ABSTRACT
Transfer RNAs (tRNAs) have been important in shaping biomolecular evolution. Initiator tRNAs (tRNAi), a special class of tRNAs, carry methionine (or its derivative, formyl-methionine) to ribosomes to start an enormously energy consuming but a highly regulated process of protein synthesis. The processes of tRNAi evolution, and selection of methionine as the universal initiating amino acid remain an enigmatic problem. We constructed phylogenetic trees using the whole sequence, the acceptor-TψC arm ('minihelix'), and the anticodon-dihydrouridine arm regions of tRNAi from 158 species belonging to all 3 domains of life. All the trees distinctly assembled into 3 domains of life. Large trees, generated using data for all the tRNAs of a vast number of species, fail to reveal the major evolutionary events and identity of the probable elongator tRNA sequences that could be ancestor of tRNAi. Therefore, we constructed trees using the minihelix or the whole sequence of species specific tRNAs, and iterated our analysis on 50 eubacterial species. We identified tRNAPro, tRNAGlu, or tRNAThr (but surprisingly not elongator tRNAMet) as probable ancestors of tRNAi. We then determined the factors imposing selection of methionine as the initiating amino acid. Overall frequency of occurrence of methionine, whose metabolic cost of synthesis is the highest among all amino acids, remains almost unchanged across the 3 domains of life. Our correlation analysis shows that its high metabolic cost is independent of many physicochemical properties of the side chain. Our results indicate that selection of methionine, as the initiating amino acid was possibly a consequence of the evolution of one-carbon metabolism, which plays an important role in regulating translation initiation.
KEYWORDS: Evolution, initiator tRNA, methionine, minihelix, protein synthesis, one-carbon metabolism, tRNA
Introduction
The present day tRNAs act as adaptor molecules to decipher the genetic code present in the mRNA to bring the corresponding amino acids to the ribosome.1 In the ‘RNA world’, the ancestral tRNA molecules, thought to have emerged as ribozymes, might have also catalyzed their own aminoacylation.2–5 Though the ‘RNA-world’ hypothesis is well accepted, the successive events in tRNA evolution are highly controversial but a coveted field of research.6-12 Of the 2 (monophyletic and polyphyletic) theories of tRNA evolution, the monophyletic theory proposes that the tRNA (and therefore the different segments in it) originated as a single unit, whereas the polyphyletic theory argues that 2 segments of the modern tRNA, the anticodon-dihydrouridine (DHU) arm (A-D), and the acceptor-TψC arm (minihelix) regions (Fig. S1), originated separately, and then over the course of evolution converged into a single entity.13-16 The monophyletic theory of tRNA origin has been considered to derive tRNA phylogenies,17,18 and while this method discerns 2 distinct classes (class I and class II) of tRNAs, it neither allows to clearly trace the ancestors of the different tRNAs nor their segregation into the 3 domains of life.19 However, the polyphyletic theory of tRNA evolution has been preferred by a large number of studies.13,20,21 In fact, it has been shown that merely the minihelix regions can also be aminoacylated.22 The polyphyletic theory also explains the co-evolution of the tRNA and aminoacyl tRNA synthetases.4 Of course, the availability of amino acids would have also played a role during tRNA diversification.23
However, none of these studies shed light on the phylogenetic lineage of tRNAi. The tRNAi possesses the characteristic 3 consecutive G:C base pairs (G29-C41, G30-C40, G31-C39) in the anticodon arm. These sequences are absent from the elongator tRNAs.24,25 Presumably, the tRNAi arose much later in the evolution of translational apparatus.26 A tempting hypothesis has been that the elongator tRNAMet might have been the precursor for the tRNAi as the 2 tRNAs are aminoacylated by a common enzyme, methionine tRNA synthetase (MetRS).27 Finding out a possible lineage of tRNAi evolution is of interest to understand regulation of translation initiation and to also understand selection of methionine as the universal initiating amino acid.
Following from the polyphyletic theory of tRNA evolution, we constructed phylogenetic trees of tRNAi taking the secondary structure elements into account. These results provide compelling evidence about the ancient nature of tRNAi arising prior to the branching of last universal common ancestor (LUCA). Interestingly, tRNAi shared closest common ancestry most frequently with tRNAPro, tRNAGlu, and tRNAThr and not with elongator tRNAMet. In addition, our analysis highlights interplay between evolution of metabolism where the high cost of methionine synthesis, would serve as an indicator of the energy status of the cell and regulate initiation of protein synthesis.
