ABSTRACT
The primary function of ribosomes is to decode mRNAs into polypeptide chains; however, this description is overly simplistic. Accumulating evidence shows that ribosomes themselves can affect the relative efficiency with which various mRNAs are translated and indicates that these effects can be modulated by ribosome heterogeneity. The notion that ribosomes have regulatory capabilities was elaborated more than a decade ago in the ribosome filter hypothesis. Various lines of evidence support this idea and have shown that the translation of some mRNAs is affected by discrete binding interactions with rRNA or ribosomal proteins. Recent work from our laboratory has demonstrated that base-pairing of the Hepatitis C Virus (HCV) internal ribosome entry site (IRES) to 18S rRNA is required for IRES function, but only in the context of more complex ribosomal interactions. The HCV IRES provides an example of the ribosome filter that involves multiple binding interactions between mRNAs and ribosomal subunits.
Keywords: Filter, HCV, Hepatitis C Virus, IRES, internal ribosome entry site, regulation, ribosome, translation
Introduction
Protein synthesis is one of the most fundamental biological processes and has been highly conserved through evolution. Ribosomes have a central role in this process, functioning as peptidyl transferases that coordinate mRNAs with aminoacyl-tRNAs to decode mRNA coding regions into polypeptides. Ribosomes also appear to have important regulatory capabilities; however, until recently the ability of ribosomes to differentially affect mRNA translation had been essentially overlooked. Perhaps this oversight was due to the enormity and importance of the ribosome's primary function in peptide synthesis. Alternatively, as suggested by Woese, it may be that many scientists considered the problem of protein synthesis to be solved when it was understood how the genetic code contained within mRNA is translated into protein.1,2
The ribosome filter hypothesis suggests that ribosomes can regulate the translation of particular sets of mRNAs through specific interactions with mRNAs.3 Ribosome heterogeneity provides a mechanism for modulating these interactions by altering rRNA accessibility or ribosomal protein composition. Since 2002, support for this model has increased; see refs.4-6 In this article, we briefly discuss the ribosome filter hypothesis and critically review some exciting new studies that give additional credence to the notion of ribosomal regulation. In addition, we discuss the mechanism used by the HCV IRES to recruit ribosomes and initiate translation as an example of ribosomal regulation that requires binding to multiple sites of the 40S ribosomal subunit.
The ribosome filter hypothesis
The filter hypothesis is based on various observations in the literature and early studies from one of the authors (VM). These early studies investigated changes in gene expression affected by interactions between brain cells, and between brain cells and extracellular matrix (ECM).7,8 The results indicated that cell adhesion affects the expression of various mRNAs, including those encoding cell-adhesion molecules. Two unexpected observations of these studies were the identification of sequences within mRNAs that resemble rRNA, in both sense and anti-sense orientations, and the different levels of accumulation of various mRNAs encoding ribosomal proteins.9 The identification of rRNA-like sequences within mRNAs suggested potential interactions with ribosomes or ribosomal proteins. At the time, the significance of these rRNA-like sequences was unclear, particularly because mRNA-rRNA base pairing was thought to be restricted to bacteria.10 The differential enrichment of mRNAs encoding ribosomal proteins suggested that the composition of ribosomes might change under different conditions or in different cell types. A review of the literature for evidence of ribosome heterogeneity revealed numerous studies showing differences in both protein composition and rRNA. Reports of ribosomal protein heterogeneity date back more than 40 years. However, technical limitations associated with ribosome purification methods cast doubt on many of these studies.11 Perhaps as a consequence of criticism, very few studies of this type were published for several years following. Indeed, a timeline of the numbers of scientific publications addressing ‘ribosome heterogeneity’ since 1969 has a U-shaped distribution; see Fig. 1 of ref.12 Interestingly, the literature has also revealed that mutations in the genes encoding various ribosomal proteins often give rise to very specific effects rather than effects consistent with global defects in translation, as might be expected from ribosomal haploinsufficiency.13
Figure 1.
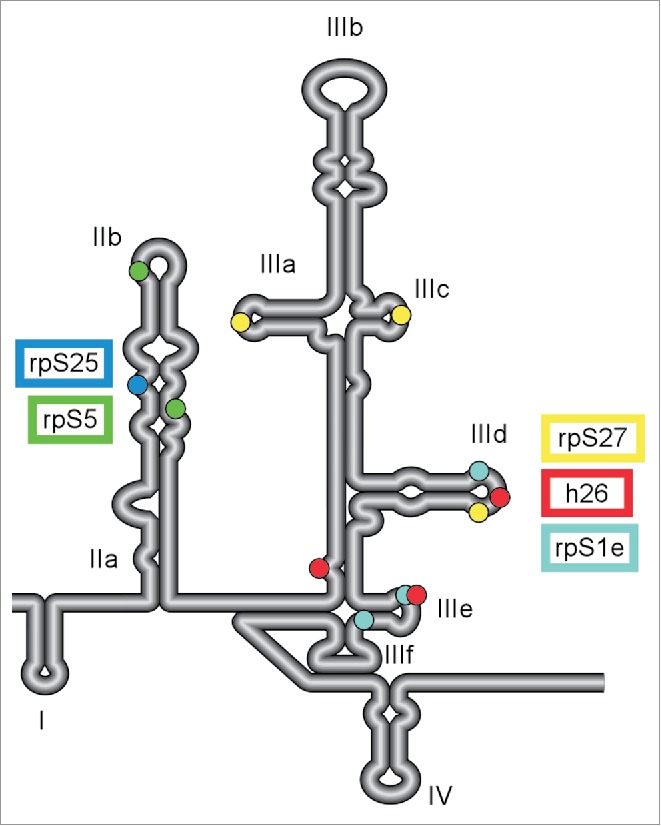
Multiple contact points between HCV IRES and 40S ribosomal subunit. Schematic diagram of the secondary structure of HCV 5′ UTR. Four distinct domains (I-IV) are labeled along with subdomains of IIa-b and IIIa-f. The IRES regions that were identified to interact with various components of 40S subunit by CryoEM analysis are indicated with color-coded dots: rpS5 (uS7; green), rpS25 (blue), rpS27 (yellow), rpS1e (cyan) and 18S rRNA helix 26 (red).40,54. Additional nucleotides 70-74 and 84-91 of HCV IRES are predicted to make contact with rpS5.39
The ribosome filter hypothesis was formulated to explain these various observations.3,4 As originally postulated, the main tenet of this hypothesis is that the ribosome is a regulatory structure that embodies mechanisms for preferentially translating different subsets of the message population. Mechanisms by which ribosomes were postulated to affect translation include specific interactions between rRNA or ribosomal proteins and cis-regulatory sequences in mRNAs. The hypothesis also postulates that ribosomes may display a continuum of regulatory effects; competition for binding sites in ribosomal subunits may affect the rate of translation of different mRNAs, and the filter may be modulated as a result of altering or masking particular binding sites on ribosomes. This modulation of the accessibility of various binding sites may be affected for example, by ribosome heterogeneity. However, ribosome heterogeneity is not required for the filter mechanism.
