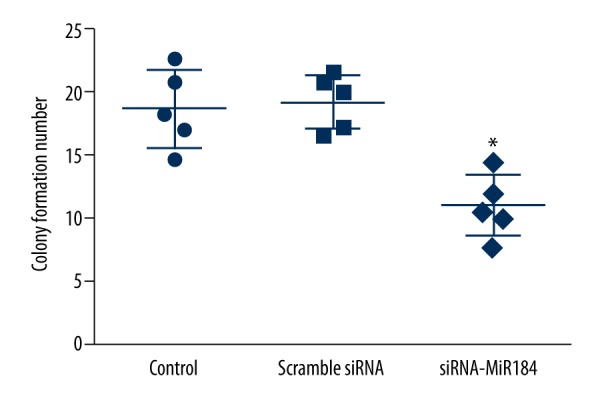
The number of colonies formed by HaCaT cells. miR-184 siRNA treatment significantly reduced in the number of colonies formed compared to untreated controls. Data expressed as mean ±SD (* P<0.05).
Cell apoptosis
In addition to the cell viability, we also examined the effects of miR-184 on the apoptosis of HaCaT cells, and the results are shown in Figure 3. Compared with the apoptosis rate in the control group and scramble siRNA group, the apoptosis rate was significantly increased in HaCaT cells transfected with siRNA-miR-184 (P<0.05).
Figure 3.
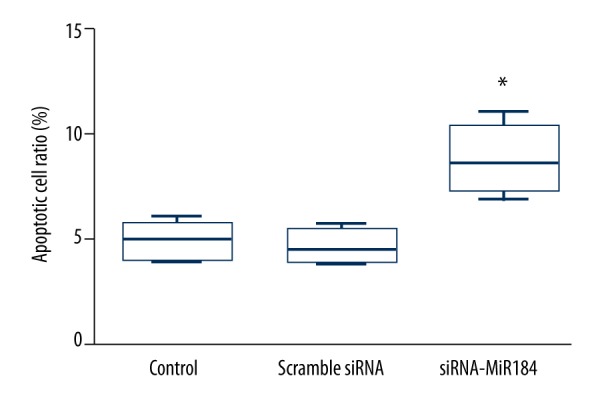
The apoptosis rate of HaCaT cells assayed by flow cytometer. MiR-184 siRNA treatment enhanced the apoptosis of HaCaT cells. Data expressed as mean ±SD (* P< 0.05).
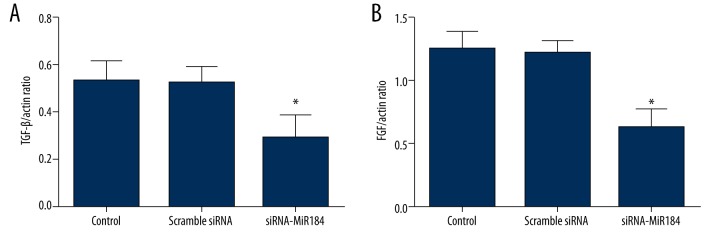
Expression of TGF-β1 and bFGF
In order to further study the molecular biological mechanisms of miR-184 on HaCaT cells, the mRNA expressions of TGF-β1 and bFGF were detected. As shown in Figure 4A, the mRNA expression of TGF-β1 decreased significantly after cells were transfected with siRNA-miR-184 (P<0.05). In addition, the down-regulation of miR-184 also significantly inhibited the mRNA expression of bFGF (P<0.05) (Figure 4B).
Figure 4.
The mRNA expressions of transforming growth factor (TGF)-β1 (A) and basic fibroblast growth factor (bFGF) (B) in HaCaT cells assayed by RT-PCR. Data expressed as mean ±SD (* P<0.05).
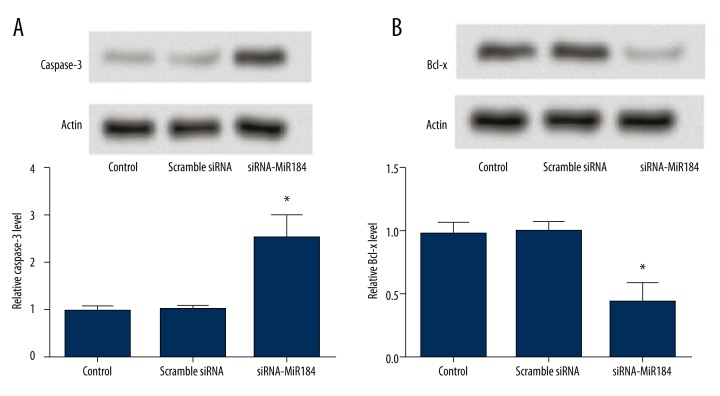
Expression of apoptosis-related protein
The expressions of apoptosis-related proteins, including pro-apoptotic protein caspase-3 and anti-apoptotic protein Bcl-x, were determined by Western blot analysis. The result showed that when HaCaT cells were transfected with siRNA-miR-184, the expression of caspase-3 increased significantly (P<0.05) (Figure 5A) and the expression of Bcl-x was significantly decreased in the siRNA-miR-184 group (P<0.05) (Figure 5B).