Results
Phylogenetic tree construction using tRNAi sequences
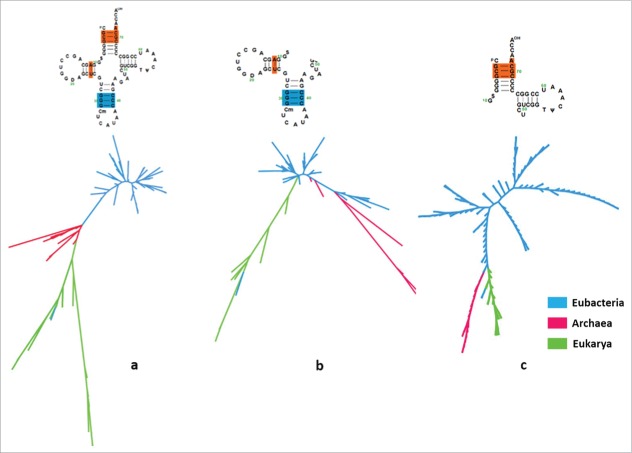
Construction of phylogenies of tRNAs poses challenges due to their short sizes, which are often susceptible to the clade-specific mutations and horizontal transfers.28 However, recent methodologies allow use of even the short sequences of tRNAs to construct phylogenies which efficiently reproduce the 3 domains of life.28 There are a number of studies where sequence alignments have been made considering the secondary structures of the tRNAs.19 Thus, we used R-COFFEE secondary structure alignment tool to construct phylogenies from, (a) the whole tRNA sequences, (b) the A-D, and (c) the minihelix regions. The trees generated from the 3 alignments assemble into the 3 distinct domains of life (Fig. 1). However, when the whole sequences were considered, there were a few outliers in the 3 clusters. Interestingly, few species of bacteria which cause infectious diseases, e. g. virulent E. coli, clustered within the eukaryotic group suggesting a possible case of horizontal gene transfer or selection pressure within eukaryotic physiological conditions. Even the tree constructed from the minihelices of the tRNAi, clustered organisms into the 3 different domains of life (Figs. 1c and 2) suggesting that it evolved before the formation of 3 branches, most possibly around the emergence of the LUCA but after the convergence of the 2 segments (the A-D, and the minihelix domains) of the tRNA. The alignment of the A-D regions also produced clusters of species of the 3 domains of life. However, the number of outliers was higher in this tree. A higher bootstrapping was observed when the whole tRNA was used (average ∼50–70) compared to the other trees (38–94) indicating probabilistic variations due to the short stretch of sequences. Nonetheless, these results validate suitability of using tRNAi, especially the minihelices as phylogenetic markers with a good degree of accuracy. In fact, similar analysis with minihelix regions of tRNAGly, tRNAPro, tRNAThr, tRNAGlu, and tRNAAla but not the elongator tRNAMet also yielded phylogenies with the 3 distinct domains of life (data not shown).
Figure 1.
Phylogenetic tree using alignments of different segments of tRNAi sequence: The phylogenetic tree obtained using tRNAi sequences from different organisms from, (a) the whole tRNAi sequences, (b) the region having anticodon and DHU arm, and (c) the region having the acceptor and TψC arm. The regions of tRNAi from E. coli is shown on the top.
Figure 2.

Small excerpt from the tree shown in Fig. 1c: The red and green branches indicate the ‘archaea’ and ‘eukarya’ domains, respectively.
Ancestor of tRNAi
The identity of the probable ancestor is lost in the common method of using all the tRNAs from a large number of organisms to construct phylogenies because many predominant clade-specific events in the elongator tRNA evolution influence the nature and branching of the tree.18 Not surprisingly, when we constructed a tree with the minihelix regions of 2758 sequences of all tRNAs from a large variety of species, the results were too complicated for a clear understanding (data not shown). Thus, to trace the evolutionary lineage of tRNAs, we generated phylogenetic trees from the species specific minihelices of tRNAs from 50 independent species of eubacteria (Table S1). In E. coli, tRNAi clustered with tRNAPro, and not with the elongator tRNAMet (Fig. 3). Similarly, in ancient Aquifex aeolicus, tRNAi clustered with tRNAPro (Fig. S2). The results were the same for other eubacterial species such as M. tuberculosis (Fig. S3), Ralstonia solanacearum, Bacillus cereus, and Lactobacillus johnsonii etc. In the eukaryote, Saccharomyces cerevisiae (Fig. S5) and the archaebacterium, Methanothermobacter thermautotrophicus (Fig. S4), tRNAi clustered with tRNALeu and tRNAAla, respectively. Overall, among the 50 eubacterial species studied (Table S1), the most commonly occurring relative of tRNAi was tRNAPro (21 species), followed by tRNAGlu (13 species) and tRNAThr (7 species) (Table 1). Surprisingly, elongator tRNAMet did not cluster with tRNAi even once. The other tRNAs also showed specific patterns in clustering. For example, elongator tRNAMet mostly clustered with tRNAIle and tRNACys (Figs. 3, S2–S5). In many cases, tRNAPhe and tRNAHis showed common ancestry (Figs. 3, S2-S5). These results further validate reproducibility of the results and strengthen the case for a confident conclusion about the origin of tRNAi.
Figure 3.

Phylogenetic tree of tRNAs from E. coli: obtained from alignment of the minihelix region of the tRNA sequences; green ellipse and red box indicate tRNAi and elongator tRNAMet, respectively. The bootstrap values are indicated in color.
Table 1.
