ABSTRACT
Cell polarity is crucial to development since apico-basal polarity conferred by the 3 polarity protein modules (or complexes) is essential during embryogenesis, namely the Par (partition defective)-, the CRB (Crumbs)-, and the Scribble-based polarity protein modules. While these protein complexes and their component proteins have been extensively studied in Drosophila and C. elegans and also other mammalian tissues and/or cells, their presence and physiological significance in the testis remain unexplored until the first paper on the Par-based protein published in 2008. Since then, the Par-, the Scribble- and the CRB-based protein complexes and their component proteins in the testis have been studied. These proteins are known to confer Sertoli and spermatid polarity in the seminiferous epithelium, and they are also integrated components of the tight junction (TJ) and the basal ectoplasmic specialization (ES) at the Sertoli cell-cell interface near the basement membrane, which in turn constitute the blood-testis barrier (BTB). These proteins are also found at the apical ES at the Sertoli-spermatid interface. Thus, these polarity proteins also play a significant role in regulating Sertoli and spermatid adhesion in the testis through their actions on actin-based cytoskeletal function. Recent studies have shown that these polarity proteins are having antagonistic effects on the BTB integrity in which the Par6- and CRB3-based polarity complexes promotes the integrity of the Sertoli cell TJ-permeability barrier, whereas the Scribble-based complex promotes restructuring/remodeling of the Sertoli TJ-barrier function. Herein, we carefully evaluate these findings and provide a hypothetic model regarding their role in the testis in the context of the functions of these polarity proteins in other epithelia, so that better experiments can be designed in future studies to explore their significance in spermatogenesis.
KEYWORDS: cell polarity, germ cells, PCP proteins, planar cell polarity, polarity protein complexes, seminiferous epithelial cycle, seminiferous tubule, Sertoli cells, spermatids, testis
Introduction
Polarity protein complexes including Par (partition defective)-, CRB (Crumbs)-, and Scribble-based protein modules are crucial to confer apico-basal (AB) polarity during embryogenesis and development; and they are also essential to cell polarity and regulating tissue and/or cell function in adult animals including spermatogenesis in mammals (for reviews, see1-4). While these proteins were initially found in Drosophila and/or C. elegans, they are well conserved across species and their homologs have since been identified and found to play similar roles in mammals. Also, studies have shown that their mutations in humans or rodents lead to different diseases and pathological conditions and they are critically involved in tumorigenesis and/or metastasis (for reviews, see5-8). The core components of the Par-based protein module are Par3, Par6, atypical protein kinase C (aPKC) and Cdc42, and the CRB-based module are CRB1, 2 or 3, Protein associated with Lin-7 1 (PALS1) and PALS1-associated tight junction protein (PATJ). These two polarity complexes are usually localized adjacent to one another near the apical region of a cell epithelium at or close to the tight junction (TJ), and these 2 protein modules are mutually exclusive regarding their spatial localization and/or functionality in reference to the basally localized Scribble protein complex comprised of Scribble, Lethal giant larvae (Lgl) and Discs large (Dlg) (for reviews, see1-3,9). The structures of Par-, CRB- and Scribble-based polarity proteins are shown in Figure 1. Since there are numerous partner proteins that can be recruited to these protein complexes, each polarity protein module can thus create a huge protein complex and due to their cellular spatial differences in localization such as between Par-/CRB-based complexes and Scribble-based complex, this thus creates apico-basal polarity during development and also confers cell polarity in adult epithelial cells and tissues. In the rat testis, all 3 polarity protein modules known to confer cell polarity in Drosophila and C. elegans including the Par-, Scribble- and CRB-based complexes including their corresponding component proteins, such as the Par3/Par6/aPKC/Cdc42 complex,10-12 the Scribble/Lgl2/Dlg1 complex,13 and CRB3/PATJ/PALS110,14 have all been identified, and their functional significance in spermatogenesis such as by conferring spermatid polarity have been studied. These earlier studies have shown that polarity proteins, besides conferring Sertoli cell and spermatid polarity, are involved in endocytic vesicle-mediated protein trafficking,10-12 actin microfilament organization at the ectoplasmic specialization (ES, an actin-rich, testis-specific adherens junction (AJ) which can be found in the apical compartment at the Sertoli-spermatid interface called apical ES vs. the basal ES restricted to the Sertoli cell-cell interface at the blood-testis barrier (BTB) near the basal compartment),13,14 thereby playing a crucial role in conferring spermatid and Sertoli cell adhesion, as well as spermatid transport during the epithelial cycle of spermatogenesis.
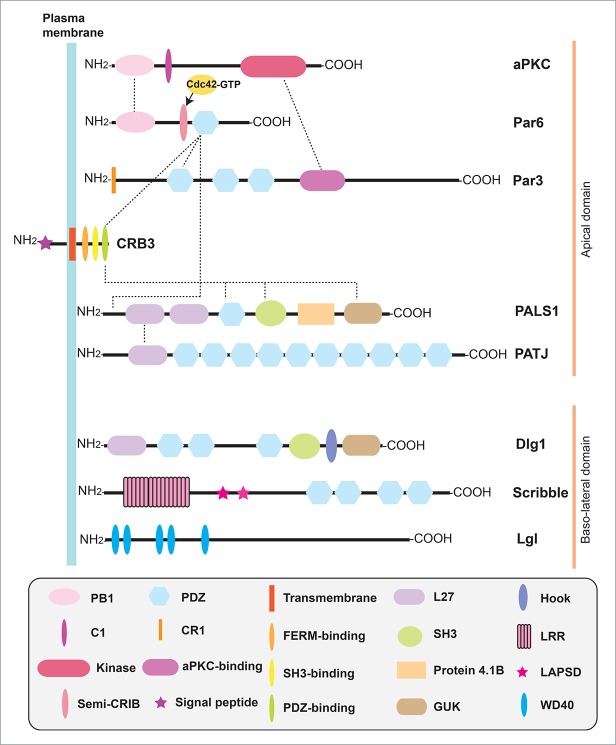
Figure 1.