Figure 5.
The expressions of apoptosis-related proteins caspase-3 (A) and Bcl-x (B) in HaCaT cells determined by Western blot analysis. Data expressed as mean ±SD (* P<0.05).
Discussion
In the present study we demonstrated that miR-184 played an important role in the growth, proliferation, and apoptosis of HaCaT cells. After miR-184 was transfected with siRNA, cell viability and colony forming ability decreased significantly, and apoptosis was significantly increased. Furthermore, the expressions of growth factors TGF-β1 and bFGF mRNAs, as well as apoptosis-related proteins Bcl-x, in HaCaT cells decreased significantly after miR-184 was transfected with siRNA, and the expression of pro-apoptotic protein caspase-3 increased significantly.
Previous studies have indicated that miR-184 is an important modulator of stem cell proliferation and growth. For instance, miR-184 has been reported to regulate germline cell proliferation in Drosophila [12]. Additionally, miR-184 as a potential onco-miR has been found to be up-regulated in tumors [13]. Wong et al. [14] found that inhibition of miR-184 in tongue squamous cell carcinoma cells reduced cell proliferation and induced apoptosis. Although the role of miR-184 in the proliferation and apoptosis of keratinocyte has not been investigated before, our study found that the cell viability and colony forming ability of HaCaT cells decreased significantly, and the apoptosis of HaCaT cells increased significantly, after miR-184 was transfected with siRNA. Therefore, we speculate that miR-184 may play an important role in the growth, proliferation, and apoptosis of keratinocyte.
Some growth factors, such as TGF-β1 and bFGF, have been suggested to play an important role in epithelial cells proliferation [15]. TGF-β1 is a polypeptide member of the transforming growth factor beta superfamily of cytokines, which performs many cellular functions, such as the control of cell growth, proliferation, and apoptosis [16]. bFGF is a member of the fibroblast growth factor family, which is known for its regulatory, mitogenic, and angiogenic abilities [17]. In addition, bFGF can increase cell survival rate and stabilize cell phenotype [18]. In the present study, the mRNA expressions of TGF-β1 and bFGF were significantly lower in the siRNA-miR-184 group than in the control and scramble siRNA groups. The results suggest that miR-184 promotes the proliferation of keratinocyte through targeting TGF-β1 and bFGF.
Apoptosis is a complex biological process that enables organisms to kill and remove unwanted cells during their development, normal homeostasis, and disease [19,20]. Apoptosis is usually regulated by the caspase family and Bcl-2 family of proteins [21,22], which are key mediators in induction of apoptosis in different cells [23]. Keratinocyte apoptosis is regulated by pro-apoptotic and anti-apoptotic proteins [23]. Caspase-3 is a member of the caspase family, which is a pro-apoptotic protein. Sequential activation of caspases in different cell types plays a central role in the execution phase of cell apoptosis [24]. Importantly, caspase-3 has been reported to be expressed in human keratinocytes [25]. Khalil et al. [26] suggested that caspase-3 is a main mediator of UV-B-induced keratinocyte apoptosis. Bcl-x is a member of the Bcl-2 family of proteins [27], which has been demonstrated to protect HaCaT cells against apoptosis [28]. Bcl-x is an anti-apoptotic protein, and its overexpression inhibits cell death [29]. Nagane et al. [30] have demonstrated that constitutive activation of epidermal growth factor receptor can increase the expression of Bcl-x, thereby leading to apoptosis resistance. In our study, the expression of caspase-3 increased and the expression of Bcl-x decreased after miR-184 was transfected, which indicates that miR-184 plays an important role in the apoptosis of keratinocytes.