Frequency of different elongator tRNAs appearing as closest relative of tRNAi as obtained from phylogenetic tree analysis of the 50 organisms listed in Table S1. The values indicate the number of trees in which a particular type of elongator tRNA had closest common ancestor with tRNAi.
| Elongator Relative | Recurrence |
|---|---|
| Pro | 21 |
| Glu | 13 |
| Thr | 7 |
| Met | 0 |
| Others | 9 |
Conserved regions of tRNAi
To elaborate on the conserved regions of tRNAi, a logo plot was generated from the alignment of the whole tRNA sequence (Fig. 4a). As expected, the minihelix and the anticodon stem showed a higher conservation compared to the D arm. The C1xA72 mismatch, showing an A1-U72 variation, was less conserved compared with the 3GC base pairs in the anticodon arm. The conserved regions are shown in Fig. 4b using a co-crystal structure of the initiator tRNA with MetRS from Aquifex aeolicus.29 The regions of tRNAi which interact with proteins are highly conserved (Fig. 4a and 4b), e.g., G2-C71 and C3-G70 (MetRS), G4-C69 (methionyl tRNA transformylase), G12 and C13 (IF2), etc. The other conserved regions especially the anticodon arm is implicated in the tRNA functions in the context of ribosome.
Figure 4.
Evolution of initiator tRNA: (a) Logo plot of tRNAi obtained from alignment of the whole of tRNAi sequences; (b) Co-crystal structure of the MetRS- tRNAi complex of Aquifex aeolicus, the tRNA is represented in blue (highly conserved in our analysis) and yellow, MetRS is represented in red (PDB ID:2CSX); (c) Distribution of tRNAi gene copies across 3 domains. The tree is a schematic of the 3 domains without reflecting precise lineages, the numbers indicate the predominantly found copy number of tRNAi gene in the adjacent taxa. The arrows indicate direction of the increase.
Why methionine as the initiating amino acid
The chemistry of the amino acids in the primordial soup was stochastically the primary driver of the evolution of central dogma and metabolic pathways. To ascertain factors that aided the selection of the initiating amino acid, a correlation analysis among some key physico-chemical features of amino acids was done. The data for the various attributes were taken from the literature.30-36 As shown in Fig. S7, the metabolic cost of Met synthesis is highest among the amino acids.30 An increase in the metabolic cost of amino acid synthesis shows a tendency of positive correlation with its decreased abundance (Fig. 5a) in the cell.32 The difference in metabolic cost of biosynthesis of amino acids shows no apparent correlation with their various intrinsic physico-chemical properties such as hydrophobicity (Fig. 5b),33 volume of the side chains (Fig. 5c),34 and side chain bond energy (Fig. 5d).35 However, there is a positive correlation between the increase in the electrostatic potential energy36 and increase in metabolic cost (Fig. 5a, a negative value indicates direction of the force field). If the abundance of methionine acts as a signal to initiate translation, it should be scarcely present in the ORF and the fraction of Met codon should be unchanged across domains and independent of GC content of the organism. Interestingly, the frequencies of Met occurrence do not change with either the GC contents of the genome or the complexity of the organisms (Fig. 6). Except Cys, Trp, and His codons, which show no change in frequency across domains, codons for all the other amino acids (Fig. 6 and S6) show considerable variations across species. Frequency of Gly, Val, Ala, Arg, Thr, and Pro codons show a clear positive correlation (Figs. S6b) with the increase in the GC content of the coding region, whereas Lys, Glu, Ser, Lys, Asn, Ile, Phe, and Tyr show a clear negative correlation (Fig. 6 and S6b).
Figure 5.
Metabolic cost of biosynthesis of amino acids and its relationship with physico-chemical properties of amino acids: correlations of metabolic cost (x axis) with (a) electrostatic potential energy (y axis on right) and experimentally measured cellular concentrations of amino acids (y axis on left), (b) with hydropathy index, (c) with volume in 3D space of the side chains, and (d) with side chain bond energy; the metabolic cost is represented in terms of number of ATP molecules required to synthesize one molecule of an amino acid.
Figure 6.

Frequency of methionine in ORF: The data for both frequency of occurrence of Met (codon usage per 1000 codons left Y axis) and GC percentage (right Y axis) in the coding region; (a) variation of abundance of Met in different organisms; (b) the correlation of GC percentage in the coding region of organisms and the occurrence of Met; the organisms are Aqui, Aquifex aeolicus; Ther, Thermotoga maritima; Bifi, Bifidobacterium longum; Borr, Borrelia burgdorferi; Agro, Agrobacterium tumefaciens; Esch, Escherichia coli; Desu, Desulfovibrio vulgaris; Camp, Campylobacter jejuni; Pyro, Pyrobaculum aerophilum; Meth, Methanothermobacter thermautotrophicus; Nano, Nanoarchaeum equitans; Sacc, Saccharomyces cerevisiae; Dros, Drosophila melanogaster; Homo, Homo sapiens.