Testing the hypothesis
Evidence for mRNA-ribosomal subunit interactions includes binding and cross-linking studies which can demonstrate specific interactions between mRNA sequence elements and ribosomal components. Functional data demonstrating an effect on translation requires different types of experiments depending on whether the mRNA-ribosomal interaction involves rRNA or protein.
Functional evidence for mRNA-rRNA base pairing requires genetic testing that includes mutation of both RNAs. Mutation of the complementary sequence in either the mRNA or rRNA should disrupt translation and compensatory mutations in the other RNA—which restore complementarity—should restore translation. With suitable controls, e.g. to show that the mutated rRNAs are incorporated into functional subunits, this type of analysis can provide compelling evidence for a base pairing interaction. This type of analysis was used to establish the Shine-Dalgarno interaction in bacteria.14 To enable comparable testing in eukaryotes, we have developed yeast and mammalian 18S rRNA expression systems.15,16 The yeast expression system was used to confirm the requirement for a specific base-pairing sequence interaction between 18S rRNA and a short sequence element in the 5' leader of the Gtx homeodomain mRNA, which functions as an IRES, a translation enhancing element (TEE), and a ribosomal shunt site.17,18 This system was also used to establish the requirement for an mRNA-rRNA base pairing interaction during translation reinitiation of an overlapping cistron in Calicivirus.19 More recently, the mammalian expression system has been used to establish the requirement for a specific base-pairing interaction between HCV IRES and 18S rRNA; discussed below and see ref.20 A potential limitation of genetic analyses of mRNA-rRNA base pairing is the susceptibility of the ribosome to various point mutations in 18S rRNA, some of which can completely disrupt subunit formation or lead to altered ribosomal activity.
Compared to an mRNA-rRNA interaction, it is significantly more difficult to obtain convincing functional evidence for an mRNA-ribosome interaction that involves binding to a ribosomal protein. The problem is that numerous ribosomal proteins are found outside of ribosomal subunits and many have extraribosomal activities that can complicate the analysis.21 In addition, it is difficult to differentiate between a direct ribosomal effect and a secondary effect, e.g., via the altered expression of other proteins or mRNAs. One way to test a putative mRNA-ribosomal protein interaction is to perform a cell-free reconstitution experiment using ribosomal subunits isolated from different sources. In these experiments, the ribosomal subunits are the only variable. All other variables remain constant, including the mRNA population. However, these types of experiments can be technically challenging. For example, ribosome heterogeneity is not necessarily restricted to differences in ribosomal subunits obtained from different cell types, but may also occur between subunits from a single cell source. In addition, other sources of ribosomal heterogeneity, i.e. independent of the ribosomal protein of interest, may arise and differentially affect translation because ribosomes from different sources co-purify with different non-ribosomal proteins or RNAs that are tightly associated with the subunits.
An example of a cell-free reconstitution study to assess the effects of rRNA pseudouridylation was performed by Penzo et al.,22 In this study, the authors showed that ribosomes purified from dyskerin-depleted human cells translated some IRES-containing mRNAs with different relative efficiencies compared to ribosomes purified from control cells.
Chicer recently published a method for performing an extensive/deep proteomic analysis of purified 48S and 80S mRNA-ribosome complexes assembled in a cell-free lysate, which provides a potential approach for overcoming some of the limitations of reconstitution experiments.23 This approach can be applied to different cell lysates to assess whether specific mRNAs form ribosomal complexes enriched for, or restricted to, ribosomal subunits that contain or lack a particular ribosomal protein. This method can be further applied to the reconstitution studies discussed above, in which a ribosome-free cell lysate is reconstituted with ribosomal subunits from different sources. In addition, by mixing ribosomal subunits purified from different sources, it may be possible to assess both specificity and competition. A limitation of the Chicer approach is that the analysis uses in vitro transcribed templates, which may differ significantly from mRNAs transcribed in a nucleus in vivo—in conformation, associated factors, and translation properties. Nevertheless, this analysis enables a level of analysis of ribosomal protein-mediated ribosomal filtering that has not yet been possible.
Terminology
Terms used to describe ribosomes as regulatory elements include ribosome filter, ribosome code, and specialized ribosomes. The authors suggest that 'ribosome filter' may be a more appropriate term as it is analogous to other types of filters, e.g. membranes that physically block some objects while letting others through, or electronic filters that enhance certain signals and reduce others. In the case of a ribosomal filter, non-canonical interactions with ribosomes are postulated to enhance the translation of some mRNAs in the population and decrease the translation of others. This regulation is hypothesized to be an intrinsic property of ribosomes that can be modulated by ribosomal heterogeneity.