A schematic drawing that illustrates some common functional domains in component proteins of the Par-, CRB- and Scribble-based polarity protein complexes. Based on studies in the testis, the Par-based polarity protein complex is constituted by Par6, Par3, aPKC and Cdc42-GTP, whereas the CRB3-based polarity complex is composed of CRB3, PALS1 and PATJ. Furthermore, there are extensive interactions between Par- and CRB3-based protein complexes via the corresponding protein domains. The Scribble-based polarity complex consists of Scribble, Lgl2 and Dlg1. Abbreviations used: Par, partitioning defective; aPKC, atypical protein kinase C; PB1 domain, Phox and Bem1 domain; C1 domain, protein kinase C conserved region 1; Kinase, kinase domain; Semi-CRIB, semi-Cdc42/Rac interactive binding motif; PDZ domain, PSD-95/Discs large/ZO-1 (PDZ) domain; CR1, conserved region 1. CRB, Crumbs; PALS1, protein associated with Lin-7 1; PATJ, PALS1 associated tight junction protein; FERM-binding domain, protein 4.1/ezrin/radixin/moesin-binding domain; L27 domain, Lin2 and Lin7 binding domain; SH3 domain, Src homology domain 3 domain; GUK domain, Guanylate kinase domain; Dlg, Discs large; Lgl, Lethal giant larvae; LRR repeats, leucine-rich repeats; LAPSD, LRR and PDZ specific domain. WD40, tryptophan-aspartic acid (WD) 40 repeats.
Other studies have shown that besides serving as polarity proteins, Scribble such as Scrib 1 is also a planar cell polarity (PCP) protein, which is working in concert with Lgl2 and other PCP proteins (e.g., Vangl2) to confer PCP.15,16 PCP, in particular the highly conserved non-canonical Wnt-Frizzled-Dishevelled PCP signaling, unlike cell polarity which confers apico-basal polarity during development, is crucial to convergent extension (CE) during embryonic development in which the tissue narrows (converge) along an axis concomitant with elongation (extension) along a perpendicular axis due to polarized cell movement to generate the anteroposterior axis.17,18 PCP proteins are also involved in the development of the nervous system (e.g., neural tube formation),19,20 the kidney,21 hair cells of the inner ear,22,23 skin epidermis,24 female reproductive tract,25 and the heart.26 PCP proteins are also involved in endocytic vesicle-mediated protein trafficking events,27 and cancer metastasis.28 In adults, PCP is known to maintain directional alignment and polarization of a field of cells within the plane of a cell epithelium, most notably in conferring PCP to the hair of wing hair cells in Drosophila, and also hair cells of the cochlear (inner ear) in mammals.22,23,29 Interestingly, PCP is less studied in adult animals under normal physiological conditions except for a report illustrating its role in hair alignment in adult mice.30 However, multiple diseases are associated with mutation and deletion of different PCP genes in humans, such as neural tube defects,31 tumorigenesis32 and metastasis,33 and skin disease.24 In the testis, the role of PCP remains virtually unexplored since no reports are found in the literature unraveling the role of PCP proteins in spermatogenesis. Yet the unique spatial alignment of polarized elongating/elongated spermatids in the plane of seminiferous epithelium during spermiogenesis in particular at stages VII-VIII of the epithelial cycle implicates the likely role of PCP proteins packaging the maximal number of developing spermatids in seminiferous tubules to support daily production of millions of sperm from an adult male.
Based on these earlier studies, we critically evaluate findings in the field herein particularly the Par-, Scribble- and CRB-based cell polarity proteins, putting forth a concept that the actin-based cytoskeleton is one of the ultrastructures in Sertoli cells which serves as the target regulated by the concerted efforts of 3 polarity protein complexes.
Why cell polarity is important during spermatogenesis?
To provide an orderly arrangement of germ cells and to maintain the proper Sertoli:germ cell ratio in the limited space of seminiferous epithelium to meet the sperm output during spermatogenesis. The daily sperm production is about 8, 70, and 200 million spermatozoa per pair testes in mouse, rat and human, respectively,34-36 indicating that an enormous number of developing spermatids are packed in the seminiferous epithelium of the testes under development to sustain the sperm output during the epithelial cycle of spermatogenesis.37,38 Thus, spermatids must be properly arranged and aligned in the limited space of the seminiferous epithelium in which their heads are pointed toward the basement membrane and their tails to the tubule lumen, so that they can be supported and nurtured by the fixed number of Sertoli cells at about 434, 30–4039-41 and 300–50042 million Sertoli cells per testis in the mouse, rat, and human, respectively. As it is known that Sertoli cells cease to divide but terminally differentiated after puberty by 15–17 dpp (day postpartum) in rodents vs. ∼12-year-old in humans, unless Sertoli cells are exposed to serum proteins such as fetal calf serum (5–10%) when cultured in vitro.43-45 The presence of serum proteins in culture medium can de-differentiate these Sertoli cells, rendering them capable of mitotic proliferation. Morphometric analysis in the rat testis has shown that each Sertoli cell can nurture the development of ∼30–50 germ cells46 simultaneously during all stages of germ cell development in the seminiferous epithelium throughout the epithelial cycle to sustain spermatogenesis following puberty.
To provide an orderly arrangement of cell organelles in Sertoli vs. germ cells in the seminiferous epithelium. Besides specific transporters, such as MCT2 (monocarboxylate transporter 2) that transports lactate from Sertoli to developing germ cells in particular spermatids for provision of energy for development (for a review, see47), the intimate contact between Sertoli cells and developing germ cells and the corresponding cellular domain compartmentalization conferred by polarity proteins are also necessary to ensure proper, timely and efficient signal or chemical communications at the Sertoli-Sertoli, germ-germ and also Sertoli-germ interface via gap junction (GJ),48,49 intercellular bridges,50 also known as tunneling nanotubes (TNTs)51,52 and ES53-56 to coordinate and/or synchronize different cellular events across the seminiferous epithelium during spermatogenesis. For instance, it is noted that elongating/elongated spermatids in the adluminal (apical) compartment during spermiogenesis are metabolically quiescent cells vs. undifferentiated spermatogonia, type A and B spermatogonia and spermatocytes in the basal compartment. As such, the precise spatial arrangements between Sertoli and germ cells in particular post-meiotic spermatids are physiologically important to sustain spermatogenesis, illustrating the significance of polarity proteins that confer cell alignment and compartmentalization of cell organelles in Sertoli and germ cells in the seminiferous epithelium to sustain spermatogenesis. Besides the obvious polarized alignment of developing spermatids in the epithelium with their heads pointing toward the basement membrane and their elongating tails toward the tubule lumen, plus the polarized development of acrosome, packaging of genetic material in the spermatid head, and elongation of the tail; organelles in the Sertoli cell are also highly polarized. For instance, Sertoli cell nucleus that serves as the command center for various cell functions is localized at the base of the Sertoli cell near the basement membrane in the tunica propria. Furthermore, many of the protein/glycoprotein synthesis ultrastructures such as Golgi apparatus for de novo protein synthesis, and phagosomes destined to undergo intracellular degradation are also found near the basement membrane. On the other hand, residual bodies to be engulfed by the Sertoli cell at late stage VIII when the release of spermatozoa takes place at spermiation57,58 are located in the adluminal (apical) compartment near the tubule lumen, which must be transported to the basal compartment for degradation as phagosomes at stage IX.59 This compartmentalization conferred by polarity protein is necessary to maintain efficacy for cell-cell communication to sustain rapid germ cell development throughout the epithelial cycle.