Discussion
Construction of the phylogenetic trees from the whole, or the minihelix, or the A-D regions of tRNAi segregated organisms into the 3 different domains of life indicating that tRNAi evolved prior to the separation of the 3 domains of life (Fig. 1). Among the 50 bacterial species studied, 21 showed tRNAPro having closest common ancestry with tRNAi. This hypothesis is supported by the fact that ProRS and MetRS can dock to the either side of the acceptor stem of the tRNA.22 So, during the transition, a common minihelix could have served as ancestor to tRNAPro and tRNAi. Class Ia and IIa of aminoacyl tRNA synthetases dock on to the either side of a single minihelix.4,22 The tRNAThr, which clustered with tRNAi in 13 species, is also a good candidate as ThrRS falls into the class IIa category of synthetases whereas the MetRS belongs to class Ia.22 If the co-evolution of the aminoacyl tRNA synthetases and tRNAs is taken into consideration, both the tRNAPro and tRNAThr make excellent candidates for having closest common ancestry with tRNAi. On the other hand, tRNAGlu clustered with tRNAi in 7 species. However, tRNAGlu (discussed later) falls in the class Ib.22 Interestingly, none of the trees showed clustering of tRNAi with elongator tRNAMet strongly suggesting against the possibility of them having a close common ancestry. Further, we have previously shown experimentally that the elongator tRNAs can also initiate37,38 and among them, tRNAThr, tRNAPro and tRNAGlu were the most efficient in initiation activity.38 The logo plot (Fig. 4a) shows that the minihelix and the anticodon regions of tRNAi are highly conserved. Notably, the highly conserved residues of G2-C71, C3-G70, G4-C69, G12 and C13 are involved in interactions with different proteins like MetRS, methionyl tRNA transformylase, and IF2. Specific residues in the minihelix interact with 70S during initiation. These residues may have evolved in a species-independent manner, as the functions are too intricate to have inter-species variation.
All the different types of the amino acids were not available or were less abundant in the primordial soup.39 The co-evolution of the metabolism with ‘RNA world’40 must have played an important role in fixing the initiating amino acid.41 It should be noted that ribosome methylations play a vital role in protein synthesis.42 In fact, methylations are important in many aspects of the cellular processes.43,44 It has been suggested that the one-carbon metabolism is a primitive metabolic process.45-47 One-carbon metabolism is essential for the synthesis of methionine.48 During the one-carbon metabolism driven geoenergetics to bioenergetics transition in prokaryotic life,45 the abundance of methionine in hydrothermal vents played a crucial role in shaping the cellular metabolic and other processes involving methylation such as rRNA-tRNA modifications and DNA methylations.49 Methionine is important for quorum-sensing.48,51–52 The intracellular concentrations of methionine and N10-formyl THF (products of one-carbon metabolism), shown to be important in protein synthesis,53 might have acted as the indicators for energy status of the cell or the richness of the surrounding environment in the prebiotic soup. Evolutionary importance of changes in the tRNA gene and energy efficiency with bacterial growth has already been well characterized.20,54,55 The occurrence of Met in proteins is very infrequent (Fig. 6a), its internal occurrence would mean a higher metabolic cost. A regulation or checkpoint based on metabolic cost is best made at the point of starting a process as supported by the ubiquitous occurrence of Met as the initiating amino acid but very infrequently at the subsequent positions in the polypeptide chain. In contrast to the other amino acids, the abundance of Met has not changed from LUCA to modern genomes.56 For evolution of a specialized elongator tRNAMet from tRNAi, it would require several drastic changes in the specific features of tRNAi making the whole phenomenon an unlikely event. Therefore, the specialized elongator tRNAMet might have evolved from other tRNAs. Although, it may be mentioned that tRNAi can act as an elongator tRNA in the modern day systems.24,57,58 In fact, some organelles possess only a single tRNAMet with the characteristics of tRNAi, which act as both the initiators and elongators.59 The tRNAGlu could have been a strong candidate as the ancestral tRNAi. From the metabolic perspective, Glu is known to play a central role in amino acid metabolism. There are multiple pathways in the cell that allow its interconversion with other amino acids.60 Thus, a high steady state accumulation of Glu could have served as an indicator of the energy sufficiency of the cell, linking initiation of protein synthesis with tRNAGlu. Later, during evolution of one-carbon metabolism, Glu changed to Met meeting the demand of the metabolic changes, and the energetic state of the cell. As mentioned, N10-formyltetrahydrofolate (required for formylation of tRNAi in eubacteria and eukaryotic organelles) is also generated by one-carbon metabolism.
The correlation analysis indicates that field effect (electrostatic potential) rather than steric effect (volume) might have played a crucial role in determining abundance of amino acids. The hydrophobicity does not correlate with metabolic cost of the amino acid synthesis. Incidentally, the metabolic cost also correlates with the electrostatic potential energy. These provide further clues about the determining factors of protein composition and its interaction with solvent and other peptides. It is evident from our data, that metabolism played more important role than any other physico-chemical attributes of the amino acids in the selection of the first amino acid in the ORF. Overexpression of tRNAi61 can change the growth pattern of the cells. As the complexities increased, the control of the initiation process became critically important. This resulted in a change in the copy number (rather than sequence) of tRNAi genes across the 3 domains of life (Fig. 4c). It has been reported that tRNAi competes with other tRNAs for binding to the P-site.37 The copy number of the initiator tRNA genes increased from 1 to multiple when more complex organisms evolved.