The ribosome code has been suggested as an analogy to the histone code, which postulates that transcription is affected by modifications to histone proteins. However, because the histone code functions at the DNA level and does not involve RNA polymerase modifications, it may be more analogous to the ribonome, which consists of RNAs and their associated regulatory factors.24
By contrast with the terms ribosome filter and ribosome code, which both seek to describe how ribosomes modulate the translation of populations of mRNAs, the term specialized ribosome infers a higher degree of specificity. In addition, confusion may arise because the same term has been used to describe synthetic ribosomes in which the rRNA has been engineered for extreme mRNA specificity. For example, see ref.14
Ribosomal regulation mediated by rRNA
rRNA is a source of ribosome heterogeneity that can arise either from differences in primary sequence or post-transcriptional modifications. Examples of regulation are predominantly at the recruitment step of initiation, but there is also evidence that mRNA-rRNA interactions can specifically affect ribosomal movement to the initiation codon and during translation reinitiation.18,19 Some recent examples of ribosomal regulation that involve rRNA include effects of rRNA methylation and mRNA-rRNA interactions that reduce ribosomal movement during elongation.
rRNA methylation
Schosserer et al., (2015) performed a genome-wide transcriptional profiling study in order to identify genetic regulators of aging.25 They identified the NSUN5 mRNA (RCM1 in yeast), which encodes a methyltransferase that is differentially regulated in various human and yeast aging models. Using yeast, worm, and fly model systems, they showed that down-regulation of this methyltransferase—using RNAi in Drosophila melanogaster and Caenorhabditis elegans—led to an increase in mean lifespan. In addition, knockout of this gene in Saccharomyces cerevisiae led to an increase in chronological lifespan. By contrast, overexpression of the methyltransferase significantly reduced lifespan in flies. In yeast knock-out cells, it was shown that this enzyme is necessary and sufficient for methylating nucleotide C2278 of 25S rRNA. The effect of the methylated nucleotide on lifespan was tested in yeast strains in which all of the rDNA repeats were deleted. Strains were complemented with plasmids that expressed either the wild-type rRNAs or a mutated 25S rRNA with a C2278G mutation that cannot be methylated. These studies showed that the strain with the C2278G mutation had a significantly extended chronological lifespan, demonstrating that this single nucleotide modification could account for the observed lifespan extension. By comparing wild-type and rcm-1-knockout cells under different oxidative stress conditions, the authors showed that the methylation status of C2278 causes the recruitment of a different set of mRNAs from the mRNA population. In stressed wild-type and unstressed rcm1 knockout cells, translation of stress-responsive mRNAs was increased. This study indicates that methylation at C2278 is the basis for a ‘specialized’ subpopulation.
mRNA-rRNA interactions facilitate ribosomal pausing during elongation
A study from the Weissman lab shows that the concept of ribosomal regulation is not limited to effects on translation initiation, but that bacterial ribosomes can also direct sites of ribosomal pausing during elongation.26 This study used ribosome profiling to identify mRNA sequences protected by ribosomes during elongation. For this analysis, elongation was blocked and ribosome protected fragments were analyzed by deep sequencing. By mapping the protected sequences onto mRNAs, the authors were able to identify sites with high ribosome density. They found little correlation between these ribosomal pause sites and the abundance of corresponding tRNAs. However, sequence analysis identified features found in Shine-Dalgarno sequences. They directly tested the notion that Shine-Dalgarno-like sequences can lead to ribosomal pausing by using an orthogonal ribosome system; these recombinant ribosomes do not contain the anti-Shine Dalgarno sequence, but contain a different ribosome binding site. These ribosomes only translate mRNAs with a corresponding orthogonal ribosome binding site. Using this system, the authors looked at ribosome occupancy of the lacZ mRNA translated by wild type or orthogonal ribosomes. The results showed that the ribosome occupancy profile was related to Shine-Dalgarno-like features when the mRNA was translated by wild-type ribosomes. However, when this same mRNA was translated exclusively by orthogonal ribosomes, the ribosome occupancy profile was very different and correlated with orthogonal-ribosome binding site features.
Ribosomal proteins
Ribosomal protein heterogeneity
Ribosome heterogeneity arising from differences in ribosomal protein composition is postulated to be a mechanism by which ribosomal regulation is modulated. Evidence from various sources continue to provide examples of heterogeneity.12 In addition, regulation of translation by ribosomes has been implicated in various systems and across kingdoms, based on different levels of evidence. For example, see refs.27-30 Some recent studies of ribosomal regulation mediated by specific ribosomal proteins are discussed below.
Ribosomal protein rpL38 and Hox mRNAs
Xue et at., (2015) have identified IRES-elements in the 5' leaders of Hox mRNAs that appear to be capable of directly mediating 80S ribosome formation.31 For the Hoxa9 IRES, normalized activity is decreased by ≈20% by disruption of a chromosomal copy of the rpL38 gene that decreases rpL38 expression by 40%. The expression directed by the Hoxa4, Hoxa5, and Hoxa9 5' leaders were similarly affected. This effect appears to be specific because the cap-dependent translation of a reporter gene and the translation initiating from the HCV IRES were not affected. This study suggests that ribosomal specificity results from a direct interaction between rpL38 and sequence elements in the 5' leaders of some Hox mRNAs. RNA pull down experiments showed that the Hoxa9 5' leader, as well as the minimal mRNA element were able to pull down ribosomal proteins from both subunits, including rpL38. However, it remains to be demonstrated that rpL38's effects are not extraribosomal and that binding of the IRES-element to 60S subunits is restricted to ribosomal subunits that contain rpL38.