To maintain polarized cell junction ultrastructures and the underlying polarized cytoskeletal elements to facilitate spermatid and organelle (e.g., phagosome) transport across the epithelium. Besides polarized arrangement of cellular organelles as noted above, various cell junction types are also polarized ultrastructures in the seminiferous epithelium. For instance, tight junction (TJ), and basal ES are co-existing ultrastructures and restrictively confined to the BTB which physically divides the seminiferous epithelium into the apical (adluminal) and basal compartments. On the other hand, desmosome and GJ are also integrated components of the BTB at the Sertoli cell-cell interface. Interestingly, desmosome and GJ are also found at the Sertoli-germ cell (including spermatogonia, spermatocytes and step 1–7 spermatids) interface. It is noted that once apical ES appears, it is the only anchoring device that replaces GJ and desmosome at the Sertoli-spermatid (step 8–19) interface in the adluminal compartment of the rat testis (for reviews, see53,55,60,61). Besides serving as an anchoring device, apical ES also confers spermatid polarity and to facilitate spermatid transport. It is noted that apical ES, similar to basal ES, is an actin-rich ultrastructure, typified by the presence of actin microfilament bundles that are sandwiched perpendicular to the Sertoli cell plasma membrane and sandwiched in-between cisternae of endoplasmic reticulum and the Sertoli cell plasma membrane.53,62 However, studies have shown that the apical ES found in the adluminal compartment is also constituted by proteins of the TJ (e.g., CAR, ZO-1), GJ (e.g., connexin 43), focal adhesion complex (FAC or focal contact, e.g., p-FAK-Tyr397, p-FAK-Tyr407), illustrating ES is a hybrid atypical AJ, possibly it can execute functions conferred by TJ, GJ and FAC (for reviews, see54,56,63). Interestingly, it is also noted that integral membrane proteins of TJ (e.g., CAR, ZO-1), ES (e.g., E-cadherin, ß-catenin), and FAC (e.g., ß1-integrin) at the apical ES utilize F-actin for attachment. Studies in the rat testis have shown that polarized proteins, such as Par6, are integrated components of the apical and basal ES, associated with PALS1, PATJ, and JAM-C,10 whereas CRB3 is restrictively expressed at the basal ES/BTB but not the apical ES.14 However, some prominent CRB3 staining is also detected as “stalk-like” structures in the adluminal compartment in stages I-VII tubules, but not in stage VIII-XIV tubules.14 Interestingly, PATJ, a component of the CRB3-based polarity complex, is prominently expressed at the apical ES, besides the basal ES, in stage VII tubules but considerably diminished at stage VIII.14 These findings thus illustrate that there are structural interactions between the Par- and CRB3-based polarity complexes to maintain ES function in the seminiferous epithelium of rat testes. On the other hand, Scribble is restrictively expressed at the basal ES/BTB in all stages of the epithelial cycle but most prominently in stages VII-VIII tubules.13 Collectively, these findings illustrate the significance of polarity proteins in conferring polarized and restrictively expression of junction proteins in the seminiferous epithelium during the epithelial cycle of spermatogenesis.
Polarized cytoskeletal elements
It is of interest to note that both apical and basal ES are localized in close proximity with the polarized actin microfilaments and microtubules (MT), which in turn serve as the track and also to provide the energy (through motor proteins such as myosin VII in F-actin and dynein and kinesin in MT) (for reviews, see64-68) to assist the transport of developing spermatids across the seminiferous epithelium, so that fully developed spermatids (i.e., spermatozoa) can line up near the tubule lumen for their release at spermiation at stage VIII of the epithelial cycle. Both actin microfilaments and MT are polarized ultrastructures with their fast growing end (plus (+)-end) in the basal compartment near the basement membrane of the tunica propria, and their slow growing (minus (−)-end) end in the apical compartment near the tubule lumen.58,64-66 It is generally accepted that MTs, probably working in concert with actin microfilaments, serve as the track to facilitate the transport of spermatids and organelles (e.g., residual bodies, phagosomes, endocytic vesicles) across the epithelium.53,66,69 Studies have shown that many polarity proteins, such as Scribble and CRB3 exert their regulatory function via their effects on F-actin organization in Sertoli cells through changes in the spatiotemporal expression of actin binding proteins (ABPs),13,14 such as Arp370 (actin-related protein 3, which together with Arp2 to form the Arp2/3 complex, which when activated by N-WASP upstream induces branched actin polymerization, effectively converting bundled actin microfilaments into a branched configuration) and Eps871 (epidermal growth factor receptor pathway substrate 8, an actin barbed end capping and bundling protein that confers actin microfilaments their bundled configuration at the ES). For instance, knockdown (KD) of CRB3 in Sertoli cells was shown to induce truncation of actin microfilament in cell cytosol, which in turn impeded the localization of TJ (e.g., CAR, ZO-1) and basal ES (e.g., N-cadherin, ß-catenin) at the Sertoli cell-cell interface, thereby destabilizing the Sertoli cell TJ-barrier function.14 In contrast, triple KD of Scribble and its component proteins Lgl2 and Dlg1 induced re-organization of actin microfilaments in Sertoli cells in which actin filaments were localized more concentrated at the cell cortical zone near the cell-cell interface,13 thereby strengthening the basal ES, promoting the TJ-permeability barrier function, making the TJ-barrier tighter.13 In short, these 2 polarity complexes have distinctive but contrasting effects on the organization of polarized actin microfilaments, illustrating their concerted efforts but antagonistic effects on the Sertoli cell TJ-barrier are important to modulate the rapid conversion of actin microfilaments between their branched/unbundled and bundled configuration. This thus confers plasticity to the actin microfilaments so that the F-actin network can be rapidly altered in response to changes of the epithelial cycle to sustain spermatogenesis.