In conclusion, the current study suggests that tRNAi evolved prior to the separation of 3 domains of life. Most probably, it has not evolved from elongator tRNAMet. The tRNAPro, tRNAThr, and tRNAGlu are more likely candidates for sharing closest ancestry with tRNAi. From the perspective of evolution of aminoacyl tRNA synthetases, tRNAPro or tRNAThr are more likely candidates. But, if evolution of metabolic pathways is taken into consideration, then tRNAGlu seems like a more probable candidate. Finally, co-evolution of metabolic pathways might have played a crucial role in determining the first amino acid in the translation of an ORF. Co-evolution of one-carbon metabolism and the process of translation make a strong case for methionine to be a cellular signal for initiation of the protein synthesis.
Materials and methods
tRNA sequence retrieval and alignment
The tRNA sequences were downloaded from tRNAdb database (http://trna.bioinf.uni-leipzig.de/DataOutput/Search).62 For many organisms, the tRNAi was already identified and annotated. For other organisms, the identification was primarily based on 3 consecutive G-C base pairs of the anticodon stem and the 1–72 mismatch in the acceptor arm. The sequences were aligned using R-COFFEE from T-COFFEE website (http://tcoffee.crg.cat/apps/tcoffee/do:rcoffee).63 When required, the alignments were modified using aliview offline (http://www.ormbunkar.se/aliview/). This was particularly useful when a stretch of sequences was to be removed from the alignment. The nucleotides 1–7 and 49–76 of E. coli metY gene were considered as minihelix. The rest of the nucleotides (9–48) were considered as A-D region.
Phylogenetic trees and logo plots
Using the alignments, phylogenetic trees were constructed using Seaview program,64 where a PHYML based phylogenetic tree construction was done using maximum likelihood method. The evolutionary model used was generalized-time reversal model (GTR). For statistical analysis, non-parametric bootstrapping was done with 100 replicates. Initiator tRNA sequence from a total of 158 species were used. The alignments were used to generate a logo plot using web-logo program (http://weblogo.berkeley.edu/logo.cgi).65
Structural model
The crystal structures were downloaded from PDB database. The structures were visualized using Chimera software, which was also used for generation of the figures showing different segments of crystal structure.66
Correlation analysis
The data for the metabolic cost30 of amino acids (in terms of number of ATP molecules required to synthesize one molecule of an amino acid) was correlated with physico-chemical parameters like electrostatic potential energy [‘www.ch.ic.ac.uk/rzepa/watoc96/abstracts/24/’ and36], experimentally measured cellular concentrations of amino acids,32 hydropathy index,33 volume in 3D space of the side chains,34 and with side chain bond energy [calculated using Table 4.1 from67]. The data for both frequency of occurrence of Met and GC percentage in the coding region were collected from ‘http://www.kazusa.or.jp/codon/’.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Madhumita Bhattacharyya (CSIR-Indian Institute of Chemical Biology, Kolkata) is acknowledged for her inputs in the phylogeny construction.
Funding
This work was supported by the Department of Science and Technology (DST) and the Department of Biotechnology (DBT), New Delhi. UV is a J. C. Bose fellow of DST. SB was supported by a senior research fellowship of the Council of Scientific and Industrial Research, New Delhi.
References
- 1.Ibba M, Becker HD, Stathopoulos C, Tumbula DL, Soll D. The adaptor hypothesis revisited. Trends Biochem Sci 2000; 25:311-6; PMID:10871880; http://dx.doi.org/ 10.1016/S0968-0004(00)01600-5 [DOI] [PubMed] [Google Scholar]
- 2.Crick FH. The origin of the genetic code. J Mol Biol 1968; 38:367-79; PMID:4887876; http://dx.doi.org/ 10.1016/0022-2836(68)90392-6 [DOI] [PubMed] [Google Scholar]
- 3.Woese CR. The genetic code: The molecular basis for genetic expression. Harper & Row 1967, New York, QH431W58, 179-95. [Google Scholar]
- 4.Ribas de Pouplana L, Schimmel P. Aminoacyl-tRNA synthetases: potential markers of genetic code development. Trends Biochem Sci 2001; 26:591-6; PMID:11590011; http://dx.doi.org/ 10.1016/S0968-0004(01)01932-6. [DOI] [PubMed] [Google Scholar]