RpL40 and translation of vesicular stomatitis virus (VSV) mRNAs
Lee et al., (2013) provide a nice example of the ribosome filter in their analysis of VSV mRNAs.32 Translation of VSV mRNAs occurs efficiently despite host shutoff induced by the virus. VSV mRNAs are structurally indistinguishable from cellular mRNAs, and it was not obvious why these mRNAs are preferentially translated. To begin to address this question, Lee and colleagues performed an siRNA screen of ribosomal proteins. As expected, most of the ribosomal proteins (t=61) are required for cell viability. Another set are not required for cell viability or VSV infection (t=16). However, 8 ribosomal proteins were identified which are specifically required for VSV infection, but not cell viability. One of these, rpL40, had a large effect on virus (vs host) translation and was chosen for further analysis. Extensive control experiments confirmed the requirement of rpL40 for virus mRNA translation, showed that ribosome biogenesis and maturation were not affected by rpL40 depletion, and established that the effects on translation were not due to a reduction in the pool of cytoplasmic ribosomes. In addition, it was confirmed that the effects of rpL40 depletion were specific to the virus and did not seem to affect bulk cellular translation or translation driven by the cricket paralysis virus IRES or the poliovirus IRES. Polysome analysis also showed that depletion of rpL40 completely abolished polysome formation on VSV mRNAs, but not on a cellular mRNA (β-actin). To establish that the effects of rpL40 are not extraribosomal, and not a consequence of other potential variables related to virus infection, in vitro translation assays were performed using yeast cytoplasmic extracts. These extracts were prepared from a strain that had both rpL40 paralogs deleted and contained an inducible rpL40. A reporter Renilla luciferase mRNA and an rVSV-Luc mRNA fusion mRNA were translated in lysates containing or lacking rpL40A. The results showed that the reporter mRNA was similarly translated in both lysates, but the rVSV-Luc mRNA was only translated in the lysate lacking rpL40. To address whether rpL40 affects translation as a ribosomal component or through extraribosomal effects, the polysomal distribution of this ribosomal protein was monitored by polysome analysis. The results showed that rpL40 was only detected in 60S, 80S, and polysome fractions. There was no detectable rpL40 in extraribosomal fractions. By using the cell-free system, the authors were able to identify a subset of the mRNA population (≈7%) that require rpL40 for polysome association. These mRNAs included stress-response mRNAs.
Non-ribosomal proteins
In addition to heterogeneity in ribosomal proteins and rRNAs, there are also examples of ribosome heterogeneity that involve non-ribosomal proteins which can affect the translation of subsets of mRNAs, without altering global translation. Non-ribosomal proteins include reaper and RACK1, which have been discussed previously.4 Another example is glycogen synthase 1 (GYS1), an enzyme involved in glycogen biosynthesis. This protein was identified in a proteomic analysis of HeLa ribosomes designed to identify non-ribosomal proteins associated with ribosomal subunits.33 The phosphorylated form of GLY1 was found to be preferentially associated with the 60S ribosomal subunit of elongating ribosomes. These studies showed that depletion of GYS1 did not significantly affect protein synthesis, but altered the expression of a subset of mRNAs. The authors suggested that GYS1 provides a mechanism to link the energy state of cells to translation.
HCV IRES: ribosome regulation mediated by multiple binding sites
The HCV IRES provides a strong case supporting ribosomal regulation due to its unique and well-established interaction with the ribosome, through which binary complex formation is achieved without the help of translation factors.34-36 The 5′ ≈340 nucleotides of the HCV RNA encompasses the untranslated region with 4 highly structured and distinct domains (I-IV; Fig. 1) where the last 3 domains (II-IV) comprise the IRES. An early cryo-electron microscopy (cryo-EM) structure study on the binary complex revealed that the IRES is bound as an extended form on the solvent side of the 40S subunit, making multiple contacts.37 Consistent with this observation, multiple domains of the IRES were protected from RNaseT1 digestion when bound with 40S subunits.35,36,38
Initial interaction of 40S subunit with domain III of HCV IRES
Various studies using methods including cryoEM, crosslinking, and fluorescent protein modification have reported that many ribosomal proteins (including rpS5 (uS7), S25, S27, S1e (S3A), S26, S28 and many others) as well as 18S rRNA serve as contact points on the 40S subunit to interact with the domains of the IRES.34,37,39-47 However, not all of the ribosomal constituents reported in these studies are required for the initial binary complex formation. A filter binding study with purified 40S subunits revealed that domains II, IV, and subdomain IIIb are dispensable for efficient binary complex formation, whereas deletion of subdomain IIIabc or mutation of subdomain IIId/IIIe abolished complex formation by orders of magnitude.36 Therefore, domain III (except subdomain IIIb) is essential for the initial binary complex formation. A recent high-resolution cryoEM structure showed that the domain III subdomains (IIIa-e except IIIb) interact with rpS1e, S27 and helix 26 of 18S rRNA. See.40 It is intriguing that all 3 ribosomal components interact with the loop of IRES domain IIId, which therefore, can be a pivotal binding site for the 40S subunit.
Among these proposed interactions, the most studied and supported is the interaction between the apical loop of HCV IRES domain IIId and helix 26 of 18S rRNA. First highlighted in a cryoEM study,41 this interaction and its importance in the binary complex formation are supported by the following biochemical and functional evidence: chemical/enzymatic protection on each apical loop of the 2 molecules was observed when the binary complex is formed.35,36,46,48 Substitution mutations of the tri-guanine stretch at the apical loop of subdomain IIId of HCV IRES led to drastic reduction of the binding affinity with purified 40S subunits,36,49 and to severely compromised IRES activity as a functional consequence in vitro as well as in cells.35,49-52
Recently, our laboratory demonstrated that the RNA-RNA interaction involves G:C base-pairing between tri-nucleotides of each apical loop. We showed that mutations in the IIId apical loop of the HCV IRES, which disrupt activity, can be functionally rescued in cells expressing a synthetic 18S rRNA with compensatory mutations in helix 26.20 These mutations in 18S rRNA allow restoration of the full Watson-Crick base-pairing interaction. Importantly, the 18S rRNA mutations seem to specifically affect the HCV IRES as no significant changes were observed on translation mediated by either the 5′-cap-structure or other viral IRESes. Ribosomes that incorporated the 18S rRNA mutations were distributed in polysome fractions in a very similar manner as endogenous ribosomes. The base-pairing interaction is also supported by phylogenetic evidence: HCV-like IRESes can be found in some genera of Flaviviridiade and Picornaviridae and strict conservation has been observed in the trinucleotide stretch of the apical loop of IRES domain IIId, as well as in the apical loop sequence in helix 26 of 18S rRNA from the virus hosts.53 These findings are supported by a recent cryo-EM study, which reveals the Watson-Crick kissing-loop interaction between the IIId domain of the HCV IRES and helix 26 of 18S rRNA at 3.9Å resolution.54 This study suggests that this interaction, together with interactions between IRES domain IIIe and adjacent bases in helix 26 serve as the central anchor point for this IRES on the 40S subunit.