Scribble- vs. CRB3- and Par6-based polarity proteins have antagonistic effects on the Sertoli cell TJ-permeability barrier function through their differential effects on actin microfilament organization at the BTB
Sertoli cells cultured in vitro with an established TJ-permeability barrier that mimics the Sertoli cell BTB in vivo have been used to examine the consequence of Scribble KD on the barrier function. A triple KD of Scribble and its 2 component proteins Dlg1 and Lgl2 in Sertoli cells by RNAi using specific siRNA duplexes (vs. non-targeting negative control siRNA duplexes as controls) without any apparent off-target effects leads to a tightening of the TJ-barrier function by promoting better organization of actin microfilaments at the Sertoli cell-cell interface.13 This thus strengthens the F-actin filament bundles at the cell cortical zone to support better basal ES/BTB function. This also facilitates better distribution of TJ proteins (e.g., occludin, ZO-1), basal ES proteins (e.g., ß-catenin) at the Sertoli cell-cell interface to promote the Sertoli cell TJ-barrier function.13 More important, the findings that a triple KD of Scribble, Dlg1 and Lgl2 that promotes the Sertoli cell TJ-barrier function are reproduced in vivo when Scribble, Dlg1 and Lgl2 are silenced in the testis. Since their simultaneously KD leads to recruitment of more TJ integral membrane protein occludin at the BTB through better organization of F-actin at the site in which F-actin forms an almost undisrupted belt surrounding the base of the seminiferous epithelium, consistent with their localization at the BTB.13 In short, a KD of the Scribble/Dlg1/Lgl2 complex by RNAi promotes the Sertoli TJ-barrier, illustrating the expression of Scribble-based protein complex at the basal ES/BTB in normal testes can likely induce de-stabilization of the Sertoli cell BTB integrity. On the other hand, a KD of CRB3 perturbs the Sertoli cell TJ-permeability barrier by causing mis-localization of TJ- (e.g., CAR, ZO-1) and basal ES- (e.g., N-cadherin, ß-catenin) proteins at the cell-cell interface, in which these proteins no longer tightly associates with the cell cortical zone but rapidly internalizes into the cell cytosol, thereby destabilizing the TJ-barrier.14 Additionally, these changes are mediated through a re-organization of actin microfilaments across the Sertoli cell cytosol in which actin filaments are grossly truncated following CRB3 KD in Sertoli cells.14 As such, the disrupted actin filament bundles no longer support proper localization of TJ- and basal ES-proteins at the BTB since these proteins utilize actin for their attachment (for a review, see61). More important, these findings in vitro are also reproduced in studies in vivo since CRB3 KD in the testis also leads to a failure in retaining basal ES proteins N-cadherin and ß-catenin at the BTB through a re-organization of F-actin which no longer highly expressed at the BTB to support the proper localization of TJ- and basal ES-proteins at the BTB.14 These phenotypes are also similar to the KD of either Par6 or Par3 in the Sertoli cell epithelium with an established functional TJ-permeability barrier in vitro in which TJ proteins JAM-A and ZO-1 as well as basal ES proteins N-cadherin and α-catenin no longer tightly localized at the Sertoli cell-cell interface, but internalized into the cell cytosol, the result of a disruption of the underlying actin cytoskeleton.10 The conclusion that Par3 or Par6 plays a role in regulating actin-based cytoskeleton is also based on the use of the adjudin model since defects in spermatid polarity (and spermatid adhesion) following adjudin treatment is associated with extensive defragmentation of actin microfilaments as well as considerable down-regulation on the expression and/or localization of Par6 at the apical ES.10 Thus, KD of the CRB3 or Par6 (or Par3) by RNAi perturbs the Sertoli cell TJ-barrier, illustrating their expression at the basal ES in normal testes is being used to promote BTB integrity. Collectively, these findings illustrate the CRB3- and Par3/6-based vs. the Scribble-based polarity complexes have antagonistic effects on the Sertoli BTB function in which the CRB3- and Par3/6-complexes promote the tightening whereas the Scribble complex promotes the disruption of the TJ-barrier. This conclusion is further supported by the differential spatiotemporal expression of the CRB3 and Par6 vs. the Scribble at the BTB during the epithelial cycle based on studies using immunohistochemistry (IHC) and immunofluorescence microscopy. For instance, it was reported that Par610 and CRB314 were expressed at the basal ES/BTB in the seminiferous epithelium since these proteins were noted to promote BTB integrity based on KD studies,10,14 however, their expression was considerably diminished at stage VIII concomitant with the remodeling/restructuring of the BTB at this stage to facilitate the transport of preleptotene spermatocytes across the immunological barrier. This pattern is in sharp contrast to the stage-specific expression of Scribble at the BTB when Scribble expression at the basal ES/BTB was at its highest at stages VII-VIII at the time of BTB remodeling/restructuring13 since the KD of Scribble and its component proteins Lgl2 and Dlg1 was found to promote BTB integrity.13 In short, it is likely that through the restrictive spatiotemporal expression of CRB3/Par6 vs. Scribble at the BTB that modulates the F-actin organization, new BTB above or below the preleptotene spermatocytes under transport at the site can be assembled or disassembled, respectively, providing a unique mechanism to modulate efficient closing or opening of the BTB. Based on these findings, we propose a model shown in Figure 2 that depicts the antagonistic effects of the CRB3- and Par3/6- vs. the Scribble-based polarity complexes to regulate BTB restructuring in which the CRB3- and the Par3/6-based protein complexes promote, whereas the Scribble/Lgl2/Dlg1 perturbs the Sertoli cell TJ-barrier function. This thus provides an efficient and novel mechanism to streamline the transport of preleptotene spermatocytes across the BTB during the epithelial cycle, possibility via stage-specific and spatiotemporal expression of these polarity proteins along the microdomains of the BTB depicted in Figure 2.
Figure 2.
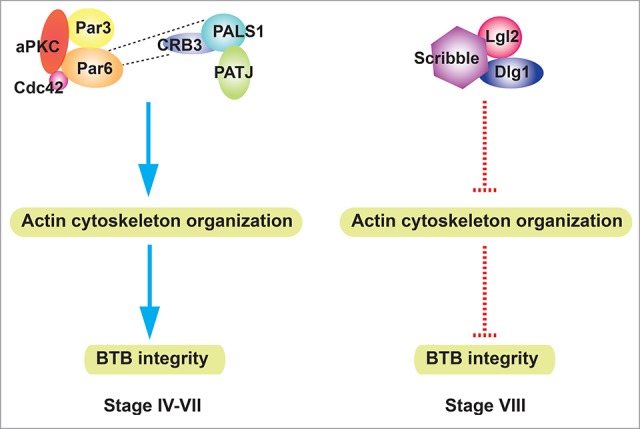
A schematic model that illustrates the antagonistic effect of CRB/Par-based polarity complex vs. Scribble-based polarity complex at the BTB. Par- and CRB3-based polarity complexes are highly expressed at the BTB from stages IV-VII. These two complexes directly interact with each other, and they both stabilize actin network and promote BTB integrity. At stage VIII, Par- and CRB3-based complexes are diminished at the BTB, whereas Scribble-based complex is highly expressed. Scribble complex disrupts actin cytoskeleton organization, thereby disrupting BTB integrity. Blue arrow, stabilization/promotion; red dash line, inhibition/disruption.