- 5.de Farias ST, do Rego TG, Jose MV. Evolution of transfer RNA and the origin of the translation system. Front Genet 2014; 5:303; PMID:25221573; http://dx.doi.org/ 10.3389/fgene.2014.00303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marck C, Grosjean H. tRNomics: analysis of tRNA genes from 50 genomes of Eukarya, Archaea, and Bacteria reveals anticodon-sparing strategies and domain-specific features. Rna 2002; 8:1189-232; PMID:12403461; http://dx.doi.org/ 10.1017/S1355838202022021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eigen M, Winkler-Oswatitsch R. Transfer-RNA, an early gene? Die Naturwissenschaften 1981; 68:282-92; PMID:7266675; http://dx.doi.org/ 10.1007/BF01047470 [DOI] [PubMed] [Google Scholar]
- 8.Eigen M, Winkler-Oswatitsch R. Transfer-RNA: the early adaptor. Die Naturwissenschaften 1981; 68:217-28; PMID:6909552; http://dx.doi.org/ 10.1007/BF01047323 [DOI] [PubMed] [Google Scholar]
- 9.Di Giulio M. The evolution of aminoacyl-tRNA synthetases, the biosynthetic pathways of amino acids and the genetic code. Orig Life Evol Biosph 1992; 22:309-19; PMID:1454354; http://dx.doi.org/ 10.1007/BF01810859 [DOI] [PubMed] [Google Scholar]
- 10.Di Giulio M. The phylogeny of tRNA molecules and the origin of the genetic code. Orig Life Evol Biosph 1994; 24:425-34; PMID:7970630; http://dx.doi.org/ 10.1007/BF01582018 [DOI] [PubMed] [Google Scholar]
- 11.Di Giulio M. The non-monophyletic origin of the tRNA molecule. J Theor Biol 1999; 197:403-14; PMID:10089150; http://dx.doi.org/ 10.1006/jtbi.1998.0882 [DOI] [PubMed] [Google Scholar]
- 12.Di Giulio M. The RNA world, the genetic code and the tRNA molecule. Trends Genet 2000; 16:17-9; PMID:10637625; http://dx.doi.org/ 10.1016/S0168-9525(99)01893-4 [DOI] [PubMed] [Google Scholar]
- 13.Di Giulio M. The ‘recently’ split transfer RNA genes may be close to merging the two halves of the tRNA rather than having just separated them. J Theor Biol 2012; 310:1-2; PMID:22749890; http://dx.doi.org/ 10.1016/j.jtbi.2012.06.022 [DOI] [PubMed] [Google Scholar]
- 14.Di Giulio M. The origin of the tRNA molecule: Independent data favor a specific model of its evolution. Biochimie 2012; 94:1464-6; PMID:22305822; http://dx.doi.org/ 10.1016/j.biochi.2012.01.014 [DOI] [PubMed] [Google Scholar]
- 15.Randau L, Munch R, Hohn MJ, Jahn D, Soll D. Nanoarchaeum equitans creates functional tRNAs from separate genes for their 5′- and 3′-halves. Nature 2005; 433:537-41; PMID:15690044; http://dx.doi.org/ 10.1038/nature03233 [DOI] [PubMed] [Google Scholar]
- 16.Lacey JC Jr., Staves MP. Was there a universal tRNA before specialized tRNAs came into existence? Orig Life Evol Biosph 1990; 20:303-8; PMID:2290687; http://dx.doi.org/ 10.1007/BF01808112 [DOI] [PubMed] [Google Scholar]
- 17.de Farias ST. Suggested phylogeny of tRNAs based on the construction of ancestral sequences. J Theor Biol 2013; 335:245-8; PMID:23871958; http://dx.doi.org/ 10.1016/j.jtbi.2013.06.033 [DOI] [PubMed] [Google Scholar]
- 18.Sun FJ, Caetano-Anolles G. Evolutionary patterns in the sequence and structure of transfer RNA: early origins of archaea and viruses. PLoS Comput Biol 2008; 4:e1000018; PMID:18369418; http://dx.doi.org/ 10.1371/journal.pcbi.1000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun FJ, Caetano-Anolles G. Evolutionary patterns in the sequence and structure of transfer RNA: a window into early translation and the genetic code. PloS One 2008; 3:e2799; PMID:18665254; http://dx.doi.org/ 10.1371/journal.pone.0002799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujishima K, Kanai A. tRNA gene diversity in the three domains of life. Front Genet 2014; 5:142; PMID:24904642; http://dx.doi.org/ 10.3389/fgene.2014.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Giulio M. The origin of the tRNA molecule: implications for the origin of protein synthesis. J Theor Biol 2004; 226:89-93; PMID:14637058; http://dx.doi.org/ 10.1016/j.jtbi.2003.07.001 [DOI] [PubMed] [Google Scholar]
- 22.Ribas de Pouplana L, Schimmel P. Two classes of tRNA synthetases suggested by sterically compatible dockings on tRNA acceptor stem. Cell 2001; 104:191-3; PMID:11269237; http://dx.doi.org/ 10.1016/S0092-8674(01)00204-5 [DOI] [PubMed] [Google Scholar]
- 23.Ribas de Pouplana L, Turner RJ, Steer BA, Schimmel P. Genetic code origins: tRNAs older than their synthetases? Proc Natl Acad Sci U S A 1998; 95:11295-300; PMID:9736730; http://dx.doi.org/ 10.1073/pnas.95.19.11295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varshney U, Lee CP, RajBhandary UL. From elongator tRNA to initiator tRNA. Proc Natl Acad Sci U S A 1993; 90:2305-9; PMID:8460138; http://dx.doi.org/ 10.1073/pnas.90.6.2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayer C, Stortchevoi A, Kohrer C, Varshney U, RajBhandary UL. Initiator tRNA and its role in initiation of protein synthesis. Cold Spring Harbor Symp Quant Biol 2001; 66:195-206; PMID:12762022; http://dx.doi.org/ 10.1101/sqb.2001.66.195 [DOI] [PubMed] [Google Scholar]