This essential interaction via mRNA-rRNA base-pairing is a part of the overall binding lattice of IRES and 40S subunit, which additionally includes the contacts mediated via the aforementioned ribosomal proteins, rpS1e and rpS27. These ribosomal proteins are positioned close to each other on the 40S subunit by sandwiching helix 26 of 18SrRNA. In fluorescent probing studies, it was shown that upon binding of HCV IRES, fluorescent labeling on both rpS1e and S27 was reduced, strongly supporting their involvement in the physical interaction.55
Ribosomal protein rpS1e was detected by crosslinking studies using HCV IRES carrying a reactive group at domains IIIe and IIId.44,47 In the 40S subunit, rpS1e is closely situated according to the cryoEM structure mapping.40 Consistently, a set of mutations in IIIe resulted in reduced binding affinity to 40S subunits,36,56 which is correlated with debilitated IRES activity.36,57,58
Ribosomal protein rpS27 was initially identified as one of many ribosomal proteins cross-linked to 4-thiouridine-containing HCV IRES RNA.43 Recent cryoEM modeling predicts that rpS27 interacts with apical loops of IRES domain IIIa, IIIc and IIId.40 However, HCV IRES RNA lacking IIIabc was still able to crosslink to rpS27, albeit with reduced efficiency.43 Consistent with this observation, the consequence of individual mutations of the apical loop of IIIa and IIIc were much less severe in reducing 40S binding affinity than mutation of the crucial tri-guanine resides.36 These results suggest that the individual binding site (IIIa, IIIb and IIId) retains its own binding capability to some extent. Recently, a frameshift mutation in rpS27 was reported in patients with Diamond-Blackfan anemia, which is a well-known ribosomopathy associated with mutations in numerous ribosomal protein genes.59
Ribosomal interactions with HCV IRES following 40S recruitment
Although not involved in the initial binary complex formation, HCV IRES domain II is required for 80S formation.49,60 Domain II appears to make contact around the E-site and to induce a conformational change of the head/body rotational orientation and the platform domain of the 40S subunit.37 Moreover, selective hydroxyl acylation analyzed by primer extension (SHAPE) has shown that IIb is responsible for inducing unfolding of IRES domain IV and setting at the decoding groove of the subunit.61 HCV IRES domain IIb was demonstrated to interact with 40S subunit via at least 2 ribosomal proteins, rpS5 and rpS25.
The interaction between IIb and rpS5 is supported by crosslinking studies and cryoEM structure modeling.39,44,45 siRNA knockdown of rpS5 in Huh7 cells or addition of anti-rpS5S antibody in cell-free lysates resulted in a significant reduction of translation mediated by HCV-IRES over cap-dependent translation.62
Situated near the top of the 40S subunit head and closely positioned with rpS5, rpS25 presents rather unique characteristics inasmuch as it is required not only in translation mediated by HCV IRES, but in translation mediated by a diverse range of IRESes of virus- and cellular-origins.63 Ribosomal protein rpS25 assembled on an active ribosome was recently demonstrated to interact with HCV IRES domain II by FRET analysis.64 The functional effect of this interaction to HCV IRES-dependent translation was demonstrated by siRNA knockdown of rpS25 as well as in rpS25-KO cells, both of which showed drastic and specific reduction of HCV IRES-dependent translation over 5′-cap mediated translation of a reporter gene.64,65
The interaction between the HCV IRES and 40S ribosomal subunits is illustrated as supporting evidence for the ribosome filter hypothesis by showing the ribosome as a regulatory structure exemplifying mechanisms for differential translation via specific interactions between rRNA or ribosomal proteins and cis-regulatory sequences in mRNAs. The interaction of the HCV IRES with 40S ribosomal subunits is based on compiling evidence showing the importance of specific components in the 40S subunit for making physical and functional contact with the IRES. In particular, the triple guanine sequence at the apical loop of helix 26 of 18S rRNA, rpS5, and rpS25; these are the 40S constituents with distinctive contributions to HCV IRES-mediated translation, but not to translation mediated by the 5′-cap structure. As with rpS25, the specific interactions of the apical loop of 18S rRNA and rpS5 may affect the translation of other mRNAs beyond HCV RNA, and contribute to the complex and dynamic regulation of translation imposed by ribosomes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Dr. Stephen A. Chappell for critical reading of the manuscript.
References
- 1.Woese CR. Translation: in retrospect and prospect. RNA 2001; 7:1055-67; PMID:11497425; http://dx.doi.org/ 10.1017/S1355838201010615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woese CR. A new biology for a new century. Microbiol Mol Biol Rev 2004; 68:173-86; PMID:15187180; http://dx.doi.org/ 10.1128/MMBR.68.2.173-186.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mauro VP, Edelman GM. The ribosome filter hypothesis. Proc Natl Acad Sci U S A 2002; 99:12031-6; PMID:12221294; http://dx.doi.org/ 10.1073/pnas.192442499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauro VP, Edelman GM. The ribosome filter redux. Cell Cycle 2007; 6:2246-51; PMID:17890902; http://dx.doi.org/ 10.4161/cc.6.18.4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert WV. Functional specialization of ribosomes? Trends Biochem Sci 2011; 36:127-32; PMID:21242088; http://dx.doi.org/ 10.1016/j.tibs.2010.