Interplay of CRB3-, Par-, and Scribble-based polarity proteins to regulate spermatid transport through changes in the organization of actin microfilaments at the apical ES
Since CRB3, Scribble/Lgl2/Dlg1 and Par6 exerts one of their regulatory effects through actin microfilament organization at the basal ES/BTB, it is envisioned that actin microfilaments at the apical ES restrictively expressed at the Sertoli-spermatid (step 8-19) interface is also one of the targets of polarity protein complexes since the ultrastructural features of the basal and apical ES are similar. Indeed, following a KD of CRB3 in the testis in vivo using Polyplus in vivo-jetPEI as a transfection medium with high transfection efficiency, F-actin organization at the apical ES was disrupted since F-actin no longer conspicuously detected in the epithelium vs. control testes transfected with the non-targeting negative control siRNA duplexes.14 These findings are also consistent with the data that rats treated with adjudin to induce defects in spermatid polarity via considerable downregulation of Par6 is mediated through defragmentation of actin microfilaments at the apical ES in vivo,10 illustrating CRB3 and Par6 are also playing similar role in maintaining the integrity of the apical ES through their action on the organization of actin microfilaments in the testis in vivo. These changes were shown to be the result of changes in the spatiotemporal expression of Arp3 and Eps8 in which these actin binding proteins no longer tightly associated with the apical ES but either considerably down-regulated or diffused away from the apical ES.14 As such, adhesion proteins ß1-integrin (a Sertoli cell-specific apical ES protein) and laminin-γ3 chain (a spermatid-specific apical ES protein) at the apical ES no longer tightly attached to the spermatid head to support spermatid anchorage following CRB3 KD in the testis,14 as these apical ES proteins utilize F-actin for their attachment.61 This thus de-stabilized the apical ES, and elongated spermatids were shown to undergo premature spermiation, since step 18 spermatids at stage VI and step 19 spermatids at stage VII were being released into the tubule lumen, reducing the frequency of stage VII tubules but more stage VIII tubules14 as stages VII and VIII tubules are mostly distinguished based on the relative location of polarized spermatids.37,72 Due to the defects of actin microfilament organization, the transport of phagosomes and spermatids were also grossly affected following CRB3 KD in the testis.14 Thus, while elongated spermatids found near the tubule lumen at stage VII tubules were induced to undergo spermiation, those spermatids embedded inside the epithelium at stage VI-VII failed to be transported to the tubule lumen, and they remained clearly visible inside the epithelium in stage IX to XII tubules.14 Furthermore, phagosomes remained to be seen near the tubule lumen even in stage IX tubules14 when they should have been transported to the base of the epithelium for degradation via the lysosomal pathway.59 While CRB3- and Par6- vs. Scribble-based polarity proteins have antagonistic effects on the BTB function, the triple Scribble/Lgl2/Dlg1 KD in the testis was also found to perturb apical ES integrity,13 similar to CRB3 KD in the testis14 (or Par6 down-regulation induced by adjudin treatment10), illustrating actin microfilaments at the basal vs. the apical ES respond differently following the knockdown of either one of the 3 polarity protein complexes. Collectively, these findings illustrate that actin microfilaments at the apical ES could be regulated via different signaling pathways vs. the basal ES. This postulate is indeed supported by the observations that p-FAK-Tyr397 is exclusively expressed at the apical ES to regulate spermatid adhesion and polarity73,74 whereas p-FAK-Tyr40774 is expressed both at the apical and basal ES/BTB stage-specifically but more important to the basal ES function at the BTB based on studies using phosphomimetic mutants.74 For instance, overexpression of p-FAK-Y407E, a constitutively active p-FAK-Tyr407 phosphomimetic mutant, or p-FAK-Y397F, a constitutively inactive p-FAK-Tyr397 non-phosphorylatable mutant, in Sertoli cell epithelium was both found to promote the Sertoli cell TJ-barrier function, making it tighter74; whereas overexpression of p-FAK-Y407F, a constitutively inactive p-FAK-Tyr407 non-phosphorylatable mutant perturbed the Sertoli TJ function.74 On the other hand, overexpression of p-FAK-Y397E, a constitutively active p-FAK-Tyr397 phosphomimetic mutant, in adult rat testes in vivo was found to promote F-actin at the apical ES by maintaining the organization of actin microfilaments at the site, thereby delaying degeneration of adhesion protein complexes, retaining elongated spermatids in the epithelium75. These findings thus support the notion that different signaling molecules/pathways are involved in regulating actin microfilament organization at the apical vs. the basal ES.
Concluding remarks and future perspectives
As reviewed herein, it took almost a decade since the late 2000s to unfold some of the function of the Par-, the Scribble- and the CRB-based polarity protein complexes in the mammalian testis. Based on these findings, an interesting concept is emerging as noted above that the Par6- and the CRB3-based polarity modules are working in concert to maintain and promote the Sertoli cell TJ-permeability barrier function (i.e., making the TJ-barrier tighter), whereas the Scribble-based complex is to promote BTB remodeling/restructuring (i.e., making the barrier leaky) during the epithelial cycle through their action on the organization of the actin microfilaments at the basal ES/BTB. Due to the antagonistic effects of the Par6-/CRB3- and the Scribble-based polarity complexes, their combined actions thus fine-tunes the dynamic restructuring of the actin microfilaments at the basal ES/BTB to support the transport of preleptotene spermatocytes, and plausibly other necessary biomolecules and/or hormones, across the immunological barrier. For instance, proteins (e.g., occludin, JAM-A, claudin 11, ZO-1) at the “old” BTB located above the preleptotene spermatocytes connected in clones via intercellular bridges under transport at the BTB can be efficiently recycled for the assembly of the “new” BTB located behind the spermatocytes. Their synchronized and concerted efforts not just streamline the transport of preleptotene spermatocytes across the BTB as summarized in Figure 2, they also maintain the integrity of the BTB during this series of cellular events since the immunological barrier must be fully functional and maintained at all stages of the epithelial cycle. However, much work is needed in future studies to better understand the precise molecular mechanism(s) by which these polarity proteins regulate ES function. These include the identification of the downstream signaling molecules and/or pathways utilized by these polarity protein complexes. For instance, are p-FAK-Tyr397 and p-FAK-Try407 being used in concert with c-Src and c-Yes as the molecular switches by these polarity proteins? Are mTORC176,77 and mTORC278 involved in the polarity protein-mediated BTB restructuring since these 2 signaling complexes are also shown to have antagonistic effects on the BTB function in the testis? Since the adaptor proteins in these polarity complexes such as PALS1 and PATJ, can recruit other protein partners including signaling protein kinases to create a giant protein complex to serve as the platform for signal transduction, it is anticipated that many of these apparently unrelated signaling proteins are physiologically linked to work in concert to modulate BTB, polarity and adhesion function in the seminiferous epithelium. More important, what is the role of PCP in these events? Are PCP proteins working in concert with polarity proteins or independently? It is likely that many of these questions will be answered in the coming decade.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the National Institutes of Health (NICHD, R01 HD056034, to C.Y.C.; U54 HD029990 Project 5, to C.Y.C.)