Conclusions
Our results suggest distinct roles of miR-184 during the growth, proliferation, and apoptosis of keratinocytes. miR-184 may be useful as a molecular target for the treatment of abnormal keratinocyte proliferation-associated diseases, such as psoriasis. Further clinical study will be done to translate the basic research on the miR-184 into practice.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Source of support: Departmental sources
References
- 1.Chigurupati S, Mughal MR, Okun E, et al. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials. 2013;34:2194–201. doi: 10.1016/j.biomaterials.2012.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai Y, Li D, Li C, et al. The antimicrobial protein REG3A regulates keratinocyte proliferation and differentiation after skin injury. Immunity. 2012;37:74–84. doi: 10.1016/j.immuni.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckert RL, Efimova T, Dashti SR, et al. Keratinocyte survival, differentiation, and death: many roads lead to mitogen-activated protein kinase. J Investig Dermatol Symp Proc. 2002;7:36–40. doi: 10.1046/j.1523-1747.2002.19634.x. [DOI] [PubMed] [Google Scholar]
- 4.McKay IA, Leigh IM. Altered keratinocyte growth and differentiation in psoriasis. Clin Dermatol. 1995;13:105–14. doi: 10.1016/0738-081x(95)93817-8. [DOI] [PubMed] [Google Scholar]
- 5.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–55. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 6.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–74. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 7.Latronico MV, Condorelli G. MicroRNAs and cardiac pathology. Nat Rev Cardiol. 2009;6:418–29. doi: 10.1038/nrcardio.2009.56. [DOI] [PubMed] [Google Scholar]
- 8.Aboobaker AA, Tomancak P, Patel N, et al. Drosophila microRNAs exhibit diverse spatial expression patterns during embryonic development. Proc Natl Acad Sci USA. 2005;102:18017–22. doi: 10.1073/pnas.0508823102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C, Teng Z-Q, Santistevan NJ, et al. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 2010;6:433–44. doi: 10.1016/j.stem.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Stallings RL. Differential patterns of microRNA expression in neuroblastoma are correlated with prognosis, differentiation, and apoptosis. Canc Res. 2007;67:976–83. doi: 10.1158/0008-5472.CAN-06-3667. [DOI] [PubMed] [Google Scholar]
- 11.Dua P, Gude RP. Antiproliferative and antiproteolytic activity of pentoxifylline in cultures of B16F10 melanoma cells. Cancer Chemother Pharmacol. 2006;58:195–202. doi: 10.1007/s00280-005-0155-8. [DOI] [PubMed] [Google Scholar]
- 12.Iovino N, Pane A, Gaul U. miR-184 has multiple roles in Drosophila female germline development. Dev Cell. 2009;17:123–33. doi: 10.1016/j.devcel.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Walter BA, Valera VA, Pinto PA, Merino MJ. Comprehensive microRNA profiling of prostate cancer. J Cancer. 2013;4:350–57. doi: 10.7150/jca.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong TS, Liu XB, Wong BY-H, et al. Mature miR-184 as potential oncogenic microRNA of squamous cell carcinoma of tongue. Clin Cancer Res. 2008;14:2588–92. doi: 10.1158/1078-0432.CCR-07-0666. [DOI] [PubMed] [Google Scholar]
- 15.Wang YW, Ren JH, Xia K, et al. Effect of mitomycin on normal dermal fibroblast and HaCat cell: An in vitro study. Journal of Zhejiang University SCIENCE B. 2012;13:997–1005. doi: 10.1631/jzus.B1200055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghadami M, Makita Y, Yoshida K, et al. Genetic mapping of the Camurati-Engelmann disease locus to chromosome 19q13. 1-q13. 3. Am J Hum Genet. 2000;66:143–47. doi: 10.1086/302728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kikuchi K, Kadono T, Takehara K. Effects of various growth factors and histamine on cultured keloid fibroblasts. Dermatology. 1995;190:4–8. doi: 10.1159/000246625. [DOI] [PubMed] [Google Scholar]
- 18.Hong RH, Lum J, Koch RJ. Growth of keloid-producing fibroblasts in commercially available serum-free media. Otolaryngol Head Neck Surg. 1999;121:469–73. doi: 10.1016/S0194-5998(99)70239-1. [DOI] [PubMed] [Google Scholar]
- 19.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–58. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korsmeyer SJ, Danial NN. Cell death: Critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 22.Galluzzi L, Vitale I, Abrams J, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–20. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narbutt J, Boncela J, Smolarczyk K, et al. Pathogenic activity of circulating anti-desmoglein-3 autoantibodies isolated from pemphigus vulgaris patients. Arch Med Sci. 2012;8:347–56. doi: 10.5114/aoms.2012.28564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brentnall M, Rodriguez-Menocal L, De Guevara RL, et al. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013;14:32. doi: 10.1186/1471-2121-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi T, Ogo M, Hibino T. Partial purification and characterization of two distinct types of caspases from human epidermis. J Invest Dermatol. 1998;111:367–72. doi: 10.1046/j.1523-1747.1998.00295.x. [DOI] [PubMed] [Google Scholar]
- 26.Khalil H, Peltzer N, Walicki J, et al. Caspase-3 protects stressed organs against cell death. Mol Cell Biol. 2012;32:4523–33. doi: 10.1128/MCB.00774-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edlich F, Banerjee S, Suzuki M, et al. Bcl-x L retrotranslocates Bax from the mitochondria into the cytosol. Cell. 2011;145:104–16. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiberio R, Marconi A, Fila C, et al. Keratinocytes enriched for stem cells are protected from anoikis via an integrin signaling pathway in a Bcl-2 dependent manner. FEBS Lett. 2002;524:139–44. doi: 10.1016/s0014-5793(02)03040-5. [DOI] [PubMed] [Google Scholar]
- 29.Youle RJ, Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 30.Nagane M, Levitzki A, Gazit A, et al. Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci USA. 1998;95:5724–29. doi: 10.1073/pnas.95.10.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]