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue S, Barna M. Specialized ribosomes: a new frontier in gene regulation and organismal biology. Nat Rev Mol Cell Biol 2012; 13:355-69; PMID:22617470; http://dx.doi.org/ 10.1038/nrm3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mauro VP, Wood IC, Krushel L, Crossin KL, Edelman GM. Cell adhesion alters gene transcription in chicken embryo brain cells and mouse embryonal carcinoma cells. Proc Natl Acad Sci USA 1994; 91:2868-72; PMID:8146202; http://dx.doi.org/ 10.1073/pnas.91.7.2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tranque P, Crossin KL, Cirelli C, Edelman GM, Mauro VP. Identification and characterization of a RING-finger gene (C-RZF) expressed in chicken embryo cells. Proc Natl Acad Sci USA 1996; 93:3105-9; PMID:8610176; http://dx.doi.org/ 10.1073/pnas.93.7.3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauro VP, Edelman GM. rRNA-like sequences occur in diverse primary transcripts: implications for the control of gene expression. Proc Natl Acad Sci USA 1997; 94:422-7; PMID:9012798; http://dx.doi.org/ 10.1073/pnas.94.2.422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson RJ. A comparative view of initiation site selection mechanisms. : Sonenberg N, Hershey JWB, Mathews MB, ed. Translational control of gene expression. Cold Spring Harbor: Cold Spring Harbor Laboratory Press, 2000:127-83. [Google Scholar]
- 11.Sherton CC, Wool IG. A comparison of the proteins of rat skeletal muscle and liver ribosomes by two-dimensional polyacrylamide gel electrophoresis. Observations on the partition of proteins between ribosomal subunits and a description of two acidic proteins in the large subunit. J Biol Chem 1974; 249:2258-67; PMID:4594498 [PubMed] [Google Scholar]
- 12.Sauert M, Temmel H, Moll I. Heterogeneity of the translational machinery: Variations on a common theme. Biochimie 2014; 114:39-47; PMID:25542647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armistead J, Triggs-Raine B. Diverse diseases from a ubiquitous process: the ribosomopathy paradox. FEBS letters 2014; 588:1491-500; PMID:24657617; http://dx.doi.org/ 10.1016/j.febslet.2014.03.024 [DOI] [PubMed] [Google Scholar]
- 14.Hui A, de Boer HA. Specialized ribosome system: preferential translation of a single mRNA species by a subpopulation of mutated ribosomes in Escherichia coli. Proc Natl Acad Sci U S A 1987; 84:4762-6; PMID:2440028; http://dx.doi.org/ 10.1073/pnas.84.14.4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dresios J, Chappell SA, Zhou W, Mauro VP. An mRNA-rRNA base-pairing mechanism for translation initiation in eukaryotes. Nat Struct Mol Biol 2006; 13:30-4; PMID:16341227; http://dx.doi.org/ 10.1038/nsmb1031 [DOI] [PubMed] [Google Scholar]
- 16.Burman LG, Mauro VP. Analysis of rRNA processing and translation in mammalian cells using a synthetic 18S rRNA expression system. Nucleic Acids Res 2012; 40:8085-98; PMID:22718970; http://dx.doi.org/ 10.1093/nar/gks530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chappell SA, Edelman GM, Mauro VP. A 9-nt segment of a cellular mRNA can function as an internal ribosome entry site (IRES) and when present in linked multiple copies greatly enhances IRES activity. Proc Natl Acad Sci USA 2000; 97:1536-41; PMID:10677496; http://dx.doi.org/ 10.1073/pnas.97.4.1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chappell SA, Dresios J, Edelman GM, Mauro VP. Ribosomal shunting mediated by a translational enhancer element that base pairs to 18S rRNA. Proc Natl Acad Sci U S A 2006; 103:9488-93; PMID:16769881; http://dx.doi.org/ 10.1073/pnas.0603597103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luttermann C, Meyers G. The importance of inter- and intramolecular base pairing for translation reinitiation on a eukaryotic bicistronic mRNA. Gen Dev 2009; 23:331-44; PMID:19204118; http://dx.doi.org/ 10.1101/gad.507609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuda D, Mauro VP. Base pairing between hepatitis C virus RNA and 18S rRNA is required for IRES-dependent translation initiation in vivo. Proc Natl Acad Sci U S A 2014; 111:15385-9; PMID:25313046; http://dx.doi.org/ 10.1073/pnas.1413472111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Nag S, Zhang X, Wang MH, Wang H, Zhou J, Zhang R. Ribosomal proteins and human diseases: pathogenesis, molecular mechanisms, and therapeutic implications. Med Res Rev 2015; 35:225-85; PMID:25164622; http://dx.doi.org/ 10.1002/med.21327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penzo M, Rocchi L, Brugiere S, Carnicelli D, Onofrillo C, Coute Y, Brigotti M, Montanaro L. Human ribosomes from cells with reduced dyskerin levels are intrinsically altered in translation. FASEB J 2015; 29(8):3472-82; PMID:25934701 [DOI] [PubMed] [Google Scholar]
- 23.Chicher J, Simonetti A, Kuhn L, Schaeffer L, Hammann P, Eriani G, Martin F. Purification of mRNA-programmed translation initiation complexes suitable for mass spectrometry analysis. Proteomics 2015; 15(14):2417-25; PMID:25914180 [DOI] [PubMed] [Google Scholar]
- 24.Mansfield KD, Keene JD. The ribonome: a dominant force in co-ordinating gene expression. Biol Cell 2009; 101:169-81; PMID:19152504; http://dx.doi.org/ 10.1042/BC20080055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schosserer M, Minois N, Angerer TB, Amring M, Dellago H, Harreither E, Calle-Perez A, Pircher A, Gerstl MP, Pfeifenberger S, et al. Methylation of ribosomal RNA by NSUN5 is a conserved mechanism modulating organismal lifespan. Nat Commun 2015; 6:6158; PMID:25635753; http://dx.doi.org/ 10.1038/ncomms7158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li GW, Oh E, Weissman JS. The anti-Shine-Dalgarno sequence drives translational pausing and codon choice in bacteria. Nature 2012; 484:538-41; PMID:22456704; http://dx.doi.org/ 10.1038/nature10965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid DW, Nicchitta CV. Diversity and selectivity in mRNA translation on the endoplasmic reticulum. Nat Rev Mol Cell Biol 2015; 16:221-31; PMID:25735911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duval M, Simonetti A, Caldelari I, Marzi S. Multiple ways to regulate translation initiation in bacteria: Mechanisms, regulatory circuits, dynamics. Biochimie 2015; 114:18-29; PMID:25792421 [DOI] [PubMed] [Google Scholar]