References
- [1].Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta 2008; 1778:614-30; PMID:18005931 [DOI] [PubMed] [Google Scholar]
- [2].Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nature Rev Mol Cell Biol 2008; 9:846-59; PMID:18946474; http://dx.doi.org/ 10.1038/nrm2521 [DOI] [PubMed] [Google Scholar]
- [3].Wong EWP, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol 2009; 278:309-53; PMID:19815182; http://dx.doi.org/ 10.1016/S1937-6448(09)78007-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yeaman C, Grindstaf K, Nelson W. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev 1999; 79:73-98; PMID:9922368 [DOI] [PubMed] [Google Scholar]
- [5].Ashraf A, Pervaiz S. Hippo circuitry and the redox modulation of hippo components in cancer cell fate decisions. Int J Biochem Cell Biol 2015; 69:20-8; PMID:26456518; http://dx.doi.org/ 10.1016/j.biocel.2015.10.001 [DOI] [PubMed] [Google Scholar]
- [6].Copp AJ, Stanier P, Greene NDE. Neural tube defects: recent advances, unsolved questions, and controversies. The Lancet Neurology 2013; 12:799-810; PMID:23790957; http://dx.doi.org/ 10.1016/S1474-4422(13)70110-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gissen P, Arias IM. Structural and functional hepatocyte polarity and liver disease. J Hepatol 2015; 63:1023-37; PMID:26116792; http://dx.doi.org/ 10.1016/j.jhep.2015.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lin WH, Asmann YW, Anastasiadis PZ. Expression of polarity genes in human cancer. Cancer Informatics 2015; 14(Suppl 3):15-28; PMID:25991909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Su WH, Mruk DD, Wong EWP, Lui WY, Cheng CY. Polarity protein complex Scribble/Lgl/Dlg and epithelial cell barriers. Adv Exp Med Biol 2012; 763:149-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA 2008; 105:9657-62; PMID:18621709; http://dx.doi.org/ 10.1073/pnas.0801527105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wong EWP, Sun S, Li MWM, Lee WM, Cheng CY. 14-3-3 protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology 2009; 150:4713-23; PMID:19608648; http://dx.doi.org/ 10.1210/en.2009-0427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wong EWP, Mruk DD, Lee WM, Cheng CY. Regulation of blood-testis barrier dynamics by TGF-b3 is a Cdc42-dependent protein trafficking event. Proc Natl Acad Sci USA 2010; 107:11399-404; PMID:20534521; http://dx.doi.org/ 10.1073/pnas.1001077107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Su WH, Wong EWP, Mruk DD, Cheng CY. The Scribble/Lgl/Dlg polarity protein complex is a regulator of blood-testis barrier dynamics and spermatid polarity during spermatogenesis. Endocrinology 2012; 153:6041-53; http://dx.doi.org/ 10.1210/en.2012-1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gao Y, Lui WY, Lee WM, Cheng CY. Polarity protein Crumbs homolog-3 (CRB3) regulates ectoplasmic specialization dynamics through its action on F-actin organization in Sertoli cells. Sci Rep 2016; 6:28589 (DOI: 10.1038/srep28589); PMID:27358069; http://dx.doi.org/ 10.1038/srep28589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Montcouguiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 2003; 423:173-7; PMID:12724779; http://dx.doi.org/ 10.1038/nature01618 [DOI] [PubMed] [Google Scholar]
- [16].Kallay LM, McNickle A, Brennwald PJ, Hubbard AL, Bralteman LT. Scribble associates with two polarity proteins, Lgl2 and Vangl2, via distinct molecular domains. J Cell Biochem 2006; 99:647-64; PMID:16791850; http://dx.doi.org/ 10.1002/jcb.20992 [DOI] [PubMed] [Google Scholar]
- [17].Goodrich LV, Strutt D. Principles of planara polarity in animal development. Development 2011; 138:1877-92; PMID:21521735; http://dx.doi.org/ 10.1242/dev.054080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Davenport D. The cell biology of planar cell polarity. J Cell Biol 2014; 207:171-9; PMID:25349257; http://dx.doi.org/ 10.1083/jcb.201408039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tissir F, Goffinet AM. Shaping the nervous system: role of the core planar cell polarity genes. Nat Rev Nruosci 2013; 14:525-35; http://dx.doi.org/ 10.1038/nrn3525 [DOI] [PubMed] [Google Scholar]
- [20].Seo JH, Ziber Y, Babayeva S, Liu J, Kyriakopoulos P, De Marco P, Merello EC, V Gros P, Torban E. Mutations in the planar cell polarity gene, Fuzzy, are associated with neural tube defects in humans. Hum Mol Genet 2011; 20:4324-33; PMID:21840926; http://dx.doi.org/ 10.1093/hmg/ddr359 [DOI] [PubMed] [Google Scholar]
- [21].Carroll TJ, Yu J. The kidney and planar cell polaroity. Curr Top Dev Biol 2012; 101:185-212; PMID:23140630; http://dx.doi.org/ 10.1016/B978-0-12-394592-1.00011-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ezan J, Montcouquiol M. Revisiting planar cell polarity in the inner ear. Semin Cell Dev Biol 2013; 24:499-506; PMID:23562830; http://dx.doi.org/ 10.1016/j.semcdb.2013.03.012 [DOI] [PubMed] [Google Scholar]
- [23].May-Simera H, Kelley MW. Planar cell polarity in the inner ear. Curr Top Dev Biol 2012; 101:111-40; PMID:23140627; http://dx.doi.org/ 10.1016/B978-0-12-394592-1.00006-5 [DOI] [PubMed] [Google Scholar]
- [24].Tellkamp F, Vorhagen S, Niessen CM. Epidermal polarity genes in health and disease. Cold Spring Harb Perspect Biol 2014; 4:a015255; http://dx.doi.org/ 10.1101/cshperspect.a015255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vandenberg LN, Chauhoud IJJH, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Cien Saude Colet 2012; 17:407-34; PMID:22267036; http://dx.doi.org/ 10.1590/S1413-81232012000200015 [DOI] [PubMed] [Google Scholar]
- [26].Henderson DJ, Chaudhry B. Getting to the heart of planar cell polarity signaling. Birth Defects Res A Clin Mol Teratol 2011; 91:460-67; PMID:21538810; http://dx.doi.org/ 10.1002/bdra.20792 [DOI] [PubMed] [Google Scholar]