- 26.Larue B, Cedergren RJ, Sankoff D, Grosjean H. Evolution of methionine initiator and phenylalanine transfer RNAs. J Mol Evol 1979; 14:287-300; PMID:537108; http://dx.doi.org/ 10.1007/BF01732496 [DOI] [PubMed] [Google Scholar]
- 27.Deniziak MA, Barciszewski J. Methionyl-tRNA synthetase. Acta Biochim Pol 2001; 48:337-50; PMID:11732605. [PubMed] [Google Scholar]
- 28.Widmann J, Harris JK, Lozupone C, Wolfson A, Knight R. Stable tRNA-based phylogenies using only 76 nucleotides. Rna 2010; 16:1469-77; PMID:20558546; http://dx.doi.org/ 10.1261/rna.726010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakanishi K, Ogiso Y, Nakama T, Fukai S, Nureki O. Structural basis for anticodon recognition by methionyl-tRNA synthetase. Nat Struct Mol Biol 2005; 12:931-2; PMID:16155581; http://dx.doi.org/ 10.1038/nsmb988 [DOI] [PubMed] [Google Scholar]
- 30.Kaleta C, Schauble S, Rinas U, Schuster S. Metabolic costs of amino acid and protein production in Escherichia coli. Biotechnol J 2013; 8:1105-14; PMID:23744758; http://dx.doi.org/ 10.1002/biot.201200267 [DOI] [PubMed] [Google Scholar]
- 31.Chou PY, Fasman GD. Empirical predictions of protein conformation. Annu Rev Biochem 1978; 47:251-76; PMID:354496; http://dx.doi.org/ 10.1146/annurev.bi.47.070178.001343 [DOI] [PubMed] [Google Scholar]
- 32.Bennett BD, Kimball EH, Gao M, Osterhout R, Van Dien SJ, Rabinowitz JD. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat Chem Biol 2009; 5:593-9; PMID:19561621; http://dx.doi.org/ 10.1038/nchembio.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol 1982; 157:105-32; PMID:7108955; http://dx.doi.org/ 10.1016/0022-2836(82)90515-0 [DOI] [PubMed] [Google Scholar]
- 34.Zamyatnin AA. Protein volume in solution. Prog Biophys Mol Biol 1972; 24:107-23; PMID:4566650; http://dx.doi.org/ 10.1016/0079-6107(72)90005-3 [DOI] [PubMed] [Google Scholar]
- 35.House JE. A survey of inorganic structures and bonding Inorganic chemistry: Academic press, Elsevier, 2008:130 [Google Scholar]
- 36.Wu H. Chemical Property Calculation through JavaScript and Applications in QSAR. Molecules 1999; 4:16-27; http://dx.doi.org/ 10.3390/40100016 [DOI] [Google Scholar]
- 37.Kapoor S, Das G, Varshney U. Crucial contribution of the multiple copies of the initiator tRNA genes in the fidelity of tRNA(fMet) selection on the ribosomal P-site in Escherichia coli. Nucleic Acids Res 2011; 39:202-12; PMID:20798174; http://dx.doi.org/ 10.1093/nar/gkq760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samhita L, Virumae K, Remme J, Varshney U. Initiation with elongator tRNAs. J Bacteriol 2013; 195:4202-9; PMID:23852868; http://dx.doi.org/ 10.1128/JB.00637-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higgs PG, Pudritz RE. A thermodynamic basis for prebiotic amino acid synthesis and the nature of the first genetic code. Astrobiology 2009; 9:483-90; PMID:19566427; http://dx.doi.org/ 10.1089/ast.2008.0280 [DOI] [PubMed] [Google Scholar]
- 40.Powner MW, Gerland B, Sutherland JD. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 2009; 459:239-42; PMID:19444213; http://dx.doi.org/ 10.1038/nature08013 [DOI] [PubMed] [Google Scholar]
- 41.de Bivort BL, Perlstein EO, Kunes S, Schreiber SL. Amino acid metabolic origin as an evolutionary influence on protein sequence in yeast. J Mol Evol 2009; 68:490-7; PMID:19357800; http://dx.doi.org/ 10.1007/s00239-009-9218-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baldridge KC, Contreras LM. Functional implications of ribosomal RNA methylation in response to environmental stress. Crit Rev Biochem Mol Biol 2014; 49:69-89; PMID:24261569; http://dx.doi.org/ 10.3109/10409238.2013.859229 [DOI] [PubMed] [Google Scholar]
- 43.Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate's role. Adv Nutr 2012; 3:21-38; PMID:22332098; http://dx.doi.org/ 10.3945/an.111.000992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet 2010; 11:204-20; PMID:20142834; http://dx.doi.org/ 10.1038/nrg2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sousa FL, Martin WF. Biochemical fossils of the ancient transition from geoenergetics to bioenergetics in prokaryotic one carbon compound metabolism. Biochim Biophys Acta 2014; 1837:964-81; PMID:24513196; http://dx.doi.org/ 10.1016/j.bbabio.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 46.Fani R, Fondi M. Origin and evolution of metabolic pathways. Phys Life Rev 2009; 6:23-52; PMID:20416849; http://dx.doi.org/ 10.1016/j.plrev.2008.12.003 [DOI] [PubMed] [Google Scholar]
- 47.Fondi M, Emiliani G, Fani R. Origin and evolution of operons and metabolic pathways. Res Microbiol 2009; 160:502-12; PMID:19465116; http://dx.doi.org/ 10.1016/j.resmic.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 48.Gophna U, Bapteste E, Doolittle WF, Biran D, Ron EZ. Evolutionary plasticity of methionine biosynthesis. Gene 2005; 355:48-57; PMID:16046084; http://dx.doi.org/ 10.1016/j.gene.2005.05.028 [DOI] [PubMed] [Google Scholar]