- 29.Zsogon A, Szakonyi D, Shi X, Byrne ME. Ribosomal Protein RPL27a Promotes Female Gametophyte Development in a Dose-Dependent Manner. Plant Physiol 2014; 165:1133-43; PMID:24872379; http://dx.doi.org/ 10.1104/pp.114.241778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Licona-Cassani C, Lim S, Marcellin E, Nielsen LK. Temporal dynamics of the Saccharopolyspora erythraea phosphoproteome. Molecular & cellular proteomics : MCP 2014; 13:1219-30; http://dx.doi.org/ 10.1074/mcp.M113.033951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue S, Tian S, Fujii K, Kladwang W, Das R, Barna M. RNA regulons in Hox 5' UTRs confer ribosome specificity to gene regulation. Nature 2015; 517:33-8; PMID:25409156; http://dx.doi.org/ 10.1038/nature14010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee AS, Burdeinick-Kerr R, Whelan SP. A ribosome-specialized translation initiation pathway is required for cap-dependent translation of vesicular stomatitis virus mRNAs. Proc Natl Acad Sci U S A 2013; 110:324-9; PMID:23169626; http://dx.doi.org/ 10.1073/pnas.1216454109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuchs G, Diges C, Kohlstaedt LA, Wehner KA, Sarnow P. Proteomic analysis of ribosomes: translational control of mRNA populations by glycogen synthase GYS1. J Mol Biol 2011; 410:118-30; PMID:21570405; http://dx.doi.org/ 10.1016/j.jmb.2011.04.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pestova TV, Shatsky IN, Fletcher SP, Jackson RJ, Hellen CUT. A prokaryotic-like mode of cytoplasmic eukaryotic ribosome binding to the initiation codon during internal translation initiation of hepatitis C and classical swine fever virus RNAs. Genes Dev 1998; 12:67-83; PMID:9420332; http://dx.doi.org/ 10.1101/gad.12.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolupaeva VG, Pestova TV, Hellen CU. An enzymatic footprinting analysis of the interaction of 40S ribosomal subunits with the internal ribosomal entry site of hepatitis C virus. J Virol 2000; 74:6242-50; PMID:10864633; http://dx.doi.org/ 10.1128/JVI.74.14.6242-6250.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kieft JS, Zhou K, Jubin R, Doudna JA. Mechanism of ribosome recruitment by hepatitis C IRES RNA. Rna 2001; 7:194-206; PMID:11233977; http://dx.doi.org/ 10.1017/S1355838201001790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spahn CM, Kieft JS, Grassucci RA, Penczek PA, Zhou K, Doudna JA, Frank J. Hepatitis C virus IRES RNA-induced changes in the conformation of the 40s ribosomal subunit. Science 2001; 291:1959-62; PMID:11239155; http://dx.doi.org/ 10.1126/science.1058409 [DOI] [PubMed] [Google Scholar]
- 38.Lytle JR, Wu L, Robertson HD. Domains on the hepatitis C virus internal ribosome entry site for 40s subunit binding. Rna 2002; 8:1045-55; PMID:12212848; http://dx.doi.org/ 10.1017/S1355838202029965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joseph AP, Bhat P, Das S, Srinivasan N. Re-analysis of cryoEM data on HCV IRES bound to 40S subunit of human ribosome integrated with recent structural information suggests new contact regions between ribosomal proteins and HCV RNA. RNA Biol 2014; 11:891-905; PMID:25268799; http://dx.doi.org/ 10.4161/rna.29545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashem Y, des Georges A, Dhote V, Langlois R, Liao HY, Grassucci RA, Pestova TV, Hellen CU, Frank J. Hepatitis-C-virus-like internal ribosome entry sites displace eIF3 to gain access to the 40S subunit. Nature 2013; 503:539-43; PMID:24185006; http://dx.doi.org/ 10.1038/nature12658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boehringer D, Thermann R, Ostareck-Lederer A, Lewis JD, Stark H. Structure of the hepatitis C virus IRES bound to the human 80S ribosome: remodeling of the HCV IRES. Structure 2005; 13:1695-706; PMID:16271893; http://dx.doi.org/ 10.1016/j.str.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto H, Unbehaun A, Loerke J, Behrmann E, Collier M, Burger J, Mielke T, Spahn CM. Structure of the mammalian 80S initiation complex with initiation factor 5B on HCV-IRES RNA. Nat Struct Mol Biol 2014; 21:721-7; PMID:25064512; http://dx.doi.org/ 10.1038/nsmb.2859 [DOI] [PubMed] [Google Scholar]
- 43.Otto GA, Lukavsky PJ, Lancaster AM, Sarnow P, Puglisi JD. Ribosomal proteins mediate the hepatitis C virus IRES-HeLa 40S interaction. Rna 2002; 8:913-23; PMID:12166646; http://dx.doi.org/ 10.1017/S1355838202022057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laletina E, Graifer D, Malygin A, Ivanov A, Shatsky I, Karpova G. Proteins surrounding hairpin IIIe of the hepatitis C virus internal ribosome entry site on the human 40S ribosomal subunit. Nucleic Acids Res 2006; 34:2027-36; PMID:16614452; http://dx.doi.org/ 10.1093/nar/gkl155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukushi S, Okada M, Stahl J, Kageyama T, Hoshino FB, Katayama K. Ribosomal protein S5 interacts with the internal ribosomal entry site of hepatitis C virus. J Biol Chem 2001; 276:20824-6; PMID:11331271; http://dx.doi.org/ 10.1074/jbc.C100206200 [DOI] [PubMed] [Google Scholar]