- [27].Eaton S, Martrin-Belmonte F. Cargo sorting in the endocytic pathway: a key regulator of cell polarity and tissue dynamics. Cold Spring Harb Perspect Biol 2014; 6:a016899; PMID:25125399; http://dx.doi.org/ 10.1101/cshperspect.a016899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Luga V, Wrana JL. Tumor-stroma interaction: Revealing fibroblast-secreted exosomes as potent regulators of Wnt-planar cell polarity signaling in cancer metastasis. Cancer Res 2013; 73:6843-7 [DOI] [PubMed] [Google Scholar]
- [29].Adler PN. The frizxzled/stan pathway wand planar cell polarity in the Drosophila wing. Curr Top Dev Biol 2012; 101:1-31; PMID:23140623; http://dx.doi.org/ 10.1016/B978-0-12-394592-1.00001-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Davenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmeteric mophogenesis of hair folicles. Nat Cell Biol 2008; 10:1257-68; PMID:18849982; http://dx.doi.org/ 10.1038/ncb1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].De Marco P, Merello E, Piatelli G, Cama A, Kibar Z, Capra V. Planar cell polarity gene mutations contribute to the etiology of human neural tube defects in our population. Birth Defects Res A Clin Mol Teratol 2014; 100:633-41; PMID:24838524; http://dx.doi.org/ 10.1002/bdra.23255 [DOI] [PubMed] [Google Scholar]
- [32].Hatakeyama J, Wald JH, Printsev I, Ho HY, Carraway KL 3rd. Vangl1 and Vangl2: planar cell polarity components with a developing role in cancer. Endocr Relat Cancer 2014; 21:R345-56; PMID:24981109; http://dx.doi.org/ 10.1530/ERC-14-0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Luga V, Wrana JL. Tumor-stroma interaction: Revealing fibroblast-secreted exosomes as potent regulators of Wnt-planar cell polarity signaling in cancer metastasis. Cancer Res 2013; 73:6843-7; PMID:24265274; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-1791 [DOI] [PubMed] [Google Scholar]
- [34].Auharek SA, Avelar GF, Lara NLM, Sharpe RM, Franca LR. Sertoli cell numbers and spermatogenic efficency are increased in inducible nitric oxide synthase (iNOS) mutant-mice. Int J Androl 2011; 34:e621-9; PMID:21831234; http://dx.doi.org/ 10.1111/j.1365-2605.2011.01209.x [DOI] [PubMed] [Google Scholar]
- [35].Johnson L, Petty CS, Neaves WB. A comparative study of daily sperm production and testicular composition in humans and rats. Biol Reprod 1980; 22:1233-43; PMID:7417656 [DOI] [PubMed] [Google Scholar]
- [36].Amann RP, Howards SS. Daily spermatozoal production and epididymal spermatozoal reserves of the human male. J Urol 1980; 124:211-5; PMID:6772801 [DOI] [PubMed] [Google Scholar]
- [37].Xiao X, Mruk DD, Wong CKC, Cheng CY. Germ cell transport across the seminiferous epithelium during spermatogenesis. Physiology 2014; 29:286-98; PMID:24985332; http://dx.doi.org/ 10.1152/physiol.00001.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].de Kretser DM, Kerr JB. The cytology of the testis In The Physiology of Reproduction. Vol. 1, 1988; eds. Knobil E, et al.. New York: Raven Press; pp 837-932. [Google Scholar]
- [39].Berndtson WE, Thompson TL. Changing relationships between testis size, Sertoli cell number and spermatogenesis in Sprague-Dawley rats. J Androl 1990; 11:429-35; PMID:2254176 [PubMed] [Google Scholar]
- [40].Wing TY, Christensen AK. Morphometric studies on rat seminiferous tubules. Am J Anat 1982; 165:13-25; PMID:7137056; http://dx.doi.org/ 10.1002/aja.1001650103 [DOI] [PubMed] [Google Scholar]
- [41].Wang ZX, Wreford NG, de Kretser DM. Determination of Sertoli cell numbers in the developing rat testis by sterological methods. Int J Androl 1989; 12:58-64; PMID:2714873; http://dx.doi.org/ 10.1111/j.1365-2605.1989.tb01285.x [DOI] [PubMed] [Google Scholar]
- [42].Johnson L, Zane RS, Petty CS, Neaves WB. Quantification of the human Sertoli cell population: its distribution, relation to germ cell numbers, and age-related decline. Biol Reprod 1984; 31:785-95; PMID:6509142; http://dx.doi.org/ 10.1095/biolreprod31.4.785 [DOI] [PubMed] [Google Scholar]
- [43].Ahmed EA, Barten-van Rijbroek AD, Kal HB, Sadri-Ardekani H, Mizrak SC, van Pelt AM, de Rooij DG. Proliferative activity in vitro and DNA repair indicate that adult mouse and human Sertoli cells are not terminally differentiated, quiescent cells. Biol Reprod 2009; 80:1084-91; PMID:19164176; http://dx.doi.org/ 10.1095/biolreprod.108.071662 [DOI] [PubMed] [Google Scholar]
- [44].Chui K, Trivedi A, Cheng CY, Cherbavaz DB, Dazin PF, Huynh ALT, Mitchell JB, Rabinovich GA, Noble-Haeusslein LJ, John CM. Characterization and functionality of proliferative human Sertoli cells. Cell Transplant 2011; 20:619-35; PMID:21054948; http://dx.doi.org/ 10.3727/096368910X536563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Xiao X, Mruk DD, Tang EI, Wong CKC, Lee WM, John CM, Turek PJ, Silvestrini B, Cheng CY. Environmental toxicants perturb human Serotli cell adhesive function via changes in F-actin organization medicated by actin regulatory proteins. Hum Reprod 2014; 29:1279-91; PMID:24532171; http://dx.doi.org/ 10.1093/humrep/deu011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Weber JE, Russell LD, Wong V, Peterson RN. Three dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli-Sertoli and Sertoli-germ cell relationships. Am J Anat 1983; 167:163-79; PMID:6613902; http://dx.doi.org/ 10.1002/aja.1001670203 [DOI] [PubMed] [Google Scholar]
- [47].Boussouar F, Benahmed M. Lactate and energy metabolism in male germ cells. Trends Endocrinol Metab 2004; 15:345-50; PMID:15350607; http://dx.doi.org/ 10.1016/j.tem.2004.07.003 [DOI] [PubMed] [Google Scholar]
- [48].Pointis G, Gilleron J, Carette D, Segretain D. Physiological and physiopathological aspects of connexins and communicating gap junctions in spermatogenesis. Philos Trans R Soc Lond B Biol Sci 2010; 365:1607-20; PMID:20403873; http://dx.doi.org/ 10.1098/rstb.2009.0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li MWM, Mruk DD, Cheng CY. Gap junctions and blood-tissue barriers. Adv Exp Med Biol 2012; 763:260-80; PMID:23397629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Weber JE, Russell LD. A study of intercellular bridges during spermatogenesis in the rat. Am J Anat 1987; 180:1-24; PMID:3661461; http://dx.doi.org/ 10.1002/aja.1001800102 [DOI] [PubMed] [Google Scholar]