- 49.Milucka J, Ferdelman TG, Polerecky L, Franzke D, Wegener G, Schmid M, et al.. Zero-valent sulphur is a key intermediate in marine methane oxidation. Nature 2012; 491:541-6; PMID:23135396; http://dx.doi.org/ 10.1038/nature11656 [DOI] [PubMed] [Google Scholar]
- 50.Hanzelka BL, Greenberg EP. Quorum sensing in Vibrio fischeri: evidence that S-adenosylmethionine is the amino acid substrate for autoinducer synthesis. J Bacteriol 1996; 178:5291-4; PMID:8752350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaefer AL, Hanzelka BL, Eberhard A, Greenberg EP. Quorum sensing in Vibrio fischeri: probing autoinducer-LuxR interactions with autoinducer analogs. J Bacteriol 1996; 178:2897-901; PMID:8631679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schaefer AL, Val DL, Hanzelka BL, Cronan JE Jr., Greenberg EP. Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc Natl Acad Sci U S A 1996; 93:9505-9; PMID:8790360; http://dx.doi.org/ 10.1073/pnas.93.18.9505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Das G, Thotala DK, Kapoor S, Karunanithi S, Thakur SS, Singh NS, et al.. Role of 16S ribosomal RNA methylations in translation initiation in Escherichia coli. EMBO J 2008; 27:840-51; PMID:18288206; http://dx.doi.org/ 10.1038/emboj.2008.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maitra A, Dill KA. Bacterial growth laws reflect the evolutionary importance of energy efficiency. Proc Natl Acad Sci U S A 2015; 112:406-11; PMID:25548180; http://dx.doi.org/ 10.1073/pnas.1421138111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yona AH, Bloom-Ackermann Z, Frumkin I, Hanson-Smith V, Charpak-Amikam Y, Feng Q, et al.. tRNA genes rapidly change in evolution to meet novel translational demands. eLife 2013; 2:e01339; PMID:24363105; http://dx.doi.org/ 10.7554/eLife.01339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brooks DJ, Fresco JR, Lesk AM, Singh M. Evolution of amino acid frequencies in proteins over deep time: inferred order of introduction of amino acids into the genetic code. Mol Biol Evol 2002; 19:1645-55; PMID:12270892; http://dx.doi.org/ 10.1093/oxfordjournals.molbev.a003988 [DOI] [PubMed] [Google Scholar]
- 57.Varshney U, Lee CP, Seong BL, RajBhandary UL. Mutants of initiator tRNA that function both as initiators and elongators. J Biol Chem 1991; 266:18018-24; PMID:1917940 [PubMed] [Google Scholar]
- 58.Varshney U, RajBhandary UL. Initiation of protein synthesis from a termination codon. Proc Natl Acad Sci U S A 1990; 87:1586-90; PMID:2406724; http://dx.doi.org/ 10.1073/pnas.87.4.1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.RajBhandary UL. More surprises in translation: initiation without the initiator tRNA. Proc Natl Acad Sci U S A 2000; 97:1325-7; PMID:10677458; http://dx.doi.org/ 10.1073/pnas.040579197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waugh DS. Genetic tools for selective labeling of proteins with α-15N-amino acids. J Biomol NMR 1996; 8:184-92; PMID:8914274; http://dx.doi.org/ 10.1007/BF00211164 [DOI] [PubMed] [Google Scholar]
- 61.Pavon-Eternod M, Gomes S, Rosner MR, Pan T. Overexpression of initiator methionine tRNA leads to global reprogramming of tRNA expression and increased proliferation in human epithelial cells. Rna 2013; 19:461-6; PMID:23431330; http://dx.doi.org/ 10.1261/rna.037507.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Juhling F, Morl M, Hartmann RK, Sprinzl M, Stadler PF, Putz J. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res 2009; 37:D159-62; PMID:18957446; http://dx.doi.org/ 10.1093/nar/gkn772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilm A, Higgins DG, Notredame C. R-Coffee: a method for multiple alignment of non-coding RNA. Nucleic Acids Res 2008; 36:e52; PMID:18420654; http://dx.doi.org/ 10.1093/nar/gkn174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gouy M, Guindon S, Gascuel O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 2010; 27:221-4; PMID:19854763; http://dx.doi.org/ 10.1093/molbev/msp259 [DOI] [PubMed] [Google Scholar]
- 65.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res 2004; 14:1188-90; PMID:15173120; http://dx.doi.org/ 10.1101/gr.849004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al.. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 2004; 25:1605-12; PMID:15264254; http://dx.doi.org/ 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- 67.House JE. Inorganic chemistry. Elsevier, 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.