- 46.Malygin AA, Kossinova OA, Shatsky IN, Karpova GG. HCV IRES interacts with the 18S rRNA to activate the 40S ribosome for subsequent steps of translation initiation. Nucleic Acids Res 2013; 41:8706-14; PMID:23873958; http://dx.doi.org/ 10.1093/nar/gkt632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babaylova E, Graifer D, Malygin A, Stahl J, Shatsky I, Karpova G. Positioning of subdomain IIId and apical loop of domain II of the hepatitis C IRES on the human 40S ribosome. Nucleic Acids Res 2009; 37:1141-51; PMID:19129232; http://dx.doi.org/ 10.1093/nar/gkn1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lukavsky PJ, Otto GA, Lancaster AM, Sarnow P, Puglisi JD. Structures of two RNA domains essential for hepatitis C virus internal ribosome entry site function. Nat Struct Biol 2000; 7:1105-10; PMID:11101890; http://dx.doi.org/ 10.1038/81951 [DOI] [PubMed] [Google Scholar]
- 49.Otto GA, Puglisi JD. The pathway of HCV IRES-mediated translation initiation. Cell 2004; 119:369-80; PMID:15507208; http://dx.doi.org/ 10.1016/j.cell.2004.09.038 [DOI] [PubMed] [Google Scholar]
- 50.Kieft JS, Zhou K, Jubin R, Murray MG, Lau JY, Doudna JA. The hepatitis C virus internal ribosome entry site adopts an ion-dependent tertiary fold. J Mol Biol 1999; 292:513-29; PMID:10497018; http://dx.doi.org/ 10.1006/jmbi.1999.3095 [DOI] [PubMed] [Google Scholar]
- 51.Jubin R, Vantuno NE, Kieft JS, Murray MG, Doudna JA, Lau JY, Baroudy BM. Hepatitis C virus internal ribosome entry site (IRES) stem loop IIId contains a phylogenetically conserved GGG triplet essential for translation and IRES folding. J Virol 2000; 74:10430-7; PMID:11044087; http://dx.doi.org/ 10.1128/JVI.74.22.10430-10437.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barria MI, Gonzalez A, Vera-Otarola J, Leon U, Vollrath V, Marsac D, Monasterio O, Perez-Acle T, Soza A, Lopez-Lastra M. Analysis of natural variants of the hepatitis C virus internal ribosome entry site reveals that primary sequence plays a key role in cap-independent translation. Nucleic Acids Res 2009; 37:957-71; PMID:19106142; http://dx.doi.org/ 10.1093/nar/gkn1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asnani M, Kumar P, Hellen CU. Widespread distribution and structural diversity of Type IV IRESs in members of Picornaviridae. Virology 2015; 478:61-74; PMID:25726971; http://dx.doi.org/ 10.1016/j.virol.2015.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quade N, Boehringer D, Leibundgut M, van den Heuvel J, Ban N. Cryo-EM structure of Hepatitis C virus IRES bound to the human ribosome at 3.9-A resolution. Nat Commun 2015; 6:7646; PMID:26155016; http://dx.doi.org/ 10.1038/ncomms8646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malygin AA, Shatsky IN, Karpova GG. Proteins of the human 40S ribosomal subunit involved in hepatitis C IRES binding as revealed from fluorescent labeling. Biochemistry Biokhimiia 2013; 78:53-9; PMID:23379559; http://dx.doi.org/ 10.1134/S0006297913010069 [DOI] [PubMed] [Google Scholar]
- 56.Odreman-Macchioli F, Baralle FE, Buratti E. Mutational analysis of the different bulge regions of hepatitis C virus domain II and their influence on internal ribosome entry site translational ability. J Biol Chem 2001; 276:41648-55; PMID:11498532; http://dx.doi.org/ 10.1074/jbc.M104128200 [DOI] [PubMed] [Google Scholar]
- 57.Psaridi L, Georgopoulou U, Varaklioti A, Mavromara P. Mutational analysis of a conserved tetraloop in the 5' untranslated region of hepatitis C virus identifies a novel RNA element essential for the internal ribosome entry site function. FEBS letters 1999; 453:49-53; PMID:10403373; http://dx.doi.org/ 10.1016/S0014-5793(99)00662-6 [DOI] [PubMed] [Google Scholar]
- 58.Kolupaeva VG, Pestova TV, Hellen CU. Ribosomal binding to the internal ribosomal entry site of classical swine fever virus. Rna 2000; 6:1791-807; PMID:11142379; http://dx.doi.org/ 10.1017/S1355838200000662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang R, Yoshida K, Toki T, Sawada T, Uechi T, Okuno Y, Sato-Otsubo A, Kudo K, Kamimaki I, Kanezaki R, et al. Loss of function mutations in RPL27 and RPS27 identified by whole-exome sequencing in Diamond-Blackfan anaemia. Br J Haematol 2015; 168:854-64; PMID:25424902; http://dx.doi.org/ 10.1111/bjh.13229 [DOI] [PubMed] [Google Scholar]
- 60.Ji H, Fraser CS, Yu Y, Leary J, Doudna JA. Coordinated assembly of human translation initiation complexes by the hepatitis C virus internal ribosome entry site RNA. Proc Natl Acad Sci U S A 2004; 101:16990-5; PMID:15563596; http://dx.doi.org/ 10.1073/pnas.0407402101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Filbin ME, Kieft JS. HCV IRES domain IIb affects the configuration of coding RNA in the 40S subunit's decoding groove. Rna 2011; 17:1258-73; PMID:21606179; http://dx.doi.org/ 10.1261/rna.2594011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhat P, Shwetha S, Sharma DK, Joseph AP, Srinivasan N, Das S. The beta hairpin structure within ribosomal protein S5 mediates interplay between domains II and IV and regulates HCV IRES function. Nucleic Acids Res 2015; 43:2888-901; PMID:25712089; http://dx.doi.org/ 10.1093/nar/gkv110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hertz MI, Landry DM, Willis AE, Luo G, Thompson SR. Ribosomal protein S25 dependency reveals a common mechanism for diverse internal ribosome entry sites and ribosome shunting. Mol Cell Biol 2013; 33:1016-26; PMID:23275440; http://dx.doi.org/ 10.1128/MCB.00879-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuchs G, Petrov AN, Marceau CD, Popov LM, Chen J, O'Leary SE, Wang R, Carette JE, Sarnow P, Puglisi JD. Kinetic pathway of 40S ribosomal subunit recruitment to hepatitis C virus internal ribosome entry site. Proc Natl Acad Sci U S A 2015; 112:319-25; PMID:25516984; http://dx.doi.org/ 10.1073/pnas.1421328111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Landry DM, Hertz MI, Thompson SR. RPS25 is essential for translation initiation by the Dicistroviridae and hepatitis C viral IRESs. Genes and Development 2009; 23:2753-64; PMID:19952110; http://dx.doi.org/ 10.1101/gad.1832209 [DOI] [PMC free article] [PubMed] [Google Scholar]