- [51].Tarakanov AO, Goncharova LB. Cell-cell nanotubes: Tunneling through several types of synapses. Commun Integr Biol 2009; 2:359-61; PMID:19721891; http://dx.doi.org/ 10.4161/cib.2.4.8289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gerdes HH, Bukoreshtliev NV, Barroso JFV. Tunneling nanotubes: A new route for the exchange of components between animal cells. FEBS Letts 2007; 581:2194-201; PMID:17433307; http://dx.doi.org/ 10.1016/j.febslet.2007.03.071 [DOI] [PubMed] [Google Scholar]
- [53].Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol 2008; 636:186-211; PMID:19856169; http://dx.doi.org/ 10.1007/978-0-387-09597-4_11 [DOI] [PubMed] [Google Scholar]
- [54].Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem Biophys Acta 2008; 1778:692-708; PMID:18068662; http://dx.doi.org/ 10.1016/j.bbamem.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 2004; 25:747-806; PMID:15466940; http://dx.doi.org/ 10.1210/er.2003-0022 [DOI] [PubMed] [Google Scholar]
- [56].Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Rev Endocrinol 2010; 6:380-95; PMID:20571538; http://dx.doi.org/ 10.1038/nrendo.2010.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis 2011; 1:14-35; PMID:21866274; http://dx.doi.org/ 10.4161/spmg.1.1.14525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cheng CY, Mruk DD. Biochemistry of Sertoli cell/germ cell junctions, germ cell transport, and spermiation in the seminiferous epithelium In Sertoli Cell Biology, 2nd Edition. 2015; Ed. Griswold MD; Amsterdam: Elsevier; pp. 333-83; DOI: http://dx.doi.org/ 10.1016/B978-0-12-417047-6.00012.0. [DOI] [Google Scholar]
- [59].Clermont Y, Morales C, Hermo L. Endocytic activities of Sertoli cells in the rat. Ann NY Acad Sci 1987; 513:1-15; PMID:3328532; http://dx.doi.org/ 10.1111/j.1749-6632.1987.tb24994.x [DOI] [PubMed] [Google Scholar]
- [60].Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol 1985; 94:177-211; PMID:3894273; http://dx.doi.org/ 10.1016/S0074-7696(08)60397-6 [DOI] [PubMed] [Google Scholar]
- [61].Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev 2002; 82:825-74; PMID:12270945; http://dx.doi.org/ 10.1152/physrev.00009.2002 [DOI] [PubMed] [Google Scholar]
- [62].Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev 2012; 64:16-64; PMID:22039149; http://dx.doi.org/ 10.1124/pr.110.002790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Siu MKY, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. BioEssays 2004; 26:978-92; PMID:15351968; http://dx.doi.org/ 10.1002/bies.20082 [DOI] [PubMed] [Google Scholar]
- [64].Tang EI, Mruk DD, Cheng CY. MAP/microtubule affinity-regulating kinases, microtubule dynamics, and spermatogenesis. J Endocrinol 2013; 217:R13-R23; PMID:23449618; http://dx.doi.org/ 10.1530/JOE-12-0586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Tang EI, Mruk DD, Lee WM, Cheng CY. Cell-cell interactions, cell polarity, and the blood-testis barrier In Cell Polarity 1. 2015; Ed. Ebnet K.; Geneva: Springer International Publishing; pp. 303-26; DOI: 10.1007/978-3-319-14463-4_13. [DOI] [Google Scholar]
- [66].O'Donnell L, O'Bryan MK. Microtubules and spermatogenesis. Semin Cell Dev Biol 2014; 30:45-54; PMID:24440897; http://dx.doi.org/ 10.1016/j.semcdb.2014.01.003 [DOI] [PubMed] [Google Scholar]
- [67].O'Donnell L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis 2014; 4:e979623; PMID:26413397; http://dx.doi.org/ 10.4161/21565562.2014.979623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Qian X, Mruk DD, Cheng YH, Tang EI, Han D, Lee WM, Wong EW, Cheng CY. Actin binding proteins, spermatid transport and spermiation. Semin Cell Dev Biol 2014; 30:75-85; PMID:24735648; http://dx.doi.org/ 10.1016/j.semcdb.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lee NPY, Cheng CY. Ectoplasmic specialization, a testis-specific cell-cell actin-based adherens junction type: is this a potential target for male contraceptive development. Human Reprod Update 2004; 10:349-69; PMID:15192055; http://dx.doi.org/ 10.1093/humupd/dmh026 [DOI] [PubMed] [Google Scholar]
- [70].Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA 2010; 107:11411-6; PMID:20534520; http://dx.doi.org/ 10.1073/pnas.1001823107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J 2009; 23:2555-67; PMID:19293393; http://dx.doi.org/ 10.1096/fj.06-070573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev 1982; 3:404-17; PMID:6295753; http://dx.doi.org/ 10.1210/edrv-3-4-404 [DOI] [PubMed] [Google Scholar]
- [73].Siu MKY, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of b1-integrin and focal adhesion complex (FAC)-associated proteins. Endocrinology 2003; 144:2141-63; PMID:12697723; http://dx.doi.org/ 10.1210/en.2002-221035 [DOI] [PubMed] [Google Scholar]
- [74].Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA 2012; 109:12562-7; PMID:22797892; http://dx.doi.org/ 10.1073/pnas.1207606109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wan HT, Mruk DD, Li SYT, Mok KW, Lee WM, Wong CKC, Cheng CY. p-FAK-Tyr397 regulates spermatid adhesion in the rat testis via its effects on F-actin organization at the ectoplasmic specialization. Am J Physiol Endocrinol Metab 2013; 305:E687-99; PMID:23880313; http://dx.doi.org/ 10.1152/ajpendo.00254.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mok KW, Mruk DD, Silvestrini B, Cheng CY. rpS6 regulates blood-testis barrier dynamics by affecting F-actin organization and protein recruitment. Endocrinology 2012; 153:5036-48; PMID:22948214; http://dx.doi.org/ 10.1210/en.2012-1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mok KW, Mruk DD, Cheng CY. rpS6 regulates blood-testis barrier dynamics through Akt-mediated effects on MMP-9. J Cell Sci 2014; 127:4870-82; PMID:25217631; http://dx.doi.org/ 10.1242/jcs.152231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Mok KW, Mruk DD, Lee WM, Cheng CY. Rictor/mTORC2 regulates blood-testis barrier dynamics via its effects on gap junction communications and actin filament network. FASEB J 2013; 27:1137-52; PMID:23288930; http://dx.doi.org/ 10.1096/fj.12-212977 [DOI] [PMC free article] [PubMed] [Google Scholar]